Abstract
BACKGROUND
Constitutively activated NFκB contributes to the development of cancer by regulating the expression of genes involved in cell survival, metastasis, and angiogenesis. We have demonstrated that MEKK3 plays a critical role in cytokine-mediated NFκB activation and that stable expression of MEKK3 in cultured cells leads to increased NFκB activity.
METHODS
MEKK3 expression in ovarian cancer cells or tumors was assessed by western blotting and real-time PCR. NFκB activities were analyzed by EMSA and luciferase reporter assays. Western blot analysis for the survival factors were also performed and correlated with MEKK3 and NFκB activities. Cell survival assays were used to determine the sensitivity of ovarian cancer cells to various chemotherapeutic agents.
RESULTS
We found that 63% of the ovarian cancers had higher MEKK3 expression than the normal ovarian epithelial cells. Ovarian cancers with high MEKK3 showed correspondingly high IKK and NFκB activity. Moreover, MEKK3 co-immunoprecipitated with Akt and cooperated with Akt to synergistically activate NFκB. Consistent with increased MEKK3 and NFκB activity in ovarian cancers, Bcl-2, Bcl-xL, survivin, and XIAP levels were increased, which correlated with increased resistance to chemotherapeutic agents. Knockdown of MEKK3 with siRNA significantly increased cancer cell sensitivity to paclitaxel.
CONCLUSIONS
MEKK3 may be aberrantly expressed in ovarian cancers and plays an important role in tumors with constitutively activated NFκB.
Keywords: MEKK3, NFκB, Akt, ovarian cancer, apoptosis
INTRODUCTION
Ovarian cancer is the fifth leading cause of cancer-related death in women in the United States with over 25,000 women newly diagnosed each year with this disease and frequently does not result in symptoms until the cancer has spread extensively (1). Despite considerable improvement in therapeutic regimens, the prognosis for patients with ovarian cancers remains poor (2, 3). In addition, ovarian cancers often develop resistance to chemotherapy (4), which may be attributed, in part, to progressive selection of cancer cells in which apoptosis is inhibited (5). As such, identification of potential therapeutic targets and characterization of their participation in cell growth, anti-apoptosis, and chemotherapy resistance remain a central issue. Among the potential targets is NFκB. Consistent with its importance in cancer development and oncogenic transformation, NFκB is frequently constitutively activated in many human cancers (6–8). In normal cells, NFκB activation by cytokines or growth factors involves a complex cascade of signaling events that utilizes adaptor molecules, protein kinases, and ubiquitination pathways (9). In resting cells, NFκB is retained in the cytoplasm through interaction with IκB, an inhibitor of NFκB. Activation of the signaling cascade ultimately leads to rapid degradation of IκB (10). NFκB is then released and translocated to the nucleus, where it regulates the expression of target genes (11).
NFκB regulates genes essential for inflammation and immune responses as well as genes that participate in cell survival, angiogenesis, and metastasis (12). Thus, elevated NFκB have been correlated with increased cell proliferation and survival and may contribute to cancer pathogenesis and metastasis (7, 11, 13). Among the protein kinases known to regulate NFκB activation are the mitogen activated protein kinase (MAPK)/extracellular signal regulated kinase (ERK) kinase kinases (MEKKs) (5, 14–17). MEKKs are members of the serine/threonine protein kinase family that function as upstream regulators of MAPKs. Expression of MEKK1-3 leads to the activation of IκB kinase (IKK), which in turn phosphorylates IκB resulting in the degradation of IκB and activation of NFκB. In gene knock-out studies, MEKK3 deficiency produced profound defects in NFκB activation (18). In MEKK3(−/−) fibroblasts, the activation of NFκB by IL-1 and TNF was severely impaired, providing strong genetic evidence that links MEKK3 to cytokine-mediated NFκB activation. Consistent with its importance in NFκB activation, cell lines that stably express MEKK3 had constitutively elevated NFκB activities and were more responsive to cytokine stimulation (5).
In addition to the MAPK pathway, the PI3-kinase-Akt pathway also contributes to the full activation of NFκB (19, 20). Activated Akt not only promotes cell survival (21) but also phosphorylates and activates IKK, which in turn activates NFκB (22). Finally, activated IKK can directly phosphorylate the p65 subunit of NFκB and confer enhanced transactivation activity (23). While dysregulation of the PI3-kinase-Akt signaling pathway has been well documented in ovarian cancers (24), it is unclear whether abnormal expression of MEKK3 may also be associated with ovarian cancers. In this study, we examined the expression of MEKK3 and show that a significant portion of the ovarian cancers express MEKK3 at high levels, which positively correlated with high NFκB activity. The significance of MEKK3 is further amplified by its functional cooperation with Akt to synergistically activate NFκB. Our findings support an important role for MEKK3 in the constitutive activation of NFκB in ovarian cancers.
RESULTS
Elevated MEKK3 expression and NFκB activity in ovarian cancer cell lines
Western blotting with anti-MEKK3 showed very low levels of MEKK3 in normal ovarian epithelial (NOE) NOE115 control cells, high levels in four ovarian cancer lines (OVCA429, Hey, DOV13, and SKOv3), and low or non-detectable levels in three lines (OVCA433, OVCA420, and OVCA432) (Fig. 1A). MEKK3 protein levels in these cell lines correlated with their MEKK3 mRNA levels, as determined by real-time RT-PCR (Fig. 1B).
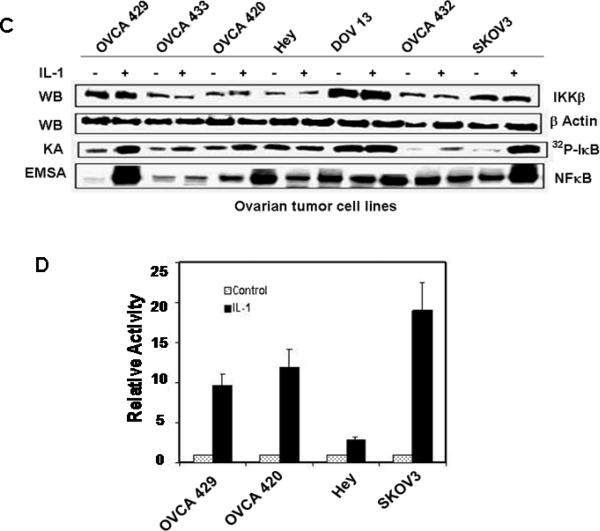
Figure 1. MEKK3 expression is directly related to NFκB activity in ovarian cancer cells.
(A) Levels of MEKK3 expression and NFκB activity in normal ovarian epithelial and ovarian cancer cells. Whole-cell extracts from NOE115 and ovarian cancer cell lines were western blotted (WB) with antibodies. Nuclear extracts were used in EMSA with 32P-labeled NFκB probe. NFκB activity is competed by wild-type (WT) but not by mutant (mt) NFκB oligonucleotides. IKK kinase activity (KA) was assayed using GST-IκB as substrates. (B) MEKK3 mRNA levels in normal ovarian epithelial and ovarian cancer cells. MEKK3 mRNA levels were measured by quantitative RT-PCR and normalized to that of NOE095. All measurements were done in triplicate and the mean value and standard deviations are presented. (C) Induction of IKK and NFκB activities. Cells were stimulated with IL-1 for 30 min and the IKKβ levels and IKK activity in the cell extracts and NFκB activity in the nuclear extracts were determined. (D) Responsiveness of ovarian cancer cells to IL-1 stimulation. Cells were transfected with p(κB)3/luciferase reporter and stimulated with IL-1. Results were normalized to the luciferase activity of the cells transfected with the reporter gene and not stimulated with IL-1.
Given the significantly elevated MEKK3 expression in the four ovarian cancer cell lines, we examined whether this increased MEKK3 activity affected NFκB-binding activity. EMSA studies showed high constitutive NFκB-binding activities in Hey, DOV13, and SKOv3 cells, low binding activities in OVCA429 and OVCA420, and no activity in the control NOE115 cells and in OVCA433 and OVCA432 cells (Fig. 1A).
Since activation of NFκB requires IKK to phosphorylate IκB, we examined whether the high constitutive activation of NFκB in ovarian cancer cells could be attributed to increased basal IKK kinase activity and if so, whether this increased kinase activity was associated with increased expression of IKKβ. Alternatively, increased basal IKK kinase activity could be attributable to increased activation of IKK complex by upstream signals without increased IKKβ expression. The IKK complex was immunoprecipitated and kinase activity was assayed in the presence of [γ-32P]ATP with GST-IκBα(1–54) as substrate. Basal IKK kinase activities were detected in all cell lines, albeit at various levels (Fig. 1A), and as expected, there is a direct relationship between kinase activity and NFκB activity. Interestingly, basal IKK kinase activities paralleled MEKK3 expression pattern better than they did IKKβ protein levels. This result suggests that increases in basal IKK kinase activities in some ovarian cancer cells were due to activation by upstream kinases such as MEKK3, rather than an increase in the amount of IKK complex. Taken together, our results indicate that several of the ovarian cancer cell lines expressed significantly higher levels of MEKK3 than NOE cells. Moreover, the high MEKK3 expression in ovarian cancer cells may contribute to higher basal IKK kinase activity, which in turn led to higher levels of active NFκB.
Responsiveness of ovarian cancer cells to stimulation
To determine whether NFκB activity can be further increased in cells with high constitutive NFκB activity, cells were stimulated with IL-1 and then assayed for IKK kinase and NFκB-binding activities. IL-1 dramatically increased NFκB binding activity in OVCA429, OVCA420, and SKOv3 cells and was accompanied by corresponding increases in IKK kinase activity with no change in IKKβ expression (Fig. 1C). In contrast, OVCA433, Hey, DOV13, and OVCA432 cells were not responsive to IL-1. Consistent with NFκB activation, transfection of p(κB)3/luciferase reporter into OVCA429, OVCA420, and SKOv3 cells also showed dramatic induction of luciferase activity, whereas transfection into Hey cells produced little response (Fig. 1D).
MEKK3 expression and NFκB activity in ovarian tumors
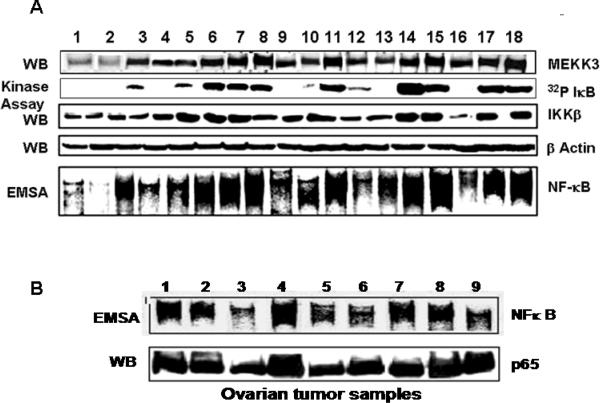
We next examined the expression of MEKK3 in human ovarian tumors that had been dissected from the surrounding stroma and kept at −70°C. Twenty seven ovarian tumors were analyzed for MEKK3 and IKKβ protein expression and IKK kinase and NFκB activities. Representative results are shown in Figure 2A. Expression of MEKK3 in these tumors could be grouped into three classes--9/27 (33%) tumors had significantly elevated MEKK3 levels (comparable to those in Hey and SKOv3 cells), 8/27 (30%) had intermediate levels, and 10/27 (37%) had very low levels (comparable to NOE115 cells). In contrast, all 27 ovarian tumors expressed IKKβ at approximately equal levels. However, IKK kinase activities ranged from un-detectable to very high levels. Interestingly, IKK kinase activity closely paralleled MEKK3 but not IKKβ protein levels. These findings are consistent with those in the ovarian cancer cell lines and support the notion that IKK activity in ovarian tumors and cell lines reflects more closely the expression of the upstream kinase MEKK3 than that of IKKβ.
Figure 2. MEKK3 is overexpressed in ovarian carcinomas and parallels with increased IKK kinase activity and constitutive NFκB activity.
(A) Whole-cell extracts from ovarian carcinomas were used for western blotting with anti-MEKK3, anti-IKKβ, and β-actin. A portion of the same tumor samples were used to prepare detergent-soluble extracts for IKK kinase assays. (B) Nuclear and whole-cell extracts from ovarian carcinomas were used for, respectively, EMSA and western blot with anti-p65 antibodies.
NFκB binding activity reflected IKK kinase activity: that is, tumor samples that had high IKK kinase activity also had high constitutive NFκB binding activity (Fig. 2A and Fig. S1). Differences in NFκB activity in these tumors were due to differential activation of NFκB rather than altered expression of NFκB proteins, as determined by the near equal levels NFκB p65 subunit in 9 representative tumor samples (Fig. 2B).
Akt and pAkt levels in ovarian cell lines and tumors
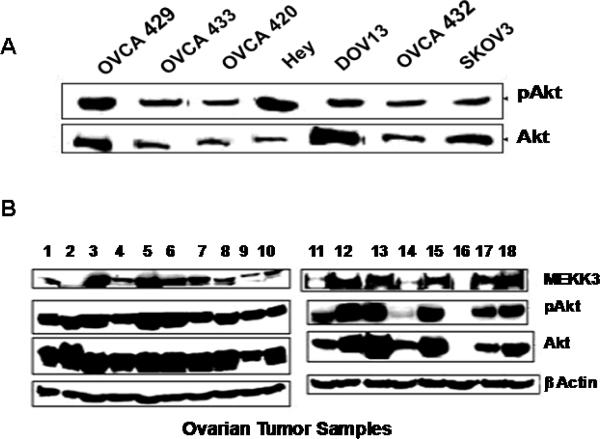
Because the PI3-kinase-Akt pathway also contributes to NFκB activation and is often dysregulated in ovarian cancers, we examined Akt expression and its phosphorylation. While Akt protein levels were much higher in DOV13 than in Hey, pAkt levels were significantly higher in Hey than in DOV13 (Fig. 3A). When ovarian tumors were examined, many showed overexpression of Akt and high constitutive pAkt levels (Fig. 3B). However, pAkt levels did not correlate with MEKK3 levels, although some samples had high levels of both pAkt and MEKK3.
Figure 3. Differential expression of Akt and pAkt in ovarian cell lines and ovarian carcinomas.
(A) Levels of Akt and pAkt in ovarian cancer cell lines were determined using anti-Akt and anti-pAkt antibodies. (B) Levels of Akt and pAkt in ovarian carcinomas. MEKK3 levels were determined for comparison, and β-actin was used as loading control.
MEKK3 does not affect the activation of Akt by IL-1
To examine whether MEKK3 has any effect on cytokine-mediated activation of Akt, we employed U373-MEKK3 cells, which overexpress MEKK3 (5) and parental U373 cells. In response to IL-1 both cell lines demonstrated equivalent increased Akt phosphorylation, suggesting MEKK3 overexpression has little effect on cytokine-mediated Akt activation (Fig. 4A). In contrast, basal JNK activity was higher in U373-MEKK3 cells than in U373 cells and can be further induced by IL-1 (Fig. 4A).
Figure 4. Activation of Akt is independent of MEKK3.
(A) Overexpression of MEKK3 activates JNK but not Akt. U373 and U373-MEKK3 stable cells were treated with IL-1. Cell extracts were probed with anti-pAkt and anti-Akt antibodies. For JNK kinase assays, JNK was immunoprecipitated and incubated with [γ-32P]ATP and GST-c-Jun. 32P-labeled GST-c-Jun was then detected by autoradiography. A portion of the immune complexes was subjected to western blotting with anti-JNK. (B) Activation of Akt is not affected by the absence of MEKK3. MEKK3(+/+) and MEKK3(−/−) MEFs were stimulated with IL-1 and pAkt and total Akt levels were determined by western blotting.
To further verify that Akt activation is independent of MEKK3, MEKK3(-/-) (18) and wildtype MEKK3(+/+) fibroblasts were stimulated with IL-1. Consistent with results from MEKK3-overexpressing cells, the absence of MEKK3 in MEKK3(-/-) cells also had no effect on Akt phosphorylation (Fig. 4B). Taken together our results indicate that while MEKK3 can enhance IL-1-mediated JNK activation, it has no direct effect on Akt activation.
Physical association and functional cooperation between MEKK3 and Akt
Since both MEKK3 and Akt participate in the activation of NFκB and are frequently overexpressed in ovarian cancers, we examined whether they can form protein complexes with each other. We immunoprecipitated MEKK3 from 11 tumor extracts and probed the immune complexes for Akt and pAkt. Various amounts of MEKK3 were detected (Fig. 5A). Akt and pAkt were also present in the immune complexes, albeit at very different levels. This result suggests that MEKK3 and Akt can form protein complexes through either direct or indirect protein-protein interactions.
Figure 5. Physical association and functional cooperation between MEKK3 and Akt.
(A) Akt co-immunoprecipitates with MEKK3. Whole-cell extracts from ovarian carcinomas were incubated with anti-MEKK3. The MEKK3 immune complexes were then probed with anti-Akt and anti-pAkt antibodies. (B) MEKK3 and Akt cooperate to activate NFκB. U373 cells were transfected with p(κB)3/luciferase reporter gene, either alone or with MEKK3- and Akt-expression vectors. Transfected cells were stimulated with IL-1 for 6 h before the cells were harvested for luciferase assays. Results were normalized to the activities of the control cells.
To determine whether this physical association leads to functional cooperation, we transfected U373 cells with an NFκB/luciferase reporter and expression vectors of MEKK3 and Akt, either individually or together and then treated the cells with IL-1. Transfection of MEKK3 or Akt individually increased luciferase activity 2- to 4-fold, but cotransfection of MEKK3 and Akt together increased luciferase activity approximately 10-fold. Moreover, luciferase activity was significantly higher in IL-1-stimulated cells that had been transfected with both MEKK3 and Akt than in those with either MEKK3 or Akt alone (Fig. 5B).
Elevated MEKK3 correlates with increased expression of anti-apoptotic proteins
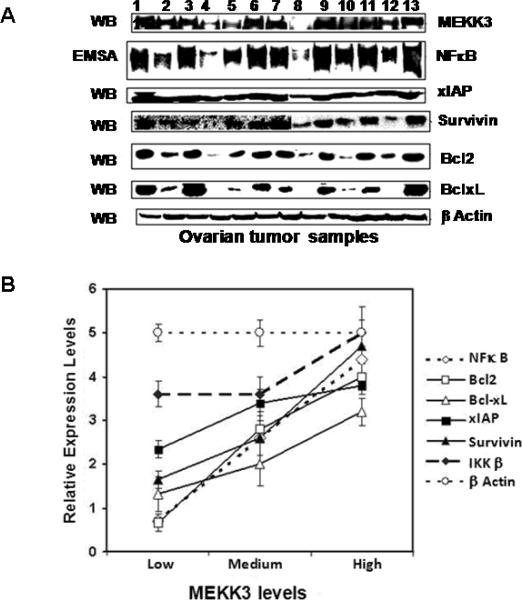
We examined whether increased MEKK3 and NFκB activities were reflected in altered expression of apoptosis-associated genes that are known targets of NFκB regulation. Western blotting of ovarian tumor extracts showed a wide spectrum of expression for Bcl-2, Bcl-xL, XIAP, and survivin, ranging from non-detectable to very high levels (Fig. 6A). Their expression levels however closely paralleled those of MEKK3 and NFκB; that is, tumors with low MEKK3 and NFκB had low expression of Bcl-2, Bcl-xL, XIAP, and survivin, whereas tumors with high MEKK3 and NFκB expressed higher levels of these anti-apoptotic genes (Fig. 6B). In contrast, the expression of IKKβ and β-actin remained constant regardless of the levels of MEKK3 and NFκB.
Figure 6. Levels of Bcl-2, Bcl-xL, survivin, and XIAP in ovarian carcinomas.
(A) Expression levels of Bcl-2, Bcl-xL, survivin, and XIAP. β-actin was included as controls and MEKK3 and NFκB activities were for comparison. (B) Levels of cell survival factors correlate with MEKK3 expression.
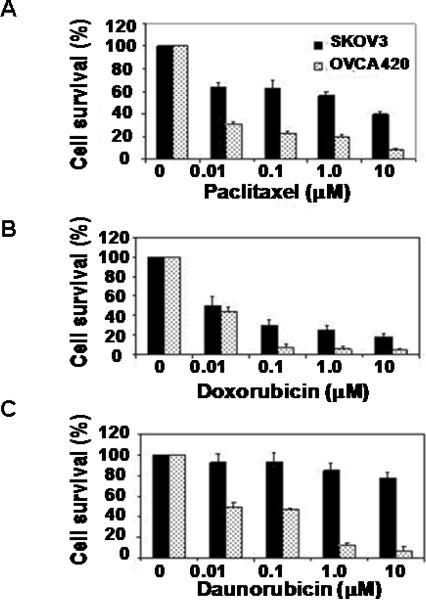
Differential sensitivity of ovarian cancer cells to therapeutic agents
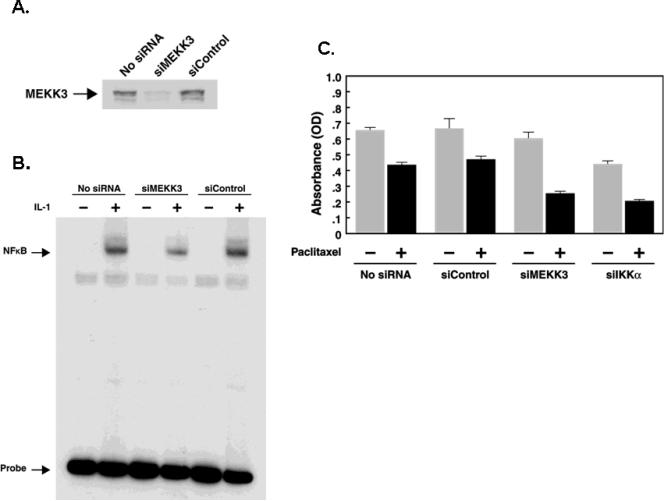
To examine whether increased MEKK3 and NFκB in ovarian cells was associated with higher resistance to apoptosis, we treated SKOv3 and OVCA420 cells with paclitaxel, doxorubicin, and daunorubicin and determined the cell survival. SKOv3 cells, which have higher levels of MEKK3 and NFκB, showed significantly greater resistance than OVCA420 cells (Fig. 7). To examine directly whether increased resistance could be attributed to MEKK3, we knockdowned MEKK3 expression with siRNA in SKOv3, OVCA420 and ES2 cells and assessed their sensitivity to paclitaxel. Transfection of MEKK3 siRNA into SKOv3 cells effectively reduced MEKK3 expression and NFκB binding activity (Fig. 8A, B). Knockdown of MEKK3 and IKKβ significantly increased the sensitivity of these cells to paclitaxel (Fig. 8C and Fig. S2) suggesting that MEKK3 may be one of the factors that contribute to chemoresistance in ovarian cancer cells.
Figure 7. SKOv3 cells are more resistant to chemotherapeutic drug-induced cytotoxicity than OVCA420 cells.
SKOv3 and OVCA420 cells were treated with indicated concentrations of (A) paclitaxel, (B) doxorubicin, and (C) daunorubicin for 72 h before they were harvested for MTT assays. Cell survivals for each treatment were normalized to the values of the untreated controls.
Figure 8. Knockdown of MEKK3 in SKOv3 ovarian cancer cells increased their sensitivity to paclitaxel.
SKOv3 cells were transfected with control siRNA (siControl) or MEKK3 siRNA (siMEKK3) and analyzed for (A) MEKK3 protein levels or (B) NFκB binding activity. (C) SKOv3 cells were seeded in triplicates in 96-well culture plates for 16 h before they were transfected with siMEKK3 and siControl. Cells were treated with paxlitaxel and subjected to crystal violet proliferation assay. Results shown are average values with standard deviations.
DISCUSSION
Many human cancers have high basal NFκB activity, which protects cancer cells from radiation-induced apoptosis, confers resistance to chemotherapeutic agents, and promotes cell survival (2, 5, 8, 10, 25). In this report, we showed that about half of the ovarian cell lines and tumors examined expressed aberrantly high levels of MEKK3. Moreover, MEKK3 physically interacted with Akt and functionally cooperated with Akt to activate NFκB. Consistent with its function, increased MEKK3 expression not only paralleled increased basal IKK kinase and NFκB activity but also increased expression of genes associated with anti-apoptosis and cell survival. Finally, ovarian cancer cells with higher levels of MEKK3 and NFκB were more resistant to chemotherapeutic agents than those with lower levels and knockdown of MEKK3 increased their drug sensitivity. Our results therefore suggest that a significant fraction of ovarian carcinomas express aberrantly high levels of MEKK3, and this increase contributes to the constitutive activation of NFκB and increases the expression of NFκB-targeted cell survival and anti-apoptotic genes.
Several studies have documented the importance of MEKK3 in regulating the activation of NFκB. Using MEKK3(-/-) fibroblasts, we initially reported that MEKK3-null cells failed to activate NFκB in response to IL-1 and TNF, thus provided strong genetic evidence for a central role of MEKK3 in cytokine-mediated activation of NFκB (18). More recently, MEKK3 was shown to be involved in the formation of the IκBα:NFκB/IKK complex and regulates the rapid phase of NFκB activation (11, 26). Finally, overexpression of MEKK3 not only increased basal NFκB activity but also potentiated cytokine-mediated NFκB activation (5). Taken together, these results raised the distinct possibility that dysregulation of MEKK3, ie., overexpression, provides a mechanism for cancer cells to achieve constitutive NFκB activation. Indeed, we found that overexpression of MEKK3 in ovarian cancers is a frequent event, with approximately two thirds of the tumor samples showed moderate to dramatic elevated MEKK3 expression. The patterns of MEKK3 expression and NFκB activity led to two striking conclusions: First, the basal NFκB activity in the ovarian cancer cell lines and tumors varied tremendously, with many samples having very high levels. Second, levels of NFκB activity closely paralleled MEKK3 expression. These findings are consistent with our MEKK3-overexpression systems in which stable expression of MEKK3 in cultured cells led to increased basal and induced NFκB activities (5), suggesting that increased NFκB activity in ovarian cancers may also be associated with increased MEKK3 expression. It is noteworthy that while many tumors with high basal NFκB activity also showed high IKK kinase activity, this increased IKK kinase activity paralleled more closely with MEKK3 expression than with the expression of IKKβ. These results further support the notion that MEKK3 may function as an important regulator of NFκB in ovarian cancers by contributing to the constitutive activation of IKK kinase activity and increased NFκB activity. Despite the close association between MEKK3 expression and NFκB activity, alteration in MEKK3 expression alone cannot account for increased NFκB activity in all tumors. Indeed, a few tumor samples showed high NFκB activity with low MEKK3 expression, suggesting that other activation mechanisms are involved.
Aberrant activity in the PI3-kinase-Akt signaling pathway has been closely associated with ovarian cancer (19, 27). It is intriguing that activated Akt co-immunoprecipitated with MEKK3. Since both Akt and MEKK3 contribute to NFκB activation, their physical interaction in a protein complex may facilitate this process. Indeed, cotransfection of Akt and MEKK3 resulted in synergistic activation of NFκB-dependent reporter gene expression. Thus, in ovarian cancer cells, high levels of MEKK3 together with elevated pAkt can facilitate their interaction and contribute to the high NFκB activities. The consequence of such interaction may significantly affect the development of cancer.
NFκB regulates a number of cell survival and anti-apoptotic genes. Consequently, cells with elevated NFκB activity are more resistant to apoptotic stimuli. The expression of several survival and anti-apoptotic genes, Bcl-2, Bcl-xL, survivin, and XIAP, in our ovarian tumor samples was high. Importantly, their expression patterns showed excellent correlation with the expression levels of MEKK3 and NFκB activity. Consistent with this proposed role of MEKK3 in activating cell survival genes through activation of NFκB, SKOv3 cells with elevated MEKK3 was significantly more resistant to killing by chemotherapeutic agents than OVCA420 cells. Conversely, knockdown of MEKK3 in ovarian cancer cells significantly increased their sensitivity to chemotherapeutic agents. While MEKK3-NFκB signaling pathway may contribute to chemosensitivity of ovarian cancer cells, other mechanisms, such as the expression of ABC transporter and multi-drug resistance proteins, may also be involved. Nevertheless, as an upstream regulator of NFκB signaling pathway, MEKK3 may be a potential therapeutic target through which the activation of NFκB and expression of cell survival genes could be restrained.
MATERIALS AND METHODS
Chemicals and reagents
Anti-MEKK3, Akt, phospho-Akt (pAkt) antibodies, and secondary sheep IgG-HRP antibody were purchased from Upstate Biotechnology, NY. Antibodies against JNK, MEKK1, IKKβ, and HRP-conjugated IgG from mouse, rabbit, and goat were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against Bcl-2, XIAP, and survivin were obtained from Cell Signaling Technology (Beverley, MA). Anti-β-actin antibody was purchased from Sigma (St. Louis, MO), and [γ-32P]ATP (7000 Ci/mmol) from ICN Pharmaceuticals, Inc. (Costa Mesa, CA). IL-1 was from the National Cancer Institute Biological Resources Branch (Bethesda, MD).
Cell lines, culture conditions, and cancer samples
Ovarian cancer cell lines and normal ovarian epithelial cells (NOE 095 and NOE 115) were cultured in DMEM with 10% fetal bovine serum (Hyclone). MEKK3(−/−) mouse embryo fibroblasts and wild-type MEKK3(+/+) cells were cultured as described (18). U373-MEKK3 cells that overexpress MEKK3 were derived by transfecting MEKK3-expression vectors into U373 cells and selecting for stable clones (5).
Epithelial ovarian cancers were frozen from 27 subjects who had undergone initial surgery at The University of Texas M. D. Anderson Cancer Center using protocols and procedures approved by our Institutional Review Board. The samples were assigned a grade based on Gynecologic Oncology Group criteria and staged according to the International Federation of Gynecology and Obstetrics (FIGO) system. Of the 27 cancers, 26 were invasive and among these 19 were serous, one serous with mixed mesodermal tumor elements, one serous with endometrioid and clear cell elements, one serous with clear cell elements, two endometrioid and two clear cell. Twenty cancers were high-grade, 3 were intermediate grade and 3 were low grade. Four cancers were removed in stage I, one in stage II, 18 in stage III and 3 in stage IV. All tissue samples were dissected from the surrounding stroma before frozen at −70°C at our institution tumor tissue bank.
Immunoprecipitation and western blot
Whole-cell extracts were prepared using lysis buffer containing 20 mM Tris, pH 7.5, 1% Triton-X, 250 mM NaCl, 2 mM EDTA, 1 mM DTT, 1 mM PMSF, 2 μg/ml leupeptin and pepstatin, and 0.5 μg/ml sodium vanadate. For immunoprecipitation, 400 μg of cell lysates were pre-cleared with protein G agarose, and then incubated at 4°C with 1 μg of anti-MEKK3 antibody in a final mixture of 400 μl. After a 2-h incubation, 25 μl of protein G-agarose beads were added, and the lysates incubated for another 2 h. Washed beads were boiled for 5 min in 2× sample buffer and the proteins were separated on 7.5% SDS-PAGE and western blotted. For comparison, some filters were stripped and reprobed with a different antibody. Quantitative measurements of western blots were done using an image quantification software (ImageQuant version 5.0). The values obtained were then grouped into 3 categories (low, medium, and high) for MEKK3 and 0 to 5 for other proteins. These values were used for calculation of mean and standard deviation.
Nuclear extracts and electrophoretic mobility shift assay (EMSA)
Cultured cells were washed with ice-cold PBS and recovered in 1 ml of PBS. After brief centrifugation, cells were resuspended in lysis buffer [10 mM KCl, 10 mM HEPES, pH 7.9, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.2 mM PMSF, 0.4 mM NaVO4, and protease inhibitor cocktail (Roche Molecular Biochemicals)], and allowed to swell on ice for 20 min. Then 10% NP-40 was added and the mixture was mixed vigorously for 10–15 s and centrifuged for 1 min. The nuclear pellets were resuspended in ice-cold extraction buffer (0.4 M NaCl, 20 mM HEPES, pH 7.9, 1 mM EDTA, 1 mM EGTA, and mixture of protease and phosphatase inhibitors) and the nuclear proteins extracted by constant mixing at 4°C for 30 min. Cell debris was then removed by centrifugation, and the nuclear extracts were either used immediately or stored at −70°C.
EMSA for NFκB was performed as described (5, 18). Nuclear extracts (6–8 μg) was incubated with 32P–labeled NFκB-binding oligonucleotide (5'-TTGTTACAAGGGACTTTCCGTGGGGACTTTCCAGGCGTGG -3'). NFκB-DNA complexes were detected using a PhosphorImager (Molecular Dynamics).
Transient transfection and luciferase reporter assay
Cells (2 × 104) were seeded in 6-well plates and transfected using Fugene 6 with 0.5 μg of p(κB)3/luciferase reporter (18). Twenty hours later, cells were treated with IL-1 (25 nM) and harvested 7 h later for luciferase assays.
Quantitative Real-time Reverse Transcription-PCR Analysis
The quantitative real-time reverse transcription-PCR (RT-PCR) analysis was done as described (28). The TaqMan probe and primers used for MEKK3 mRNA were 5'-AATGTGCCAACCAAGTCTCC-3' and 5'-TCCAGAGCACTCACCTCCTT-3'. The human GAPDH gene was used as an endogenous control. All measurements were done in triplicate. Amplification data were analyzed with an ABI Prism Sequence Detection Software version 2.1 (ABI). To normalize the relative expression of MEKK3 to the GAPDH control, standard curves were prepared for MEKK3 and for GAPDH in each experiment.
JNK kinase assay
Cells (3 ×106) were stimulated with IL-1 for 30 min and lysed in buffer containing 20 mM HEPES, pH 7.4, 250 mM NaCl, 2 mM EDTA, 1 mM PMSF, 1 mM DTT, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 0.5 μg/ml benzamidine. Cell extracts (200 μg) were then incubated with anti-JNK antibody at 4°C for 1 h. Protein G agarose was added and incubated for an additional 45 min. After incubation, the agarose beads were collected and washed with lysis buffer and then with kinase buffer (20 mM HEPES, pH 7.4, 1 mM DTT, and 25 mM NaCl). Washed beads were added to kinase reaction mixtures containing 20 mM HEPES, pH 7.4, 10 mM MgCl2, 1 mM DTT, 2 μg GST-c-Jun, and 10 μCi [γ-32P]ATP, and incubated at 30°C for 15 min. After electrophoresis, the 32P-labeled GST-c-Jun detected with a PhosphoImager.
In vitro kinase assay for IKK
Cells (3 × 106) were serum starved for 3 h before stimulation with IL-1 for 15 min. Cells were washed with low salt buffer and lysed with lysis buffer [20 mM HEPES, pH 7.4, 250 mM NaCl, 1 mM EDTA, 1% NP40, 1 mM DTT, 1 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 20 mM β-glycerophosphate, 20 mM paranitrophenyl phosphate (PNPP), and 0.1 mM sodium vanadate] for 30 min on ice. For frozen ovarian tumor samples, the tumor tissues were cut into small pieces before homogenization in lysis buffer. The whole-cell extracts or the crude tissue homogenates were clarified by centrifugation before used for subsequent analyses.
To immunoprecipitate the IKK complex, 300 μg of cell extracts were precleared with 30 μl of protein G agarose, and the supernatants were then incubated with 1 μg of anti-IKKγ for 2 h at 4°C. The immune complexes were then precipitated with protein G agarose and washed with lysis buffer followed by kinase buffer (20 mM HEPES, pH 7.6, 20 mM MgCl2, 2 mM DTT, 1 mM EDTA, 20 mM β-glycerophosphate, and 2 mM PNPP). The IKK kinase reaction was initiated by the addition of kinase buffer containing 20 μM ATP, 10 μCi [γ-32P]ATP, and 1 μg GST-IκBα(1–54) and incubated at 30°C for 20 min. 32P-labeled IκB signals were detected with a PhosphoImager.
siRNA for MEKK3
All siRNAs and transfection reagents were from Dharmacon (Lafayette, CO.). A reverse transfection format was used to knockdown MEKK3. SMARTpool MEKK3 siRNA (siMEKK3) was from Dharmacon. siControl non-targeting siRNA pool was used as the negative control. Mixture of siRNAs and DharmaFECT 4 (DF4) transfection reagent were prepared in a 96-well culture plate in triplicates. The final concentrations of siRNAs and DF4 were 15 nM and 12.5 nM, respectively. OVCA420 and ES2 cells were chosen for their high transfection efficiency. Pilot experiments were performed to determine the optimal seeding cell densities for OVCA420 (1.5 × 105) and ES2 (1.25 × 105) cells and the optimal concentrations of paclitaxel (Bristol-Myers Squibb) for each cell line (100 nM for OVCA420 and 12.5 nM for ES2). Appropriate amount of paclitaxel was added to the wells containing the siRNA-DF4 complexes. The transfection was left in 37°C incubator for overnight before the supernatants were removed and replaced with fresh complete media. Cells were cultured for additional 48 h and subjected to a crystal violet proliferation assay (33).
Condensed Abstract.
MEKK3 is aberrantly expressed in ovarian cancers and may play an important role in tumors with constitutively activated NFκB.
Acknowledgements
This work was supported in part with funds from Pharmacia and Ovarian SPORE (W.S.L.), Ovarian SPORE NCI P50 CA83639 (R.C.B. Jr.), and by an Institutional Research Grant (IRG)(A.K.S.).
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Stuart GC. First-line treatment regimens and the role of consolidation therapy in advanced ovarian cancer. Gynecol Oncol. 2003;90:S8–S15. doi: 10.1016/s0090-8258(03)00472-4. [DOI] [PubMed] [Google Scholar]
- 3.Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol. 2003;90:390–396. doi: 10.1016/s0090-8258(03)00278-6. [DOI] [PubMed] [Google Scholar]
- 4.Samanta AK, Huang HJ, Bast RC, Jr, Liao WS. Overexpression of MEKK3 confers resistance to apoptosis through activation of NFkappaB. J Biol Chem. 2004;279:7576–7583. doi: 10.1074/jbc.M311659200. [DOI] [PubMed] [Google Scholar]
- 5.Basséres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 6.Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–361. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- 7.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 8.Sun Z, Andersson R. NF-kappaB activation and inhibition: a review. Shock. 2002;18:99–106. doi: 10.1097/00024382-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Karin M. The IkappaB kinase - a bridge between inflammation and cancer. Cell Res. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738–747. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 11.Boone DL, Lee EG, Libby S, Gibson PJ, Chien M, Chan F, Madonia M, Burkett PR, Ma A. Recent advances in understanding NF-kappaB regulation. Inflamm Bowel Dis. 2002;8:201–212. doi: 10.1097/00054725-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Darnell JE., Jr Transcription factors as targets for cancer therapy. Nat Rev Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 13.Di Y, Li S, Wang L, Zhang Y, Dorf ME. Homeostatic interactions between MEKK3 and TAK1 involved in NF-kappaB signaling. Cell Signal. 2008;20:705–713. doi: 10.1016/j.cellsig.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matitau AE, Scheid MP. Phosphorylation of MEKK3 at threonine 294 promotes 14-3-3 association to inhibit nuclear factor kappaB activation. J Biol Chem. 2008;283:13261–13268. doi: 10.1074/jbc.M801474200. [DOI] [PubMed] [Google Scholar]
- 15.Tojima Y, Fujimoto A, Delhase M, Chen Y, Hatakeyama S, Nakayama K, Kaneko Y, Nimura Y, Motoyama N, Ikeda K, Karin M, Nakanishi M. NAK is an IkappaB kinase-activating kinase. Nature. 2000;404:778–782. doi: 10.1038/35008109. [DOI] [PubMed] [Google Scholar]
- 16.Weston CR, Lambright DG, Davis RJ. Signal transduction. MAP kinase signaling specificity. Science. 2002;296:2345–2347. doi: 10.1126/science.1073344. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Lin Y, Guo Z, Cheng J, Huang J, Deng L, Liao W, Chen Z, Liu Z, Su B. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol. 2001;2:620–624. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- 18.Crowell JA, Steele VE. AKT and the phosphatidylinositol 3-kinase/AKT pathway: important molecular targets for lung cancer prevention and treatment. J Natl Cancer Inst. 2003;95:252–253. doi: 10.1093/jnci/95.4.252. [DOI] [PubMed] [Google Scholar]
- 19.Nair AS, Shishodia S, Ahn KS, Kunnumakkara AB, Sethi G, Aggarwal BB. Deguelin, an Akt inhibitor, suppresses IkappaBalpha kinase activation leading to suppression of NF-kappaB-regulated gene expression, potentiation of apoptosis, and inhibition of cellular invasion. J Immunol. 2006;177:5612–5622. doi: 10.4049/jimmunol.177.8.5612. [DOI] [PubMed] [Google Scholar]
- 20.Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, Hideshima T, Treon SP, Munshi NC, Richardson PG, Anderson KC. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene. 2002;21:5673–5683. doi: 10.1038/sj.onc.1205664. [DOI] [PubMed] [Google Scholar]
- 21.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Zhu GD. Targeting serine/threonine protein kinase B/Akt and cell-cycle checkpoint kinases for treating cancer. Curr Top Med Chem. 2002;2:939–971. doi: 10.2174/1568026023393318. [DOI] [PubMed] [Google Scholar]
- 23.Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, Thomas RJ, Phillips WA. The phosphatidylinositol 3'-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426–7429. [PubMed] [Google Scholar]
- 24.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 25.Carter RS, Geyer BC, Xie M, Acevedo-Suárez CA, Ballard DW. Persistent activation of NF-kappa B by the tax transforming protein involves chronic phosphorylation of IkappaB kinase subunits IKKbeta and IKKgamma. J Biol Chem. 2001;276:24445–24458. doi: 10.1074/jbc.C000777200. [DOI] [PubMed] [Google Scholar]
- 26.Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, Tsang BK, Cheng JQ. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 27.Le XF, Arachchige-Don AS, Mao W, Horne MC, Bast RC., Jr Roles of human epidermal growth factor receptor 2, c-jun NH2-terminal kinase, phosphoinositide 3-kinase, and p70 S6 kinase pathways in regulation of cyclin G2 expression in human breast cancer cells. Mol Cancer Ther. 2007;6:2843–2857. doi: 10.1158/1535-7163.MCT-07-0109. [DOI] [PubMed] [Google Scholar]
- 28.Le XF, Claret FX, Lammayot A, Tian L, Deshpande D, LaPushin R, Tari AM, Bast RC., Jr The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J Biol Chem. 2003;278:23441–23450. doi: 10.1074/jbc.M300848200. [DOI] [PubMed] [Google Scholar]