Abstract
Wing geometric morphometry of Triatoma infestans (Klug) (Hemiptera: Reduviidae) populations in northwestern Argentina showed that individual collection sites represent the discrete unit where metric differentiation took place. Here we studied temporal variations in wing size and shape of T. infestans populations from defined capture sites on three occasions between 2000 and 2003. Bugs collected from domiciles and/or storerooms had significantly larger right-wing centroid size than bugs collected at goat and/or pig corrals by the end of summer 2000 for both sexes. Conversely, male bugs collected from domiciles and/or storerooms had significantly smaller centroid size than bugs collected from pig corrals in spring 2002. The inversion in wing centroid size between seasons was consistent between sexes. Wing shape analysis from the south-central extreme of the study village showed divergence between collection dates for both sexes. Wing shape divergence was highly significant between male bugs collected by the end of summer 2000 and those collected in spring 2002 and by the end of summer 2003. For females, wing shape divergence was marginally significant between the end of summer 2000 and spring 2002, and significant between spring 2002 and the end of summer 2003. Unlike season-related variations in wing centroid size, shape differentiation was related to the time period elapsed between sample collections and suggested genetic influences acting on shape. Simultaneous consideration of wing size and shape provided complementary information on the direction and timing of bug dispersal. Morphological studies may allow determining the degree of relatedness of different bug populations and to associate morphological heterogeneity with temporal patterns of reinfestation.
Keywords: geometric morphometry, seasonal variations, dispersal, size, Triatoma infestans
Triatoma infestans (Klug) (Hemiptera: Reduviidae), the principal vector of Trypanosoma cruzi, occurs almost exclusively in domestic or peridomestic habitats except in Bolivia (Noireau et al. 2005). The domestic environment (human habitations) is where the transmission of T. cruzi to humans mostly occurs as a result of the interaction between people and domestic triatomine bugs, dogs, cats, and chickens (Cohen and Gürtler 2001). The peridomestic rural environment usually includes a wide array of structures, which in northern Argentina are frequently infested by T. infestans and other triatomine species (Gürtler et al. 1999). Peridomestic sites apparently were the most important sources of T. infestans that reinvaded the house after residual spraying of insecticides (Cecere et al. 2002, 2004, 2006). Consideration of peridomestic habitats and a clear understanding of the reinfestation process are necessary for designing scientifically based control programs, especially because the ongoing regional elimination program of T. infestans has experienced less success in the Gran Chaco region than elsewhere (Gürtler et al. 2007). These and other concerns focusing on population structuring and individual bug dispersal may be addressed by quantitative studies using different genetic and phonetic markers.
Body size has dramatic effects on fitness and its correlates (Davidowitz et al. 2004 and references therein). Phenotypic plasticity of body size is an important mechanism by which organisms can increase fitness in response to short-term environmental variation. Size variation is more influenced by environmental factors, whereas shape variation has a stronger genetic component (Klingenberg et al. 2004, Dujardin and Slice 2007). Therefore, the main premise of shape variation studies is that a statistical analysis of population differentiation expressed in shape characters could be a measure of genetic heterogeneity (Patterson et al. 2001). Recent studies on the geometric morphometry of male T. infestans wings in the rural village of Amamá (northwestern Argentina) showed that individual sites represented the discrete unit where metric differentiation took place and that bugs from the northern part of the community differed from those in the south-central part (Schachter-Broide et al. 2004). This pattern was corroborated with microsatellite markers (Marcet et al. 2008) and is consistent with the spatial and temporal reinfestation patterns by T. infestans observed over a 5-yr period, in which the northern infestation source seemed to be independent of the southern sources (Cecere et al. 2004). In the absence of residual insecticide spraying of the study sites and of significant bug dispersal within a rather limited time window relative to the bugs’ generation length, we expected little or no shape variations in established bug populations carefully georeferenced to the collection site level. In this study, our main objective was to investigate temporal variations in wing size and shape of peridomestic and domestic populations of T. infestans over a 3-yr period.
Materials and Methods
Study Area and Insects
The insects were captured in the rural village of Amamá (27.18° S, 63.08° W), Moreno Department, Province of Santiago del Estero, Argentina, in March 2000, October 2002, and March 2003. The area and its history of infestation by T. infestans were described by Gürtler et al. (1999, 2007). Amamá had 88% of houses with domestic areas infested by T. infestans before being sprayed with 2.5% suspension concentrate deltamethrin at 25 mg (AI)/m2 by the National Chagas Service (NCS) in September 1985 and again in October 1992. Reinfested sites were selectively sprayed with deltamethrin by NCS between 1992 and 1995 or by householders themselves between 1996 and 2002 (Cecere et al. 2004). Most domiciles had adobe walls and thatched roofs. Peridomestic sites were those that did not share a roof with bedroom areas, such as storerooms (where chickens usually nested, also used by dogs and cats) and goat or pig corrals.
We analyzed a total of 199 male and 116 female right wings of T. infestans from Amamá; 88 male and 60 female right wings were collected in March (end of summer) 2000; 56 male and 27 female right wings in October (spring) 2002; and 55 male and 29 female right wings in March (end of summer) 2003 (Table 1). To increase the sample size of each ecotope, we included bugs from two neighboring communities (Trinidad and Mercedes) 6 km away from Amamá, which had experienced the same insecticide treatments. Bugs from both villages were collected in March 2000 (30 male and 19 female right wings) and in October 2002 (38 male and 30 female right wings) from the same ecotopes as in Amamá and were pooled with the latter.
Table 1.
Numbers of male and female T. infestans right wings examined for geometric morphometry according to capture ecotope and date of collection
| Sex | Ecotopes | Mar. 2000 | Oct. 2002 | Mar. 2003 |
|---|---|---|---|---|
| Males | Domicile | 8 | 2 | 0 |
| Storeroom | 25 | 29 | 3 | |
| Goat corral | 45 | 46 | 38 | |
| Pig corral | 40 | 17 | 14 | |
| Total | 118 | 94 | 55 | |
| Females | Domicile | 11 | 9 | 0 |
| Storeroom | 27 | 13 | 0 | |
| Goat corral | 21 | 26 | 21 | |
| Pig corral | 20 | 9 | 8 | |
| Total | 79 | 57 | 29 |
Numbers are the no. sites for each ecotope. Amamá and nearby villages, 2000–2003.
Metric Data
The steps to obtain the metric data and the position of each landmark were described elsewhere (Schachter-Broide et al. 2004). In this study, only 11 of the 13 landmarks were used; type II landmarks (which correspond to the tip and the base of the wing and not to vein intersection) were excluded from the analysis because of the low precision achieved in digitizing them. The geometric coordinates of each landmark were digitized using tpsDig version 1.4 (Rohlf 2004).
Size Variation
For comparison of overall wing size between populations, we used the isometric estimator known as centroid size (CS) derived from coordinates data. CS is defined as the square root of the sum of the squared distances between the center of the configuration of landmarks, and each individual landmark (Bookstein 1991) was extracted from each matrix using mog version 74a (Dujardin 2006a). Because size is strongly influenced by environmental factors, we analyzed wing size variations between collection units (i.e., ecotope) that shared similar characteristics (construction, temperature, and resident host species) and capture occasion using analysis of variance (ANOVA) implemented in Statistica version 98.
Variation of wing size between ecotopes was compared for March (end of summer) 2000 and October (spring) 2002 samples for both sexes for all communities pooled together. In March 2003, T. infestans bugs were collected only in Amamá, and only a few came from storerooms; therefore, it was not possible to make comparisons between all ecotopes for March 2003. Analysis of temporal variations among the three collection dates was conducted only for males collected from an identified pig corral not sprayed with insecticides (from house A44), which was the only site detectably infested throughout the study period. The few female bugs collected in this site did not allow us to perform the same type of analysis as for males.
Shape Variation
Shape variables (partial warps) were obtained using the Generalized Procrustes Analysis superimposition algorithm (Rohlf 2004) using mog version 74a (Dujardin 2006a). Because individual sites represented the discrete unit where metric differentiation took place, the optimal way of studying temporal variation would take individual collection sites as sample units. Only the pig corral at house A44 had a persistent bug population with sufficient number of adult male bugs throughout the observation period. We analyzed nine right wings collected in March 2000; 16 right wings collected in October 2002, and 14 collected in March 2003. To compare temporal variations of wing shape at a higher spatial scale (the community), we computed shape variables relative to the total consensus of all right wings of bugs collected only from south-central Amamá (see maps in Cecere et al. 2004 and Marcet et al. 2008), because this subpopulation differed in wing shape from bugs in the northern section (Schachter-Broide et al. 2004). We analyzed a total of 180 male right wings and 99 female right wings, which included 70 males and 47 females collected in March 2000; 55 male and 23 females collected in October 2002; and 55 males and 29 females collected in March 2003. Shape variations among groups were quantified using Euclidian distances between the consensus shapes of each group. This approach was preferred to one based on discriminant analysis because we were more interested in detecting changes in shape and evaluating its amount over time rather than in classifying groups. Euclidian distances based on partial warps are more closely correlated to Procrustes distances than Mahalanobis distances computed by discriminant analysis. We compared collection dates at two geographic scales: the village and a single collection site. Temporal variations at the single site were analyzed only for males because there were very few female bugs to conduct a valid analysis.
Euclidian distances were tested for significance by nonparametric permutation tests (1,000 runs each) after Bonferroni correction using cov version 25a (Dujardin 2006b). These distances were used in an unweighted pair-group method with arithmetic average (UPGMA) cluster analysis to produce a dendrogram using the NEIGHBOR module of the PHYLIP package (Felsenstein 2005). The relationship between CS and shape variation (residual allometry) was examined by nonparametric tests after multivariate regression (Good 2000) using cov version 25a (Dujardin 2006b).
Results
Size Variation
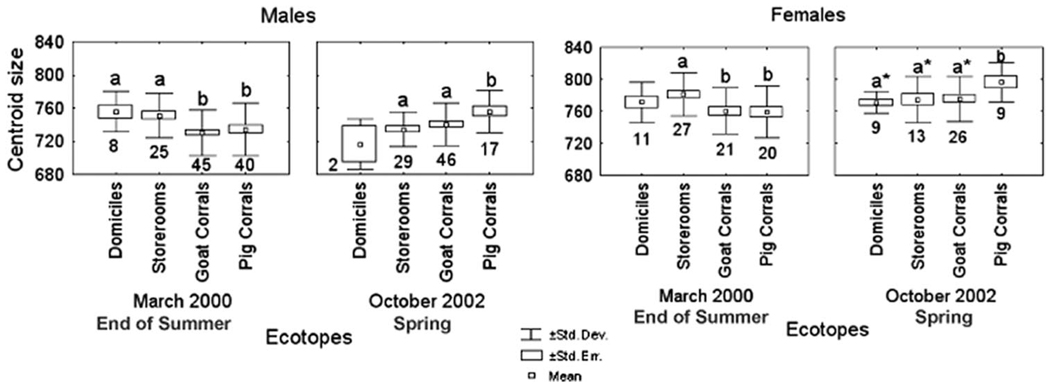
The right wing CS of T. infestans collected from domiciles and/or storerooms were significantly larger than those from bugs collected at goat and/or pig corrals by the end of summer 2000 for males (F = 4.06; df = 3,114; P < 0.009) and females (F = 3.05; df = 3,75; P < 0.034; Fig. 1). Conversely, bugs collected from domiciles and/or storerooms had significantly smaller CS than bugs collected from pig corrals in spring 2002 for males (F = 3.42; df = 3,90; P < 0.021); females showed a similar, although marginally significant, pattern. This inversion in CS between seasons tended to be consistent in males and females and was caused by a significant reduction of CS in male bugs collected from storerooms and by a significant increase of CS in bugs from both sexes collected from pig corrals.
Fig. 1.
Variation of wing CS of T. infestans between ecotopes and collection dates for the study communities (Amamá, Mercedes, and Trinidad) for both sexes. Box plots show isometric size differences of wings between ecotopes for each collection date. Different letters above the box plot indicate statistically significant differences; the absence of a letter above the box plot indicates lack of differences with other categories, and the asterisk denotes marginally significant differences; the number below the bottom whisker shows the number of wings analyzed.
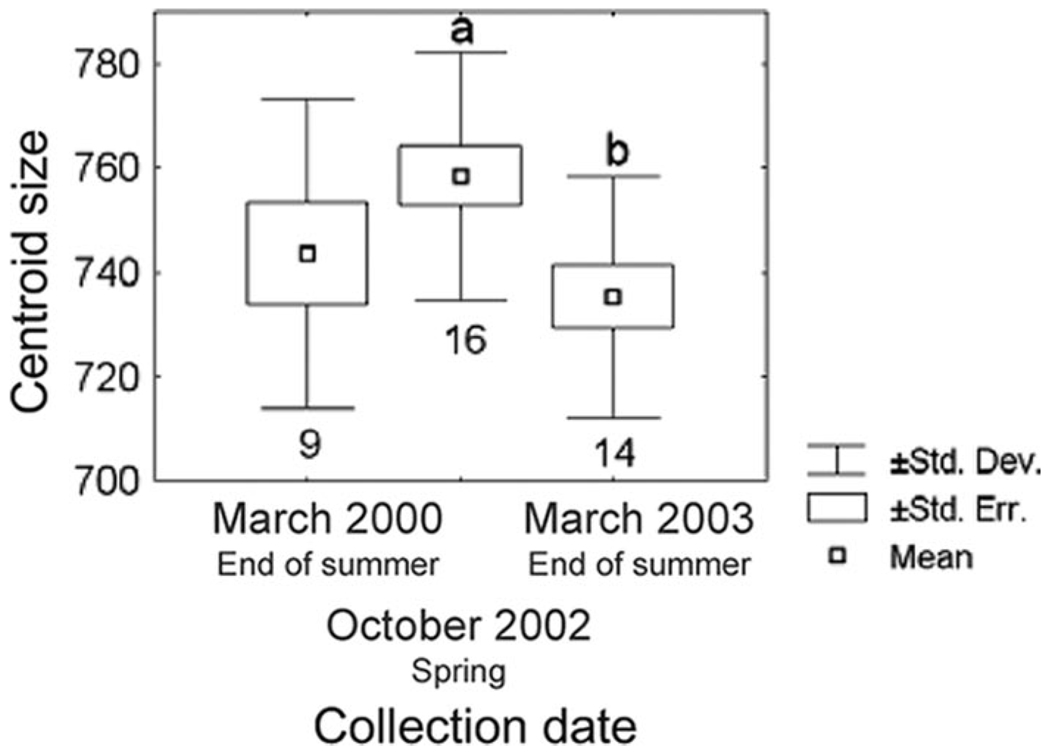
The wing CS of males captured at the A44 pig corral differed significantly among the three collection dates (F = 3.29; df = 2,36; P < 0.049). Bugs collected on both summer surveys had smaller CS than bugs collected in spring (Fig. 2); a posteriori comparisons showed significant differences (P < 0.016) only between spring 2002 and the end of summer 2003. The absence of statistically significant differences between the end of summer 2000 and spring 2002 may have been caused by the small sample size, because using both wings yielded significant differences between the end of summer 2000 and spring 2002 (P < 0.02) and between spring 2002 and the end of summer 2003 (P < 0.005). Differences in wing CS between the end of summer (March) and spring (October) bug collections were larger than differences between samples collected by the end of summers 2000 and 2003, suggesting seasonal effects rather than purely time-dependent effects.
Fig. 2.
Variations of wing CS between collection dates of male T. infestans captured in the A44 pig corral. Box plots show isometric size differences of wings between collection dates. Different letters above the box plot indicate statistically significant differences; the absence of a letter above the box plot indicates lack of differences with other categories; the number below the bottom whisker shows the number of wings analyzed.
Shape Variation
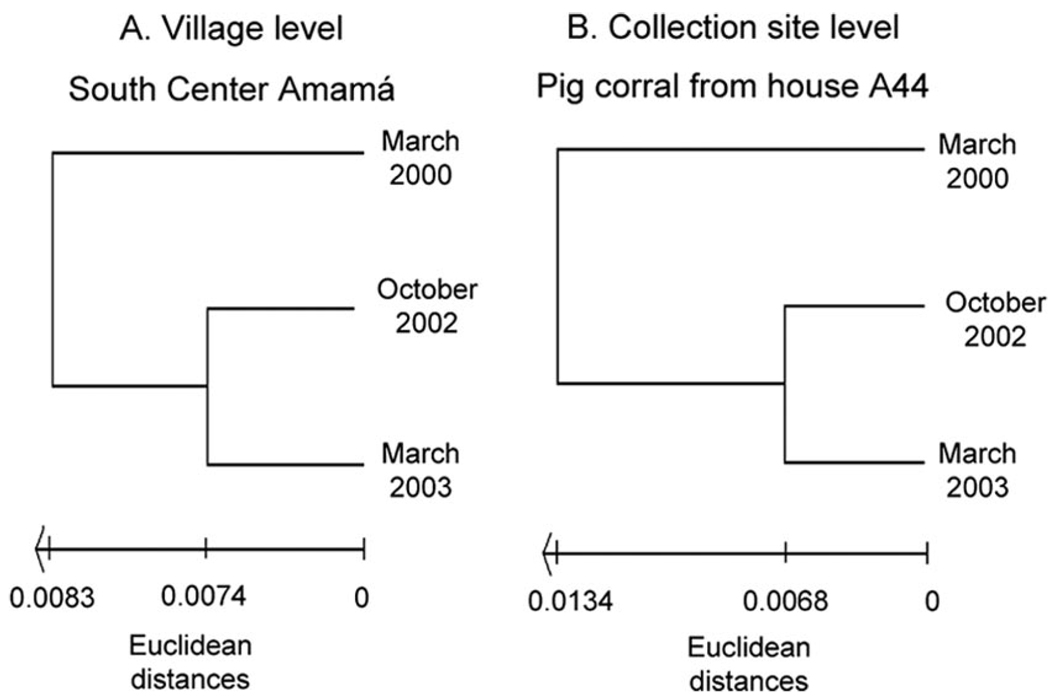
Wing shape analysis of bugs from south-central Amamá showed divergence between dates of bug collection for both sexes. Wing shape divergence was highly significant (P < 0.0001) between male bugs collected by the end of summer 2000 and those collected in spring 2002 and by the end of summer 2003. The UPGMA tree showed that male bug populations collected in spring 2002 and by the end of summer 2003 were clustered together, whereas the summer 2000 population was more external (Fig. 3A). For females, shape divergence was statistically significant (P < 0.005) only between bug populations collected in spring 2002 and by the end of summer 2003 and marginally significant (P < 0.019) between bug populations collected by the end of summer 2000 and spring 2002 (UPGMA tree not shown). The multivariate regression of shape variables on isometric size was not statistically significant for both sexes, thus indicating that shape variation was not a secondary effect of size changes.
Fig. 3.
Unweighted pair-group method with arithmetic average dendrograms derived from Euclidean distances, comparing (A) collection dates for village level (south-central Amamá) and (B) collection site level (A44 pig corral).
Male bugs collected at the A44 pig corral did not show significantly different wing shapes among dates, although the Euclidian distances between bug collections in the ends of summer 2000 and summer 2003 were marginally significant (P < 0.079). The multivariate regression of shape variables on isometric size was not statistically significant. The UPGMA tree paralleled the previous one, showing that bug populations collected in spring 2002 and by the end of summer 2003 clustered together, and the end of summer 2000 population was more external (Fig. 3B).
Discussion
The inversion in wing CS over time was unexpected and suggested different hypotheses. The smaller triatomine bugs collected in domiciles and/or storerooms than in corrals in spring samples supports the general hypothesis that natural selection would favor larger phenotypes in less favorable (more unstable or sylvatic) habitats such as corrals, possibly because of a greater capacity to resist temporary food shortages, whereas smaller individuals apparently survive better under laboratory or domestic conditions where host availability is more stable (Dujardin et al. 1997, Schofield et al. 1999). This may explain in part why species of Triatominae grown under controlled laboratory conditions tend to show a reduction in size relative to their sylvatic counterparts (Dujardin et al. 1997, Schofield et al. 1999). However, other mechanisms, such as inbreeding, different host-feeding patterns, and/or developmental times may also explain such size variations.
The control of body size is not so much a control of growth but a control of when to stop growing (Nijhout 2003 and references therein). For organisms with determinate growth, final body size is determined by the duration of the period available for growth. As the number of days above the developmental zero of the species increase, developmental times decrease, and smaller specimens are produced. In our study case, microsite temperatures of adobe-and-thatch habitations and storerooms are significantly dampened with respect to outdoor temperatures and likely provide more days above the estimated developmental zero (16°C) of T. infestans than the thinner thatched roofs of goat or pig corrals (Vazquez-Prokopec et al. 2002). Such damping effects increase the time window for bug blood feeding indoors with respect to less protected outdoor ecotopes, even extending over a significant fraction of the winter season in northern Argentina. Thus, populations of T. infestans in adobe-and-thatch habitations and storerooms may experience a shorter generation time than bugs developing in corrals, and this may lead to smaller specimens.
Blood-meal size may also affect developmental times in triatomine bugs. In insects, the cessation of growth is caused by the secretion of ecdysteroids (Nijhout 2003), and therefore the mechanism that controls the timing of ecdysteroid secretion controls body size. Ecdysteroids are secreted in response to the prothoracicotropic hormone (PTTH). The actual stimulus for PTTH secretion is not well known except for a few Hemiptera, in which it is controlled by abdominal stretch receptors that are activated when the insect reaches a particular critical size (Nijhout 1981) or by distention of the abdomen with food. In bloodsucking Hemiptera, such as Rhodnius prolixus and Dipetalogaster maxima, the required abdominal stretch is achieved by a single large blood meal (Nijhout 1984 and references therein). In the fifth-instar stage, such a large blood meal causes the insect to secrete ecdysteroids and initiate a premature metamorphosis that leads to a miniature adult. Triatomine bugs from laboratory colonies have a higher probability of achieving a single large blood meal early in the fifth-instar stage than their field counterparts (Zeledón 1981, Catalá 1994). Therefore, different blood-meal sizes may also explain size variations between field and laboratory-reared bugs.
Developmental times, blood-meal sizes and feeding frequency may be modified by the relative density of triatomine bugs and hosts, which in turn may affect the distribution of body length and weight (Schofield 1980). As bug density increases, the mean nutritional status of the bug population would decline as a result of competition for the available blood-meal sources, and developmental times would increase. Low bug densities may cause fewer disturbances to their hosts and thus the bugs may feed longer and reach repletion more often.
The size distribution of adult bug populations in the field may also be affected by differential dispersal given their open nature. The observed size pattern in T. infestans bug populations collected in spring (October) may be explained by assuming that the insects captured at identified sites had developed there and that no immigration occurred during or immediately after winter. The latter assumption is supported by light trap collections showing peak flight dispersal of T. infestans in summer and no or little dispersal in winter and spring, respectively (Vazquez-Prokopec et al. 2006). Triatomine bugs that developed in domiciles and/or storerooms through winter (under a higher average minimum temperature than in peridomestic corrals) would have a shorter developmental time, a greater probability of achieving large blood meals, and improved survival of small specimens than bugs that developed in corrals during the same season. All these factors would lead to producing smaller bugs in domiciles and storerooms by the end of winter.
The observed inversion in wing CS by the end of summer (March) may also be influenced by size-biased flight dispersal between ecotopes. Invasion of domestic sites by adult T. infestans after community-wide insecticide spraying most likely originated from infested peridomestic sites in our study area (Gürtler et al. 1999; Cecere et al. 2002, 2004; Vazquez-Prokopec et al. 2006). The higher local abundance and lower nutritional status of T. infestans populations infesting corrals suggested that these bugs were more likely to disperse by flight during summer than the well-fed bugs associated with chickens in storerooms (Ceballos et al. 2005). Furthermore, flight initiation experiments under natural climatic conditions showed that flights occurred above 23°C at sunset, with some T. infestans adult bugs always flying while others never doing so regardless of its nutritional status (Gurevitz et al. 2006, 2007). In these experiments, male fliers had significantly bigger wing CS than nonfliers (unpublished data). If such selective flight dispersal of larger bugs also occurs in the field, this would cause a decrease in mean adult bug size in corrals (sources) and a concomitant increase in storerooms or domestic sites (targets) during summer, when the bugs are more likely to disperse.
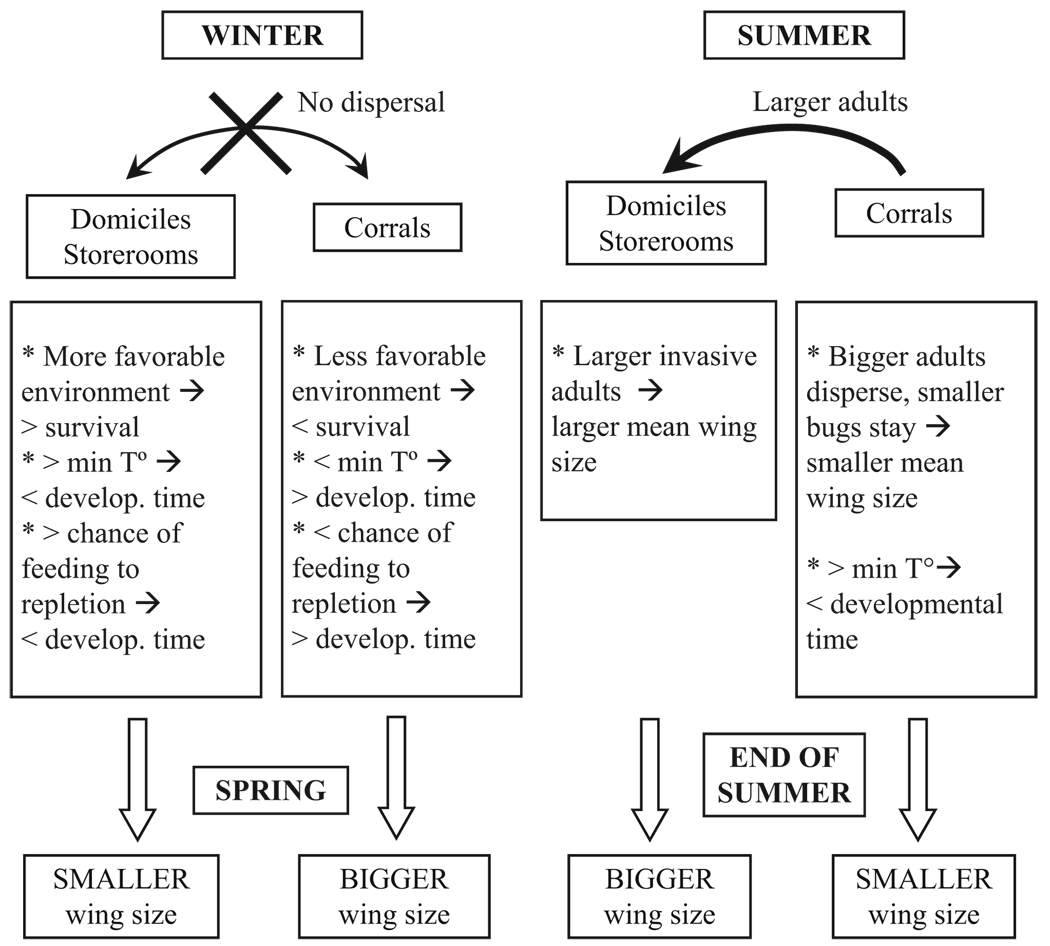
Variations in wing CS between the end of summer and spring bug collections at the A44 pig corral were larger than the differences between samples collected by the end of summers 2000 and 2003, suggesting a seasonal rather than a cumulative temporal effect. Lower temperatures result in slower growth and larger body sizes in the majority of ectotherms (Atkinson 1994). The smaller wing CS of bugs from the A44 pig corral in both summer surveys in comparison with bugs collected in spring may reflect such temperature effects, because late summer bugs most likely developed during spring-summer, whereas spring-collected bugs most likely developed between the previous summer and early spring (Cecere et al. 2003). Because bug population size was relatively small and host abundance high in the study pig corral throughout the observation period, these factors were unlikely to influence wing size variations. In conclusion, the inversion in wing CS over time may be caused by the combined effects of temperature variations across seasons, the proportion of bugs achieving large blood meals, and size-biased flight dispersal between ecotopes (Fig. 4).
Fig. 4.
Diagram of the hypothetical sequence of events and processes that may explain wing size variations of T. infestans populations in northwestern Argentina.
The wing shape of male bugs collected in different seasons (end of summer and spring) closer in time (6 mo apart) differed less than in samples collected in the same season (end of summer) 3 yr apart. In open bug populations, temporally closer groups are more related genetically than temporally distant groups, suggesting that genetic influences on wing shape are stronger than environmental ones (Klingenberg et al. 2004, Dujardin and Slice 2007). This is also supported by the fact that wing shape was successfully used to assign individual Triatoma protracta to its parental line in insectary bug populations (Dujardin and Slice 2007, Dujardin et al. 2007). It is also consistent with the hypothesis that size differences between seasons are caused by size-biased flight dispersal of large bugs from within the population (sites within the same house compound) rather than by immigration from bug populations elsewhere in the village or outside of it. The results on shape variation were essentially the same when the spatial scale considered decreased from a section of the village to a single collection site. Established bug populations from the A44 pig corral differed between collection dates with regard to wing shape. This site was close to several infested sites within the observed flight range of T. infestans (<1.5 km) (Schweigmann et al. 1988, Schofield et al. 1992), which may have acted as putative sources. However, to explain the observed wing shape differences between spring 2002 and the end of summer 2003, significant bug immigration into these corrals should have occurred within a 6-mo period, as suggested by flight dispersal experiments (Vazquez-Prokopec et al. 2006, Gurevitz et al. 2007), spatiotemporal analysis of reinfestation patterns (Cecere et al. 2004, zu Dohna et al. 2007) and genetic studies with microsatellite markers support (Marcet et al. 2008).
Our study shows that variation of wing size was related to seasonal variations, whereas wing shape differentiation was related to the length of time between bug collections, suggesting more genetic influence acting on shape and more environmental ones acting on size. Simultaneous consideration of size and shape may provide complementary information on the direction and timing of bug dispersal. Morphological studies may be used to determine the relatedness of different bug populations and to associate morphological heterogeneities with temporal patterns of reinfestation.
Acknowledgments
We thank E. Angrisano, E. Dotson, C. Cecere, and the ECLAT network for helpful discussions. This project was supported by Grant R01 TW05836 to U.K. and R.E.G.; in part by Grant 1 C06 RR 16515 to University of Illinois at Urbana-Champaign College of Veterinary Medicine both from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); by the Agencia Nacional de Promoción Científica y Técnica (Argentina) and the University of Buenos Aires to R.E.G. R.E.G. is a member of CONICET Researcher’s Career.
Footnotes
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- Atkinson D. Temperature and organism size—a biological law for ectotherms. Adv. Ecol. Res. 1994;25:1–58. [Google Scholar]
- Bookstein FL. Morphometric tools for landmark data: geometry and biology. New York: Cambridge University Press; 1991. [Google Scholar]
- Catalá S. Bloodmeal size and nutritional status of Triatoma infestans under natural climatic conditions. Med. Vet. Entomol. 1994;8:104–106. doi: 10.1111/j.1365-2915.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Ceballos LA, Vazquez-Prokopec GM, Cecere MC, Marcet PL, Gürtler RE. Feeding rates, nutritional status and flight dispersal potential of peridomestic populations of Triatoma infestans in rural northwestern Argentina. Acta Trop. 2005;95:149–159. doi: 10.1016/j.actatropica.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Cecere MC, Gürtler RE, Canale D, Chuit R, Cohen JE. Effects of partial housing improvement and insecticide spraying on the reinfestation dynamics of Triatoma infestans in rural northwestern Argentina. Acta Trop. 2002;84:101–116. doi: 10.1016/s0001-706x(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Cecere MC, Canale DM, Gürtler RE. Effects of refuge availability on the population dynamics of Triatoma infestans in central Argentina. J. Appl. Ecol. 2003;40:742–756. [Google Scholar]
- Cecere MC, Vazquez-Prokopec GM, Gürtler RE, Kitron U. Spatio-temporal analysis of reinfestation by Triatoma infestans (Hemiptera: Reduviidae) following insecticide spraying in a rural community in northwestern Argentina. Am. J. Trop. Med. Hyg. 2004;71:803–810. [PMC free article] [PubMed] [Google Scholar]
- Cecere MC, Vazquez-Prokopec GM, Gürtler RE, Kitron U. Reinfestation sources for Chagas disease vector, Triatoma infestans, Argentina. Emerg. Infect. Dis. 2006;12:1096–1102. doi: 10.3201/eid1207.051445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JE, Gürtler RE. Modeling household transmission of American trypanosomiasis. Science. 2001:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- Davidowitz G, D’Amico JL, Nijhout HF. The effects of environmental variation on a mechanism that controls insect body size. Evol. Ecol. Res. 2004;6:49–62. [Google Scholar]
- Dujardin JP. MOG. Versión 74a. Unité de Recherches 062-Unité Mixte de Recherches UMR9926, Institut de Recherches pour le Développement (IRD, France) 2006a ( http://www.mpl.ird.fr/morphometrics/)
- Dujardin JP. COV. Versión 25a. Unité de Recherches 062-Unité Mixte de Recherches UMR9926, Institut de Recherches pour le Développement (IRD, France) 2006b ( http://www.mpl.ird.fr/morphometrics/)
- Dujardin JP, Slice D. Modern methodologies. New York: Wiley; 2007. Contributions of morphometrics to medical entomology. Encyclopedia of infectious diseases. [Google Scholar]
- Dujardin JP, Bermuú dez H, Casini C, Schofield CJ, Tibayrenc M. Metric differences between sylvatic and domestic Triatoma infestans (Hemiptera: Reduviidae) in Bolivia. J. Med. Entomol. 1997;34:544–551. doi: 10.1093/jmedent/34.5.544. [DOI] [PubMed] [Google Scholar]
- Dujardin JP, Beard CB, Ryckman R. The relevance of wing geometry in entomological surveillance of Triatominae, vectors of Chagas disease. Infect. Genet. Evol. 2007;7:161–167. doi: 10.1016/j.meegid.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP (phylogeny inference package) version 3.6. Seattle, WA: Department of Genome Sciences, University of Washington; 2005. [Google Scholar]
- Good P. Permutation tests: a practical guide to resampling methods for testing hypotheses. New York: Springer; 2000. [Google Scholar]
- Gurevitz JM, Ceballos LA, Kitron U, Gürtler RE. Flight initiation of Triatoma infestans (Hemiptera: Reduviidae) under natural climatic conditions. J. Med. Entomol. 2006;43:143–150. doi: 10.1603/0022-2585(2006)043[0143:fiotih]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitz JM, Kitron U, Gürtler RE. Flight muscle dimorphism and heterogeneity in flight initiation of field-collected Triatoma infestans (Hemiptera: Reduviidae) J. Med. Entomol. 2007;44:186–191. doi: 10.1603/0022-2585(2007)44[186:fmdahi]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürtler RE, Cecere MC, Canale DM, Castanñ era MB, Chuit R, Cohen JE. Monitoring house reinfestation by vectors of Chagas disease: a comparative trial of detection methods during a four-year follow-up. Acta Trop. 1999;72:213–234. doi: 10.1016/s0001-706x(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Gürtler RE, Kitron U, Cecere MC, Segura EL, Cohen JE. Sustainable vector control and management of Chagas disease in the Gran Chaco, Argentina. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16194–16199. doi: 10.1073/pnas.0700863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg CP, Leamy LJ, Cheverud JM. Integration and modularity of quantitative trait locus effects on geometric shape in the mouse mandible. Genetics. 2004;166:1909–1921. doi: 10.1534/genetics.166.4.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcet PL, Mora MS, Cutrera AP, Jones L, Gürtler RE, Kitron U, Dotson EM. Genetic structure of Triatoma infestans populations in rural villages of Santiago del Estero, northern Argentina. Infect. Genet. Evol. 2008;8:835–846. doi: 10.1016/j.meegid.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout HF. Physiological control of molting in insects. Am. Zool. 1981;21:631–640. [Google Scholar]
- Nijhout HF. Abdominal stretch reception in Dipetalogaster maximus (Hemiptera, Reduviidae) J. Insect Physiol. 1984;30:629–633. [Google Scholar]
- Nijhout HF. The control of body size in insects. Dev. Biol. 2003;261:1–9. doi: 10.1016/s0012-1606(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Noireau F, Cortez MG, Monteiro FA, Jansen AM, Torrico F. Can wild Triatoma infestans foci in Bolivia jeopardize Chagas disease control efforts? Trends Parasitol. 2005;21:7–10. doi: 10.1016/j.pt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Patterson JS, Schofield CJ, Dujardin JP, Miles MA. Population morphometric analysis of the tropicopolitan bug Triatoma rubrofasciata and relationships with Old World species of Triatoma: evidence of New World ancestry. Med. Vet. Entomol. 2001;15:443–451. doi: 10.1046/j.0269-283x.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ. TPSDIG. Version 2.04. Stony Brook, NY: Department of Ecology and Evolution, State University of New York; 2004. [Google Scholar]
- Schachter-Broide J, Dujardin JP, Kitron U, Gürtler RE. Spatial structuring of Triatoma infestans (Hemiptera, Reduviidae) populations from northwestern Argentina using wing geometric morphometry. J. Med. Entomol. 2004;41:643–649. doi: 10.1603/0022-2585-41.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield CJ. Nutritional status of domestic populations of Triatoma infestans. Trans. R. Soc. Trop. Med. Hyg. 1980;74:770–778. doi: 10.1016/0035-9203(80)90197-2. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Lehane MJ, McEwen P, Catala SS, Gorla DE. Dispersive flight by Triatoma infestans under natural climatic conditions in Argentina. Med. Vet. Entomol. 1992;6:51–56. doi: 10.1111/j.1365-2915.1992.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Diotaiuti L, Dujardin JP. The process of domestication in Triatominae. Mem. Inst. Oswaldo Cruz. 1999;94:375–375. doi: 10.1590/s0074-02761999000700073. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM, Ceballos LA, Cecere MC, Gürtler RE. Seasonal variations of microclimatic conditions in domestic and peridomestic habitats of Triatoma infestans in rural northwest Argentina. Acta Trop. 2002;84:229–238. doi: 10.1016/s0001-706x(02)00204-8. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prokopec GM, Ceballos LA, Marcet PL, Cecere MC, Cardinal MV, Kitron U, Gürtler RE. Seasonal variations in active dispersal of natural populations of Triatoma infestans in rural north-western Argentina. Med. Vet. Entomol. 2006;20:1–6. doi: 10.1111/j.1365-2915.2006.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeledón R. El Triatoma dimidiata y su relación con la enfermedad de Chagas. San José, Costa Rica: Editorial Universidad Estatal a Distancia (EUNED); 1981. [Google Scholar]
- zu Dohna H, Cecere MC, Gürtler RE, Kitron U, Cohen JE. Re-establishment of local populations of vectors of Chagas disease after insecticide spraying. J. Appl. Ecol. 2007;44:220–227. doi: 10.1111/j.1365-2664.2006.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]