Abstract
Objective
To determine the incidence and disease-specific predictors of clinically recognized psoriatic arthritis (PsA) in patients with psoriasis.
Methods
We identified an incidence cohort of psoriasis subjects age ≥18 years diagnosed between January 1, 1970 and December 31, 1999 in a population-based setting. Psoriasis diagnoses were validated by confirmatory diagnosis in the medical record. Incident and clinically recognized PsA subjects were classified according to the Classification of Psoriatic Arthritis (CASPAR) criteria. Cox proportional hazards models were used to identify predictors of PsA within the psoriasis cohort.
Results
The psoriasis incidence cohort comprised 1,633 subjects. Of these, 40 were diagnosed with PsA concurrently with psoriasis and were excluded from analysis. The remaining 1,593 psoriasis subjects had a mean age of 43 years and 50% were men. Over 20,936 person-years of followup, 57 subjects were clinically recognized with new-onset PsA, with a cumulative incidence of 1.7% (95% confidence interval [95% CI] 1.0–2.3%), 3.1% (95% CI 2.2– 4.1%), and 5.1% (95% CI 3.7–6.6%) at 5, 10, and 20 years following psoriasis incidence, respectively. Psoriasis features associated with higher risk of PsA were scalp lesions (hazard ratio [HR] 3.89, 95% CI 2.18–6.94), nail dystrophy (HR 2.93, 95% CI 1.68–5.12), and intergluteal/perianal lesions (HR 2.35, 95% CI 1.32–4.19). Calendar year was not associated with risk of PsA (P = 0.15), indicating that the likelihood of PsA in psoriasis subjects did not change over time.
Conclusion
In this population-based study, <10% of patients with psoriasis developed clinically recognized PsA during a 30-year period. Psoriasis features associated with a higher likelihood of PsA were nail dystrophy, scalp lesions, and intergluteal/perianal psoriasis.
INTRODUCTION
Individuals with psoriasis are at an increased risk for developing psoriatic arthritis (PsA) in their lifetime (1). First studied by Bourdillon in France, PsA was considered a manifestation of rheumatoid arthritis (RA) until the mid 20th century (2). PsA is now recognized as a distinct clinical entity, reportedly affecting 7–40% of patients with psoriasis (1,3–5). Despite treatment advances with newly developed immune modulating agents such as tumor necrosis factor α (TNFα) inhibitors, patients with PsA still experience significant morbidity, including progressive joint destruction, functional disability, and increased health care costs (6–9). Therefore, early diagnosis and treatment of PsA is recommended to avoid disease-associated disability and loss of work place productivity (10,11).
Although several studies have documented risk factors for development and progression of PsA, relatively little is known about the clinical features of psoriasis associated with PsA onset (3,7,10,12–18). In general, patients with severe psoriasis may have a higher risk of inflammatory arthritis than patients with mild psoriasis (19,20). In addition, oral corticosteroid exposure within 2 years prior to PsA diagnosis increases the risk of PsA, but pregnancy has a negative association with onset of PsA (17,18,21–23). Recently, physical trauma in patients with psoriasis ascribed as the deep Koebner phenomenon, as well as acute life stressors, were found to be associated with onset of PsA (17,21).
However, studies to date concerning the incidence and risk factors for PsA have been limited by small cross-sectional studies, selective study populations, limited followup, and PsA classification criteria lacking diagnostic sensitivity. More recently, the Classification of Psoriatic Arthritis (CASPAR) group developed classification criteria for PsA with a sensitivity of 91.4% and a specificity of 98.7% (24). Application of the new, validated diagnostic criteria offers a unique opportunity to document the incidence and factors predictive for the development of PsA in patients with psoriasis. Therefore, the objective of our study was to determine the cumulative incidence and clinical predictors for clinically recognized PsA in a stable, population-based incidence cohort of subjects with psoriasis followed over a 30-year period.
SUBJECTS AND METHODS
This was a longitudinal, retrospective, population-based cohort study of subjects with psoriasis performed using the resources of the Rochester Epidemiology Project medical records linkage system in Olmsted County, Minnesota. Population-based epidemiologic research is facilitated in Olmsted County because medical care is virtually self-contained within the community, and comprehensive medical records have been available for all residents seeking medical care from all health care providers for over half a century (25,26). Using the resources of the Rochester Epidemiology Project, nearly all clinically-recognized cases of psoriasis and PsA can be captured, along with access to the complete medical records for data collection.
Data collection and analysis
We identified and reviewed the medical records of subjects age ≥18 years with diagnoses consistent with psoriasis between January 1, 1970 and December 31, 1999 according to the following International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes: 696.1 psoriasis not otherwise specified, 696.2 psoriasiform dermatitis, 696.3 pityriasis rosea, 696.5 other and unspecified pityriasis, and 696.8 other psoriasis and similar disorders (27). Psoriasis diagnosis was confirmed either by documentation in the medical record by a dermatologist or by review of the medical record by the dermatologist investigator (MTM). A standardized, computerized data abstraction form was used to obtain information on demographics, clinical manifestations, type of psoriasis, site of psoriasis lesions, nail involvement, skin biopsy results, and dermatologist confirmation. The type of psoriasis was determined based on physician diagnosis and/or description of characteristic lesions in the medical records, and classified as chronic plaque psoriasis, guttate psoriasis, erythrodermic psoriasis, pustular psoriasis, and sebopsoriasis. If the type of psoriasis was not specified in the medical record, then it was assumed to be chronic plaque psoriasis.
The medical records of patients with a potential diagnosis of arthritis (both within and outside the psoriasis cohort) between January 1, 1970 and December 31, 1999 were reviewed by the first author (FCW) using a standardized, computerized data abstraction form (28). These patients were clinically suspected cases of PsA and were identified using the following ICD-9 and HICDA (Hospital Adaptation of International Classification of Diseases) diagnostic codes: arthralgias, arthritis, monarthritis, oligoarthritis, polyarthritis, spondylitis, ankylosing spondylitis, arthropathy, psoriatic arthropathy, spondylarthropathy, and seronegative spondylarthropathy. Data collection included demographics, family and personal history of psoriasis, clinical manifestations such as joint pattern and presence of dactylitis, laboratory data such as presence of rheumatoid factor (RF), and radiographic features typical of PsA in the hand and wrist, foot, and spine. Only subjects who satisfied the CASPAR criteria with a score of ≥3 were included in the study (24). The CASPAR criteria requires a diagnosis of inflammatory articular disease (joint, spine, entheseal) documented by either a primary care physician or a rheumatologist, and achievement of a score of ≥3 of the following 5 CASPAR criteria: 1) current psoriasis (score of 2), personal history of psoriasis, or family history of psoriasis; 2) nail dystrophy such as onycholysis, pitting, or hyperkeratosis; 3) a negative RF; 4) current dactylitis or a personal history of dactylitis as recorded by a physician; and 5) radiographic evidence of psoriatic bone changes of the hand or foot, such as juxta-articular new bone formation on plain films.
Statistical analysis
Descriptive statistics including means, medians, and SDs were used to summarize the data. Subjects with missing data (e.g., no documentation in medical record) for a particular feature were excluded from percentage calculations for that feature. Chi-square and rank sum tests were used to test for differences between groups. Subjects with PsA diagnosed concurrently with psoriasis (at the same time or up to 90 days following psoriasis diagnosis) were excluded from analyses. Cumulative incidence of PsA over time was calculated using Kaplan-Meier methods (29). The log rank test was used to compare the cumulative incidence curves for men and women. Cox proportional hazards models were used to identify the clinical characteristics of psoriasis associated with PsA. Subjects with no affected site of psoriasis specified were categorized as unknown for site and number of affected sites. The multivariable model, adjusted for age, sex, and calendar year, was developed using both forward and backward selection algorithms. P values less than 0.05 were considered statistically significant.
RESULTS
The study population comprised a psoriasis incident cohort of 1,633 subjects first diagnosed with psoriasis between 1970 and 2000. In this cohort, we identified 125 potential PsA subjects and validated PsA using CASPAR criteria in 97 subjects. The 28 subjects who did not fulfill CASPAR criteria were mostly cases of osteoarthritis or RA, or had trauma-associated or mechanical musculoskeletal pain. A total of 40 (2.4%) subjects were diagnosed with PsA at the same time as the psoriasis diagnosis (or within 90 days) and were excluded from analyses. The mean ± SD age at psoriasis incidence of the remaining 1,593 subjects was 43.3 ± 17.1 years, and 50% were women (Table 1). The most common type of psoriasis was plaque psoriasis in 1,258 (79%) subjects, followed by guttate psoriasis in 130 (8.2%), sebopsoriasis in 86 (5.4%), and pustular psoriasis in 53 (3.3%). The most common site for psoriasis at initial presentation was the scalp in 662 (42%) subjects, followed by elbows and knees in 551 (35%) and the trunk in 312 (20%). Almost half of the subjects (45%) presented with multiple affected sites. A total of 83 (86%) subjects with PsA presented with inflammatory joint disease, followed by enthesopathy in 29 (30%) subjects and inflammatory back pain in 7 (7%) subjects. The most common enthesopathy sites were in the plantar fascia (9%), finger flexor tendons (7%), and Achilles tendon (7%). Thirteen (13%) patients with PsA presented with a combination of inflammatory sites. At PsA incidence, 49% of subjects had oligoarticular and 39% had polyarticular involvement.
Table 1.
Clinical characteristics of 1,593 incident psoriasis subjects (January 1, 1970 through December 31, 1999, Olmsted County, Minnesota) without PsA at time of psoriasis diagnosis*
| Total | |
|---|---|
| Age, years | |
| Mean ± SD | 43.3 ± 17.1 |
| Median (minimum, maximum) | 39.8 (18.0, 98.2) |
| Sex | |
| Men | 796 (50) |
| Women | 797 (50) |
| Type of psoriasis | |
| Plaque | 1,258 (79) |
| Guttate | 130 (8.2) |
| Sebopsoriasis | 86 (5.4) |
| Pustular | 53 (3.3) |
| Unknown | 97 (6.1) |
| Site of psoriasis | |
| Scalp | 662 (42) |
| Extremities | 551 (35) |
| Trunk | 312 (20) |
| Intergluteal/perianal | 220 (14) |
| Face | 171 (11) |
| Palms and/or soles | 117 (7.3) |
| Axilla/groin | 61 (3.8) |
| Unknown | 126 (7.9) |
| Nail dystrophy | 224 (14) |
Values are the number (percentage) unless otherwise indicated.
PsA = psoriatic arthritis.
Cumulative incidence of PsA in psoriasis
The entire psoriasis cohort had 20,936 person-years of followup, with a mean ± SD of 13.1 ± 8.8 years per subject. A total of 901 (56%) and 353 (22%) subjects were under observation at 10 and 20 years, respectively, after psoriasis incidence. During the 20,936 person-years of followup, 57 subjects developed new-onset PsA, corresponding to a rate of 2.7 per 1,000 person-years (95% confidence interval [95% CI] 2.1–3.5). The cumulative incidence of PsA at 5, 10, and 20 years following psoriasis incidence was 1.7% (95% CI 1.0–2.3%), 3.1% (95% CI 2.2–4.1%), and 5.1% (95% CI 3.7–6.6%), respectively (Figure 1). When we also considered 40 subjects who had PsA at psoriasis incidence, cumulative incidence at 20 years reached 7.5%. The cumulative incidence of PsA at 20 years was slightly higher in men (6.4%) than in women (3.9%), but the difference was not statistically significant (log rank P = 0.11) (Figure 2).
Figure 1.
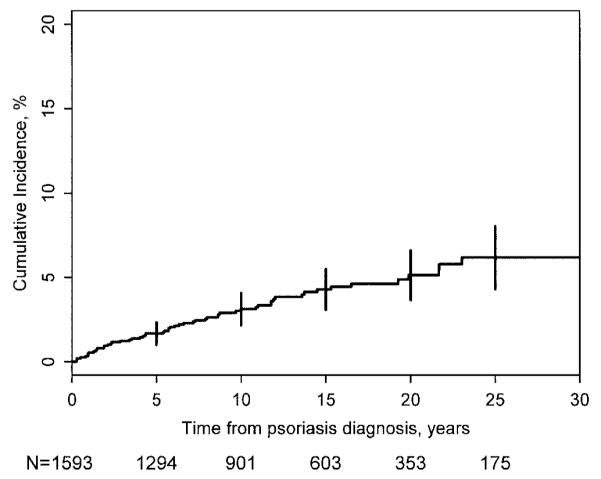
Cumulative incidence of psoriatic arthritis in an incidence cohort of subjects with psoriasis. The number of subjects with psoriasis under observation at different time points are shown at the bottom of the x-axis.
Figure 2.
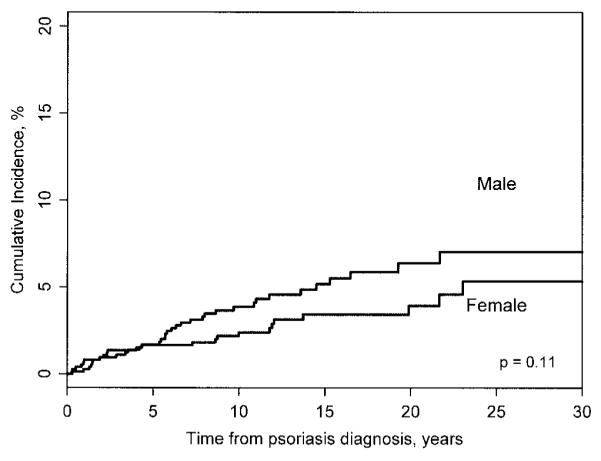
Cumulative incidence of psoriatic arthritis by sex.
Clinical predictors for development of PsA
The clinical characteristics associated with the risk of PsA in the psoriasis cohort are shown in Table 2. Calendar year was not associated with increased risk of PsA (P = 0.15), indicating that the likelihood of developing PsA among subjects with psoriasis did not increase over the 3 decades. For example, a newly diagnosed psoriasis subject in the 1990s is not more likely to develop PsA than a psoriasis subject in the 1970s. Younger age at psoriasis onset was associated with a slightly decreased risk of PsA (hazard ratio [HR] 0.91, 95% CI 0.77–1.07) but this was not statistically significant (P = 0.25).
Table 2.
Predictors of psoriatic arthritis within the incidence cohort of psoriasis subjects*
| Univariate | Multivariate | |
|---|---|---|
| Age, years† | 0.91 (0.77–1.07) | 0.92 (0.78–1.09) |
| Men (vs. women) | 1.53 (0.90–2.59) | 1.35 (0.78–2.33) |
| Calendar year† | 0.78 (0.56–1.09) | 0.76 (0.54–1.08) |
| Type of psoriasis | ||
| Plaque | 1.72 (0.81–3.63) | |
| Guttate | 0.36 (0.09–1.48) | |
| Sebopsoriasis | 1.96 (0.78–4.93) | |
| Pustular | – | |
| Unknown | 0.82 (0.26–2.63) | |
| Site of psoriasis | ||
| Scalp | 3.89 (2.18–6.94) | 3.75 (2.09–6.71) |
| Extremities | 0.83 (0.47–1.45) | |
| Trunk | 0.80 (0.40–1.58) | |
| Intergluteal/perianal | 2.35 (1.32–4.19) | 1.95 (1.07–3.56) |
| Face | 1.15 (0.52–2.53) | |
| Palms and/or soles | 0.20 (0.03–1.47) | |
| Axilla/groin | 1.40 (0.44–4.48) | |
| Unknown | 0.88 (0.32–2.44) | |
| No. of affected sites‡ | ||
| Unknown | 1.06 (0.36–3.07) | |
| 2 sites | 0.77 (0.37–1.64) | |
| ≥3 sites | 2.24 (1.23–4.08) | |
| Nail dystrophy | 2.93 (1.68–5.12) | 2.24 (1.26–3.98) |
Values are the hazard ratio (95% confidence interval).
Per 10-year increase.
Versus 1.
Psoriasis features that were significantly associated with a higher risk of PsA were scalp lesions, nail dystrophy, and intergluteal/perianal lesions. The risk of PsA was 3.89-fold higher among psoriasis subjects with scalp lesions (HR 3.89, 95% CI 2.18–6.94) compared with those without scalp lesions. Similarly, psoriasis subjects with nail dystrophy were almost 3 times more likely to develop PsA (HR 2.93, 95% CI 1.68–5.12) than subjects without nail dystrophy. Finally, the risk of PsA was 2.35-fold higher among psoriasis subjects with intergluteal/perianal lesions (HR 2.35, 95% CI 1.32–4.19) compared with those without these lesions. Furthermore, the risk of PsA increased with the number of affected sites; the risk of PsA in psoriasis subjects with ≥3 affected sites was 2.24 (95% CI 1.23–4.08). These strong associations remained strong and significant in a multivariable model that included all significant characteristics from the univariate models (Table 2).
Comparison of PsA characteristics
We then examined the demographic, clinical, and radiographic characteristics of 40 subjects with PsA who initially presented with psoriasis versus 57 subjects who developed PsA several years after the onset of psoriasis (Table 3). PsA subjects who presented concurrently with psoriasis were younger, more likely to be male, and more likely to have a family history of psoriasis compared with PsA subjects who developed PsA after psoriasis.
Table 3.
Clinical characteristics of psoriatic arthritis within the incidence cohort of psoriasis subjects*
| Onset concurrent with psoriasis (n = 40) |
Onset following psoriasis (n = 57) |
P | |
|---|---|---|---|
| Age at diagnosis, mean ± SD years | |||
| Psoriasis | 37.5 ± 15.1 | 40.3 ± 13.5 | 0.18 |
| Psoriatic arthritis | 37.5 ± 15.0 | 47.7 ± 13.1 | < 0.001 |
| Men | 32 (80) | 34 (60) | 0.03 |
| Family history of psoriasis | 10 (25) | 8 (14) | 0.17 |
| Inflammatory disease joint | 31 (78) | 52 (91) | 0.06 |
| Enthesitis | 14 (35) | 15 (26) | 0.36 |
| Back | 3 (7.5) | 4 (7.0) | 0.93 |
| Clinical features | |||
| Negative rheumatoid factor† | 35 (100) | 47 (98) | 0.39 |
| Radiographic findings‡ | 15 (45) | 31 (56) | 0.07 |
| Nail dystrophy | 15 (38) | 24 (42) | 0.65 |
| Dactylitis | 11 (28) | 12 (21) | 0.46 |
| Radiographic erosions | 8 (53) | 19 (61) | 0.61 |
Values are the number (percentage) unless otherwise indicated. Psoriatic arthritis classified according to the Classification of Psoriatic Arthritis (CASPAR) criteria (27).
14 charts did not provide information on patients who had onset of arthritis before or after psoriasis diagnosis.
9 charts did not provide information on patients who had onset of arthritis before or after psoriasis diagnosis.
DISCUSSION
This study provides a comprehensive description of the cumulative incidence and clinical predictors of clinically recognized PsA over an extended followup period of a population-based inception cohort of subjects with psoriasis. We have extended the findings of previous cross-sectional studies by describing the occurrence of PsA over 30 years in psoriasis subjects using the CASPAR criteria. In our study, PsA was clinically recognized in <10% of psoriasis patients during their lifetime, and scalp psoriasis, nail dystrophy, and intergluteal/perianal psoriasis were significantly associated with an increased PsA risk. Furthermore, the risk of PsA increases in subjects with ≥3 psoriasis sites, suggesting that the risk of PsA is higher in psoriasis subjects with more extensive disease.
One of the major conundrums in the epidemiology of psoriasis and PsA is the prevalence of PsA in psoriasis patients. Estimates range widely from 7–40%, but it is unclear whether these estimates are reflective of the true incidence and prevalence of PsA in a population of psoriasis subjects representative of the entire disease spectrum (1,3,4,19,30–34). There are 3 main reasons for the wide variability of these estimates. First, selection bias may be a major problem: the majority of psoriasis study populations have been ascertained at referral centers, so these patients may reflect only severe psoriasis cases. Second, the definitions used to identify PsA subjects have been variable with the use of self-reporting methods and/or nonspecific definitions. Third, the majority of the studies have been cross-sectional and not truly reflective of the evolution of PsA from the time of psoriasis diagnosis to death.
Our study overcomes these limitations by identifying both psoriasis and PsA subjects over time, and by examining the incidence of clinically recognized PsA over a 10-year period in a population-based cohort representative of the entire psoriasis spectrum, including mild and moderate cases of psoriasis occurring in the community. Based upon this stable population, our findings indicate that the incidence of clinically recognized PsA in psoriasis subjects is at most 7.5%, substantially less than earlier cross-sectional estimates. However, this estimate does not include clinically unrecognized cases of PsA that never came to medical attention. Our average 13 years of followup period may be too short to capture all PsA cases. Furthermore, our psoriasis cohort did not include childhood-onset psoriasis cases who may have a higher risk of PsA than adult-onset psoriasis cases. Alternatively, the demographics of several epidemiologic studies included slightly more men than women in the 30–40-year age range, a group with a higher prevalence of PsA than our study (1,5).
Furthermore, our findings suggest that the risk for PsA may be slightly higher in men than in women. A limited number of previous studies have addressed the potential role of hormonal influences in the pathogenesis of PsA (18,22,23). In one study of 33 women with PsA, 6 developed PsA in the postpartum period and 8 patients had a postpartum flare, presumably when estrogen levels were lowest. Also, 8 women had postmenopausal onset of PsA, 5 of them within 2 years of menopause (22). In addition, Østensen reported that 8 out of 10 patients with PsA improved during pregnancy and half of the patients flared postpartum (23). Additionally, Thumboo and colleagues found that women were less likely to develop PsA if they had a pregnancy within 2 years of psoriasis onset (18). These studies suggest that estrogen levels may protect against the development of PsA; alternatively, a relative increase in testosterone with declining estrogen levels may serve as an effect modifier toward PsA development. Sex-specific PsA incidence trends also provide indirect support for hormonal influences in the etiopathogenesis of PsA (28). However, these epidemiologic observations are insufficient to elucidate the immunomodulating effects of estrogens and other sex hormones. More research is needed to understand the interactions between the hormonal and inflammatory pathways in the etiology and course of psoriasis and PsA, involving both men and women at different stages of the human life span, including pregnancy and menopause (35,36).
Although various studies have examined the risk factors for the development and progression of PsA, none have explored the psoriasis-specific disease features associated with an increased risk of PsA. In our study, we demonstrated that scalp lesions, intergluteal/perianal psoriasis, and nail dystrophy, as well as the involvement of 3 or more sites, were significantly associated with an increased risk of developing PsA. Our findings are, in part, consistent with previous studies suggesting that larger, more severe psoriatic lesions are associated with PsA (19,37,38). The proinflammatory cytokine TNFα has been shown to be expressed in the synovium, synovial lining, and skin lesions of PsA patients; therefore, it is plausible that larger psoriatic lesions may result in increased systemic levels of TNFα and therefore play a role in PsA development (39,40). Alternatively, larger psoriatic plaques are more likely to be colonized by microorganisms, serving as entry portals for microbial byproducts to “trigger … a sustained but inadequate immune response,” thus providing a mechanism for chronic inflammation and PsA development (39). Therefore, scalp lesions and intergluteal/perianal lesions may be associated with PsA, given the predominance of microbial flora in those areas. However, the precise pathophysiologic mechanism between PsA and psoriasis has yet to be elucidated because PsA flares and eruption of skin disease do not always correspond (41).
It is unclear why nail dystrophy may be associated with a higher risk of PsA. Previous studies have also suggested that nail dystrophy may be associated with an increased risk for PsA, although not necessarily correlated with disease severity (18,41–44). It is plausible that dystrophic nails may serve as a marker for increased immunoreactivity leading to PsA in a subset of psoriasis patients. A recent study by Scarpa and colleagues suggests that nail involvement is present in almost all PsA, even though it may not always be clinically evident, and distal interphalangeal joint involvement may be secondary to nail involvement (45). The study raises the possibility that nail dystrophy in psoriasis patients can be an indicator of ongoing involvement of the distal phalanx. Clinicians should therefore consider nail dystrophy as highly informative toward predicting the development of PsA.
A significant conclusion in our study is that the CASPAR criteria can be used to retrospectively identify incident PsA subjects, provided that detailed documentation of clinical characteristics and radiographic results are available. However, we may have missed PsA cases that presented with little or no skin disease because inflammatory arthritis may precede psoriasis in ~15% of subjects (46). In addition, some PsA cases may not be clinically recognized and/or evaluated for presence of inflammatory arthropathy, resulting in an underestimation of the true incidence of PsA in psoriasis. In our study, 124 (84.4%) of the 147 patients with PsA had a rheumatologist’s confirmation of PsA. The only way to define the true incidence of PsA in psoriasis is to prospectively screen all psoriasis patients for an inflammatory arthritis. However, due to the extended study period and availability of near-complete medical history in this population, we expected that most PsA cases would have been ascertained. Other potential limitations common to most retrospective reviews include detection/reviewer bias and variability in clinician documentation in the medical record. To minimize potential errors, the majority of data abstraction was performed by one of the study investigators (FCW) using a standardized, pretested data abstraction form that outlined variable definitions from the CASPAR criteria, and questions that arose were discussed with other coinvestigators and resolved by consensus. In addition, psoriasis features examined in this study are limited to baseline disease features and do not take into account the evolution of psoriasis-specific disease features and treatment over time. Furthermore, the radiographic results in earlier years of the study were available only in paper format, with reports of “erosions typical of psoriatic arthritis” with no further elaboration. Hard copies of radiographic films were not reviewed for detailed confirmation of presence or absence of individual radiographic features. Finally, the population of Olmsted County, Minnesota is a predominantly (90%) white population, limiting the generalization of our findings for racially diverse populations.
In summary, our population-based data demonstrates that <10% of psoriasis patients develop PsA over a 30-year period. Significant predicting factors for the development of PsA in psoriasis include scalp lesions, intergluteal/perianal lesions, and nail dystrophy. Clinicians should therefore be cognizant that certain clinical features may be highly associated with the development of PsA in psoriasis patients.
ACKNOWLEDGMENTS
We wish to acknowledge Mitch Bais for his assistance in obtaining medical records, David Tines for preparing the computerized data abstraction screens, and Justin Carlin for data management.
Supported by an unrestricted research grant from Amgen.
Floranne C. Wilson, MD, Murat Icen, MD, Cynthia S. Crowson, MS, Marian T. McEvoy, MD, Sherine E. Gabriel, MD, MSc, Hilal Maradit Kremers, MD, MSc: The Mayo Clinic, College of Medicine, Rochester, Minnesota.
REFERENCES
- 1.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–7. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neill T, Silman AJ. Psoriatic arthritis: historical background and epidemiology. Baillieres Clin Rheumatol. 1994;8:245–61. doi: 10.1016/s0950-3579(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 3.Gladman DD. Natural history of psoriatic arthritis. Baillieres Clin Rheumatol. 1994;8:379–94. doi: 10.1016/s0950-3579(94)80024-3. [DOI] [PubMed] [Google Scholar]
- 4.Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol. 2003;4:441–7. doi: 10.2165/00128071-200304070-00001. [DOI] [PubMed] [Google Scholar]
- 5.Gisondi P, Girolomoni G, Sampogna F, Tabolli S, Abeni D. Prevalence of psoriatic arthritis and joint complaints in a large population of Italian patients hospitalised for psoriasis. Eur J Dermatol. 2005;15:279–83. [PubMed] [Google Scholar]
- 6.Ackermann C, Kavanaugh A. Economic burden of psoriatic arthritis. Pharmacoeconomics. 2008;26:121–9. doi: 10.2165/00019053-200826020-00003. [DOI] [PubMed] [Google Scholar]
- 7.McHugh NJ, Balachrishnan C, Jones SM. Progression of peripheral joint disease in psoriatic arthritis: a 5-yr prospective study. Rheumatology (Oxford) 2003;42:778–83. doi: 10.1093/rheumatology/keg217. [DOI] [PubMed] [Google Scholar]
- 8.Bond SJ, Farewell VT, Schentag CT, Gladman DD. Predictors for radiological damage in psoriatic arthritis: results from a single centre. Ann Rheum Dis. 2007;66:370–6. doi: 10.1136/ard.2006.056457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kane D, Stafford L, Bresnihan B, FitzGerald O. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford) 2003;42:1460–8. doi: 10.1093/rheumatology/keg384. [DOI] [PubMed] [Google Scholar]
- 10.Mease P. Psoriatic arthritis update. Bull NYU Hosp Jt Dis. 2006;64:25–31. [PubMed] [Google Scholar]
- 11.Kavanaugh AF, Ritchlin CT. Systematic review of treatments for psoriatic arthritis: an evidence based approach and basis for treatment guidelines. J Rheumatol. 2006;33:1417–21. [PubMed] [Google Scholar]
- 12.Gladman DD, Farewell VT, Nadeau C. Clinical indicators of progression in psoriatic arthritis: multivariate relative risk model. J Rheumatol. 1995;22:675–9. [PubMed] [Google Scholar]
- 13.Gladman DD, Farewell VT, Wong K, Husted J. Mortality studies in psoriatic arthritis: results from a single outpatient center. II. Prognostic indicators for death. Arthritis Rheum. 1998;41:1103–10. doi: 10.1002/1529-0131(199806)41:6<1103::AID-ART18>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 14.Kammer GM, Soter NA, Gibson DJ, Schur PH. Psoriatic arthritis: a clinical, immunologic and HLA study of 100 patients. Semin Arthritis Rheum. 1979;9:75–97. doi: 10.1016/s0049-0172(79)80001-3. [DOI] [PubMed] [Google Scholar]
- 15.Wong K, Gladman DD, Husted J, Long JA, Farewell VT. Mortality studies in psoriatic arthritis: results from a single out-patient clinic. I. Causes and risk of death. Arthritis Rheum. 1997;40:1868–72. doi: 10.1002/art.1780401021. [DOI] [PubMed] [Google Scholar]
- 16.Queiro-Silva R, Torre-Alonso JC, Tinture-Eguren T, Lopez-Lagunas I. A polyarticular onset predicts erosive and deforming disease in psoriatic arthritis. Ann Rheum Dis. 2003;62:68–70. doi: 10.1136/ard.62.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pattison EJ, Harrison BJ, Griffiths CE, Silman AJ, Bruce IN. Environmental risk factors for the development of psoriatic arthritis: results from a case control study. Ann Rheum Dis. 2008;67:672–6. doi: 10.1136/ard.2007.073932. [DOI] [PubMed] [Google Scholar]
- 18.Thumboo J, Uramoto K, Shbeeb MI, O’Fallon WM, Crowson CS, Gibson LE, et al. Risk factors for the development of psoriatic arthritis: a population based nested case control study. J Rheumatol. 2002;29:757–62. [PubMed] [Google Scholar]
- 19.Gelfand JM, Gladman DD, Mease PJ, Smith N, Margolis DJ, Nijsten T, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53:573. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 20.Scarpa R, Oriente P, Pucino A, Torella M, Vignone L, Riccio A, et al. Psoriatic arthritis in psoriatic patients. Br J Rheumatol. 1984;23:246–50. doi: 10.1093/rheumatology/23.4.246. [DOI] [PubMed] [Google Scholar]
- 21.Scarpa R, Del Puente A, di Girolamo C, della Valle G, Lubrano E, Oriente P. Interplay between environmental factors, articular involvement, and HLA-B27 in patients with psoriatic arthritis. Ann Rheum Dis. 1992;51:78–9. doi: 10.1136/ard.51.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McHugh NJ, Laurent MR. The effect of pregnancy on the onset of psoriatic arthritis. Br J Rheumatol. 1989;28:50–2. doi: 10.1093/rheumatology/28.1.50. [DOI] [PubMed] [Google Scholar]
- 23.Ostensen M. Pregnancy in psoriatic arthritis. Scand J Rheumatol. 1988;17:67–70. doi: 10.3109/03009748809098763. [DOI] [PubMed] [Google Scholar]
- 24.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, the CASPAR Study Group Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–73. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 25.Melton L. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 26.Kremers H Maradit, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30:819–34. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Kremers H Maradit. Trends in incidence of psoriasis over three decades: a population based study. J Am Acad Dermatol. 2009 doi: 10.1016/j.jaad.2008.10.062. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers H Maradit. Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: a population-based study. J Rheumatol. doi: 10.3899/jrheum.080691. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 30.Alenius GM, Stenberg B, Stenlund H, Lundblad M, Dahlqvist SR. Inflammatory joint manifestations are prevalent in psoriasis: prevalence study of joint and axial involvement in psoriatic patients, and evaluation of a psoriatic and arthritic questionnaire. J Rheumatol. 2002;29:2577–82. [PubMed] [Google Scholar]
- 31.Salvarani C, Lo Scocco G, Macchioni P, Cremonesi T, Rossi F, Mantovani W, et al. Prevalence of psoriatic arthritis in Italian psoriatic patients. J Rheumatol. 1995;22:1499–503. [PubMed] [Google Scholar]
- 32.Stern RS. The epidemiology of joint complaints in patients with psoriasis. J Rheumatol. 1985;12:315–20. [PubMed] [Google Scholar]
- 33.Green L, Meyers OL, Gordon W, Briggs B. Arthritis in psoriasis. Ann Rheum Dis. 1981;40:366–9. doi: 10.1136/ard.40.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Romunde LK, Valkenburg HA, Swart-Bruinsma W, Cats A, Hermans J. Psoriasis and arthritis. I. A population study. Rheumatol Int. 1984;4:55–60. doi: 10.1007/BF00541197. [DOI] [PubMed] [Google Scholar]
- 35.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 36.Cutolo M, Capellino S, Sulli A, Serioli B, Secchi ME, Villaggio B, et al. Estrogens and autoimmune diseases. Ann N Y Acad Sci. 2006;1089:538–47. doi: 10.1196/annals.1386.043. [DOI] [PubMed] [Google Scholar]
- 37.Leonard DG, O’Duffy JD, Rogers RS. Prospective analysis of psoriatic arthritis in patients hospitalized for psoriasis. Mayo Clin Proc. 1978;53:511–8. [PubMed] [Google Scholar]
- 38.Molin L. Psoriasis: a study of the course and degree of severity, joint involvement, socio-medical conditions, general morbidity and influences of selection factors among previously hospitalized psoriatics. Acta Derm Venereol Suppl (Stockh) 1973;53:1–125. [PubMed] [Google Scholar]
- 39.Anandarajah AP, Ritchlin CT. Pathogenesis of psoriatic arthritis. Curr Opin Rheumatol. 2004;16:338–43. doi: 10.1097/01.bor.0000129718.13939.81<. [DOI] [PubMed] [Google Scholar]
- 40.Hueber AJ, McInnes IB. Immune regulation in psoriasis and psoriatic arthritis: recent developments. Immunol Lett. 2007;114:59–65. doi: 10.1016/j.imlet.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA): an analysis of 220 patients. Q J Med. 1987;62:127–41. [PubMed] [Google Scholar]
- 42.Wright V, Roberts MC, Hill AG. Dermatological manifestations in psoriatic arthritis: a follow-up study. Acta Derm Venereol. 1979;59:235–40. [PubMed] [Google Scholar]
- 43.Williamson L, Dalbeth N, Dockerty JL, Gee BC, Weatherall R, Wordsworth BP. Extended report: nail disease in psoriatic arthritis: clinically important, potentially treatable and often overlooked. Rheumatology (Oxford) 2004;43:790–4. doi: 10.1093/rheumatology/keh198. [DOI] [PubMed] [Google Scholar]
- 44.Serarslan G, Guler H, Karazincir S. The relationship between nail- and distal phalangeal bone involvement severity in patients with psoriasis. Clin Rheumatol. 2007;26:1245–7. doi: 10.1007/s10067-006-0476-y. [DOI] [PubMed] [Google Scholar]
- 45.Scarpa R, Soscia E, Peluso R, Atteno M, Manguso F, Del Puente A, et al. Nail and distal interphalangeal joint in psoriatic arthritis. J Rheumatol. 2006;33:1315–9. [PubMed] [Google Scholar]
- 46.Leung Y, Lim K. Apsoriatic psoriatic arthritis. APLAR J Rheumatol. 2007;10:264–9. [Google Scholar]


