Babbette LaMarca
As early as twenty weeks of gestation, preeclamptic women develop new onset hypertension with proteinuria and display increased circulating factors ranging from metabolic, proinflammatory to antiangiogenic in nature. These factors have been shown in various experimental models to possibly contribute to the development of hypertension in response to placental ischemia.1-4. A major focus of preeclamptic research has been the identification a molecular marker that could be used to predict early in gestation the development of this disease. Two potential factors associated with the development of preeclampsia is the imbalance of angiogenic factors (VEGF/PlGF) and the anti-angiogenic factor (sFlt-1) as well as agonistic autoantibody to the angiotensin II type I receptor (AT1-AA) 1-5.
The AT1-AA has been purified and specificity for the second extracellular loop of the angiotensin II type I receptor (AT1R) has been demonstrated by western blotting, colocalization, and coimmunoprecipitation experiments5. The AT1-AA induces signaling in vascular cells including activating protein-1, calcineurin, reactive oxygen species and nuclear factor kappa B activation which are blocked by an AT1R antagonist 5-8. In addition the AT1-AA appear to be responsible for other effects among different tissues including stimulation of IL-6 production from mesangial cells and most recently our laboratory has demonstrated AT1-AA activation of the endothelin pathway in human endothelial cells and in pregnant rats9,10.
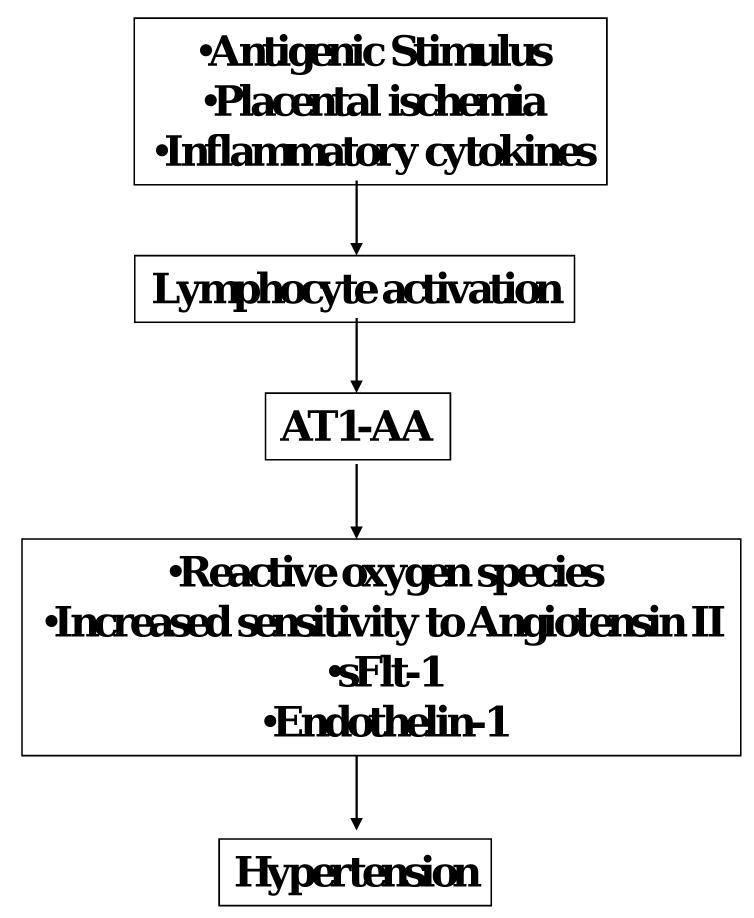
Clinical studies indicate that both plasma and amniotic fluid concentrations as well as placental sFlt-1 mRNA are increased in preeclamptic patients2. Moreover, increases in plasma levels of sFlt-1 in pregnant rodent models lead to phathophysiological alterations that mimic many of the characteristics observed in women with preeclampsia2,3. Thus, these studies suggest that sFlt-1 may contribute to the pathophysiology observed in preeclampsia. However, the exact mechanisms responsible sFlt-1 overexpression has yet to be clearly elucidated. (Figure 1)
Figure 1. Potential role for AT1-AA in the pathophysiology of preeclampsia.
Agonistic AT1-AA stimulated in response to placental ischemia play an important role in the generation of of ROS, production of sFlt-1 and enhanced ET-1 and ANG II sensitivity thereby contributing to the development of hypertension during pregnancy
Previous studies by Xia and Kellems et al demonstrated AT1-AA from preeclamptic women induces sFlt-1 production via AT1R and calcineurin/nuclear factor of activated T-cells signaling 11,12. The authors demonstrated by injecting the IgG or affinity-purified AT1-AA from women into pregnant mice caused hypertension, proteinuria, glomerular endotheliosis, placental abnormalities, IUGR and elevated sFlt-112. The onset of these symptoms were prevented by AT1R antagonist or an AT1-AA neutralizing seven-amino-acid epitope binding peptide12. Most recently, in agreement with the Xia laboratory, we have confirmed that AT1-AA infusion increased blood pressure and plasma sFlt-1 in pregnant rats13.
While these studies suggest a potential interaction between AT1-AA and sFlt-1, a clear association between AT1-AA, sFlt-1 and severity of the disease in women has never been fully established. Much uncertainty about this relationship was only heightened by recent clinical studies by Stepan et al., who found that while most preeclamptic patients expressed high sFlt-1 and the AT1-AA, in a population of patients characterized by reduced uterine perfusion and no other pregnancy complications, there was no association between the AT1-AA and sFlt-114. In these cases, sFlt-1 was not elevated when AT1-AA was frequently present.
In this issue of Hypertension, Xia and colleagues clearly demonstrate that the titer of AT1-AA not only correlate to the severity of the disease but that there was a strong correlation between AT1-AA activity to sFlt-1 in severe preeclamptics. In this study, the authors utilize a newly developed sensitive and high throughput luciferase bioassay in order to determine the presence of the AT1-AA. In contrast to previous publications from our laboratories, both LaMarca and Dechend 4-7, 10, 13, in which we utilized the cardiomyocyte contraction assay to detect the presence of AT1-AA among preeclamptic women and several rat models of preeclampsia, Xia et al reported increased luciferase activity from IgG treated CHO.AT1.luc cells indicating AT1R activation mediated by elevated AT1-AA. Both assays utilize the 7 amino acid blocking peptide inhibiting the antibody interaction with the epitope binding sequence of the AT1R.
Utilizing this sensitive bioassay to quantify AT1-AA activity in patients, Xia and colleagues provide compelling evidence that AT1-AA is present in nearly all women diagnosed with preeclampsia. Importantly, the authors distinguish greater AT1-AA activity in patients with severe preeclampsia compared to those with mild preeclampsia. However, since the AT1-AA was only measured at one stage of gestation it is uncertain whether measurement of the AT1-AA could be used early in gestation as a marker for the disease. Furthermore, in contrast to previous publications by Dechend and colleagues, Xia et al demonstrate the presence of AT1-AA, average stimulation of 14±3 % over basal, in half of pregnant non-hypertensive patients examined in the study. The increased sensitivity of this new bioassay could be one potential weakness that could lead to false positives in this patient population. Importantly, Xia and colleagues report the AT1-AA in women with gestational hypertension in the absence of sFlt-1, thus indicating a possible association of the AT1-AA with other pregnancy related hypertensive disorders. Future studies are critical in determining the presence of AT1-AA among normal pregnant non-hypertensive women not only to corroborate their preliminary findings but also to determine the assays utility to measure the AT1-AA among preeclamptics. Thus future studies utilizing either of these bioassays to determine the AT1-AA early in gestation and possibly its relevance as a potential marker to identify women that could develop pregnancy related hypertensive disorders are critical for this area research.
Although the findings of Xia and colleagues demonstrate a significant correlation of AT1-AA activity with severity of the disease in humans concurs with previous recent experimental studies demonstrating that AT1-AA induces features of preeclampsia, many unanswered questions still exist. While we have reported that placental ischemia and inflammatory cytokines are important stimuli for AT1-AA, the antigenic stimulus for AT1-AA production is still unknown. (Figure 1) Moreover, the pathway of production of the AT1-AA, such as T cell dependent verses T cell independent, has yet to be elucidated. Furthermore, it is unclear how early in gestation the onset of AT1-AA production occurs. Studies inhibiting the production of the AT1-AA in pregnant animal models of preeclampsia are also necessary to advance our understanding of the pathophysiological role of the autoantibody during pregnancy. A better understanding of the pathophysiology of AT1-AA production in preeclampsia may lead to novel therapeutic targets for the treatment of the disease and /or a marker for predicting patient risk of developing preeclampsia.
Acknowledgments
Sources of Funding American Heart Association Scientist Development Grant 0835472N
Footnotes
Disclosures: NONE
References
- 1.Roberts JM, Lain KY. Recent 1nsights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 2.Maynard S, Min JY, Mercahn J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble Fms-like tyrosine kinase 1 (sFlt-1) may contribute to endothelial dysfunction, hypertension and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert JS, Babcock SA, Ganger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased sFlt-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 4.LaMarca BB, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Auoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008;52:1168–1172. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 7.Dechend R, Homuth V, Wallukat G, Müller DN, Krause M, Dudenhausen J, Haller H, Luft FC. Agonistic antibodies directed at the angiotensin II, AT1 receptor in preeclampsia. J Soc Gynecol Investig. 2006;13:79–86. doi: 10.1016/j.jsgi.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC, 3rd, Xia Y, Kellems RE. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation. 2004;110:1612–1619. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- 9.Bobst SM, Day MC, Gilstrap LC, 3rd, Xia Y, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. American Journal of Hypertension. 2005;18:330–336. doi: 10.1016/j.amjhyper.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 10.LaMarca B, Parrish M, Ray L, Murphy S, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: Role of endothelin-1. Hypertension. 2009 Aug 24; doi: 10.1161/HYPERTENSIONAHA.109.137935. Epub 19704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou CC, Ahmad S, Mi T, Abbasi S, Xia L, Day MC, Ramin SM, Ahmed A, Kellems RE, Xia Y. Autoantibody from women with preeclampsia induces soluble Fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T-cells signaling. Hypertension. 2008;51:1010–1019. doi: 10.1161/HYPERTENSIONAHA.107.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, xia Y. Angiotensin receptor agonistic autantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parrish MR, Murphy SR, Keiser S, Ray LF, Dechend R, Martin JN, Granger JP, LaMarca B. Central Association of Obstetricians and Gynecologists. Maui, Hawaii: 2009. Soluble fms-like tyrosine-1 (sFlt-1) production is enhanced during hypertension in response to Tumor Necrosis Factor- alpha (TNF-α) and agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA) Abstract. [Google Scholar]
- 14.Stepan H, Faber R, Wessel N, Wallukat G, Schultheiss HP, Walther T. Relation between circulating angiotensin II type 1 receptor agonistic autoantibodies and soluble fms-like tyrosine kinase 1 in the pathogenesis of preeclampsia. J Clin Endocrinol Metab. 2006;91:2424–2427. doi: 10.1210/jc.2005-2698. [DOI] [PubMed] [Google Scholar]