SUMMARY
Embryonic stem cells (ESCs) are an attractive source for tissue regeneration and repair therapies because they can be differentiated into virtually any cell type in the adult body. However, for this approach to succeed, the transplanted ESCs must survive long enough to generate a therapeutic benefit. A major obstacle facing the engraftment of ESCs is transplant rejection by the immune system. Here we show that blocking leukocyte costimulatory molecules permits ESC engraftment. We demonstrate the success of this immunosuppressive therapy for mouse ESCs (mESC), human ESCs (hESC), mouse induced pluripotent stem cells (miPSC), human iPSCs (hiPSC), and more differentiated ESC/iPSC-derivatives. Additionally, we provide evidence describing the mechanism by which inhibition of costimulatory molecules suppresses T-cell activation. This report describes a short-term immunosuppressive approach capable of inducing engraftment of transplanted ESCs and iPSCs, providing a significant improvement in our mechanistic understanding of the critical role costimulatory molecules play in leukocyte activation.
Keywords: ESC, iPSC, stem cell, transplantation, immunogenicity
INTRODUCTION
In recent years, there is much interest in using hESCs to regenerate tissues and organs. However, despite the potential of hESCs, important issues surrounding immunogenicity have not been fully addressed and strategies to avoid rejection remain largely untested. Previous studies have demonstrated that traditional immunosuppressive therapies (e.g., tacrolimus, sirolimus, and mycophenolate mofetil) provide only marginal improvements in ESC survival, with little evidence of cell engraftment past 3–4 weeks post transplantation (Swijnenburg et al., 2008; Toriumi et al., 2009). Furthermore, traditional immunosuppression requires chronic administration, leaving the host immune system impaired and vulnerable to opportunistic infections. Thus the ideal therapy should involve only a brief period of immunosuppression, but be able to induce a specific long-lasting tolerance to the donor cells (Chidgey et al., 2008). With this goal in mind, we tested whether a brief course of treatment with three costimulatory receptor blocking agents — cytotoxic T-lymphocyte-associated antigen 4 (CTLA4)-Ig, anti-CD40-ligand (anti-CD40L), and anti-lymphocyte function-associated antigen-1 (anti-LFA-1) — could induce long-term allogeneic and xenogeneic ESC engraftment. We investigated these agents because blocking various combinations of these costimulatory molecules has demonstrated promise for hESCs in the immune-privileged environment of the testis (Grinnemo et al., 2008) and has been demonstrated to prolong the engraftment of cardiac (Larsen et al., 1996), pancreatic islet cell (Lenschow et al., 1992) and bone marrow grafts (Kurtz et al., 2009; Pan et al., 2003).
An optimal T cell response requires two-signals, ligation of the antigen-specific T cell receptor (TCR) (signal 1) and an accessory signal from a non-antigen specific costimulatory molecule (signal 2) (Jenkins, 1994). When only signal 1 is provided without signal 2, T cell activation is disturbed and the cell may adopt a state of anergy, undergo apoptosis, abortive proliferation, or immunoregulation (Ford and Larsen, 2009; Wood and Sakaguchi, 2003). Among the most important costimulatory interactions for T-cell activation are CD80/CD86 on antigen presenting cells (APC) interacting with CD28 on T-cells and CD40 on APCs engaging CD40-ligand on T-cells (Lafferty et al., 1983). Negatively regulating costimulatory molecules have also been described, particularly CTLA4, which is expressed by activated T-cells and binds to CD80/CD86 with 10-20-fold greater affinity than CD28 (Thompson and Allison, 1997). Upon engagement, CTLA4 delivers an inhibitory signal to the T-cell. Lastly, LFA-1 is involved in the formation of the immunological synapse as well as the trafficking and costimulation of T-cells (Van Seventer et al., 1990; Zuckerman et al., 1998).
RESULTS
Blockade of leukocyte costimulatory molecules permits long-term engraftment of mESCs transplanted across allogeneic barriers
Finding or creating the right techniques to evaluate transplanted cell survival is essential for the accurate assessment of immunologic rejection and drug discovery (Niu and Chen, 2008). Until recently, the majority of studies evaluating ESC survival depended on immunohistochemical staining for β-galactosidase (LacZ) (Caspi et al., 2007) or detection of GFP (Li et al., 2004). But these methods only provide a “snapshot” of cell survival. In contrast, in vivo bioluminescent imaging (BLI) provides longitudinal evaluation of the spatiotemporal kinetics of ESC rejection. In this study, mESCs and hESCs were transduced with a double fusion (DF) reporter gene construct carrying firefly luciferase (Fluc) and enhanced green florescent protein (eGFP) (Figure 1A). ESCs robustly expressed Fluc, which correlated with ESC number (r2 = 0.99) and displayed a tight cluster morphology with robust GFP expression (Figure S1A).
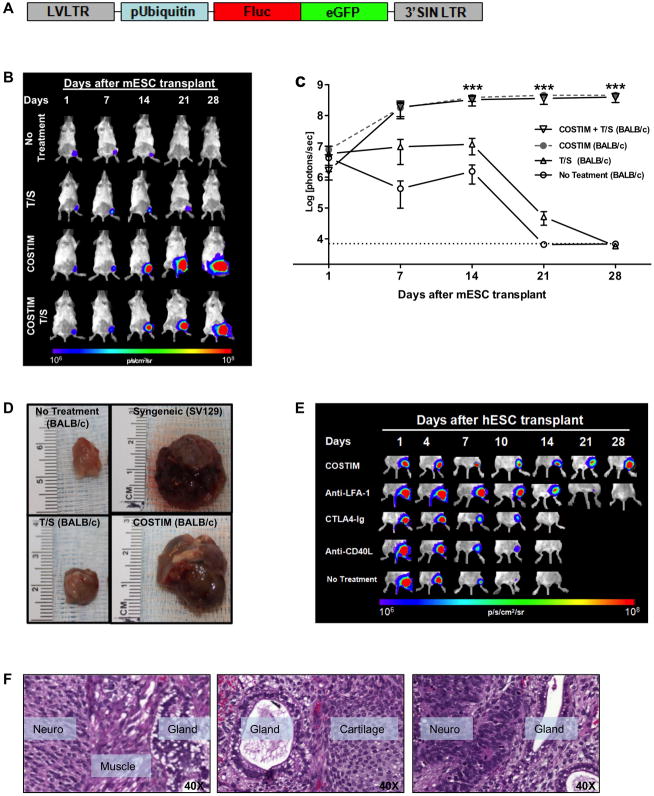
Figure 1. Blockade of leukocyte costimulatory molecules mitigates allogeneic and xenogeneic transplantation rejection of undifferentiated ESCs.
(a) Schema of the DF reporter gene construct containing Fluc and eGFP driven by a constitutive human ubiquitin promoter, using a self-inactivating (SIN) lentiviral vector. (b) Representative bioluminescence images and (c) quantitative bioluminescence intensity of mESC-transplanted mice that received either no treatment, tacrolimus + sirolimus (T/S), CTLA4-Ig + anti-LFA-1 + anti-CD40L (COSTIM), or COSTIM + T/S. n = 5 per group, ***P<0.001. (d) Representative images of gastrocnemius muscles 28 days after transplantation of non-transduced mESCs. (e) Representative bioluminescence images of xenogeneic hESC-transplanted mice that received no treatment, monotherapy, or a combination of all three costimulatory blockade agents (COSTIM). n = 5–8 per group. (f) Histopathological evaluation of HE-stained muscle sections from COSTIM treated mice demonstrating hESC-derived teratoma formation. All values are expressed as mean ± SEM. Color scale bars are in photons per second per centimeter squared per steradian (p/s/cm2/sr). H&E, hematoxylin and eosin stain. For further characterization of the ESCs and iPSCs, see Figure S1.
We next investigated longitudinal survival after intramuscular (gastrocnemius muscle) transplantation of mESCs into syngeneic (SV129, H-2kb) and allogeneic (BALB/c, H-2kd) mice by in vivo BLI. mESC survival was significantly limited in allogeneic compared to syngeneic mice (P<0.001), with BLI signal decreasing to background levels in allogeneic animals by 21 days post-transplantation. In contrast, syngeneic hosts accepted mESC grafts, resulting in teratoma formation (Figures 1B, 1C). Having previously demonstrated that the immune response to hESCs is primarily CD4+ T-cell mediated, we therefore investigated the efficacy of immunosuppressive agents that largely target T-cells (Swijnenburg et al., 2008). Two immunosuppressive agents were chosen based on different mechanisms of action; specifically, calcineurin inhibitors (tacrolimus; TAC) and target of rapamycin inhibitors (sirolimus; SIR) (Supplementary Table S1). Additionally, three costimulatory receptor blocking antibodies (CTLA4-Ig, anti-LFA-1, anti-CD40L) were evaluated in an attempt to induce immune tolerance. Importantly, costimulatory blockade was only administered for short-term on days 0, 2, 4, and 6 post-transplantation. Whereas daily administration of TAC/SIR prolonged mESC survival only out to 28 days post-transplantation, a surprisingly brief course of costimulatory blockade was sufficient to prevent mESC rejection at all time points assayed (P<0.001 costimulatory blockade treatment vs. TAC/SIR or no treatment) (Figures 1B, 1C). To exclude the possibility that the immune reaction was exclusively targeted towards antigens produced by the DF reporter genes, we transplanted non-transduced mESCs that do not express Fluc-eGFP. Similar to mESCs expressing Fluc-eGFP, costimulatory blockade treatment permitted engraftment of non-transduced mESCs. Survival of non-transduced mESCs was limited in both untreated and TAC/SIR-treated allogeneic hosts with no evidence of transplanted mESC survival at 28 days post-transplantation (Figure 1D).
Xenogeneic immune rejection of undifferentiated, in vivo differentiated, and hESC-derived endothelial cells is mitigated by costimulatory blockade
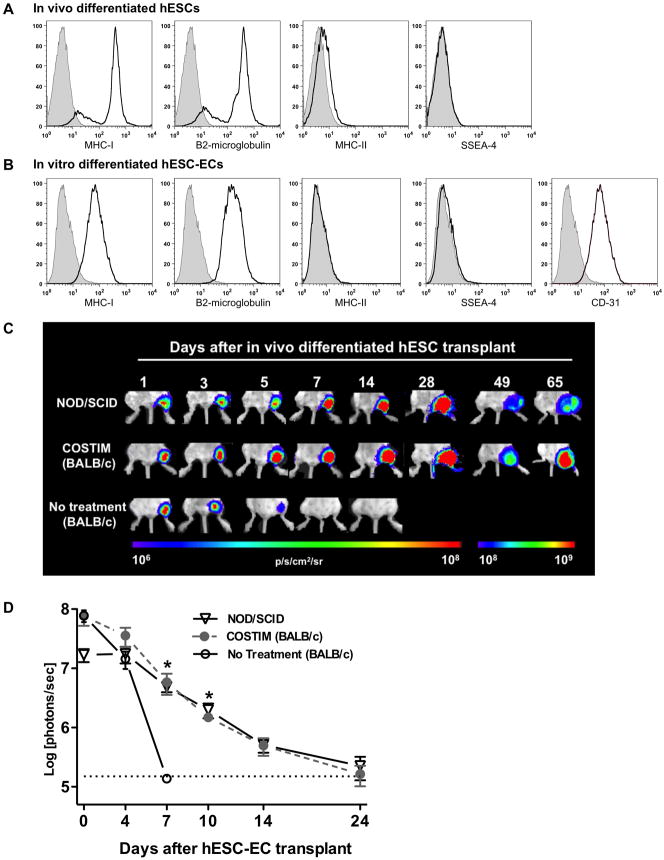
We next investigated if costimulatory blockade could prevent immune rejection of hESCs in the more hostile xenogeneic transplantation environment. Without immunosuppression, hESC survival was significantly limited as BLI signal reached background intensity by day 10–14, whereas BLI signal steadily increased at each time point assayed in the costimulatory blockade treatment group (P<0.01, Figure 1E). Consistent with BLI data, histological evaluation of the graft site at 5 days following hESC transplantation demonstrated a robust infiltration of CD3 T-cells surrounding GFP+ hESCs, which was severely diminished in the costimulatory blockade treated group (Figure S1B). At 28 days there was no histological evidence of hESC survival in untreated animals, whereas animals treated with costimulatory blockade demonstrated teratoma formation (Figure 1F). Having shown that the combination of three costimulatory blockade agents is capable of inducing hESC engraftment, we next tested whether monotherapy is sufficient. By day 28, BLI signal decreased to background intensity in all monotherapy groups, with the greatest prolongation of hESC survival observed in the anti-LFA-1 group (Figure 1E). Undifferentiated ESCs have low levels of MHC expression (Figure S1C), which increases upon differentiation (Figure 2A, 2B). These differentiated ESC-derivatives may have impaired survival capacity compared to undifferentiated ESCs when transplanted across histocompatibility barriers. This represents a problem, as it is unlikely that ESC-based therapy will utilize an undifferentiated cell population because of safety concerns regarding potential teratoma formation or uncontrolled cellular proliferation. It is likely that prior to transplantation, cells will need to be differentiated into a lineage appropriate for their intended therapy and thus may encounter a heightened immune response. We therefore tested the ability of costimulatory blockade to permit engraftment of an (1) in vivo spontaneously differentiated cell population isolated from an explanted hESC-derived teratoma and (2) in vitro differentiated hESC-derived endothelial cells (hESC-ECs). Both cell populations demonstrated increased MHC-I expression relative to undifferentiated cells (Figures 2A, 2B). Immunosuppressive treatment with costimulatory blockade permitted engraftment of in vivo differentiated cells (P<0.01 untreated vs. costimulatory blockade treated, Figures 2C, S2A) and in vitro differentiated hESC-ECs comparable to that observed in immunodeficient NOD/SCID mice (P<0.05 untreated vs. costimulatory blockade treated, Figures 2D, S2B). Transplantation of in vivo differentiated hESCs without immunosuppression resulted in limited cell survival as indicated by BLI signal diminishing to background level by 7 to 14 days. In contrast, treatment with costimulatory blockade permitted engraftment of in vivo differentiated hESC as indicated by steadily increasing BLI signal at every time point assayed. Transplantation of hESC-ECs demonstrated limited survival in all groups tested, including the immunodeficient NOD/SCID mice. At day 4 following hESC-EC transplantation, the BLI signal was 18.5±6.0% of baseline in the untreated group was, compared to 46.7±16% in the costimulatory blockade treated group. By day 7, the BLI signal decreased to background intensity in the untreated group compared to 7.9±3.1% in the costimulatory blockade treated group (P<0.05, Figures 2D, S2B). Finally, we extended our analysis of the immunosuppressive efficacy of costimulatory blockade to include the transplantation of bone marrow mononuclear stem cells (BMMCs). This cell type was chosen because it represents a well-characterized and potentially clinically relevant stem cell population (Assmus et al., 2006). Mouse BMMCs were rejected by untreated allogeneic recipients by 10 days following transplantation, whereas costimulatory blockade treated mice demonstrated persistent BLI signal at 100 days following transplantation (P<0.01, Figure S2C).
Figure 2. Leukocyte costimulatory molecule blockade permits engraftment of differentiated hESC-derivatives.
Mean fluorescence intensity of MHC antigens, pluripotency (SSEA-4), and endothelial (CD31) markers on (a) in vivo differentiated hESCs isolated from explanted teratoma and (b) in vitro differentiated hESC-ECs. Filled histograms represent isotype control antibodies. (c) BLI of the survival of in vivo differentiated hESCs transplanted into immunodeficient (NOD/SCID) and immunocompetent (BALB/) mice that received either costimulatory blockade (COSTIM) or no immunosuppressive treatment, n = 3–4 per group. (d) Bioluminescence photon intensities representing the survival of in vitro differentiated hESC-ECs after transplantation into immunodeficient, costimulatory blockade (COSTIM) treated, or non-treated immunocompetent (BALB/c) mice, n = 4 per group, *P<0.05. For additional engraftment data regarding differentiated ESCs, see Figure S2.
Allogeneic and xenogeneic transplantation of iPSCs results in immune rejection which can be prevented by costimulatory blockade
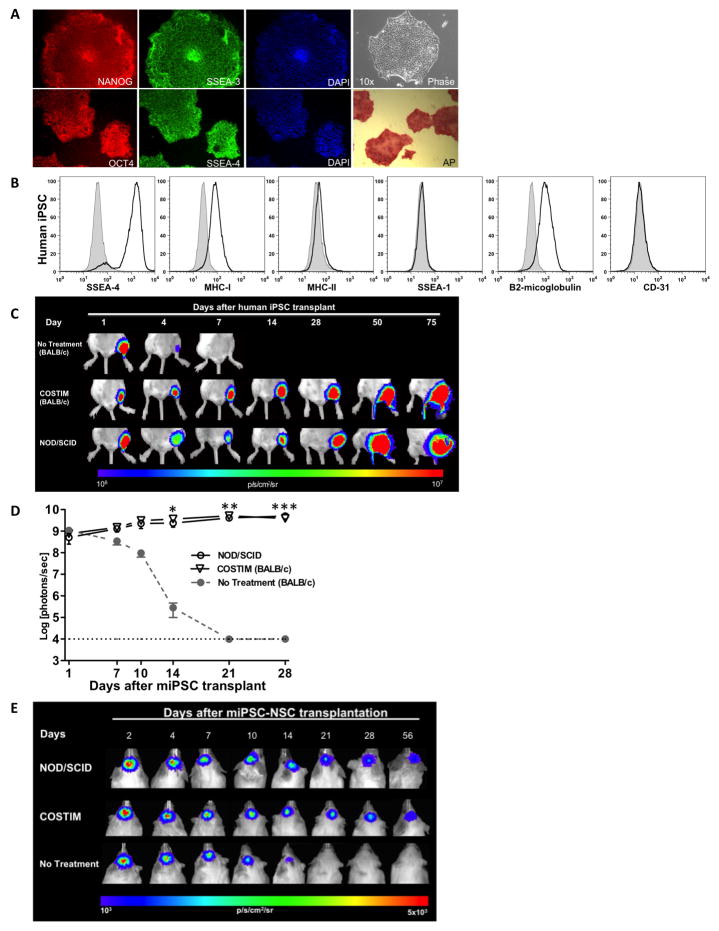
For regenerative medicine purposes, an alternative source of pluripotent cells is human induced pluripotent stem cells (hiPSC). hiPSCs can be generated by delivering transcription factors to reprogram somatic cells towards a state of pluripotency (Takahashi et al., 2007; Yu et al., 2007). To assess the immunogenic properties of hiPSCs and the efficacy of costimulatory blockade to induce long-term engraftment of hiPSCs, we created four hiPSC lines from human adipose stem cells (hASC) isolated from four different patients. These hiPSC colonies stained positive for the pluripotency markers, alkaline phosphatase (AP), Nanog, SSEA-3, SSEA-4, and Oct4 (Figure 3A). Compared to undifferentiated hESCs, the hiPSCs demonstrated similar surface expression levels of pluripotency marker SSEA-4, lack of MHC-II, and slightly higher levels of MHC-I (Figure 3B). We performed microarray gene expression analyses which demonstrated that the four hiPSC lines are similar to H7 hESCs (Wicell) and distinct from hASCs (Figures S3A). The pluripotency of hiPSCs was examined through the formation of embryoid bodies (EBs). hiPSC-EBs expressed multiple markers corresponding to each of the three embryonic germ layers (Figure S3B). The hiPSC-EBs demonstrated the capacity for multi-lineage differentiation as we were able to derive neurons, endothelial cells, and beating cardiomyocytes (Figure S3B, S3C, Supplementary Movie S1). Upon transplantation into immunocompetent mice, hiPSC survival was significantly limited in untreated compared with costimulatory blockade treated mice as the BLI signal decreased to background levels in untreated animals by 7 days post-transplantation, whereas engraftment with steadily increasing BLI signal and teratoma formation were observed in costimulatory blockade treated animals (P<0.01, Figures 3C, S3D). To assess efficacy of costimulatory blockade in an allogeneic transplant model, we generated miPSCs from FVB (H-2kq) mice and followed survival in BALB/c (H-2kd) mice by in vivo BLI. In the absence of immunosuppression, transplanted miPSC survival was significantly limited to 14 to 21 days post transplantation. However, when allogeneic mice were treated with costimulatory blockade, prolonged engraftment with steadily increasing BLI signal and teratoma formation were observed in all animals (Figures 3D, S3E). Similar to ESC-based therapy, iPSC-based therapy will likely utilize a differentiated rather than undifferentiated cell population. Hence we generated miPSC-derived neural stem cells (miPSC-NSC) (Figure S3C) to investigate the survival of this cell population in untreated and costimulatory blockade treated allogeneic recipients. Survival of miPSC-NSCs was significantly limited in untreated compared to costimulatory blockade treated mice (P<0.01, Figure 3E). At day 14 following transplantation, the BLI signal in the untreated group was 24.7±6.8% of the initial BLI intensity, compared to 60.9±6.5% in the costimulatory blockade treated group (P<0.01). By day 21, the BLI signal in the untreated group had diminished to background intensity whereas the BLI signal was 51.1±5.3% of the initial BLI intensity in the costimulatory blockade treated group (P<0.001).
Figure 3. Leukocyte costimulatory molecule blockade permits xenogeneic and allogeneic engraftment of hiPSC, miPSC, and differentiated miPSC-derivatives.
(a) Characterization of hiPSCs by immunostaining with pluripotency markers such as Nanog, Oct4, SSEA-3, SSEA-4, and alkaline phosphatase (AP). (b) Mean fluorescence intensity of MHC antigens and pluripotency markers on undifferentiated hiPSCs. Filled histograms represent isotype control antibodies. BLI and bioluminescence photon intensities representing the survival of (c) hiPSCs and (d) miPSCs transplanted into the gastrocnemius muscle of immunodeficient (NOD/SCID) and immunocompetent mice receiving costimulatory blockade (COSTIM) or no treatment, n = 3–5 per group, *P<0.05, **P<0.01, ***P<0.001. (e) In vitro differentiated miPSC-NSCs transplanted into the subcortical area of the brain in immunodeficient (NOD/SCID) and immunocompetent mice. n = 3–4 per group. For additional characterization and engraftment data regarding miPSCs and hiPSCs, see Figure S3 and Movie S1.
Costimulatory blockade inhibits allogeneic leukocyte proliferation with limited systemic toxicity
To address the mechanism by which costimulatory blockade permits engraftment of pluripotent cells and their differentiated derivatives, we next examined the effect of costimulatory blockade on both the ESCs and the host. One possible mechanism by which the agents support engraftment is to stimulate increased ESC proliferation. To test this hypothesis, we transplanted undifferentiated hESCs into immunodeficient mice randomized to receive either costimulatory blockade or saline as control. Between the two groups, we observed no significant difference in the kinetics of hESC proliferation and teratoma formation (Figure 4A), suggesting these agents do not improve survival by stimulating increased cell proliferation. We next investigated the effect of costimulatory blockade on ESC viability by comparing the percentage of ESCs undergoing early versus late apoptosis. There was no significant difference between ESCs exposed to costimulatory blockade versus unexposed controls (Figure S2D). To evaluate the toxicity of the costimulatory blockade agents on the host, we compared hematologic, renal, hepatic, and metabolic parameters between costimulatory blockade and untreated mice. For all parameters assayed, costimulatory blockade mice demonstrated similar laboratory values as untreated mice (Supplementary Table S2). The low toxicity of costimulatory blockade immunosuppression highlights another advantage of costimulatory blockade over traditional immunosuppressive approaches (e.g., TAC and SIR). Another advantage is that costimulatory blockade requires only a short period of administration. However, if costimulatory blockade diminishes the ability of the host to mount a robust immune response to future antigens, then the potential for clinical translation of this approach would be severely decreased. To address the ability of costimulatory blockade treated hosts to reject third party antigens, hESCs were injected into immunocompetent mice which had previously accepted miPSC-NSC grafts. The transplanted hESCs were rejected, indicating that despite previous costimulatory blockade treatment, the mice were fully capable of rejecting third party antigens (Figure S4A).
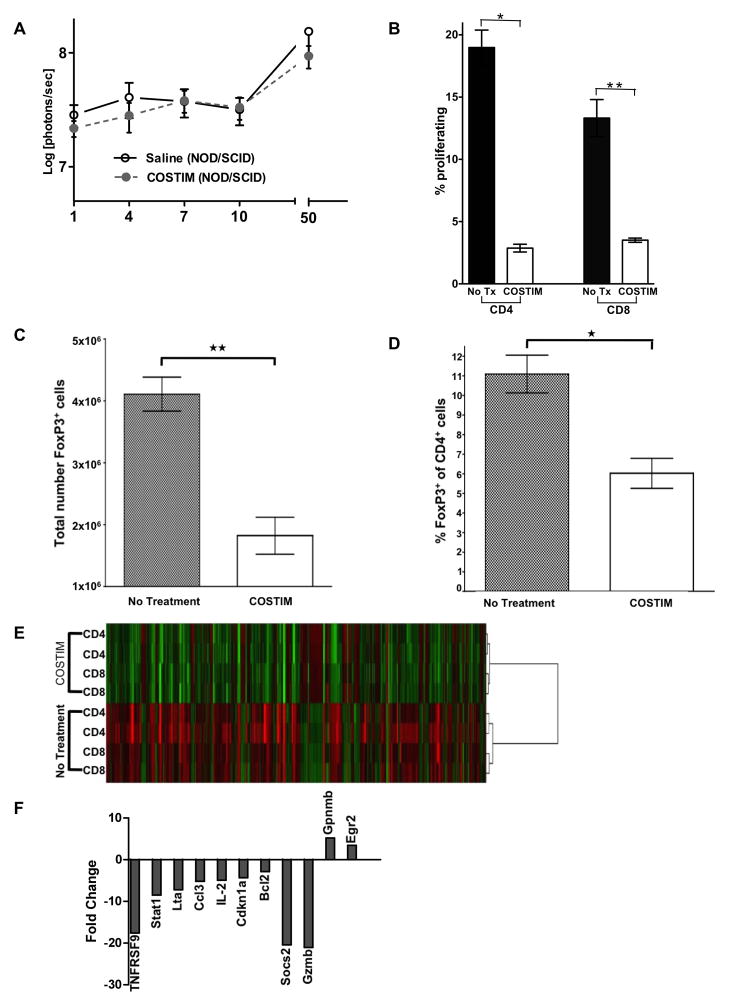
Figure 4. Gene expression and functional characterization of leukocytes treated with costimulatory molecule blockade.
(a) Bioluminescence photon intensities representing the survival of hESCs in immunodeficient (NOD/SCID) mice treated with COSTIM or saline as control. n = 5 per group. (b) Mixed lymphocyte reaction comparing the proliferation of COSTIM-treated and untreated T-cell subsets stimulated by allogeneic splenocytes. *P<0.0001, **P=0.0002. Shown is a representative trial chosen from three independent trials demonstrating similar results. (c) Comparison of the total number of CD4+FoxP3+ T cells and (d) percent of CD4+ cells that are FoxP3+ isolated from mice treated with COSTIM or saline as control. n = 6 COSTIM, n = 3 untreated control, *P=0.006, **P=0.002. (e) Hierarchical clustering of T-cells stimulated by allogeneic splenocytes reveals distinct gene expression clusters between COSTIM-treated and untreated T cells. Biological duplicates for each group are shown. (f) Gene expression fold change of COSTIM-treated relative to untreated T-cells. For additional characterization of the costimulatory blockade treated responder T-cells see Figure S4.
To characterize the effect of costimulatory blockade on the host immune response, we next performed mixed lymphocyte reactions (MLR) with MHC mismatched splenocytes as stimulators and responders. Relative to untreated controls, costimulatory blockade significantly mitigated both CD4+ (P<0.0001) and CD8+ (P=0.0002) T cell proliferation (Figure 4B). To determine the contribution of T regulatory (Treg) cells towards the costimulatory blockade induced survival of hESCs, we compared the absolute number of CD4+FoxP3+ T cells in costimulatory blockade and untreated mice 21 days after hESC transplantation. Relative to untreated controls, costimulatory blockade significantly decreased the total number of CD4+FoxP3+ T cells (P=0.002) (Figure 4C), as well as the percent of CD4+ T cells that were CD4+FoxP3+ cells (P=0.006) (Figure 4D). To assess the immunosuppressive ability of the Treg cells which develop in costimulatory blockade treated mice, MLRs were performed as described above, with or without the inclusion of CD4+CD25hi T cells. The inclusion of CD4+CD25hi T cells significantly mitigated the proliferation of CD8+ T cells (P=0.0005) (Figure S4B). However, the CD4+CD25hi T cells isolated from costimulatory blockade mice did not possess a significantly different immunosuppressive potency than CD4+CD25hi T cells isolated from untreated mice (P=NS).
Gene expression characterization of leukocytes treated with costimulatory molecule blockade
Certain genetic regulatory programs have previously been described for anergic (Safford et al., 2005) or for optimally activated T-cells (Diehn et al., 2002). To elucidate the gene expression “footprint” of costimulatory blockade treated T-cells, we performed microarray gene expression analysis comparing the expression profile of costimulatory blockade treated and untreated responder T-cells. Relative to untreated, the costimulatory blockade treated group had 96 and 40 genes significantly (P<0.05) down- and upregulated, respectively (Figures 4E and Supplementary Table S3). Next we analyzed these genes in terms of their functional relationships using Ingenuity Network software which correlates these significantly expressed genes to the signaling and metabolic pathways, molecular networks, and biological processes that are most significantly affected in the costimulatory blockade treated group (Supplementary Table S3). The key genes implicated in the establishment of costimulatory blockade induced allograft tolerance and host anergy were identified thereafter. Figure 4F represents the fold change of those genes, which include Egr2, GPNMB, BCL2, IL-2, Ccl3, Lta, Stat1, Cdkn1a, Socs2, Gzmb, and TNFRSF9. Finally, we predicted the probable gene regulatory network that is responsible for inhibiting T-cell activation, proliferation, and survival in the costimulatory blockade treated group (Figure S4C).
DISCUSSION
The field of regenerative medicine is quickly advancing. Therapeutic applications of hESC-derived oligodendrocyte progenitor cells (www.geron.com) and hESC-derived retinal pigment epithelial cells (www.advancedcell.com) have recently been initaited in patients with acute spinal cord injury and Stargardt’s macular dystrophy, respectively. More Phase I clinical trials are expected within the next 5–10 years (Lomax et al., 2007). One issue critical to the realization of such goals is the elimination of the immunologic barrier that presently precludes the successful application of cell based regenerative therapy (Carpenter et al., 2009; Chidgey et al., 2008). The focus of this study was to characterize the immunogenic properties of ESCs, iPSCs, and their differentiated derivatives, and to evaluate the efficacy of blockade of leukocyte costimulatory molecules as a way to induce transplanted cell engraftment and survival.
Future clinical applications of pluripotent cells for regenerative therapy will likely involve allogeneic transplantation setting. However, at the present time a comprehensive study of hESC immunogenicity in humans is not yet feasible due to ethical reasons and safety constrains. As a next best option, we initially focused on the allogeneic transplantation scenario. We demonstrated that costimulatory blockade is an effective approach to induce engraftment of mESCs in a murine host. However, conclusions drawn from mESCs possibly may not reliably be extrapolated to hESCs. One major difference between the two cell population is that in the undifferentiated state mESCs express undetectably low levels of MHC-I (H2-Kb) (Abdullah et al., 2007; Bonde and Zavazava, 2006), whereas hESCs demonstrate low but detectable levels of MHC-I expression. Similarly, differentiation of hESCs induces increased MHC-I expression. For these reasons, it was important to also demonstrate the immunosuppressive efficacy of costimulatory blockade to prevent the rejection of undifferentiated hESCs as well as spontaneously differentiated hESCs and in vitro differentiated hESC-ECs and miPSC-NSCs.
Both undifferentiated and spontaneously differentiated hESCs were rejected in the absence of immunosuppression and demonstrated stable engraftment at all time points assayed in the presence of costimulatory blockade treatment. In the absence of immunosuppression, hESC-ECs were rejected by day 7, whereas treatment with costimulatory blockade permitted hESC-EC survival similar to NOD/SCID mice. Overall, costimulatory blockade is more advantageous than more common forms of immunosuppression (e.g., tacrolimus, sirolimus) because it involves only a brief period of administration, produces minimal systemic toxicity, and induces superior long-term engraftment of murine and human pluripotent cells.
As an alternative approach to circumvent cellular rejection following transplantation, the use of hiPSCs has been suggested because they can be derived from the recipient and thus may not provoke an immune response (Byrne, 2008). However, it may not be economically feasible to offer this type of treatment to the population at large, nor logistically feasible to safely develop autologous hiPSCs for transplantation in patients with acute injury such as spinal cord trauma, stroke, or myocardial infarction. In the future, it is possible that allogeneic hiPSC transplantation would be necessary in certain scenarios, which therefore would necessitate the development of immunotolerance strategies. At present, the immunogenic properties of hiPSCs remain largely unknown, as no data exist regarding the immune response towards hiPSCs. The only prior study to investigate the immune properties of iPSCs focused on miPSCs and their susceptibility to NK-cell mediated immune rejection (Dressel et al., 2010). To our knowledge, this is the first study investigating the immunogenic properties of hiPSCs. We demonstrate that xenogeneic hiPSCs are rejected under similar kinetics as hESCs and that immunosuppression with costimulatory blockade successfully mitigates this immune rejection. Similarly, allogeneic transplantation of undifferentiated miPSCs or differentiated miPSC-NSCs results in immune rejection by 21 days post transplantation, whereas engraftment in animals treated with costimulatory blockade was similar to NOD/SCID mice. This is important because if future clinical applications of iPSC-based therapies involve an allogeneic transplantation setting, costimulatory blockade may be a viable immunosuppressive approach to mitigate the allogeneic immune response.
In summary, this study demonstrates that a short course of costimulatory blockade treatment is sufficient to induce engraftment of allogeneic mESCs and miPSCs as well as xenogeneic hESCs, hiPSCs, and their differentiated derivates. Our data suggest that costimulatory blockade permits transplanted cell engraftment by decreasing the expression of pro-inflammatory cytokines (e.g., IL-2, Tnfrsf9), decreasing the polarization of naive T cells towards a type I phenotype, increasing the establishment of a pro-apoptotic phenotype, and inducing clonal anergy. Further demonstrations of successful management of transplant rejection as shown here will help realize the full potential of stem cell-based regenerative therapies in the future.
EXPERIMENTAL PROCEDURES
Transduction, transplantation, and in vivo tracking of pluripotent cells
Formation of miPSCs and hiPSCs was performed as previously described (Kim et al., 2009; Sun et al., 2009). H7 hESCs (Wicell), mouse ES-D3 cells (ATCC), miPSCs and hiPSCs were transduced with a Fluc-eGFP double fusion construct by lentivirus based techniques as previously described (Cao et al., 2008). Differentiation of hESCs into hESC-ECs and miPSCs into miPSC-NSCs was performed as previously described (Li et al., 2009; Naka et al., 2008). For cell transplantation experiments, 1×106 human derived and 5×105 mouse derived cells were injected into the gastrocnemius muscle of recipient mice. Transplanted cell survival was longitudinally monitored via BLI using the Xenogen In Vivo Imaging System (Caliper Life Sciences). Briefly, D-Luciferin (Promega) was administered intraperitoneally at a dose of 375 mg/kg of body weight. Animals were placed in a light-tight chamber, and photons emitted from luciferase expressing cells were collected with integration times of 5 sec to 2 min, depending on the intensity of the bioluminescence emission. BLI signal was quantified in maximum photons per second per centimeter square per steradian (p/s/cm2/sr) and presented as log10[photons per second]. For immunosuppressive therapy protocol, female BALB/c mice (8–10 weeks old) were randomized to receive tacrolimus (TAC; Sigma- Aldrich), sirolimus (SIR; Rapamune oral solution; Sigma- Aldrich), anti-CD40L (MR-1), anti-LFA-1 (M17/4), or CTLA4-Ig (BioXCell). TAC and SIR were administered once daily by oral gavage, 4 mg/kg/d for TAC, and 3 mg/kg/d for SIR. Anti-CD40L, anti-LFA-1, and CTLA4-Ig were administered at a dose of 20 mg/kg on days 0, 2, 4, and 6 after transplantation. For statistical analysis, comparisons between groups were done by independent sample t tests or ANOVA with LSD post-hoc or Bonferroni post-tests, where appropriate. Differences were considered significant for P < 0.05. All procedures performed were approved by the Animal Care and Use Committee of Stanford University. For microarray data analysis and functional annotation, the RNA samples were hybridized to the Affymetrix Mouse 430_2 chips. Data sets were analyzed using GeneSpring GX 10.0 software as detailed in the supplemental experimental procedures. Data normalization was followed by Student’s t-test (P-value <0.05; fold-change cut off of 2.0) and hierarchical clustering to obtain the significantly expressed genes. Their functional annotation was carried out using Ingenuity IPA pathway analysis software.
More detailed protocol information is available in the supplementary experimental procedures.
Supplementary Material
Acknowledgments
The authors would like to thank Marcel Daadi for assistance with stereotactic transplantation of murine neural progenitor cells. This work was supported by a NIH AI085575, NIH HL089027, NIH DP2OD004437 (JCW), and a Howard Hughes Medical Institute research training fellowship (JIP). MMD is supported by grants from the Howard Hughes Medical Institute, the Ellison Medical Foundation, and the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullah Z, Saric T, Kashkar H, Baschuk N, Yazdanpanah B, Fleischmann BK, Hescheler J, Kronke M, Utermohlen O. Serpin-6 expression protects embryonic stem cells from lysis by antigen-specific CTL. J Immunol. 2007;178:3390–3399. doi: 10.4049/jimmunol.178.6.3390. [DOI] [PubMed] [Google Scholar]
- Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- Bonde S, Zavazava N. Immunogenicity and engraftment of mouse embryonic stem cells in allogeneic recipients. Stem Cells. 2006;24:2192–2201. doi: 10.1634/stemcells.2006-0022. [DOI] [PubMed] [Google Scholar]
- Byrne JA. Generation of isogenic pluripotent stem cells. Hum Mol Genet. 2008;17:R37–41. doi: 10.1093/hmg/ddn053. [DOI] [PubMed] [Google Scholar]
- Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, Lee A, Li RA, Gambhir SS, Weissman IL, et al. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MK, Frey-Vasconcells J, Rao MS. Developing safe therapies from human pluripotent stem cells. Nat Biotechnol. 2009;27:606–613. doi: 10.1038/nbt0709-606. [DOI] [PubMed] [Google Scholar]
- Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Chidgey AP, Layton D, Trounson A, Boyd RL. Tolerance strategies for stem-cell-based therapies. Nature. 2008;453:330–337. doi: 10.1038/nature07041. [DOI] [PubMed] [Google Scholar]
- Diehn M, Alizadeh AA, Rando OJ, Liu CL, Stankunas K, Botstein D, Crabtree GR, Brown PO. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci U S A. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel R, Nolte J, Elsner L, Novota P, Guan K, Streckfuss-Bomeke K, Hasenfuss G, Jaenisch R, Engel W. Pluripotent stem cells are highly susceptible targets for syngeneic, allogeneic, and xenogeneic natural killer cells. FASEB J. 2010 doi: 10.1096/fj.09-134957. [DOI] [PubMed] [Google Scholar]
- Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev. 2009;229:294–306. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnemo KH, Genead R, Kumagai-Braesch M, Andersson A, Danielsson C, Mansson-Broberg A, Dellgren G, Stromberg AM, Ekberg H, Hovatta O, et al. Costimulation blockade induces tolerance to HESC transplanted to the testis and induces regulatory T-cells to HESC transplanted into the heart. Stem Cells. 2008;26:1850–1857. doi: 10.1634/stemcells.2008.0111. [DOI] [PubMed] [Google Scholar]
- Jenkins MK. The ups and downs of T cell costimulation. Immunity. 1994;1:443–446. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Kim JB, Zaehres H, Arauzo-Bravo MJ, Scholer HR. Generation of induced pluripotent stem cells from neural stem cells. Nat Protoc. 2009;4:1464–1470. doi: 10.1038/nprot.2009.173. [DOI] [PubMed] [Google Scholar]
- Kurtz J, Raval F, Vallot C, Der J, Sykes M. CTLA-4 on alloreactive CD4 T cells interacts with recipient CD80/86 to promote tolerance. Blood. 2009;113:3475–3484. doi: 10.1182/blood-2008-01-133736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KJ, Prowse SJ, Simeonovic CJ, Warren HS. Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Annu Rev Immunol. 1983;1:143–173. doi: 10.1146/annurev.iy.01.040183.001043. [DOI] [PubMed] [Google Scholar]
- Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- Li L, Baroja ML, Majumdar A, Chadwick K, Rouleau A, Gallacher L, Ferber I, Lebkowski J, Martin T, Madrenas J, et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22:448–456. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- Li Z, Wilson KD, Smith B, Kraft DL, Jia F, Huang M, Xie X, Robbins RC, Gambhir SS, Weissman IL, et al. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS One. 2009;4:e8443. doi: 10.1371/journal.pone.0008443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax GP, Hall ZW, Lo B. Responsible oversight of human stem cell research: the California Institute for Regenerative Medicine’s medical and ethical standards. PLoS Med. 2007;4:e114. doi: 10.1371/journal.pmed.0040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka H, Nakamura S, Shimazaki T, Okano H. Requirement for COUP-TFI and II in the temporal specification of neural stem cells in CNS development. Nat Neurosci. 2008;11:1014–1023. doi: 10.1038/nn.2168. [DOI] [PubMed] [Google Scholar]
- Niu G, Chen X. Has molecular and cellular imaging enhanced drug discovery and drug development? Drugs R D. 2008;9:351–368. doi: 10.2165/0126839-200809060-00002. [DOI] [PubMed] [Google Scholar]
- Pan Y, Luo B, Sozen H, Kalscheuer H, Blazar BR, Sutherland DE, Hering BJ, Guo Z. Blockade of the CD40/CD154 pathway enhances T-cell-depleted allogeneic bone marrow engraftment under nonmyeloablative and irradiation-free conditioning therapy. Transplantation. 2003;76:216–224. doi: 10.1097/01.TP.0000069602.30162.A1. [DOI] [PubMed] [Google Scholar]
- Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, Blackford A, Horton MR, Drake C, Schwartz RH, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci U S A. 2009;106:15720–15725. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K, Sheikh AY, Haddad M, Connolly AJ, Davis MM, Robbins RC, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci U S A. 2008;105:12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- Toriumi H, Yoshikawa M, Matsuda R, Nishimura F, Yamada S, Hirabayashi H, Nakase H, Nonaka J, Ouji Y, Ishizaka S, et al. Treatment of Parkinson’s disease model mice with allogeneic embryonic stem cells: necessity of immunosuppressive treatment for sustained improvement. Neurol Res. 2009;31:220–227. doi: 10.1179/016164108X339378. [DOI] [PubMed] [Google Scholar]
- Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990;144:4579–4586. [PubMed] [Google Scholar]
- Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zuckerman LA, Pullen L, Miller J. Functional consequences of costimulation by ICAM-1 on IL-2 gene expression and T cell activation. J Immunol. 1998;160:3259–3268. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.