Abstract
Purpose/Objectives
To examine the feasibility, relationships among variables, and preliminary outcomes of a self-directed complementary modality, cranial electrical stimulation (CES), for symptom management in women receiving chemotherapy for breast cancer.
Design
Biobehavioral pilot feasibility study.
Setting
Two university-based cancer centers.
Sample
36 women with stage I-IIIA breast cancer scheduled to receive chemotherapy.
Methods
Data were collected via interview, questionnaires, and interactive voice technology (IVR). Biomarkers were measured from a blood sample taken prior to the initial chemotherapy.
Main Research Variables
Symptoms of depression, anxiety, fatigue, pain, and sleep disturbances; biomarkers (proinflammatory cytokines interleukin-6, tumor necrosis factor alpha [TNF-α0], interleukin-1 beta) and C-reactive protein [CRP]); and CES.
Findings
CES appears to be a safe and acceptable modality during chemotherapy. Recruitment and retention were adequate. IVR was associated with missing data. Symptoms of depression, anxiety, fatigue, and sleep disturbances were highly correlated with each other, and most symptoms were correlated with CRP at baseline. Depression and TNF-α had a positive, significant relationship. Levels of depression increased over time and trended toward less increase in the CES group; however, the differences among groups were not statistically significant.
Conclusions
The data support the feasibility of CES. Further testing in larger samples is needed to examine the efficacy of CES for symptom management of multiple, concurrent symptoms and to further develop the biobehavioral framework.
Implications for Nursing
Interventions that are effective at minimizing more than one target symptom are especially needed to provide optimal symptom management for women with breast cancer.
Although breast cancer mortality rates have declined, partly as a result of multidrug systemic chemotherapy, the morbidity associated with breast cancer and its treatments remains a significant public health problem. Patients with breast cancer experience multiple concurrent symptoms, particularly during chemotherapy. Although symptom management research in oncology traditionally has targeted the reduction of individual symptoms, current research has focused on the phenomenon of symptom clusters, defined as three or more concurrent symptoms (Dodd, Miaskowski, & Lee, 2004) that may share a common biologic mechanism (Miaskowski & Aouizerat, 2007). This article reports the results from a biobehavioral pilot study that examined the feasibility of the protocol (safety, acceptability, and ability to recruit and retain study participants) and the preliminary outcomes of cranial electrical stimulation (CES) for reducing symptoms of depression, anxiety, fatigue, pain, and sleep disturbances in women receiving chemotherapy for breast cancer. Secondary aims were to explore the inter-relationships at baseline (prior to chemotherapy) of inflammatory biomarkers (proinflammatory cytokines interleukin-6 [IL-6], tumor necrosis factor alpha [TNF-α], and interleukin-1 beta [IL-1β]) and C-reactive protein (CRP) and symptoms of depression, anxiety, fatigue, pain, and sleep disturbances.
Background and Literature Review
Concurrent Symptoms
The symptoms of pain, depression, and fatigue commonly co-occur in patients with cancer (Agency for Healthcare Research and Quality, 2002). Such symptoms have been called “sentinel symptoms” because they are the most prevalent symptoms across cancer types, and as more of these symptoms are present, negative patient outcomes become more likely (Barsevick, 2007). Pain, depression, and fatigue do not appear to be cancer type- or stage-specific, and the symptoms have been noted in patients with cancer undergoing treatments and in cancer survivors (Reyes-Gibby, Aday, Anderson, Mendoza, & Cleeland, 2006). In women with breast cancer, anxiety and sleep disturbances also may be present during the chemotherapy treatment phase. The prevalence of depressive disorders in patients with breast cancer ranges from 0%–46% (Kissane et al., 2004). In addition to elevated depressive symptoms, symptoms of anxiety are increased in patients receiving chemotherapy. Depression and anxiety are highly correlated in women with breast cancer (Badger, Segrin, Dorros, Meek, & Lopez, 2007) and may adversely affect quality of life. Cancer treatment-related fatigue has been reported in 58%–99% of women diagnosed with breast cancer during treatment and often for months afterward (Bower et al., 2005). Before, during, and after chemotherapy, patients with breast cancer experience worse fatigue than women with no cancer history (Jacobsen et al., 1999). In fact, severe fatigue remains a problem after treatment for nearly 40% of breast cancer survivors (Servaes, Verhagen, & Bleijenberg, 2002), with patients who formerly received adjuvant chemotherapy reporting more severe fatigue and worse quality of life because of fatigue (Broeckel, Jacobsen, Horton, Balducci, & Lyman, 1998). Cancer-related sleep disturbances are significantly correlated with fatigue severity (Anderson et al., 2003). Although alterations in sleep have been understudied in oncology research, an estimated 45% of people with cancer have some type of sleep disturbance (Theobald, 2004). In a heterogeneous sample of women with breast cancer (N = 72; 19 received precancer treatment, 29 received cancer treatment, and 23 received postcancer treatment), 61% had significant sleep problems (Fortner, Stepanski, Wang, Kasprowicz, & Durrence, 2002). Sleep disturbances in people with cancer include difficulty falling asleep, early awakening, and daytime sleepiness (Lee, Cho, Miaskowski, & Dodd, 2004).
Along with fatigue and sleep disturbances, most patients with cancer experience pain during the disease trajectory (Anderson et al., 2003). Prevalence rates of pain in women with nonmetastatic breast cancer range from 33%–52% (Dow, Ferrell, Leigh, Ly, & Gulasekaram, 1996). Women with early-stage breast cancer may have surgical pain from a lumpectomy, mastectomy, or axillary lymph node dissection. Pain also may be nonspecific to cancer surgery. A longitudinal study of women with stage I–II breast cancer found that, prior to the first chemotherapy treatment, women reported sleep disturbances and pain most frequently, and both symptoms persisted after chemotherapy (Byar, Berger, Bakken, & Cetak, 2006).
Biobehavioral Framework
A biobehavioral framework permits a better understanding of the role of biologic processes in behavioral outcomes (Grady, 2006). Inflammatory mediators have been proposed as putative mechanisms of behavioral alterations and symptoms in patients with cancer (Miller, Ancoli-Israel, Bower, Capuron, & Irwin, 2008). Evidence from animal models supports a cytokine-induced “sickness behavior” model, first described as a syndrome in laboratory animals in which responses to laboratory-induced cytokine production caused lethargy, anorexia, and somnolence (Cleeland et al., 2003). The sickness behavior model is based on the premise that immune system activation resulting from insults such as acute viral or bacterial infections, autoimmune disease, and cancer induces an inflammatory cascade that results in increased production of pro-inflammatory cytokines, including IL-6, TNF-α, and IL-1β (Brebner, Hayley, Zacharko, Merali, & Anisman, 2000; Miller, 2003). IL-6, in particular, initiates the acute-phase response and stimulates the synthesis of CRP, which is a widely used marker of systemic inflammation that has been associated with depression and anxiety in multiple populations. Cytokines play an important role in regulation of the immune system and normal central nervous system function, including neural cell repair and the metabolism of the neurotransmitters dopamine and serotonin, both of which play a critical role in regulating mood and energy levels. Animal studies have consistently demonstrated that systemically or centrally administered cytokines lead to nonspecific sickness behaviors such as lethargy, fatigue, and decreased food intake (Cleeland et al., 2003; Dunn, Swiergiel, & de Beaurepaire, 2005). Recently, a model testing the effects of chemotherapy on cytokines and sickness behaviors found that the administration of the cancer chemotherapy drug etoposide rapidly increased serum levels of IL-6 in healthy mice and induced sickness-like behaviors as evidenced by decreases in food intake, body weight, hemoglobin level, and voluntary wheel-running activity (Wood et al., 2006). That study was one of the first to extend the sickness behavior model to the administration of cancer chemotherapy. Associations between cytokines and sickness behaviors in humans have been less consistent, except for studies in which cytokines were directly infused as part of medical treatment. For example, in humans who are administered interferon-gamma, a pro-inflammatory cytokine used for immunotherapy in melanoma treatment, fatigue, depressed mood, pain, gastrointestinal symptoms, tension, and irritability are common. However, studies have had inconsistent findings, resulting in a need for further research to better clarify the relationships in patients with cancer.
Cranial Electrical Stimulation
At least 70%–80% of patients with breast cancer use complementary and alternative medicine (CAM) for treatment (Matthews, Sellergren, Huo, List, & Fleming, 2007), mostly to reduce symptoms. CES is a CAM modality in the category of “veritable energy medicine” (National Institutes of Health, n.d.) and is recognized by the U.S. Food and Drug Administration (FDA) in the category of medical devices for the treatment of depression, anxiety, insomnia, and pain. Although CES has not been tested for use in patients with cancer, many well-established indications exist for the use of bioelectric and bio-magnetic energy fields to treat neurologic and psychiatric symptoms. Electrical stimulation—in a variety of modes such as vagal nerve stimulation, transcranial magnetic stimulation, and deep brain stimulation—is emerging as treatment for psychiatric symptoms and is being incorporated into standard mental healthcare practices (Wagner, Valero-Cabre, & Pascual-Leone, 2007). CES has demonstrated safety and efficacy in relieving depressive and anxiety symptoms, fatigue, sleep disturbances, and pain; however, many of the studies had design limitations. In a meta-analysis of 18 randomized, controlled trials of CES versus sham treatment in a variety of conditions, CES was significantly more effective than sham treatment (p < 0.05) in reducing symptoms of anxiety (Klawansky et al., 1995). Also, CES has been used successfully to treat anxiety in patients withdrawing from alcohol (Schmitt, Capo, & Boyd, 1986) and in patients receiving dental treatments (Winick, 1999). As part of a multimodal strategy, CES was effective in reducing symptoms of anxiety and depression (Rogers, Ei, Rogers, & Cross, 2007). In a double-blinded study, individuals with fibromyalgia were randomly assigned to three one-hour daily CES treatments or three one-hour daily sham CES treatments, or they were held as wait-listed controls. Treated patients showed significant improvements in pain, sleep, well-being, and quality of life, and no placebo effect was found among the sham-treated controls (Lichtbroun, Raicer, & Smith, 2001). The use of CES for symptom reduction in patients with cancer has several important potential advantages as compared to pharmacotherapy and other CAM modalities that require group attendance or scheduled appointments with practitioners. First, CES does not introduce any foreign substance into the body; thus, adverse effects are rare. Second, energy therapies do not pass through metabolic pathways in the liver, which may be taxed in patients receiving cancer treatments. Third, CES is an energy modality that is self-managed and can be used in the home. In-home use is a great advantage during the period of active chemotherapy, when attending meetings may add a burden to a patient’s busy schedule and possibly expose an immunosuppressed individual to potential pathogens. Therefore, in light of the lack of adverse effects and the potential benefits of CES for reducing symptoms commonly experienced by women undergoing breast cancer treatment, the authors conducted a randomized pilot feasibility trial of the potential efficacy of CES for symptom management in women receiving chemotherapy for breast cancer.
Methods
Design
The design was a prospective, three-group, randomized, double-blinded, longitudinal pilot feasibility study. The three groups were the CES device group, the sham CES device group, and the usual comparison group.
Sample and Setting
Participants included women with stage I–IIIA breast cancer receiving adjuvant chemotherapy or neoadjuvant therapy with an anthracycline-containing chemotherapy regimen. Participants were recruited from two academic health science centers in Virginia. Potential study participants were identified by their healthcare providers and were approached in the clinic by study personnel. Interested individuals were scheduled for a baseline assessment prior to the initial chemotherapy infusion. Participants were female, aged 18 years or older, able to read and speak English, and scheduled to receive at least four cycles of an anthracycline-containing chemotherapy regimen. Patients with any major psychiatric conditions were excluded, as were individuals taking antidepressant or anxiolytic medications. Because of the potential for the CES device to interfere with electrical devices, patients with implanted devices such as cardiac pacemakers were excluded.
Intervention
The CES device used in this study was the Alpha-Stim® Stress Control System (SCS) (Electromedical Products International, Inc.) (see Figure 1). The Alpha-Stim SCS device is available in the United States by prescription and sold over the counter in Europe. The CES device delivers a programmed and measurable level of electrical stimulation via electrodes attached to the earlobes, with a stimulus intensity of less than 1.0 milliampere at 100 Hz from a 9-volt battery source. The FDA recognizes the Alpha-Stim SCS device in its category of medical devices for the treatment of depression, anxiety, insomnia, and pain. Although CES may be used for 30–60 minutes per day, devices in this study were set at a subsensory intensity, and a timer turned them off automatically at 60 minutes.
Figure 1. Alpha-Stim® Device and Ear Clip.
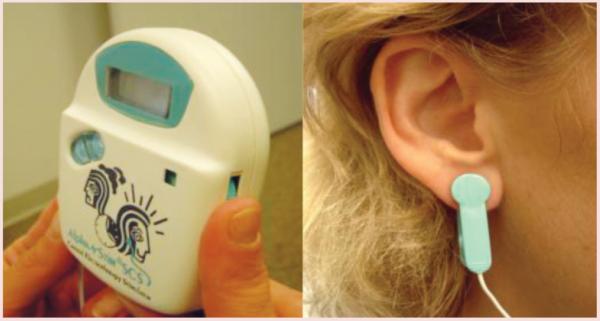
Note. Ear clips are worn on both ears.
Participant Enrollment and Assignment
The institutional review boards of the University of Virginia and Virginia Commonwealth University approved the protocol for this study. Written informed consent was obtained from all participants. Individuals who gave informed consent were randomized to ensure balance in the three groups: (a) the active Alpha-Stim SCS group, (b) the sham Alpha-Stim SCS group, and (c) the usual care group. Sham Alpha-Stim SCS devices were constructed for the placebo treatment with nonconductive wires; otherwise, the device, settings, and batteries were identical in the actual and sham groups. To ensure blinding of researchers and participants, the current was set at a subsensory level of 100 microamperes. The study statistician developed random assignment codes and kept them in a secure area in the general clinical research center. The principal investigator called the general clinical research center and received the group assignment each time a study participant was enrolled. The assignment codes were not broken until completion of the preliminary data analysis.
Procedures
Symptom reports were collected from participants in person at baseline by a research associate (RA) and weekly over an interactive voice response (IVR) telephone system. At baseline, information relevant to the type of breast cancer surgery and clinical stage and grade of cancer were obtained from the participants’ medical records. Adverse event reports related to the CES device were collected by patient report during the weekly IVR collection in addition to questioning by an RA in the cancer clinic prior to the second and third chemotherapy infusions. All participants were trained in the IVR system and given a toll-free telephone number to call on a weekly basis to complete symptom reports. Participants in the two device groups were trained in the use of the mechanisms and received extra 9-volt batteries and conduction liquid to apply to the earlobe electrodes. After instruction from an RA and a return demonstration, participants initiated use of the devices on the first infusion day. Participants in the device groups brought the devices back to the clinic for inspection and battery changes prior to the second and third chemotherapy infusions and completed device usage two weeks after the third infusion. The devices were visually inspected at the before-chemotherapy study visits, and batteries were replaced at each clinic visit. For participants receiving chemotherapy every three weeks, the total duration of CES use was eight weeks. For participants receiving chemotherapy every two weeks, the total duration of use was six weeks. After completing the protocol, participants completed a brief follow-up interview with study personnel. Participants in the device groups were asked about their satisfaction with the device and suggestions for use in further study.
Measures
Feasibility
Feasibility of the study protocol was assessed with data regarding the safety and acceptability of the CES device during the chemotherapy period and the ability to recruit and retain study participants. Adverse event data were collected over the study period. Feasibility of recruitment to the study was assessed as proportion of eligible to interested people, and feasibility of retention to the protocol was assessed as proportion of enrolled participants who completed the study.
Symptom ratings
Participants scored the severity of their symptoms on four scales. The Hospital Anxiety and Depression Scale (HADS) is a brief, 14-item, self-report questionnaire developed to detect the presence and severity of anxiety and depressive symptoms at the time of collection (Zigmond & Snaith, 1983). Because the HADS was developed for use in medically ill patients, it does not rely upon somatic symptoms of depression and anxiety such as pain and weight loss; instead, it focuses on cognitive symptoms of anxiety and depression. Each question is rated on a scale of 0–3, with a possible score of 0–21 for depression and anxiety each and a possible total score of 0–42. The HADS has well-established reliability and validity for depression and anxiety in women with breast cancer (Osborne, Elsworth, Sprangers, Oort, & Hopper, 2004). The Cronbach alpha in this study was 0.77 for the depression subscale and 0.81 for the anxiety subscale.
The Brief Pain Inventory–Short Form (BPI) (Cleeland & Ryan, 1994) is a pain assessment tool that has well-established reliability and validity for adult patients with no cognitive impairment in trajectory studies of cancer and its symptoms. It may be used as a self-report or via interview or an IVR telephone system. The BPI assesses the severity of pain, location of pain, pain medications, amount of pain relief in the prior 24 hours or the prior week, and the effect of pain on daily functions. The estimated time for completion of the BPI is five minutes. In the present study, “worst pain” or the arithmetic mean of the four severity items was used as a measure of pain severity, and the arithmetic mean of the seven interference items was used as a measure of pain interference. In widespread testing, the Cronbach alpha reliability has ranged from 0.7–0.91. The Cronbach alpha in this study was 0.89.
The Brief Fatigue Inventory (BFI) is a simple, nineitem scale that taps into a single dimension of fatigue severity and the interference fatigue creates in daily life (Mendoza et al., 1999). The BFI is a clinically validated tool used to assess cancer-related fatigue and its effect on daily functioning. The BFI uses simple numeric rating scales from 0–10 that are easily understood. On the BFI, severe fatigue is defined as a score of 7 or higher. The BFI has demonstrated excellent reliability in clinical trials, with Cronbach alpha ranging from 0.82–0.97 (Mendoza et al., 1999). The Cronbach alpha for the BFI in this study was 0.92.
The General Sleep Disturbance Scale (GSDS) is a 21-item tool (Lee, 1992). In the present study, participants rated the frequency of sleep problems over the prior week on a 0 (never) to 7 (every day) scale. The GSDS has well-established reliability and validity and has demonstrated robust psychometric properties, particularly in women. In the current study, the Cronbach alpha was 0.8.
Inflammatory biomarkers were cytokines (IL-6, TNF-α, IL-1β) and CRP. A 10 cc blood sample was collected from each participant without anticoagulant into serum separator vacutainers and allowed to coagulate for 20–30 minutes at room temperature. Sera were separated by centrifugation, and all specimens were aliquoted immediately, frozen, and stored in a −70°C freezer. Plasma concentrations of cytokines were measured with a bioplex assay. After incubation, contents of each microplate well were drawn into the Bio-Plex array reader, and precision fluidics aligned the beads in a single file through a flow cell where two lasers excited the beads individually. High-speed digital signal processors and Bio-Plex Manager software (Bio-Rad) recorded the fluorescent signals simultaneously for each bead. Levels of CRP were determined with a high-sensitivity enzyme-linked immunosorbent assay.
Data Analysis
Baseline descriptive statistics were computed for symptoms and biomarkers in the overall sample. Normality of each measure was assessed with the Shapiro-Wilk statistic. The researchers chose a nonparametric test instead of transforming each symptom measure because symptom measures with a value of zero are not defined for the log transformation. Differences in symptom levels between groups were examined at baseline (prior to the first chemotherapy) through three chemotherapy cycles. Changes in symptoms over time were assessed with random effects regression models. Because measurements were repeated within subject, random effects models were used to examine the trajectory of each symptom and biologic marker over time. Each symptom was the dependent variable, and time was the independent variable. The models account for repeated measurements on subjects over time by modeling a pattern of change (slope) in the individual and aggregating this change over time to the overall sample. All computations were completed with SAS® version 9.1, and significance level was set at α = 0.05. The intention-to-treat approach was adopted for the analysis.
Results
Sample Characteristics
The study originally included 36 women who were randomly assigned at baseline to the actual CES group, the sham CES group, or the standard care group. One of the 36 participants withdrew prior to completing the baseline data collection. The average participant was aged 48.3 ± 7.9 years (see Table 1). Sixty percent (n = 21) of the sample was Caucasian, 30% (n = 11) were African American, and 10% (n = 3) were other races. Eighteen women (51%) were postmenopausal, 16 (46%) premenopausal, and 1 (3%) perimenopausal.
Table 1.
Sample Characteristics
| Characteristic | SD | |
|---|---|---|
| Age (years) | 48.3 | 7.9 |
| Characteristic | n | % |
|---|---|---|
| Race | ||
| Caucasian | 21 | 60 |
| African American | 11 | 30 |
| Other | 3 | 10 |
| Menopausal status | ||
| Premenopausal | 16 | 46 |
| Perimenopausal | 1 | 3 |
| Postmenopausal | 18 | 51 |
| Treatment regimen | ||
| Two weeks | 16 | 46 |
| Three weeks | 19 | 54 |
| Chemotherapy | ||
| Adjuvant | 31 | 89 |
| Neoadjuvant | 4 | 11 |
N = 35
All 35 women received an anthracyline-based regimen, 31 adjuvant chemotherapy and 4 neo-adjuvant therapy. Chemotherapy intervals (time between chemotherapy infusions) were approximately balanced, with 16 (46%) receiving chemotherapy every two weeks and 19 (54%) receiving treatment every three weeks.
Measures of feasibility indicated that CES is a safe and acceptable modality during chemotherapy treatment. No adverse events were reported related to use of CES devices over the course of the study. Of the estimated 50 women who were identified by their healthcare providers as meeting the study criteria, 36 consented to be enrolled in the study, yielding a participation rate of 72%. Because of changes in interpretation of the privacy rule of the Health Insurance Portability and Accountability Act that occurred during the recruitment phase of the study, the researchers may not have captured the exact number of patients who were eligible for the study or who were informed by their healthcare providers about the study and were not interested in participating. Thirty-four of 36 completed the study through three cycles of chemotherapy. The feasibility and acceptability of CES devices during chemotherapy treatment were examined by participants’ reports of whether they stopped using their devices during the on-study period or indicated difficulty with using their devices. None of the participants reported stopping daily use of the CES device during the on-study period or indicated difficulty with using their devices.
At baseline, most participants had mild to moderate symptoms. Significant positive correlations existed among all symptoms except pain and anxiety (see Table 2). Symptoms of depression were most strongly correlated with other symptoms, whereas anxiety had more modest correlations with other symptoms. Significant and moderate positive correlations existed between symptoms of depression and TNF-α (r = 0.38, p = 0.03) and CRP (r = 0.52, p = 0.001). Symptoms of pain (r = 0.5, p = 0.003) and fatigue (r = 0.47, p = 0.004) also were strongly correlated with CRP, indicating that a common biologic mechanism may underlie these symptoms. Table 3 offers median values for symptoms over time. Levels of anxiety, sleep, and pain did not increase significantly over time, but symptoms of depression and fatigue did increase over time (p = 0.01 and 0.05, respectively). The median level of depressive symptoms in week 6 suggests that depressive symptoms went from mild to a potentially clinically significant level. The partially missing data complicated statistical analysis and the researchers’ ability to make valid inferences of the longitudinal data.
Table 2.
Baseline Descriptive Statistics for Symptoms and Cytokines by Group Assignment
| Variable | SD | Median | IQR | |
|---|---|---|---|---|
| Intervention group (n = 13) | ||||
| Age (years) | 47.54 | 9.1 | 50 | 8 |
| Depression | 2.08 | 2.97 | 1 | 2.5 |
| Anxiety | 4.42 | 3.29 | 3.5 | 4.5 |
| Fatigue | 1.19 | 1.47 | 0.78 | 2 |
| Sleep | 47.08 | 15.78 | 45 | 25 |
| Pain | 1.34 | 1.52 | 0.25 | 2.5 |
| Interleukin-6 (pg/ml) | 219.92 | 155.21 | 174 | 240.5 |
| Tumor necrosis factor-α (pg/ml) | 164.83 | 66.02 | 163.5 | 100 |
| Interleukin-1-β (pg/ml) | 101.38 | 86.03 | 70.5 | 51.5 |
| C-reactive protein (pg/ml) | 3,752.45 | 3,941.18 | 2,038.56 | 3,997 |
| Standard care group (n = 12) | ||||
| Age (years) | 50.5 | 8.28 | 52 | 9 |
| Depression | 3.83 | 3.3 | 3 | 5 |
| Anxiety | 7.92 | 3.6 | 8 | 4.5 |
| Fatigue | 3.27 | 2.32 | 3.44 | 4.78 |
| Sleep | 48.67 | 23.47 | 47 | 22 |
| Pain | 2.14 | 2.48 | 1.38 | 1.88 |
| Interleukin-6 (pg/ml) | 269.46 | 305.61 | 165.25 | 172.75 |
| Tumor necrosis factor-α (pg/ml) | 271.42 | 305.09 | 191 | 31.5 |
| Interleukin-1-β (pg/ml) | 219.71 | 457.35 | 57 | 86.5 |
| C-reactive protein (pg/ml) | 5,753.77 | 5,135.67 | 2,941.35 | 9,888 |
| Sham device group (n = 10) | ||||
| Age (years) | 46.6 | 5.64 | 49 | 9 |
| Depression | 4.33 | 3 | 5 | 5 |
| Anxiety | 6.2 | 4.13 | 6.5 | 5 |
| Fatigue | 2.4 | 2.5 | 2 | 3.22 |
| Sleep | 38.2 | 11.35 | 37.5 | 15 |
| Pain | 0.93 | 1.31 | 0.25 | 2 |
| Interleukin-6 (pg/ml) | 435.5 | 895.81 | 102 | 74 |
| Tumor necrosis factor-α (pg/ml) | 171.72 | 60.73 | 163 | 53.5 |
| Interleukin-1-β (pg/ml) | 248.39 | 463.93 | 64.5 | 83 |
| C-reactive protein (pg/ml) | 4,714.78 | 5,376.04 | 2,215.2 | 5,531 |
IQR—interquartile range
Note. Depression was statistically significant at p = 0.08.
Table 3.
Median Values for Symptoms Over Time
| Symptom | N | Median | IQR | p |
|---|---|---|---|---|
| Depression | 0.008 | |||
| Baseline | 33 | 2 | 5 | |
| Week 3 | 22 | 3.5 | 7 | |
| Week 6 | 15 | 8 | 9 | |
| Anxiety | 0.7 | |||
| Baseline | 34 | 6 | 5 | |
| Week 3 | 22 | 4.5 | 9 | |
| Week 6 | 15 | 6 | 7 | |
| Fatigue | 0.045 | |||
| Baseline | 33 | 1.22 | 2.89 | |
| Week 3 | 22 | 2 | 4.11 | |
| Week 6 | 17 | 2.56 | 3.22 | |
| Sleep | 0.58 | |||
| Baseline | 34 | 44 | 24 | |
| Week 3 | 22 | 38.5 | 17 | |
| Week 6 | 15 | 47 | 19 | |
| Pain | 0.99 | |||
| Baseline | 33 | 1 | 2.5 | |
| Week 3 | 20 | 0.25 | 2.13 | |
| Week 6 | 16 | 1.25 | 4.13 |
IQR—interquartile range
Although the use of a random effects models to account for repeated measurements as well as distinguish between treatment group differences was desired, the missing data did not provide enough power to adequately fit such models. Because of missing data, a more simplistic approach was taken in which the change in symptom scores over time was examined at three weeks. This time point was chosen in part because it was the midpoint of the study period with an average sample size of at least seven per treatment group (the highest number of differences per treatment group computable). Group differences in the change from baseline at three weeks were tested with the Kruskal-Wallis test for non-normal measures and analysis of variance if the measures were normal. A trend in differences among symptoms in the three groups was noted over time (see Figure 2). After three weeks, greater increases occurred in the symptoms of depression and sleep disturbances in the sham and standard care groups than occurred in the CES intervention group; however, the differences were not statistically significant.
Figure 2. Post Three-Week Change in Median Level of Symptoms by Assigned Group.
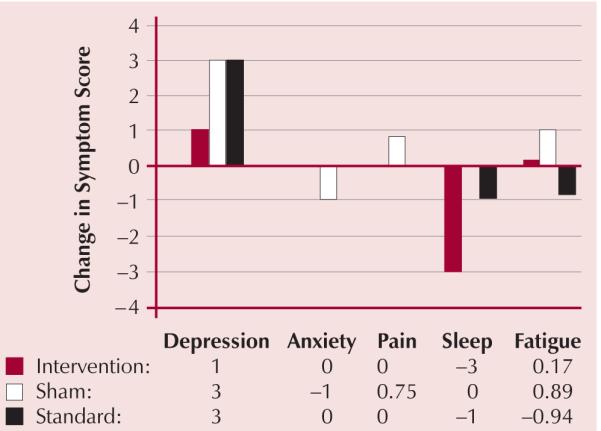
Discussion
The findings from this pilot study provide preliminary data on the feasibility of CES during chemotherapy treatment in women with breast cancer. The study design, except for the IVR, was supported by the feasibility parameters. Approximately three-quarters of eligible patients enrolled in the trial. None of the participants reported a problem or adverse events related to the CES devices. Collecting serum samples was feasible and did not appear to add participant burden. Study retention rates were excellent. However, the study design can be improved with closer monitoring of weekly symptom data instead of an IVR system. Although IVR systems have been used in cancer trials and have the potential advantages that participants can assess their symptoms at home and that the data are entered directly into a database (Cleeland, 2000), the authors noted several difficulties with IVR. The IVR system was not set up to be accessed by wireless phones; this was a problem because many participants did not have landline, touch-tone telephones. In 2006, 17% of adults with household incomes below 200% of the federal poverty thresholds, 25% of young adults aged 18–29 years, and 32% of young adults with low income lived in households with only cell phones (Galesic, Tourangeau, & Couper, 2006). Thus, the IVR system could have led to under-reporting of symptoms in a nonrandom manner.
The baseline results indicate a continued need for symptom management research in women with breast cancer. Prior to the initiation of chemotherapy, symptoms of anxiety, depression, fatigue, sleep disturbances, and pain are prevalent in women receiving treatment for breast cancer. Many of the symptoms worsened over time. Findings provide some support that the symptoms may form a cluster in women with breast cancer during chemotherapy (Dodd et al., 2004). The relationships among biomarkers of proinflammatory activation and symptoms at baseline indicate that the biobehavioral conceptual framework may be useful and potentially enlightening for further research. The findings have implications for the design of a future randomized clinical trial of CES use by women receiving chemotherapy for breast cancer. With design modifications suggested by the feasibility study, the authors recommend that data collection not rely on IVR systems. In person or telephone-supported data collection may work better during the busy chemotherapy period. A larger trial with adequate power to reliably define the effect of the CES intervention is warranted to further test the efficacy of this safe, patient-delivered CAM modality for women during the chemotherapy phase of treatment for breast cancer. Further exploration of nonpharmacologic modalities such as CES to decrease symptoms for patients with cancer is an area of continued need in symptom management research. As compared to other CAM modalities, CES has the advantage of being a self-managed modality that can be used in the home, sparing immunosuppressed patients from contact with others during this vulnerable period.
Implications for Nursing
The findings have implications for oncology nursing. The baseline data indicate a continued need for symptom management research in women with breast cancer. Prior to chemotherapy, anxiety, depression, fatigue, sleep disturbances, and pain are prevalent in women receiving treatment for breast cancer. Many of the symptoms worsened over time. Because women who were diagnosed with depression or were taking antidepressant or anti-anxiety medication were excluded from the study, the levels of symptoms may have been truncated. Thus, examining the symptom levels of all women receiving chemotherapy is needed, even those without histories suggesting vulnerability to depression and anxiety. Interventions that are effective at minimizing more than one target symptom are especially needed to provide optimal symptom management for women with breast cancer.
References
- Agency for Healthcare Research and Quality Management of cancer symptoms: Pain, depression, and fatigue. 2002 Retrieved from http://archive.ahrq.gov/clinic/epcsums/cansympsum.pdf.
- Anderson KO, Getto CJ, Mendoza TR, Palmer SN, Wang XS, Reyes-Gibby CC, Cleeland CS. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. Journal of Pain and Symptom Management. 2003;25:307–318. doi: 10.1016/s0885-3924(02)00682-6. doi: 10.1016/S0885-3924(02)00682-6. [DOI] [PubMed] [Google Scholar]
- Badger T, Segrin C, Dorros SM, Meek P, Lopez AM. Depression and anxiety in women with breast cancer and their partners. Nursing Research. 2007;56:44–53. doi: 10.1097/00006199-200701000-00006. [DOI] [PubMed] [Google Scholar]
- Barsevick AM. The concept of symptom cluster. Seminars in Oncology Nursing. 2007;23:89–98. doi: 10.1016/j.soncn.2007.01.009. doi: 10.1016/j.soncn.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Brebner K, Hayley S, Zacharko R, Merali Z, Anisman H. Synergistic effects of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha: Central monoamine, corticosterone, and behavioral variations. Neuropsychopharmacology. 2000;22:566–580. doi: 10.1016/S0893-133X(99)00166-9. doi: 10.1016/S0893-133X(99)00166-9. [DOI] [PubMed] [Google Scholar]
- Broeckel JA, Jacobsen PB, Horton J, Balducci L, Lyman GH. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. Journal of Clinical Oncology. 1998;16:1689–1696. doi: 10.1200/JCO.1998.16.5.1689. [DOI] [PubMed] [Google Scholar]
- Byar KL, Berger AM, Bakken SL, Cetak MA. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life [Online exclusive] Oncology Nursing Forum. 2006;33:E18–E26. doi: 10.1188/06.ONF.E18-E26. doi: 10.1188/06.ONF.E18-E26. [DOI] [PubMed] [Google Scholar]
- Cleeland CS. Cancer-related symptoms. Seminars in Radiation Oncology. 2000;10:175–190. doi: 10.1053/srao.2000.6590. doi: 10.1016/S1053-4296(00)80036-3. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, Lee BN. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- Dodd MJ, Miaskowski C, Lee KA. Occurrence of symptom clusters. Journal of the National Cancer Institute. Monographs, 2004. 2004;32:76–78. doi: 10.1093/jncimonographs/lgh008. doi: 10.1093/jncimonographs/lgh008. [DOI] [PubMed] [Google Scholar]
- Dow KH, Ferrell BR, Leigh S, Ly J, Gulasekaram P. An evaluation of the quality of life among long-term survivors of breast cancer. Breast Cancer Research and Treatment. 1996;39:261–273. doi: 10.1007/BF01806154. doi: 10.1007/BF01806154. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: What can we learn from animal studies? Neuroscience and Biobehavioral Reviews. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. Journal of Pain and Symptom Management. 2002;24:471–480. doi: 10.1016/s0885-3924(02)00500-6. [DOI] [PubMed] [Google Scholar]
- Galesic M, Tourangeau R, Couper MP. Complementing random-digit-dial telephone surveys with other approaches to collecting sensitive data. American Journal of Preventive Medicine. 2006;31:437–443. doi: 10.1016/j.amepre.2006.07.023. doi: 10.1016/j.amepre.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Grady PA. Biobehavioral research at NINR and NIH. Nursing Outlook. 2006;54:300–302. doi: 10.1016/j.outlook.2006.04.004. doi: 10.1016/j.outlook.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: Characteristics, course, and correlates. Journal of Pain and Symptom Management. 1999;18:233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- Kissane DW, Grabsch B, Love A, Clarke DM, Bloch S, Smith GC. Psychiatric disorder in women with early stage and advanced breast cancer: A comparative analysis. Australian and New Zealand Journal of Psychiatry. 2004;38:320–326. doi: 10.1080/j.1440-1614.2004.01358.x. doi: 10.1111/j.1440-1614.2004.01358.x. [DOI] [PubMed] [Google Scholar]
- Klawansky S, Yeung A, Berkey C, Shah N, Phan H, Chalmers TC. Meta-analysis of randomized controlled trials of cranial electrostimulation. Efficacy in treating selected psychological and physiological conditions. Journal of Nervous and Mental Disease. 1995;183:478–484. doi: 10.1097/00005053-199507000-00010. [DOI] [PubMed] [Google Scholar]
- Lee K, Cho M, Miaskowski C, Dodd M. Impaired sleep and rhythms in persons with cancer. Sleep Medicine Reviews. 2004;8:199–212. doi: 10.1016/j.smrv.2003.10.001. doi: 10.1016/j.smrv.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992;15:493–498. doi: 10.1093/sleep/15.6.493. [DOI] [PubMed] [Google Scholar]
- Lichtbroun AS, Raicer MM, Smith RB. The treatment of fibromyalgia with cranial electrotherapy stimulation. Journal of Clinical Rheumatology. 2001;7(2):72–78. doi: 10.1097/00124743-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Matthews AK, Sellergren SA, Huo D, List M, Fleming G. Complementary and alternative medicine use among breast cancer survivors. Journal of Alternative and Complementary Medicine. 2007;13:555–562. doi: 10.1089/acm.2007.03-9040. doi: 10.1089/acm.2007.03-9040. [DOI] [PubMed] [Google Scholar]
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. doi: 10.1002/(SICI)1097-0142 (19990301)85:5<1186::AID-CNCR24>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Aouizerat BE. Is there a biological basis for the clustering of symptoms? Seminars in Oncology Nursing. 2007;23:99–105. doi: 10.1016/j.soncn.2007.01.008. doi: 10.1016/j.soncn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Miller AH. Cytokines and sickness behavior: Implications for cancer care and control. Brain, Behavior, and Immunity. 2003;17(Suppl. 1):S132–S134. doi: 10.1016/s0889-1591(02)00080-6. [DOI] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. Journal of Clinical Oncology. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health Health information: Understanding alternative and complementary medicine. n.d. Retrieved from http://www.nccam.nih.gov.
- Osborne RH, Elsworth GR, Sprangers MA, Oort FJ, Hopper JL. The value of the Hospital Anxiety and Depression Scale (HADS) for comparing women with early onset breast cancer with population-based reference women. Quality of Life Research. 2004;13:191–206. doi: 10.1023/B:QURE.0000015292.56268.e7. doi: 10.1023/B:QURE.0000015292.56268.e7. [DOI] [PubMed] [Google Scholar]
- Reyes-Gibby CC, Aday LA, Anderson KO, Mendoza TR, Cleeland CS. Pain, depression, and fatigue in community-dwelling adults with and without a history of cancer. Journal of Pain and Symptom Management. 2006;32:118–128. doi: 10.1016/j.jpainsymman.2006.01.008. doi: 10.1016/j.jpainsymman.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DR, Ei S, Rogers KR, Cross CL. Evaluation of a multi-component approach to cognitive-behavioral therapy (CBT) using guided visualizations, cranial electrotherapy stimulation, and vibroacoustic sound. Complementary Therapies in Clinical Practice. 2007;13:95–101. doi: 10.1016/j.ctcp.2006.10.002. doi: 10.1016/j.ctcp.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Schmitt R, Capo T, Boyd E. Cranial electrotherapy stimulation as a treatment for anxiety in chemically dependent persons. Alcoholism, Clinical and Experimental Research. 1986;10(2):158–160. doi: 10.1111/j.1530-0277.1986.tb05064.x. [DOI] [PubMed] [Google Scholar]
- Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: Prevalence, correlates and interventions. European Journal of Cancer. 2002;38:27–43. doi: 10.1016/s0959-8049(01)00332-x. [DOI] [PubMed] [Google Scholar]
- Theobald DE. Cancer pain, fatigue, distress, and insomnia in cancer patients. Clinical Cornerstone. 2004;6(Suppl. 1D):S15–S21. doi: 10.1016/s1098-3597(05)80003-1. [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annual Review of Biomedical Engineering. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Winick RL. Cranial electrotherapy stimulation (CES): A safe and effective low cost means of anxiety control in a dental practice. General Dentistry. 1999;47:50–55. [PubMed] [Google Scholar]
- Wood LJ, Nail LM, Perrin NA, Elsea CR, Fischer A, Druker BJ. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biological Research for Nursing. 2006;8:157–169. doi: 10.1177/1099800406290932. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]


