Abstract
Aquaporin 3 (AQP3) is an aquaglyceroporin that transports water and glycerol and is expressed in the epidermis, among other epithelial tissues. We have recently shown that there is an association between this glycerol channel and phospholipase D2 (PLD2) in caveolin-rich membrane microdomains. While PLD2 is able to hydrolyze membrane phospholipids to generate phosphatidic acid, this enzyme also catalyzes, in the presence of primary alcohols, a transphosphatidylation reaction to produce a phosphatidylalcohol. We have proposed that AQP3 associated with PLD2 provides the physiological primary alcohol glycerol to PLD2 for use in the transphosphatidylation reaction to generate phosphatidylglycerol (PG). Further, we have proposed that PG functions as a signaling molecule to mediate early epidermal keratinocyte differentiation, and manipulation of this signaling module inhibits keratinocyte proliferation and enhances differentiation. In contrast, other investigators have suggested a proliferative role for AQP3 in keratinocytes. In addition, AQP3 knockout mice exhibit an epidermal phenotype, characterized by dry skin, decreased elasticity and delayed barrier repair and wound healing, which can be corrected by glycerol but not other humectants. AQP3 levels have also been found to be altered in human skin diseases. In this article the evidence supporting a role for AQP3 in the epidermis will be discussed.
Aquaporins (AQPs), as the name implies, are channels that facilitate the entry of water into cells, and currently 13 family members have been identified to date (AQP0-AQP12). However, there exists a subset of AQPs termed aquaglyceroporins that also transport small neutral molecules such as glycerol ([1] and reviewed in [2, 3]). Aquaporin-3 (AQP3) is a member of this subfamily of aquaglyceroporins, and in fact, studies in the Xenopus laevis oocyte expression system have shown that AQP3 not only has high water permeability but functions efficiently as a glycerol (and to a lesser extent urea) transporter [4-6]. AQP3 protein has been shown to be expressed in epithelial tissues exposed to conditions of possible water loss, including cells of the epidermis, corneal epithelium, respiratory system, gastrointestinal tract, and kidney [7]. In the epidermis AQP3 is abundantly expressed and localized to the basal and spinous layers of the epidermis [8]. Several studies have contributed to the idea that AQP3 is important in regulating epidermal structure and function, as will be discussed in this article.
Evidence in vitro
Keratinocyte proliferation and differentiation are regulated by the extracellular calcium concentration, as was first demonstrated by Yuspa et al. in vitro [9] and perhaps more importantly by Menon and colleagues in vivo [10]. In vitro, calcium is one of the most potent means of stimulating epidermal differentiation, with cells maintaining a basal-like state in low calcium concentrations and expressing differentiation markers upon elevation of the medium calcium level [9, 11]. In vivo, the epidermis shows a gradient of calcium with the lowest concentrations in the basal layer, and highest concentrations in the granular layer [12, 13], where proteins critical for barrier function are produced. In the skin the calcium gradient helps maintain the differentiated function of the epidermis, including the permeability barrier. Disruption of this barrier leads to loss of the calcium gradient followed by increased proliferation and decreased expression of various differentiation markers (loricrin, profilaggrin, and involucrin) [10, 14].
Keratinocyte differentiation induced by elevated extracellular calcium concentrations is tightly linked to a rise in intracellular free calcium levels [15], an effect mediated by the calcium-sensing receptor (CaR) [16-18]. The CaR is a G-protein-coupled receptor for which calcium ions serve as the ligand. After calcium combines with the CaR, phosphatidylinositol 4,5-bisphosphate (PIP2) is hydrolyzed to yield 1,4,5-inositol trisphosphate (IP3) and diacylglycerol (DAG) through phospholipase C (PLC)-β and -γ1 [19, 20]. IP3, which releases calcium from intracellular stores, and DAG, which activates DAG-binding enzymes such as protein kinase C family members, then function as second messengers to mediate keratinocyte differentiation. Keratinocytes have a full-length CaR, but as the cells differentiate, they express an alternatively spliced variant of the CaR (CaRalt), which lacks exon 5 [17], and lose their acute response to calcium [17]. Keratinocytes lacking the CaR also do not respond to extracellular calcium acutely [21], and the epidermis of mice lacking the CaR does not differentiate normally [16].
We have previously shown that AQP3 expression and activity are modulated by extracellular calcium levels [22], an effect that has been observed in some other aquaporin family members (e.g., AQP0 [23] and AQP2 [24]). Thus, elevation of extracellular calcium from a value of 25 μM, which maintains keratinocytes in a proliferative state, to 1 mM calcium, which drives cells to late differentiation, decreases AQP3 mRNA and protein levels and reduces glycerol uptake [22, 25]. On the other hand, a more moderate calcium increase to approximately 100 to 120 μM induces a more physiological program of differentiation, with maximal expression of both early (spinous) and late (granular) differentiation markers, such as keratin 1, involucrin, filaggrin and loricrin [9]. In order to determine the effect of a more natural program of keratinocyte differentiation induced by a more physiological change in extracellular calcium levels, we treated primary mouse keratinocytes for 24 hours with a moderately elevated calcium concentration (125 μM) and isolated light membrane fractions (in which AQP3 resides [25]) by sucrose density ultracentrifugation, as in [25]. As shown in Figure 1, this moderately elevated calcium concentration decreases the protein levels of the lower AQP3 band (unglycosylated AQP3 at ∼28 kDa) but increases levels of the upper band of AQP3 (the ∼40 kDa glycosylated AQP3) [26]. Note that the aquaporins are extensively glycosylated, resulting in the multiple bands observed by western analysis for AQP3 and other aquaporins (e.g., [27-29]). The rise in glycosylated AQP3 levels with increased extracellular calcium levels is consistent with the protein expression observed in human skin with immunohistochemistry, in that AQP3 protein often localizes intracellularly in basal cells, as might be expected for immature protein, but is found on the plasma membrane in spinous keratinocytes [8]. Indeed, we hypothesize that glycosylation may help to regulate the cellular localization of AQP3, thereby influencing its biological effects (i.e., promotion of proliferation versus induction of differentiation; please see below). In addition to regulation by extracellular calcium, AQP3 is also modulated by osmotic stress in keratinocytes in vitro [30], again suggesting a possible role for this channel in epidermal physiology.
Figure 1. AQP3 Glycosylation is Regulated by Extracellular Ca2+ Concentration.
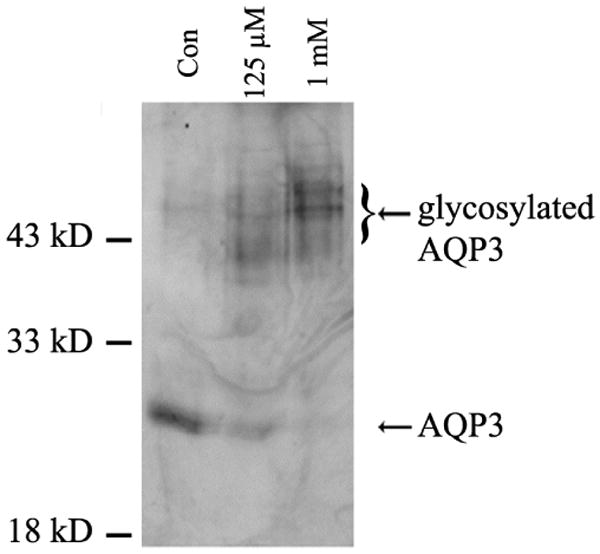
Keratinocytes were pre-treated with low 25 μM Ca2+- (control), 125 μM Ca2+- or 1 mM Ca2+-containing medium for 24 hours and then lysed using sodium carbonate. Light membrane fractions (fractions 4 and 5 [25]) were isolated by sucrose gradient ultracentrifugation, analyzed by western blotting with anti-AQP3 and visualized by enhanced chemiluminescence. The results shown are representative of three separate experiments. Note that unglycosylated AQP3 is partially down-regulated by elevated Ca2+, but glycosylated AQP3 levels are increased.
Hara-Chikuma and Verkman [31] have also investigated the role of AQP3 in human keratinocytes and AQP3 knockout mouse keratinocytes in vitro. Using RNA interference with siRNA, these authors demonstrated that AQP3 knock down resulted in decreased cell proliferation and migration in human keratinocytes [31]. Similarly, AQP3 knockout mouse keratinocytes exhibited reduced proliferation, migration and scratch wound healing compared to wild-type cells. Adenovirus-mediated re-expression of either AQP3 or AQP1 in AQP3 knockout mouse keratinocytes restored migration, suggesting water transport as the important parameter in regulating migration [31]. In contrast, the defect in proliferation observed in the AQP3 null mouse keratinocytes was corrected by providing glycerol, suggesting the importance of AQP3's glycerol transport function in modulating this parameter. ATP levels were also reduced in AQP3 knockout mouse keratinocytes, and glycerol replacement corrected these impaired ATP levels [31]. These authors therefore proposed a role of AQP3 in keratinocyte proliferation and migration.
On the other hand, Kim and Lee [32] performed similar siRNA-mediated knockdown experiments in human keratinocytes and demonstrated that keratinocytes with reduced AQP3 levels exhibit inhibited keratin 10 up-regulation in response to an elevated calcium concentration. This result is consistent with an involvement of AQP3 in inducing early keratinocyte differentiation, as we have proposed previously ([33, 34] and see below). These authors also showed an association of AQP3 with adherens junction complexes, such that siRNA-mediated knockdown of AQP3 levels results in reduced levels of E-cadherin, β- and γ-catenins and phosphorylated (active) phosphoinositide 3-kinase (PI3K) [32]. Since activation of PI3K and its downstream effector Akt is critical for the survival of differentiating keratinocytes [35, 36] and is linked to adherens junctions [21, 36], these results provide an explanation for the reduced keratinocyte survival observed with AQP3 knockdown [32] and argue for an important role for this aquaglyceroporin in maintaining keratinocyte viability during the differentiation process. Thus, the exact role of AQP3 in regulating keratinocyte proliferation versus differentiation is as yet unclear and requires additional investigation.
Evidence in vivo
The functional role of AQP3 in skin physiology has recently been demonstrated by the observed phenotype of AQP3 knockout mice. Mice lacking AQP3 display selectively reduced glycerol content and water holding capacity in the epidermis [37], which is consistent with the glycerol and water permeability of this aquaporin. Furthermore, AQP3 deletion results in decreased skin elasticity and delayed wound healing [38], as might be expected based on the findings in vitro demonstrating decreased cell proliferation, migration and scratch wound healing in keratinocytes deficient in AQP3 [31]. In addition to the decreased epidermal glycerol content [37], both water and glycerol permeability are significantly reduced in the epidermis of AQP3 knockout mice, with normal glycerol concentrations in the dermis and serum, suggesting reduced glycerol transport from the blood into the epidermis in the absence of AQP3. Moreover, Hara and Verkman [39] showed that in AQP3 knockout mice, skin conductance is similar to that in wild type mice after exposure to 10% humidity; furthermore, glycerol replacement corrected the skin hydration and mechanical defects observed in AQP3-deficient mice. These results suggest that water transport through AQP3 is not a rate-limiting factor in transepidermal water loss and that the glycerol transporting function of AQP3 is responsible for the skin abnormalities. Indeed, several of the defects in epidermal physiology in these AQP3 null mice could be corrected by topical or oral application of glycerol, but not glycerol analogs [39]. Glycerol can enter cells by simple diffusion in the absence of the facilitated transport function of AQP3, albeit less efficiently, thus necessitating the supply of pharmacological levels of glycerol. Thus, these observations provide a scientific explanation for the use of glycerol in cosmetics and skin lotions used to treat multiple skin disorders [40].
On the other hand, these authors observed no effect of AQP3 ablation on the general structure of the epidermis (although the subcutaneous fat layer was reduced in AQP3 knockout mice) [37] nor on epidermal differentiation markers [41]. In addition to the effects of AQP3 ablation on epidermal conductance, skin elasticity and wound healing, AQP3 knockout mice exhibit delayed barrier recovery after disruption of the stratum corneum [38]. For this measurement, the stratum corneum barrier is removed by repeated application and removal of tape (i.e., tape-stripping) to yield an increase in trans-epidermal water loss, as can be measured using an evaporimeter. The recovery of the barrier can then be followed by monitoring the decrease in trans-epidermal water loss over time after barrier disruption. As indicated above, the time required for AQP3 knockout mice to repair their permeability barrier after tape-stripping is increased [38]. We recently generated a transgenic mouse model in which AQP3 is over-expressed in the suprabasal epidermis under the control of the human keratin 1 promoter using the construct shown in Figure 2. These mice overexpress AQP3 in the epidermis as determined in situ via immunohistochemistry and in vitro in isolated keratinocytes by western analysis (Figure 3). Consistent with the delay in barrier recovery in AQP3 knockout mice [38], transgenic male mice overexpressing AQP3 demonstrate significantly accelerated barrier recovery relative to matched non-transgenic littermates [35 ± 6 (mean ± SEM; n=14 sites, 5 mice) versus 13 ± 7 (n=9 sites, 3 mice) percent recovery after 3 to 3.5 hours] following disruption of the permeability barrier by tape-stripping (Figure 4). Together these results indicate the importance of AQP3 in skin physiology, in particular in helping to maintain the epidermal permeability barrier, presumably through its ability to transport glycerol. Nevertheless, the question remains: how does the glycerol transported by AQP3 mediate its actions on skin function?
Figure 2. The Construct Used to Generate a Mouse Model Transgenic for Aquaporin 3 Overexpression in the Suprabasal Epidermis.
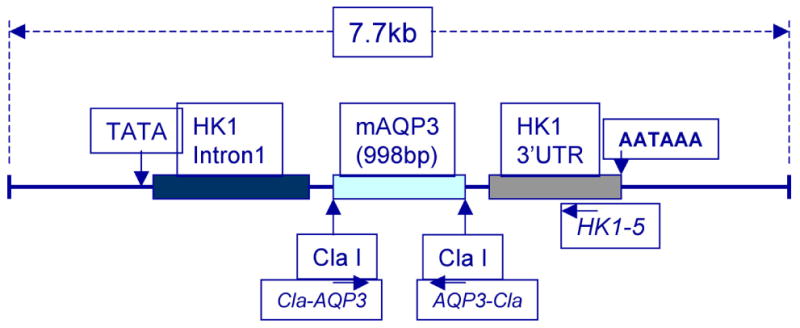
Shown is the construct used to generate the HK1-AQP3 transgenic mouse model, in which AQP3 is overexpressed in the suprabasal epidermis under the control of the human keratin 1 promoter. Also illustrated are the primers used to genotype the mice.
Figure 3. HK1-AQP3 Transgenic Mice Exhibit Enhanced Aquaporin 3 Immunoreactivity in Epidermal Sections and in Isolated Primary Epidermal Keratinocytes.
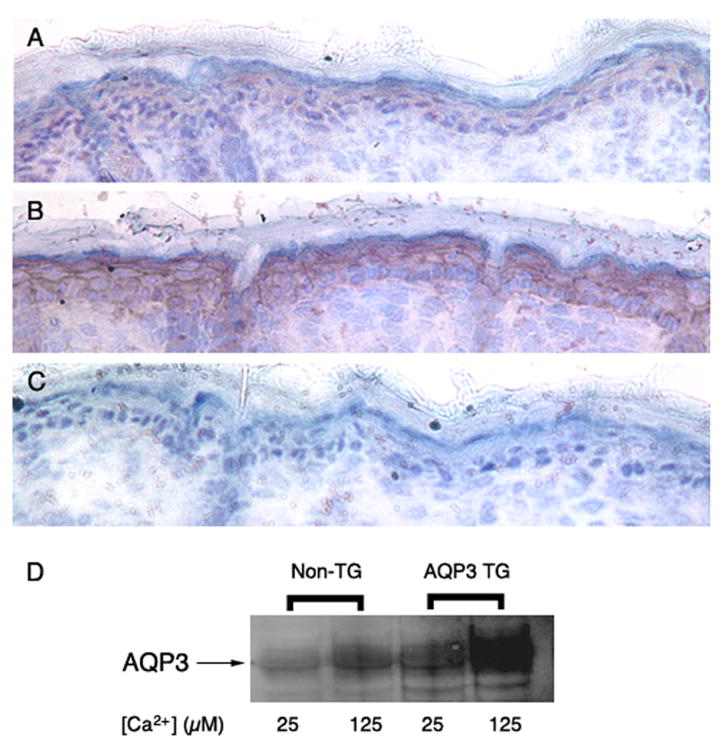
Skin from non-transgenic (Panel A) and transgenic (Panel B) neonatal littermates was dissected, placed in OCT and frozen in dimethylpentane cooled with liquid nitrogen. Cryosections were cut and incubated with hydrogen peroxide (to eliminate endogenous peroxidase activity) and blocking buffer. All sections were then stained using the ABC immunohistochemical staining kit from Santa Cruz Biotech and a primary antibody to AQP3 (Alomone Labs, Israel). Panel C illustrates a negative control in which the primary antibody was omitted. An additional control in which primary antibody was preabsorbed with antigen prior to incubation with the sections also showed minimal staining (data not shown). These results are representative of a total of 7 mice (5 transgenic newborn mice and 2 non-transgenic littermate controls). In Panel D is shown a western blot analysis of AQP3 immunoprecipitated from lysates (equal amounts of protein) of primary epidermal keratinocytes cultured from non-transgenic (Non-TG) and transgenic (AQP3 TG) littermates and treated with control medium (25 μM Ca2+) or medium containing 125 μM Ca2+ (to increase keratin 1 promoter-driven expression of AQP3) as indicated. Results are representative of three experiments. An additional experiment in which AQP3 was immunoprecipitated from lysates of whole transgenic and non-transgenic epidermis showed similarly enhanced AQP3 levels in the transgenic epidermis by subsequent western analysis (data not shown).
Figure 4. HK1-AQP3 Transgenic Mice Exhibit Accelerated Permeability Barrier Repair following Disruption by Tape-Stripping.
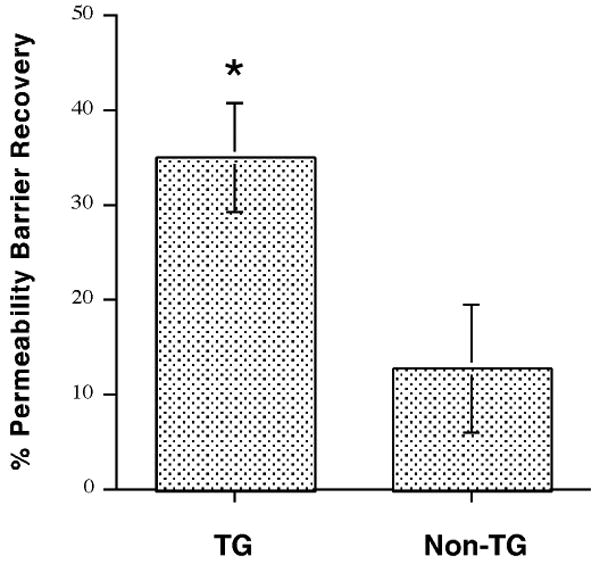
HK1-AQP3 mice and non-transgenic littermates were subjected to tape-stripping to disrupt the epidermal permeability barrier and increase trans-epidermal water loss (TEWL) as in [69]. Barrier recovery was then measured (by monitoring TEWL) at 3 to 3.5 hours. Values represent the means of 9 (non-transgenic) and 15 (transgenic) separate sites on 5 transgenic mice and 3 non-transgenic matched littermates (male mice were used); *p<0.05 versus the non-transgenic littermates. We observed that the HK1-AQP3 male mice exhibited significantly accelerated barrier recovery relative to matched non-transgenic littermates.
Glycerol has a number of effects on skin, acting to increase stratum corneum hydration, improve barrier function and repair, enhance stratum corneum plasticity and distensibility, accelerate desquamation and inhibit skin irritation (reviewed in [42]). In addition, glycerol is an important intermediate of energy metabolism and a substrate for the biosynthesis of various lipids [43]. Impaired epidermal cell proliferation has been reported in AQP3-null epidermis, and this decrease was related to decreased metabolism: lower glycerol, glucose and ATP content, and reduced CO2 production [44]. Glycerol replacement largely abolishes the proliferation defect in AQP3-deficient keratinocytes [31].
In another study Elias' group examined the skin in a stearyl-CoA desaturase-1 (scd-1) mutant mouse that lacks sebaceous glands. Sebaceous glands produce sebum, a lipid-rich holocrine secretion, the function of which is largely unknown [45]. This asebia mouse model was found to exhibit reduced epidermal glycerol content and an epidermal phenotype, including hyperproliferation, which again could be corrected by application of glycerol, but not other humectants [45]. In fact, these authors proposed that the function of the sebaceous glands is to provide triglycerides and other lipids as glycerol precursors in the skin [45]. Elias and colleagues [46] extended their sebum study to humans, examining stratum corneum hydration in relation to epidermal glycerol content and sebaceous gland abundance in particular skin regions. These authors found that sebaceous gland-enriched sites, such as the forehead or upper back, are better hydrated than sebaceous gland-deficient sites (e.g., the shins), although the correlation with glycerol content is better than that with sebum content. Stratum corneum hydration correlates well with epidermal glycerol content even in sebaceous gland-deficient sites; the presence of glycerol in these sebaceous gland-impoverished sites suggests that epidermal glycerol is derived both from sebum and from non-sebum sources (perhaps from the circulation?). Finally, glycerol levels decline following either bathing (without soap) or water immersion, indicating the presence of a water-extractable pool of glycerol that influences hydration in the stratum corneum [46]. Thus, these results provide a possible explanation for the paradoxical finding that stratum corneum hydration actually decreases following exposure to water. On the other hand, the fact that only glycerol, and not other osmotically active agents, corrects the phenotype of the asebia mouse model suggests that glycerol's role in the skin is not simply as an osmolyte.
Evidence in skin disease
In addition to the evidence linking glycerol, transported by AQP3, to skin hydration, there are data to support an involvement of altered AQP3 levels in the pathology of certain skin diseases. Thus, increased AQP3 expression has been reported in atopic eczema and was proposed to mediate the enhanced trans-epidermal water loss seen in this disorder [47]. Elevated AQP3 expression and levels in atopic dermatitis lesions in patients, as well as in two mouse models of this disease, were recently reported by Nakahigashi et al. [48], who also observed increased transepidermal water loss. Enhanced AQP3-mediated water loss may also explain the skin dryness observed with all-trans retinoic acid (atRA) treatment of the epidermis, as atRA has been shown to up-regulate AQP3 levels [49]. Interestingly, nicotinamide inhibits the atRA-induced increase in AQP3 protein expression and is also reported to reverse the side effects of atRA [49].
In contrast, Boury-Jamot et al. [26] demonstrated a down-regulation of AQP3 in eczema and, in particular, an absence of AQP3 protein expression in regions of lesional eczema skin that also exhibit spongiosis. Reduced AQP3 levels were also observed in keratinocytes from the depigmented epidermis of vitiligo [32]. This decrease in AQP3 levels is accompanied by reductions in the levels of E-cadherin, β- and γ-catenins and phosphorylated (active) phosphoinositide 3-kinase (PI3K), particularly in the plasma membrane, and was suggested to underlie a loss of keratinocytes and keratinocyte-derived growth factors leading to the passive cell death of melanocytes observed in vitiligo lesions [32]. The reduced AQP3 levels in vitiligo are also consistent with the recent finding that barrier recovery is delayed in this disease [50], since, at least in mice, AQP3 ablation results in a decreased rate [38, 39], and overexpression of AQP3 in an increased rate (Figure 4), of barrier recovery.
Verkman and colleagues have also demonstrated AQP3 immunoreactivity in human squamous cell carcinoma [44]. These authors suggested that AQP3 protein expression is correlated with proliferation in squamous cell carcinoma because the channel is colocalized with keratin 14 [44], a marker of basal keratinocytes. Verkman further indicated that AQP3 levels are increased in squamous cell carcinoma and correlate with the hyperproliferation observed in this disease [51]. However, despite the fact that keratin 14 expression is considered a marker of basal, proliferating keratinocytes, increased keratin 14 immunoreactivity is increased in the more differentiated areas of squamous cell carcinoma [52], suggesting that keratin 14 protein expression cannot be used as a marker of proliferation in these tumors. We examined AQP3 immunoreactivity in squamous cell carcinoma and found it to be non-homogeneous (i.e., “patchy”): regions of the tumor showing reduced AQP3 levels exhibiting positivity for Ki67 [53], a marker of proliferating cells, whereas areas of the tumor with significant AQP3 levels are negative for Ki67 immunoreactivity. Moreover, AQP3 levels are reduced in basal cell carcinoma, in comparison with the overlying normal-appearing epidermis [53]. Thus, our results in non-melanoma skin cancers (basal and squamous cell carcinoma) in humans suggest that decreased AQP3 levels are associated with keratinocyte proliferation in tumorigenesis. On the other hand, Verkman and colleagues [44] compared AQP3 knockout mice with wild-type mice in a two-stage tumorigenesis protocol in which mouse skin is initiated with the carcinogen 7,12-dimethylbenz[a]anthracene (DMBA) and promoted by repeated exposure to phorbol ester tumor promoters. These authors found that AQP3 knockout mice are resistant to epidermal tumor formation. However, the experiments performed did not distinguish between cell autonomous (i.e., due to the lack of AQP3 in the keratinocytes themselves) and non-cell autonomous (for instance, related to changes in other cells in the skin in these mice with a global deletion of AQP3). Indeed, the inflammatory response observed with this protocol is known to be a determinant of the ability to produce tumors in mouse skin (discussed in [54]), and a recent report indicates that the AQP3 mice have an impaired hapten-induced contact hypersensitivity response [55]. Therefore, the resistance of the AQP3 knockout mice to epidermal tumorigenesis may be related to impaired inflammation and/or immune cell activation rather than to effects in the keratinocytes themselves.
Increased AQP3 expression has also been observed in the epidermis of a mouse model in which the gene for 3β-hydroxysterol-Δ24 reductase (DHCR24) was ablated [56]. DHCR24 encodes the enzyme that catalyzes the synthesis of cholesterol from desmosterol. Lack of this gene in humans results in desmosterolosis, an autosomal recessive disorder characterized by high desmosterol and low cholesterol levels [56]. In knockout mice, the loss of DHCR24, in addition to the upregulation of AQP3 levels, results in a thickened epidermis, abnormal differentiation and increased trans-epidermal water loss, leading to perinatal death [57]. Seo and colleagues [56] suggested that the increased AQP3 expression may be the cause of the enhanced trans-epidermal water loss, although the mechanism by which enhanced desmosterol and/or reduced cholesterol leads to the defect in the permeability barrier or the upregulation of AQP3 seen in these mice is as yet unclear. Cholesterol is a component of the lamellar body lipids that comprise the water permeability barrier (reviewed in [58]); thus, reduced levels might impair barrier function. Membrane microdomains (lipid rafts) are also enriched in cholesterol (reviewed in [59]), and it seems possible that lack of this important membrane component could alter the function of enzymes and proteins residing in these membrane patches, such as AQP3 and PLD2 [25].
Possible mechanisms of AQP3's effects
In addition to its role in metabolism, glycerol can be utilized by the enzyme phospholipase D to transphosphatidylate phosphatidylcholine to yield phosphatidylglycerol (Figure 5 and [22]). Our novel findings of an association of AQP3 and PLD2 in caveolin-rich membrane microdomains of keratinocytes [25] and their functional association to generate the proposed lipid signaling molecule phosphatidylglycerol (PG) [22] provides another possible mechanism by which AQP3 could regulate epidermal function. The idea that AQP3 and PLD2 are coupled is based on immunocytochemical colocalization and their ability to co-immunoprecipitate [25]. We have also recently shown that AQP3 and PLD2 co-immunoprecipitate in Sf9 cells overexpressing both proteins (data not shown). As a result, we have proposed that in normal keratinocytes provision of glycerol to PLD2 by AQP3 results in the generation of PG, which, in turn, stimulates an effector enzyme to induce growth arrest and/or expression of early keratinocyte differentiation markers in rapidly dividing cells [33, 34]. Moreover, manipulation of this signaling module appears to inhibit keratinocyte proliferation and trigger early differentiation [34]. Thus, increasing exogenous glycerol to increase glycerol transport through AQP3 results in an inhibition of proliferation.
Figure 5. Phospholipase D Catalyzes Both a Hydrolysis and a Transphosphatidylation Reaction.
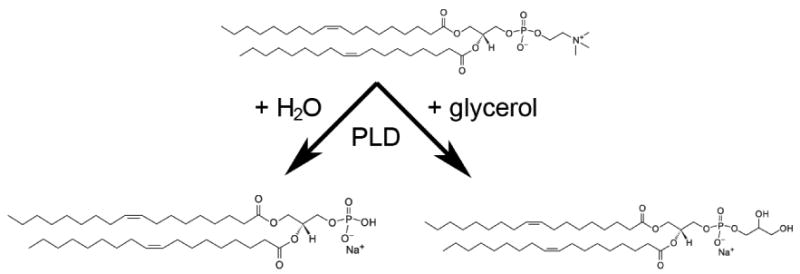
Phospholipase D (PLD) hydrolyzes phosphatidylcholine in the presence of water to yield phosphatidic acid (PA). In the presence of a primary alcohol, such as glycerol, ethanol or 1-butanol, this enzyme can also catalyze a transphosphatidylation reaction to form the corresponding phosphatidylalcohol (e.g., phosphatidylglycerol, phosphatidylethanol or phosphatidylbutanol).
Other data supporting a role for AQP3 in regulating early keratinocyte differentiation derives from experiments in which AQP3 (or an empty vector) is co-overexpressed with reporter constructs in which luciferase expression is driven by the promoters for keratin 5, a marker of basal proliferating keratinocytes, keratin 10, an early keratinocyte differentiation marker and involucrin, a marker of intermediate differentiation (as well as a Renilla luciferase plasmid for normalizing transfection efficiencies) [34]. Co-overexpression of AQP3 results in decreased keratin 5 promoter activity, increased keratin 10 promoter activity and enhanced involucrin promoter activity in the presence of an elevated extracellular calcium concentration. The effect of co-overexpression of AQP3 on keratin 5 promoter activity is augmented by exogenous glycerol [34]. Finally, direct provision of proposed product of the AQP3/PLD2 signaling module, phosphatidylglycerol (PG) in the form of liposomes composed of egg-derived PG, inhibits proliferation and enhances the effect of an elevated extracellular calcium level on involucrin levels in rapidly proliferating keratinocytes [34]. On the other hand, egg PG stimulated the proliferation of slowly dividing, presumably contact-inhibited keratinocytes, suggesting an ability of PG to normalize keratinocyte growth [34]. These results suggest that AQP3-mediated glycerol transport in skin is involved in a complex regulation of cell proliferation and differentiation, which are central features of epidermal homeostasis and regeneration, and it is possible that AQP3 may mediate different effects depending on whether or not this aquaglyceroporin is associated with PLD2.
In subsequent experiments, we demonstrated that different species of PG exert the disparate effects on proliferation, with PG species containing saturated and monounsaturated fatty acids promoting keratinocyte proliferation and those possessing polyunsaturated fatty acids inhibiting cell growth [60]. We have hypothesized that the PG effector enzyme is PKCβII [33], a known PG-responsive enzyme [61], and experiments are in progress to investigate this possibility. Another potential effect of PG relates to mitochondrial function, as PG and diphosphatidylglycerol (more commonly known as cardiolipin) are known to be important lipids in these organelles (reviewed in [62]). For instance, the addition of PG and cardiolipin restores mitochondrial membrane potential in depleted mitochondria [63]. Since mitochondrial membrane potential is known to be important in apoptotic cell death, it is possible that this pathway is involved in regulating apoptosis. Indeed, during apoptosis cardiolipin mediates the targeting of proapoptotic tBid to mitochondria, the permeabilization function of Bax on outer mitochondrial membranes and subsequent cytochrome c release [64-66]. Moreover, the ability of tBid to promote cytochrome c release from lipid vesicles is modulated by the inclusion of PG in the vesicles [66, 67]. Finally, PG protects retinal pigment epithelial cells from apoptosis [68]. Alternatively and/or additionally, the PG lipid signal may function through other, as yet unidentified, PG effector enzymes.
Multiple lines of evidence indicate that the aquaglyceroporin AQP3 is important in skin function. AQP3 has the ability to transport water and glycerol within the epidermis. The transported water is likely to play a role in epidermal hydration and hydrostatic pressure involved in migration. Glycerol, on the other hand, acts as an energy source for ATP production, a precursor for fat and phospholipid synthesis and an osmotically active agent. Glycerol can also be used by phospholipase D to generate phosphatidylglycerol, a potential lipid messenger regulating keratinocyte proliferation and differentiation. Although the mechanism by which AQP3 exerts its effects is not entirely clear, the ample data supporting an involvement of this aquaglyceroporin in keratinocyte biology and epidermal physiology indicate that further investigation is warranted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yamaji Y, Valdez DM, Jr, Seki S, Yazawa K, Urakawa C, Jin B, Kasai M, Kleinhans FW, Edashige K. Cryoprotectant permeability of aquaporin-3 expressed in Xenopus oocytes. Cryobiology. 2006;53:258–267. doi: 10.1016/j.cryobiol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am J Physiol Renal Physiol. 2000;278:F13–F28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- 3.Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- 4.Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper MA. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol. 1995;269:F663–672. doi: 10.1152/ajprenal.1995.269.5.F663. [DOI] [PubMed] [Google Scholar]
- 5.Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1-5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- 6.Echevarria M, Windhager EE, Frindt G. Selectivity of the renal collecting duct water channel aquaporin-3. J Biol Chem. 1996;271:25079–25082. doi: 10.1074/jbc.271.41.25079. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. Water channel protein AQP3 is present in epithelia exposed to the environment of possible water loss. J Histochem Cytochem. 1999;47:1275–1286. doi: 10.1177/002215549904701007. [DOI] [PubMed] [Google Scholar]
- 8.Sougrat R, Morand M, Gondran C, Barre P, Gobin R, Bonte F, Dumas M, Verbavatz JM. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J Invest Dermatol. 2002;118:678–685. doi: 10.1046/j.1523-1747.2002.01710.x. [DOI] [PubMed] [Google Scholar]
- 9.Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted calcium concentrations in vitro. J Cell Biol. 1989;109:1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon GK, Elias PM, Lee SH, Feingold KR. Localization of calcium in murine epidermis folowing disruption and repair of the permeability barrier. Cell Tissue Res. 1992;270:503–512. doi: 10.1007/BF00645052. [DOI] [PubMed] [Google Scholar]
- 11.Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 12.Menon GK, Elias PM. Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch Dermatol. 1991;127:57–63. [PubMed] [Google Scholar]
- 13.Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985;84:508–512. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- 14.Elias PM, Ahn SK, Denda M, Brown BE, Crumrine D, Kimutai LK, Komuves L, Lee SH, Feingold KR. Modulations in epidermal calcium regulate the expression of differentiation-specific markers. J Invest Dermatol. 2002;119:1128–1136. doi: 10.1046/j.1523-1747.2002.19512.x. [DOI] [PubMed] [Google Scholar]
- 15.Pillai S, Bikle DD. Role of intracellular-free calcium in the cornified envelope formation of keratinocytes: differences in the mode of action of extracellular calcium and 1,25 dihydroxyvitamin D3. J Cell Physiol. 1991;146:94–100. doi: 10.1002/jcp.1041460113. [DOI] [PubMed] [Google Scholar]
- 16.Oda Y, Tu CL, Chang W, Crumrine D, Komuves L, Mauro T, Elias PM, Bikle DD. The calcium sensing receptor and its alternatively spliced isoform in murine epidermal differentiation. J Biol Chem. 2000;275:1183–1190. doi: 10.1074/jbc.275.2.1183. [DOI] [PubMed] [Google Scholar]
- 17.Oda Y, Tu CL, Pillai S, Bikle DD. The calcium sensing receptor and its alternatively spliced form in keratinocyte differentiation. J Biol Chem. 1998;273:23344–23352. doi: 10.1074/jbc.273.36.23344. [DOI] [PubMed] [Google Scholar]
- 18.Bikle DD, Ratnam A, Mauro T, Harris J, Pillai S. Changes in calcium responsiveness and handling during keratinocyte differentiation: Potential role of the calcium receptor. J Clin Invest. 1996;97:1085–1093. doi: 10.1172/JCI118501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaken S, Yuspa SH. Early signals for keratinocyte differentiation: role of Ca2+-mediated inositol lipid metabolism in normal and neoplastic epidermal cells. Carcinogenesis. 1988;9:1033–1038. doi: 10.1093/carcin/9.6.1033. [DOI] [PubMed] [Google Scholar]
- 20.Xie Z, Bikle DD. Phospholipase C-gamma1 is required for calcium-induced keratinocyte differentiation. J Biol Chem. 1999;274:20421–20424. doi: 10.1074/jbc.274.29.20421. [DOI] [PubMed] [Google Scholar]
- 21.Tu CL, Chang W, Bikle D. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J Biol Chem. 2001;276:41079–41085. doi: 10.1074/jbc.M107122200. [DOI] [PubMed] [Google Scholar]
- 22.Zheng X, Ray S, Bollag WB. Modulation of phospholipase D-mediated phosphatidylglycerol formation by differentiating agents in primary mouse epidermal keratinocytes. Biochim Biophys Acta. 2003;1643:25–36. doi: 10.1016/j.bbamcr.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Nemeth-Cahalan KL, Hall JE. pH and calcium regulate the water permeability of aquaporin 0. J Biol Chem. 2000;275:6777–6782. doi: 10.1074/jbc.275.10.6777. [DOI] [PubMed] [Google Scholar]
- 24.Bustamante M, Hasler U, Leroy V, de Seigneux S, Dimitrov M, Mordasini D, Rousselot M, Martin PY, Feraille E. Calcium-sensing receptor attenuates AVP-induced aquaporin-2 expression via a calmodulin-dependent mechanism. J Am Soc Nephrol. 2008;19:109–116. doi: 10.1681/ASN.2007010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng X, Bollag WB. Aquaporin 3 colocates with phospholipase D2 in caveolin-rich membrane microdomains and is regulated by keratinocyte differentiation. J Invest Dermatol. 2003;121:1487–1495. doi: 10.1111/j.1523-1747.2003.12614.x. [DOI] [PubMed] [Google Scholar]
- 26.Boury-Jamot M, Sougrat R, Tailhardat M, Le Varlet B, Bonte F, Dumas M, Verbavatz JM. Expression and function of aquaporins in human skin: Is aquaporin-3 just a glycerol transporter? Biochim Biophys Acta. 2006;1758:1034–1042. doi: 10.1016/j.bbamem.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Gundry RL, Raginski K, Tarasova Y, Tchernyshyov I, Bausch-FLuck D, Elliott ST, Boheler KR, Van Eyk JE, Wollscheid B. The mouse C2C12 myoblast cell surface N-linked glycoproteome: identification, glycosite occupancy, and membrane orientation. Mol Cell Proteomics. 2009;8:2555–2569. doi: 10.1074/mcp.M900195-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendriks G, Koudijs M, van Balkom BWM, Oorschot V, Klumperman J, D PMT, van der Sluijs P. Glycosylation is importnat for cell surface expression of the water channel aquaporin-2 but is not essential for tetramerization in the endoplasmic reticulum. J Biol Chem. 2004;279:2975–2983. doi: 10.1074/jbc.M310767200. [DOI] [PubMed] [Google Scholar]
- 29.Calamita G, Mazzone A, Cho YS, Valenti G, Svelto M. Expression and localization of the aquaporin-8 water channel in rat testis. Biol Reproduct. 2001;64:1660–1666. doi: 10.1095/biolreprod64.6.1660. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama Y, Ota Y, Hara M, Inoue S. Osmotic stress up-regulates aquaporin-3 expression in cultured human keratinocytes. Biochim Biophys Acta. 2001;1522:82–88. doi: 10.1016/s0167-4781(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 31.Hara-Chikuma M, Verkman AS. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J Mol Med. 2008;86:221–231. doi: 10.1007/s00109-007-0272-4. [DOI] [PubMed] [Google Scholar]
- 32.Kim NH, Lee AY. Reduced aquaporin3 expression and survival of keratinocytes in the depigmented epidermis of vitiligo. J Invest Dermatol. 2010;130:2231–2239. doi: 10.1038/jid.2010.99. [DOI] [PubMed] [Google Scholar]
- 33.Bollag WB, Zheng X. Phospholipase D and keratinocyte biology. In: Robinson JW, editor. Trends in Protein Research. New York: Nova Science Publishers, Inc.; 2005. pp. 79–118. [Google Scholar]
- 34.Bollag WB, Xie D, Zhong X, Zheng X. A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: Production of a novel phosphatidylglycerol lipid signal. J Invest Dermatol. 2007;127:2823–2831. doi: 10.1038/sj.jid.5700921. [DOI] [PubMed] [Google Scholar]
- 35.Calautti E, Li J, Saoncella S, Brissette JL, Goetinck PF. Phosphoinositide 3-kinase signaling to Akt promotes keratinocyte differentiation versus death. J Biol Chem. 2005;2890:32856–32865. doi: 10.1074/jbc.M506119200. [DOI] [PubMed] [Google Scholar]
- 36.Thrash BR, Menges CW, Pierce RH, McCance DJ. AKT1 provides an essential survival signal required for differentiation and stratification of primary human keratinocytes. J Biol Chem. 2006;281:12155–12162. doi: 10.1074/jbc.M512116200. [DOI] [PubMed] [Google Scholar]
- 37.Ma T, Hara M, Sougrat R, Verbavatz JM, Verkman AS. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J Biol Chem. 2002;277:17147–17153. doi: 10.1074/jbc.M200925200. [DOI] [PubMed] [Google Scholar]
- 38.Hara M, Ma T, Verkman AS. Selectively reduced glycerol in skin of aquaporin-3 deficient mice may account for impaired skin hydration, elasticity and barrier recovery. J Biol Chem. 2002;277:46616–46621. doi: 10.1074/jbc.M209003200. [DOI] [PubMed] [Google Scholar]
- 39.Hara M, Verkman AS. Glycerol replacement corrects defective skin hydration, elasticity, and barrier function in aquaporin-3-deficient mice. Proc Natl Acad Sci USA. 2003;100:7360–7365. doi: 10.1073/pnas.1230416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldsmith LA. Clinical Snippets. J Invest Dermatol. 2003;120:iv. doi: 10.1046/j.1523-1747.2003.12079.x. [DOI] [PubMed] [Google Scholar]
- 41.Hara-Chikuma M, Takahashi K, Chikuma S, Verkman AS, Miyachi Y. The expression of differentiation markers in aquaporin-3 deficient epidermis. Arch Dermatol Res. 2009;301:245–252. doi: 10.1007/s00403-009-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fluhr JW, Darlenski R, Surber C. Glycerol and the skin: holistic approach to its origin and function. Br J Dermatol. 2008;159:23–34. doi: 10.1111/j.1365-2133.2008.08643.x. [DOI] [PubMed] [Google Scholar]
- 43.Brisson D, Vohl MC, St-Pierre J, Hudson TJ, Gaudet D. Glycerol: a neglected variable in metabolic processes? Bioessays. 2001;23:534–542. doi: 10.1002/bies.1073. [DOI] [PubMed] [Google Scholar]
- 44.Hara-Chikuma M, Verkman AS. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol. 2008;28:326–332. doi: 10.1128/MCB.01482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fluhr JW, Mao-Qiang M, Brown BE, Wertz PW, Crumrine D, Sundberg JP, Feingold KR, Elias PM. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J Invest Dermatol. 2003;120:728–737. doi: 10.1046/j.1523-1747.2003.12134.x. [DOI] [PubMed] [Google Scholar]
- 46.Choi EH, Man MQ, Wang F, Zhang X, Brown BE, Feingold KE, Elias PM. Is endogenous glycerol a determinant of stratum corneum hydration in humans? J Invest Dermatol. 2005;125:288–293. doi: 10.1111/j.0022-202X.2005.23799.x. [DOI] [PubMed] [Google Scholar]
- 47.Olsson M, Broberg A, Jernas M, Carlsson L, Rudemo M, Suurküla M, Svensson PA, Benson M. Increased expression of aquaporin 3 in atopic eczema. Allergy. 2006;61:1132–1137. doi: 10.1111/j.1398-9995.2006.01151.x. [DOI] [PubMed] [Google Scholar]
- 48.Nakahigashi K, Kabashima K, Ikoma A, Verkman AS, Miyachi Y, Hara-Chikuma M. Upregulation of aquaporin-3 is involved in keratinocyte proliferation and epidermal hyperplasia. J Invest Dermatol. 2010 Dec 30; doi: 10.1038/jid.2010.395. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 49.Song X, Xu A, Pan W, Wallin B, Kivlin R, Lu S, Cao C, Bi Z, Wan Y. Nicotinamide attenuates aquaporin 3 overexpression induced by retinoic acid through inhibition of EGFR/ERK in cultured human keratinocytes. Int J Mol Med. 2008;22:229–236. [PubMed] [Google Scholar]
- 50.Liu J, Man WY, Lv CZ, Song SP, Shi YJ, Elias PM, Man MQ. Epidermal permeability barrier recovery is delayed in vitiligo-involved sites. Skin Pharmacol Physiol. 2010;23:193–200. doi: 10.1159/000288166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verkman AS. A cautionary note on cosmetics containing ingredients that increase aquaporin-3 expression. Exp Dermatol. 2008;17:871–872. doi: 10.1111/j.1600-0625.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- 52.Perkins W, Campbell I, Leigh IM, MaKie RM. Keratin expression in normal skin and epidermal neoplasms demonstrated by a panel of monoclonal antibodies. J Cutan Pathol. 1992;19:476–482. doi: 10.1111/j.1600-0560.1992.tb01600.x. [DOI] [PubMed] [Google Scholar]
- 53.Voss KE, Bollag RJ, Fussell N, By C, Sheehan DJ, Bollag WB. Abnormal aquaporin-3 protein expression in hyperproliferative skin disorders. Arch Dermatol Res. doi: 10.1007/s00403-011-1136-x. Manuscript in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bollag WB. Protein kinase Calpha puts the hand cuffs on epidermal keratinocyte proliferation. J Invest Dermatol. 2009;129:2330–2332. doi: 10.1038/jid.2009.165. [DOI] [PubMed] [Google Scholar]
- 55.Hara-Chikuma M, Chikuma S, Kabashima K, Nakahigashi K, Kamegawa A, Fujiyoshi Y, Verkman AS, Sugiyama Y, Inoue S, Miyachi Y. A controlled aquaporin-3 expression in T lymphocytes regulate their migration and trafficking in cutaneous immune reaction. J Invest Dermatol. 2010;130:S116a. [Google Scholar]
- 56.Mirza R, Hayasaka S, Kambe F, Maki K, Kaji T, Murata Y, Seo H. Increased expression of aquaporin-3 in the epidermis of DHCR24 knockout mice. Br J Dermatol. 2007;158:679–684. doi: 10.1111/j.1365-2133.2007.08424.x. [DOI] [PubMed] [Google Scholar]
- 57.Mirza R, Hayasaka S, Takagishi Y, Kambe F, Ohmori S, Maki K, Yamamoto M, Murakami K, Kaji T, Zadworny D, et al. DHCR24 gene knockout mice demonstrate lethal dermopathy with differentiation and maturation defects in the epidermis. J Invest Dermatol. 2006;126:638–647. doi: 10.1038/sj.jid.5700111. [DOI] [PubMed] [Google Scholar]
- 58.Elias PM. Stratum corneum defensive functions: An integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 59.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 60.Xie D, Podolsky R, Seremwe M, Edwards JG, George MV, Bollag WB. Distinct effects of phosphatidylglycerol species on mouse keratinocyte proliferation. doi: 10.1371/journal.pone.0107119. Manuscript in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murray NR, Fields AP. Phosphatidylglycerol is a physiologic activator of nuclear protein kinase C. J Biol Chem. 1998;273:11514–11520. doi: 10.1074/jbc.273.19.11514. [DOI] [PubMed] [Google Scholar]
- 62.McMillin JB, Dowhan W. Cardiolipin and apoptosis. Biochim Biophys Acta. 2002;1585:97–107. doi: 10.1016/s1388-1981(02)00329-3. [DOI] [PubMed] [Google Scholar]
- 63.Piccotti L, Marchetti C, Migliorati G, Roberti R, Corazzi L. Exogenous phospholipids specifically affect transmembrane potential of brain mitochondria and cytochrome C release. J Biol Chem. 2002;277:12075–12081. doi: 10.1074/jbc.M200029200. [DOI] [PubMed] [Google Scholar]
- 64.Lutter M, Fang M, Luo X, Nishijima M, Xie Xs, Wang X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nature Cell Biol. 2000;2:754–756. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 65.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 66.Zhai D, Miao Q, Xin X, Yang F. Leakage and aggregation of phospholipid vesicles induced by the BH3-only Bcl-2 family member, BID. Eur J Biochem. 2001;268:48–55. doi: 10.1046/j.1432-1327.2001.01841.x. [DOI] [PubMed] [Google Scholar]
- 67.Epand RF, Martinou JC, Fornallaz-Mulhauser M, Hughes DW, Epand RM. The apoptotic protein tBid promotes leakage by altering membrane curvature. J Biol Chem. 2002;277:32632–32639. doi: 10.1074/jbc.M202396200. [DOI] [PubMed] [Google Scholar]
- 68.Shaban H, Borras C, Vina J, Richter C. Phosphatidylglycerol potently protects human retinal pigment epithelial cells against apoptosis induced by A2E, a compound suspected to cause age-related macula degeneration. Exp Eye Res. 2002;75:99–108. doi: 10.1006/exer.2001.1192. [DOI] [PubMed] [Google Scholar]
- 69.Man MQ, Barish GD, Schmuth M, Crumrine D, Barak Y, Chang S, Jiang Y, Evans RM, Elias PM, Feingold KR. Deficiency of PPARbeta/delta in the epidermis results in defective cutaneous permeability barrier homeostasis and increased inflammation. J Invest Dermatol. 2008;128:370–377. doi: 10.1038/sj.jid.5701026. [DOI] [PubMed] [Google Scholar]


