Abstract
Cardiac-directed expression of AC6 has pronounced favorable effects on cardiac function possibly not linked with cAMP production. To determine rigorously whether cAMP generation is required for the beneficial effects of increased AC6 expression, we generated a catalytically inactive AC6 mutant (AC6mut) that has markedly diminished cAMP generating capacity by replacing aspartic acid with alanine at position 426 in the C1 domain (catalytic region) of AC6. Gene transfer of AC6 or AC6mut (adenovirus-mediated) in adult rat cardiac myocytes resulted in similar expression levels and intracellular distribution, but AC6mut expression was associated with marked reduction in cAMP production. Despite marked reduction in cAMP generation, AC6mut influenced intracellular signaling events similarly to that observed after expression of catalytically intact AC6. For example, both AC6 and AC6mut reduced phenylephrine-induced cardiac myocyte hypertrophy and apoptosis (p < 0.001), expression of cardiac ankyrin repeat protein (p < 0.01), and phospholamban (p < 0.05). AC6mut expression, similar to its catalytically intact cohort, was associated with increased Ca2+ transients in cardiac myocytes after isoproterenol stimulation. Many of the biological effects of AC6 expression are replicated by a catalytically inactive AC6 mutant, indicating that the mechanisms for these effects do not require increased cAMP generation.
Introduction
Adenylyl cyclase (AC) is the effector molecule for β-adrenergic receptor and other G-protein-coupled receptors in cardiac myocytes and other cells. AC regulates the conversion of ATP to cAMP and initiates a variety of intracellular signaling events that influence heart function (Post et al., 1995; Hanoune et al., 1997; Tesmer and Sprang, 1998; Hurley, 1999). Nine isoforms of mammalian AC have been identified so far, all possessing a short intracellular amino terminus and two large cytoplasmic domains (C1 and C2) separated by two transmembrane domains (M1 and M2), each containing six transmembrane spans (Sunahara et al., 1996; Hanoune et al., 1997; Smit and Iyengar, 1998; Tesmer and Sprang, 1998; Hurley, 1999). The C1 and C2 domains form the catalytic core of AC and have activity with or without the two transmembrane domains (Tang and Gilman, 1995; Whisnant et al., 1996; Yan et al., 1996). The catalytic activity of AC is regulated by many factors: GTP-binding proteins, ATP, Mg2+, glycosylation, and phosphorylation (Iwami et al., 1995; Dessauer and Gilman, 1996; Dessauer et al., 1997, 2002; Tesmer et al., 1997; Wu et al., 2001; Lin et al., 2002; Chen-Goodspeed et al., 2005). However, intracellular AC seems to interact with intracellular proteins and influence signaling independently of its catalytic activity. For example, interaction of AC type 6 (AC6) with a PH-domain leucine-rich phosphatase protein 2 (PHLPP2) inhibits PHLPP2 activity, which leads to increased Akt phosphorylation and activity (Brognard et al., 2007; Gao et al., 2009).
β-adrenergic receptor activation or pharmacological reagents that increase cAMP have deleterious effects on the heart (Gaudin et al., 1995; Communal et al., 1998; Engelhardt et al., 1999, 2001; Bisognano et al., 2000; Singh et al., 2001). However, increased expression of AC6, which increases agonist-stimulated cAMP, has beneficial effects (Gao et al., 1999, 2002; Roth et al., 1999, 2002; Lai et al., 2000, 2004, and 2008; Takahashi et al., 2006), suggesting that AC6 expression has biological effects that may be unrelated to cAMP generation. Using pharmacological inhibitors, our previous data support this notion (Gao et al., 2004 and 2008). Because of the inherent limitations of studies using pharmacological inhibition, we generated a catalytically inactive AC6 mutant (AC6mut) molecule by substitution of alanine for aspartic acid at position 426 in the C1 domain of the catalytic core. Based on the crystal structure of the C1C2 catalytic core resolved by Tesmer et al. (1997), this amino acid is required for magnesium binding but is not critical for the overall structure of the catalytic core. This catalytically inactive mutant of AC6 enabled us to determine whether the beneficial effects of AC6 on intracellular signaling, calcium handling, and cardiac myocyte hypertrophy and apoptosis are cAMP-independent.
Materials and Methods
Adult Rat Cardiac Myocyte Culture and Gene Transfer.
Adult rat cardiac myocytes were isolated from 10-week-old Sprague-Dawley rats as described previously (Patel et al., 2006). Isolated cardiac myocytes were suspended in M199 medium (Invitrogen, Carlsbad, CA) supplemented with 1% bovine serum albumin (HyClone, Logan, UT), 100 IU/ml penicillin, and 100 mg/ml streptomycin (Invitrogen, Carlsbad, CA), and were plated on laminin-coated dishes. The culture medium was changed to fresh medium to remove the damaged myocytes that failed to attach after 2 h in the plate. At this point, approximately 70 to 80% of myocytes were viable and rod-shaped. Gene transfer was performed by infecting cells with E1-deleted adenoviruses encoding murine AC6 (Ad.AC6) or AC6 mutant (Ad.AC6mut) at 2 × 107 virus particles per well of a 12-well plate. An adenovirus vector lacking a transgene insert (Ad.Null; Invitrogen, Carlsbad, CA) was used as an additional control.
AC Activity.
Adult rat cardiac myocytes were seeded in 12-well plates at 15 × 103 cells/well. After washing and changing media, approximately 12 × 103 cells remain in the well. Cells were incubated with Ad.AC6mut or Ad.AC6 for 44 h and stimulated for 10 min with isoproterenol (10 μM; Sigma) or forskolin (10 μM; Sigma) or were not stimulated (basal). Cells were lysed in lysis buffer 1B (2.5% dodecyltrimethyammonium bromide, 0.05 M sodium acetate, pH 5.8, and 0.02% bovine serum albumin). The amount of cAMP in the supernatant was measured using the cAMP Biotrak Enzymeimmunoassay System (GE Healthcare, Waukesha, WI) according to the instructions from the provider.
Calcineurin Activity.
Calcineurin activity was assayed using a “Calcineurin cellular activity assay kit” from Enzo Life Sciences AG (Lausen, Switzerland). In brief, cells were lysed in lysis buffer containing protease inhibitors, passed through an 18-gauge needle, and centrifuged at 16,000g at 4°C for 60 min. Supernatant was desalted by gel filtration. After the protein concentration was determined, the same amount of protein (1.1 μg) was used in calcineurin activity assay. The results (i.e., the amount of phosphate released by calcineurin) were calculated using slope and intercept obtained from standard curve.
Detection of mRNA and Protein.
Immunoblotting, immunoprecipitation, and immunofluorescence staining were performed as described previously (Gao et al., 2004, 2008).
Cardiac Myocyte Hypertrophy and Apoptosis.
To determine how AC6 and AC6mut influence cardiac myocyte response to a hypertrophic stimulus, isolated adult cardiac myocytes were infected with Ad.AC6, Ad.AC6mut, or Ad.Null for 24 h followed by incubation with phenylephrine (PE; 20 μM; Sigma) for 44 h. In alternative experiments, PE was incubated with cardiac myocytes for 24 h followed by incubation with Ad.AC6, Ad.AC6mut, or Ad.Null for 24 h. Cardiac myocyte size was measured from imaged photos using MetaMorph software (Molecular Devices, Sunnyvale, CA), an integrated morphometry analysis, stipulating a cell length of ≥107 μm. Dead cardiac myocytes, defined as small, dense, and rounded cells (Merkus et al., 2005) were counted, confirmed by exclusion of trypan blue (Zaugg et al., 2002), and expressed as a percentage of total cells. Cardiac myocyte apoptosis was determined using terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL). Total cells (untreated, 700–800; PE-treated, 500–600) and apoptotic cells were counted and expressed as a percentage of total cells. The expression of fetal genes was determined with the use of quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
Measurements of Cytoplasmic Ca2+.
Cardiac myocytes were isolated from adult rat and plated on laminin-coated 25-mm glass coverslips. Cells were infected with Ad.Null, Ad.AC6, or Ad.AC6mut for 48 h and stimulated with isoproterenol (10 μM) for 20 h. Cytoplasmic Ca2+ was measured as described previously (Tang et al., 2008).
Statistical Analysis.
Data represent mean ± S.D.; group differences were tested for statistical significance using one-way ANOVA, followed by Bonferroni t testing. The null hypothesis was rejected when p < 0.05.
Results
Expression and Location of AC6mut
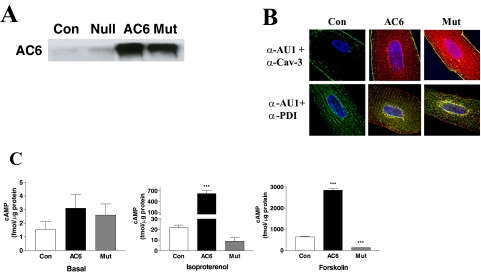
Both transgene AC6 and AC6mut proteins were increased 20- to 25-fold over endogenous AC6 in uninfected and Ad.Null controls (Fig. 1A) and showed distribution similar to that of plasma membrane/caveolae and sarcoplasmic reticulum (Fig. 1B).
Fig. 1.
AC6 and AC6mut expression, localization, and activity. A, expression of AC6 and AC6mut proteins. Adult cardiac myocytes were incubated with Ad.AC6mut, Ad.AC6, or Ad.Null (control) for 40 h. AC6 and AC6mut proteins were detected by anti-AC5/6 antibody in immunoblotting. B, location of transgene proteins. Double immunofluorescence staining of AC6 and AC6mut by anti-AU1 antibody (red), anti-caveolin 3 (Cav-3) antibody (green), and anti-protein disulfide-isomerase (PDI) antibody (green). Hoechst dye was used to identify the nucleus (blue). There were no apparent group differences in cellular distribution: AC6 and AC6mut proteins were present in plasma membrane (associated with caveolin), nuclear envelope, and sarcoplasmic reticulum. C, AC activity. cAMP was measured in uninfected (Con) and Ad.AC6- and Ad.AC6mut-infected cardiac myocytes before (basal) and after 10-min stimulation with isoproterenol (Iso; 10 μM) or forskolin (Fsk; 10 μM). As expected, AC6 increased cAMP generation in response to isoproterenol and forskolin stimulation. AC6mut was associated with reduced cAMP generation in response to isoproterenol (a 59% reduction) and forskolin (an 80% reduction). Bars in the graphs denote mean ± S.D. (***, p < 0.001) derived from triplicates in three independent experiments.
AC6 and AC6mut Activity
Neither AC6 nor AC6mut expression altered basal cAMP (AC6, 3 ± 3 fmol/μg; AC6mut, 3 ± 2 fmol/μg; control, 2 ± 1 fmol/μg.) AC6 gene transfer was associated with increased cAMP production when stimulated with isoproterenol (AC6, 623 ± 223 fmol/μg; control, 22 ± 6 fmol/μg; p < 0.001) and forskolin (AC6, 2842 ± 133 fmol/μg; control, 636 ± 25 fmol/μg; p < 0.001) (Fig. 1C and Table 1). However, AC6mut expression was associated with a 59% reduction in cAMP production stimulated by isoproterenol (AC6mut, 9 ± 8 fmol/μg; control, 22 ± 6 fmol/μg; p > 0.05) and with an 80% reduction in forskolin stimulated cAMP production (AC6mut, 130 ± 5 fmol/μg; control, 636 ± 25 fmol/μg; p < 0.001; Fig. 1C and Table 1). These data demonstrate that AC6mut expression markedly reduces cAMP generating capacity and may interfere with endogenous AC activity.
TABLE 1.
Summary of findings
The entries reflect summary of data from triplicate experiments in each case.
| Ad.AC6 vs. Control | Ad.AC6mut vs. Control | |
|---|---|---|
| Iso-stimulated cAMP | ↑ 29-fold | ↓ 59-fold |
| Fsk-stimulated cAMP | ↑ 4.5-fold | ↓ 80-fold |
| PE-induced hypertrophy | ↓ 36% | ↓ 30% |
| PE-induced cell death | ↓ 57% | ↓ 55% |
| PE-associated CARP protein | ↓ 59% | ↓ 51% |
| SR Ca2+ handling | ↑ 2.4-fold | ↑ 1.7-fold |
| PLB protein | ↓ 46% | ↓ 41% |
| Basal, P-Ser16-PLB | ↑ 7-fold | N.C. |
| Iso-stimulated, P-Thr17-PLB | ↑ 2-fold | N.C. |
| Iso-stimulated, P-23/24-TnI | ↑ 1.6-fold | ↑ 1.4-fold |
N.C., no change; Fsk, forskolin; Iso, isoproterenol.
Cardiac Myocyte Death, Apoptosis, and Hypertrophy
Cell Death.
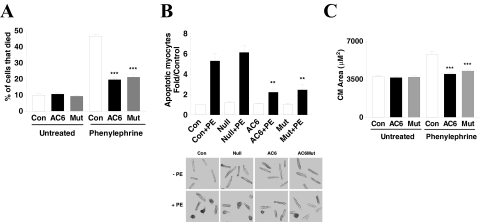
Cultured adult cardiac myocytes contain 10% dead cells (morphological evaluation plus trypan blue confirmation) 3 days after plating. Gene transfer of AC6 and AC6mut did not change cell death rate (Fig. 2A), but PE increased cardiac myocyte death by 4.7-fold (control, 10 ± 2%; control + PE, 47 ± 2%; p < 0.001). AC6 and AC6mut gene transfer reduced PE-induced myocyte death (AC6 + PE, 20 ± 1%; AC6mut, 21 ± 3%; control + PE, 47 ± 2.0%; p < 0.001; Fig. 2A).
Fig. 2.
Cardiac myocyte cell death, apoptosis, and hypertrophy. A, phenylephrine-induced cell death. Dead cardiac myocytes were counted and expressed as a percentage of total cardiac myocytes. AC6 and AC6mut reduced PE-associated cardiac myocyte death. Bars in graphs are mean values (***, p < 0.001) derived from assessment of 800 to 1000 cells for each condition; experiments were repeated three times. B, phenylephrine-induced myocyte apoptosis. Top, apoptotic cardiac myocytes were detected using TUNEL staining and expressed as -fold increase over untreated myocytes. Bottom, TUNEL-positive cells. AC6 and AC6mut reduced PE-associated cardiac myocyte apoptosis. Bars in graphs are mean values derived from assessment of 500 to 800 cells for each condition; experiments were repeated three times. The p values are from post hoc Bonferroni t testing after one-way ANOVA (**, p < 0.01). C, phenylephrine-induced hypertrophy. Cardiac myocytes infected with Ad.AC6 or Ad.AC6mut were incubated with phenylephrine (20 μM, 44 h), and cardiac myocytes were imaged and cell area (micrometers squared) measured using MetaMorph, an integrated morphometry analysis program. AC6 and AC6mut inhibited PE-induced cardiac myocyte hypertrophy. The p values indicate comparison with control condition with PE. Bars in graphs are mean values (***, p < 0.001) derived from more than 50 cells per condition; experiments were repeated three times.
Apoptosis.
PE stimulation increased cardiac myocyte apoptosis (detected by TUNEL) 5.8 ± 1.2-fold over untreated controls (both uninfected and Ad.Null-infected; p < 0.001). AC6 and AC6mut gene transfer was associated with a >60% reduction in PE-induced myocyte apoptosis compared with Ad.Null-infected cells (p < 0.001; Fig. 2B). These data indicate that AC6-related attenuation of cardiac myocyte hypertrophy and apoptosis does not require cAMP generation.
Hypertrophy.
PE (20 μM for 44 h) increased cardiac myocyte area by 2.4-fold (Ad.Null + PE, 8955 ± 2940 μm2; Ad.Null (alone), 3740 ± 887 μm2; p < 0.001) without changing cell length. Gene transfer of AC6 or AC6mut did not influence cardiac myocyte area (Ad.AC6, 3643 ± 962 μm2; Ad.AC6mut, 3696 ± 990 μm2; Ad.Null, 3740 ± 887 μm2). However, both Ad.AC6 and Ad.AC6mut attenuated PE-induced cardiac myocyte hypertrophy by 36 and 31%, respectively (Ad.AC6 + PE, 5762 ± 1400 μm2; Ad.AC6mut + PE, 6153 ± 1655 μm2; control + PE: 8955 ± 2940 μm2; p < 0.01; Fig. 2C and Table 1).
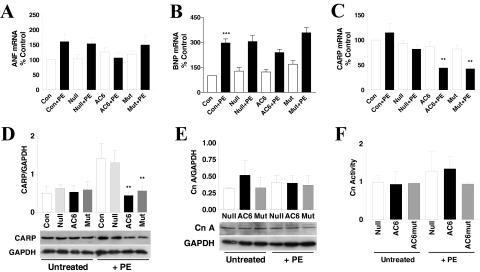
In uninfected or Ad.Null-infected cells, PE increased atrial natriuretic factor (ANF) mRNA expression by 1.5-fold (p > 0.05). Increased AC6 reduced ANF expression after PE treatment, but not significantly. AC6mut acted similarly to Ad.Null control (Fig. 3A). PE increased B-type natriuretic peptide (BNP) mRNA by 2-fold (p < 0.001). Increased AC6 and AC6mut expression did not alter PE-induced BNP expression compared with Ad.Null-infected and uninfected cardiac myocytes (Fig. 3B).
Fig. 3.
Phenylephrine-induced hypertrophy: phospholamban, CARP, and calcineurin. A, ANF mRNA was detected by qRT-PCR. AC6, but not AC6mut, reduced ANF mRNA expression after PE treatment. B, BNP mRNA was detected by qRT-PCR. BNP mRNA was increased by PE treatment in all conditions without group differences. C, CARP mRNA expression. Real-time RT-PCR was used to determine the expression of CARP, and mRNA copy number was expressed as a percentage over control (uninfected and without PE). Gene transfer of AC6 and AC6mut did not affect CARP mRNA expression. However, after incubation with PE, CARP expression was reduced by AC6 and AC6mut expression compared with the Ad.Null control (p < 0.05) or with uninfected control (**, p < 0.01). The p values are from post hoc Bonferroni t testing after one-way ANOVA. D, CARP protein expression. CARP protein was detected using anti-CARP antibody in immunoblotting and showed no group differences in the absence of PE. After PE treatment, AC6 and AC6mut expression reduced expression of CARP protein compared with both uninfected and Ad.Null controls (**, p < 0.01). E, calcineurin A protein expression. Calcineurin A protein was detected using anti-calcineurin A antibody in immunoblotting. There were no differences among groups in the basal or PE-treated conditions. F, calcineurin activity. Calcineurin activity was determined using the calcineurin cellular activity assay kit from Enzo Life Sciences. There were no differences among groups in the basal or PE-treated conditions. In all graphs, bars represent mean values of three to four experiments; error bars denote 1 S.D.
CARP Expression
Next, we examined the effects of AC6 and AC6mut expression on cardiac ankyrin repeat protein (CARP), a fetal gene activated during cardiac myocyte hypertrophy (Aihara et al., 2000; Maeda et al., 2002). Ad.Null, Ad.AC6, or Ad.AC6mut gene transfer did not alter CARP expression. After PE treatment, CARP mRNA was increased in uninfected cells, unchanged in Ad.Null-infected cells, but markedly reduced in Ad.AC6- and Ad.AC6mut-infected cells. CARP protein expression was also reduced by increased AC6 and AC6mut expression (Fig. 3, C and D and Table 1). Clearly increased AC6 and AC6mut expression reduce PE-induced CARP expression, through unknown mechanisms.
Calcineurin Expression and Activity
Increased expression of calcineurin A has been described in the setting of cardiac myocyte hypertrophy (De Windt et al., 2000; Bousette et al., 2010). However, we found that PE changed neither calcineurin A expression nor calcineurin activity in all groups (Fig. 3, E and F). These data suggest that AC6 and AC6mut induced reduction in cardiac myocyte hypertrophy but not through suppression of calcineurin signaling pathway.
SR Ca2+ Storage
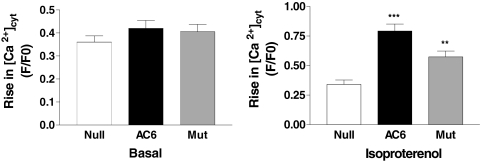
To determine whether AC6 or AC6mut expression was associated with alterations in SR [Ca2+], cardiac myocytes were analyzed by real-time [Ca2+]cyt imaging. No group differences in caffeine-stimulated Ca2+ transients were present (Fig. 4A). However, when stimulated with isoproterenol (1 μM, 24 h), caffeine-stimulated Ca2+ transients were increased by 2.4-fold in Ad.AC6-infected cardiac myocytes (P < 0.001; Fig. 4B and Table 1) and by 1.7-fold in Ad.AC6mut-infected cardiac myocytes (p < 0.01; Fig. 4B). An apparent difference between AC6 and AC6mut did not reach statistical significance. These data indicate that despite impaired cAMP generation, AC6mut has the ability to increase cardiac myocyte SR Ca2+ storage.
Fig. 4.
Calcium signaling. Ad.AC6mut- and Ad.AC6-infected adult rat cardiac myocytes, unstimulated or stimulated with isoproterenol (1 μM, 24 h), were analyzed by real-time [Ca2+]cyt imaging. Representative Ca2+ transients in response to caffeine stimulation were recorded with Fura-2 fluorescence. Left, unstimulated cardiac myocytes expressing AC6 or AC6mut showed no change in [Ca2+]cyt in response to caffeine (10 mM). Number of cardiac myocytes assessed: control, 34; AC6, 24; AC6mut, 31. Right, isoproterenol-stimulated cardiac myocytes expressing AC6 or AC6mut showed increases in the peak amplitude of Ca2+ transient in response to caffeine stimulation. Number of cardiac myocytes assessed: control, 19; AC6, 24; AC6mut, 17 cells. (AC6 versus Null, ***, p < 0.001; AC6mut versus Null, **, p < 0.01).
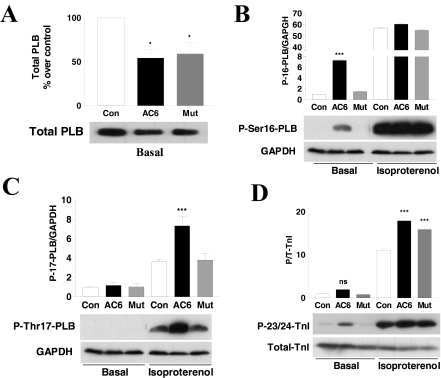
Expression and Phosphorylation of Phospholamban.
To determine possible mechanisms for the increased Ca2+ handling seen after AC6 and AC6mut gene transfer, we examined the effects of AC6 and AC6mut expression on phospholamban (PLB) and troponin I (TnI) expression and phosphorylation. Gene transfer of AC6 or AC6mut reduced PLB expression by 46 ± 18% (p < 0.05) and 41 ± 23% (p < 0.05), respectively (Fig. 5A and Table 1). The extent of PLB phosphorylation at the Ser16 or Thr17 sites in adult rat cardiac myocytes is low. Gene transfer of AC6, but not AC6mut, increased basal PLB phosphorylation at Ser16 by 7.3-fold (p < 0.001; Fig. 5B and Table 1). After isoproterenol stimulation, phospho-Ser16 was increased, but there were no group differences (Fig. 5B). AC6 and AC6mut gene transfer did not increase basal PLB phosphorylation at the Thr17 site. After isoproterenol stimulation, AC6, but not AC6mut, increased PLB phosphorylation at the Thr17 site by 2-fold (Fig. 5C and Table 1).
Fig. 5.
Expression and phosphorylation of PLB and cTnI proteins. A, immunodetection of total PLB protein using anti-PLB antibody in cell homogenates of adult rat cardiac myocytes. AC6 and AC6mut reduced expression of PLB. B and C, immunodetection of PLB phosphorylation at Ser16 and Thr17 using their specific antibodies in cell homogenates of adult rat cardiac myocytes unstimulated or stimulated with isoproterenol (10 μM, 24 h). AC6, but not AC6mut, increased basal PLB phosphorylation at the Ser16 site but not the Thr17 site. After isoproterenol stimulation, PLB phosphorylation at Ser16 was increased similarly in all conditions, and phosphorylation at Thr17 was increased in all conditions but was greater in AC6- than in AC6mut-infected cardiac myocytes. D, immunodetection of troponin I phosphorylation at the Ser23/24 sites using anti-phospho-cTnI antibody in cell homogenates of adult rat cardiac myocytes, unstimulated or stimulated with isoproterenol (10 μM, 24 h). AC6 and AC6mut did not increase basal cTnI phosphorylation, but isoproterenol treatment was associated with increased cTnI phosphorylation of similar degrees. Bars in all graphs show mean values from three or more experiments; error bars denote 1 S.D. (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Cardiac Troponin-I Expression and Phosphorylation.
AC6 or AC6mut expression was not associated with alterations in cTnI expression or basal phosphorylation at p23/24 protein kinase A phosphorylation sites. After isoproterenol stimulation, phosphorylation of TnI phosphorylation was increased by expression of AC6 (1.6-fold, p < 0.001) and AC6mut (1.4-fold, p < 0.001; Fig. 5D and Table 1).
Discussion
In this study we have shown that a single amino acid substitution in the catalytic domain (C1) of AC6 resulted in marked reduction of catalytic activity. We used this catalytically inactive mutant to determine whether the benefits of AC6 gene expression are cAMP-dependent. The most important findings are that both AC6 and AC6mut attenuated PE-induced cardiac myocyte hypertrophy and cell death, reduced CARP and PLB expression, and increased Ca2+ handling. The striking similarity in the direction and degree of these changes between a catalytically active (AC6) and a catalytically inactive mutant (AC6mut) indicates that such alterations do not require increased cAMP. We conclude that many of the beneficial biological effects of AC6 do not require increases in cAMP.
We previously have shown that increased expression of AC6 in neonatal rat cardiac myocytes alters intracellular signaling events (Gao et al., 2004, 2008, 2010), but the precise mechanism has remained elusive. In the present study, we confirmed that most of the effects of AC6 expression seen in neonatal rat cardiac myocytes (Gao et al., 2004, 2008, 2009, 2010) also are present in adult rat cardiac myocytes. More importantly, in the present study, exploiting a catalytically inactive AC6 enabled us to identify the effects of AC6 expression that might be cAMP-independent. This strategy was productive and provided novel information.
cAMP-Dependent Effects.
It is important to point out that, as anticipated, not all of the effects of AC6 were cAMP-independent. For example, 1) after AC6 gene transfer, PE stimulation was associated with increased PLB phosphorylation at the Ser16 and Thr17 sites (Fig. 3, A and B), a cAMP-dependent process that did not occur after AC6mut expression; 2) expression of AC6, but not AC6mut, increased isoproterenol-induced phosphorylation of PLB at Thr17, indicating CamKII activation; and 3) AC6 and AC6mut expression did not prevent isoproterenol-stimulated increases in protein kinase A-mediated signaling (PLB phosphorylation at Ser16 or TnI phosphorylation at p23/24) or CamKII-mediated signaling (PLB phosphorylation at Thr17), indicating that activation of other endogenous ACs is not inhibited by AC6mut.
cAMP-Independent Effects.
How do AC6 and AC6mut influence intracellular signaling independently of increases in cAMP generation? Based on our previous studies of AC6 expression in neonatal cardiac myocytes, we speculate that increased expression of AC6 and AC6mut enable interactions with intracellular proteins that previously were inaccessible. For example, coimmunoprecipitation and immunohistology indicate close associations of intracellular AC6 with a specific phosphatase (Brognard et al., 2007; Gao et al., 2009). Others have found that the N terminus of AC6 interacts with snapin protein, linking AC6 to snapin-binding protein complexes (Wang et al., 2009), which may enable AC6 and AC6mut to function independently of cAMP. We recently discovered that increased AC6 and AC6mut expression are associated with α-B-crystallin (data not shown), although the biological consequence of this interaction is presently unknown. It is also plausible that AC6 and AC6mut are interfering with normal signaling by preferentially associating with key intracellular signaling proteins such as G protein-coupled receptors, Gαs, Gβγ, and scaffold proteins such as A-kinase anchoring proteins, thus serving a dominant-negative function, which could also influence the hypertrophic response. For example, we found that AC6mut inhibited forskolin-stimulated AC activity (Fig. 1C). Although forskolin is a direct AC activator, its effectiveness is influenced by the presence of Gαs. In cells lacking Gαs, forskolin-stimulated cAMP production was reduced (Darfler et al., 1982). Our data suggest that AC6mut acts as a dominant-negative mutant by interacting with Gαs to reduce the effects of Gαs on endogenous AC responsiveness to forskolin. Identification of AC6-interacting proteins and the consequences of such interactions in adult cardiac myocytes is a focus of study in our laboratories.
Inhibition of Hypertrophy.
PE, an α1-adrenergic receptor agonist, induces cardiac myocyte hypertrophy (Simpson, 1985) and cell death. Many genes are involved in PE-induced cardiac myocyte hypertrophy. For example, transcription of CARP is increased in PE-induced hypertrophy (Pinson et al., 1993). In the present study, expression of AC6 or AC6mut reduced CARP expression and attenuated hypertrophy through unknown mechanisms. Increased expression of AC6 in vivo was not associated with altered expression of βAR, Gαs, or Giα2 (Gao et al., 1999), so the attenuation of hypertrophy observed after expression of AC6 or AC6mut in the present study is not likely to be mediated through the β-adrenergic receptor-signaling pathway. Moreover, AC6mut and AC6, which have opposite effects on cAMP production, have similar effects on the attenuation of hypertrophy.
Adult rat cardiac myocyte hypertrophy was confirmed by measuring protein synthesis using [3H]leucine incorporation. As expected, when uninfected and Ad.Null-infected cardiac myocytes were treated with phenylephrine, we saw increased apoptosis and necrosis. Protein synthesis was decreased by 20% compared with cardiac myocytes not treated with phenylephrine. In essence, the confounding elements of increases in apoptosis and cell death make protein synthesis measurements less desirable than evaluating hypertrophy based on size of viable cells in this model. Nevertheless, in PE-treated groups, AC6 and AC6mut gene transfer decreased cell size and reduced protein synthesis by 25 and 16% respectively.
AC6 (but not AC6mut) expression increased PLB phosphorylation at Ser16 and Thr17 in response to PE stimulation (Fig. 3, A and B). These data suggest that protein kinase A and CamKII are not involved in attenuation of PE-induced hypertrophy. Expression of AC6 or AC6mut did not alter the expression of hypertrophy-associated genes such as ANF and BNP, did not increase calcineurin A expression, and did not influence the extracellular signal-regulated kinase signaling pathway (data not shown), but inhibited CARP expression (Fig. 3, C and D), which is likely to contribute to the attenuation of hypertrophy.
We recently reported that AC6 deletion reduced left ventricular hypertrophy in pressure-overloaded female mice (Tang et al., 2010), which seems counter to what we found regarding cardiac myocyte hypertrophy in the present study. However, because of the influence of targeted expression and deletion of specific genes on nontargeted proteins, it is not axiomatic that increased expression of AC6 should result in directionally opposite effects versus AC6 deletion. A simple example illustrates this point. In the AC6 targeted deletion lines, we saw a marked diminution in cardiac AC5 protein levels. AC5 mRNA expression was unchanged, but the deletion of AC6 was associated with marked increases in AC5 protein degradation (Tang et al., 2008). AC5 deletion is associated with reduced LV hypertrophy in pressure overload (Yan et al., 2007)—its absence in AC6-deleted lines may explain reduced hypertrophy in pressure overload (Tang et al., 2010). Second, by increasing the expression of AC6 (or AC6mut), the likelihood of interactions with intracellular proteins (not usually accessible to endogenous levels of AC6) is increased—a condition not present in the setting of AC6 deletion. Furthermore, the responses of cardiac myocytes in vitro to short-term changes in milieu are often not replicated by cardiac myocytes in vivo, which naturally reflect more long-term responses to alterations in physiological conditions. Finally, regarding the physiological correlates of the findings of the current studies in relation to hypertrophic responses in vivo, we see improved LV function after pressure overload hypertrophy in mice with cardiac-directed expression of AC6 (N. C. Lai and H. K. Hammond, unpublished data), although LV hypertrophy is similar to that observed in transgene negative siblings.
Ca2+ Handling.
AC6 expression (but not AC6mut expression) was associated with isoproterenol-induced PLB phosphorylation at Thr17. Expression of AC6 and AC6mut was associated with reduced PLB expression and increased cytoplasmic Ca2+ concentration in response to caffeine stimulation (Fig. 4). Although AC6mut expression did not increase isoproterenol-stimulated PLB phosphorylation at Thr17, its beneficial effects on Ca2+ handling are likely to reflect reduced PLB expression, which was also seen after AC6 expression. Previous studies have established the tight linkage between levels of AC6 and calcium handling in the heart. For example cardiac-directed expression of AC6 was associated with increased SR Ca2+ uptake (Tang et al., 2004). In contrast, hearts from AC6-deleted mice show substantially impaired Ca2+ handling (Tang et al., 2008).
Conclusions
Expression of a catalytically inactive mutant of AC6 exhibited many of the beneficial biological effects seen with expression of the catalytically active normal AC6. Both AC6 and AC6mut reduced PLB expression, increased Ca2+ handling, and reduced PE-induced cardiac myocyte hypertrophy and cell death. These data indicate that the favorable effects associated with cardiac AC6 expression are not solely dependent upon increases in cAMP generation.
Acknowledgments
We are grateful to Drs. James Feramisco and Kersi Pestonjamasp for imaging and Diana Huang for isolation of adult rat cardiac myocytes.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants 5P01-HL066941, HL081741, HL088426-01]; a Department of Veterans Affairs merit grant; and American Heart Association Beginning Grant-In-Aid Awards [0765064Y, 0865147F].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.067298.
ABBREVIATIONS:
- AC
- adenylyl cyclase
- AC6
- adenylyl cyclase type 6
- AC6mut
- adenylyl cyclase type 6 mutant
- PHLPP2
- pleckstrin homology domain leucine-rich repeat protein phosphatase 2
- Ad.AC6
- E1-deleted adenoviruses encoding murine AC6
- Ad.AC6mut
- E1-deleted adenoviruses encoding murine AC6 mutant
- PE
- phenylephrine
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick-end labeling
- qRT-PCR
- quantitative reverse transcription-polymerase chain reaction
- BNP
- B-type natriuretic peptide
- CARP
- cardiac ankyrin repeat protein
- PLB
- phospholamban
- TnI
- troponin I
- cTnI
- cardiac troponin-I
- CamKII
- calcium/calmodulin-dependent protein kinase II
- ANF
- atrial natriuretic factor
- LV
- left ventricular
- SR
- sarcoplasmic reticulum
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Gao, T. Tang, Lai, Yuan, and Hammond.
Conducted experiments: Gao, R. Y. Tang, Guo, and Firth.
Contributed new reagents or analytic tools: Miyanohara.
Performed data analysis: Gao.
Wrote or contributed to the writing of the manuscript: Gao and Hammond
Other: Gao and Hammond acquired funding for the research.
References
- Aihara Y, Kurabayashi M, Saito Y, Ohyama Y, Tanaka T, Takeda S, Tomaru K, Sekiguchi K, Arai M, Nakamura T, et al. (2000) Cardiac ankyrin repeat protein is a novel marker of cardiac hypertrophy: role of M-CAT element within the promoter. Hypertension 36:48–53 [DOI] [PubMed] [Google Scholar]
- Bisognano JD, Weinberger HD, Bohlmeyer TJ, Pende A, Raynolds MV, Sastravaha A, Roden R, Asano K, Blaxall BC, Wu SC, et al. (2000) Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J Mol Cell Cardiol 32:817–830 [DOI] [PubMed] [Google Scholar]
- Bousette N, Chugh S, Fong V, Isserlin R, Kim KH, Volchuk A, Backx PH, Liu P, Kislinger T, MacLennan DH, et al. (2010) Constitutively active calcineurin induces cardiac endoplasmic reticulum stress and protects against apoptosis that is mediated by alpha-crystallin-B. Proc Natl Acad Sci USA 107:18481–18486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. (2007) PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell 25:917–931 [DOI] [PubMed] [Google Scholar]
- Chen-Goodspeed M, Lukan AN, Dessauer CW. (2005) Modeling of Galpha(s) and Galpha(i) regulation of human type V and VI adenylyl cyclase. J Biol Chem 280:1808–1816 [DOI] [PubMed] [Google Scholar]
- Communal C, Singh K, Pimentel DR, Colucci WS. (1998) Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation 98:1329–1334 [DOI] [PubMed] [Google Scholar]
- Darfler FJ, Mahan LC, Koachman AM, Insel PA. (1982) Stimulation of forskolin of intact S49 lymphoma cells involves the nucleotide regulatory protein of adenylate cyclase. J Biol Chem 257:11901–11907 [PubMed] [Google Scholar]
- Dessauer CW, Chen-Goodspeed M, Chen J. (2002) Mechanism of Galpha i-mediated inhibition of type V adenylyl cyclase. J Biol Chem 277:28823–28829 [DOI] [PubMed] [Google Scholar]
- Dessauer CW, Gilman AG. (1996) Purification and characterization of a soluble form of mammalian adenylyl cyclase. J Biol Chem 271:16967–16974 [DOI] [PubMed] [Google Scholar]
- Dessauer CW, Scully TT, Gilman AG. (1997) Interactions of forskolin and ATP with the cytosolic domains of mammalian adenylyl cyclase. J Biol Chem 272:22272–22277 [DOI] [PubMed] [Google Scholar]
- De Windt LJ, Lim HW, Taigen T, Wencker D, Condorelli G, Dorn GW, 2nd, Kitsis RN, Molkentin JD. (2000) Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: An apoptosis-independent model of dilated heart failure. Circ Res 86:255–263 [DOI] [PubMed] [Google Scholar]
- Engelhardt S, Grimmer Y, Fan GH, Lohse MJ. (2001) Constitutive activity of the human beta(1)-adrenergic receptor in beta(1)-receptor transgenic mice. Mol Pharmacol 60:712–717 [PubMed] [Google Scholar]
- Engelhardt S, Hein L, Wiesmann F, Lohse MJ. (1999) Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci USA 96:7059–7064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao MH, Bayat H, Roth DM, Yao Zhou J, Drumm J, Burhan J, Hammond HK. (2002) Controlled expression of cardiac-directed adenylylcyclase type VI provides increased contractile function. Cardiovasc Res 56:197–204 [DOI] [PubMed] [Google Scholar]
- Gao MH, Lai NC, Roth DM, Zhou J, Zhu J, Anzai T, Dalton N, Hammond HK. (1999) Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice. Circulation 99:1618–1622 [DOI] [PubMed] [Google Scholar]
- Gao MH, Miyanohara A, Feramisco JR, Tang T. (2009) Activation of PH-domain leucine-rich protein phosphatase 2 (PHLPP2) by agonist stimulation in cardiac myocytes expressing adenylyl cyclase type 6. Biochem Biophys Res Commun 384:193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao MH, Tang T, Guo T, Miyanohara A, Yajima T, Pestonjamasp K, Feramisco JR, Hammond HK. (2008) Adenylyl cyclase type VI increases Akt activity and phospholamban phosphorylation in cardiac myocytes. J Biol Chem 283:33527–33535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao MH, Tang T, Guo T, Sun SQ, Feramisco JR, Hammond HK. (2004) Adenylyl cyclase type VI gene transfer reduces phospholamban expression in cardiac myocytes via activating transcription factor 3. J Biol Chem 279:38797–38802 [DOI] [PubMed] [Google Scholar]
- Gao MH, Tang T, Miyanohara A, Feramisco JR, Hammond HK. (2010) beta(1)-Adrenergic receptor vs adenylyl cyclase 6 expression in cardiac myocytes: differences in transgene localization and intracellular signaling. Cell Signal 22:584–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin C, Ishikawa Y, Wight DC, Mahdavi V, Nadal-Ginard B, Wagner TE, Vatner DE, Homcy CJ. (1995) Overexpression of Gs alpha protein in the hearts of transgenic mice. J Clin Invest 95:1676–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoune J, Pouille Y, Tzavara E, Shen T, Lipskaya L, Miyamoto N, Suzuki Y, Defer N. (1997) Adenylyl cyclases: structure, regulation and function in an enzyme superfamily. Mol Cell Endocrinol 128:179–194 [DOI] [PubMed] [Google Scholar]
- Hurley JH. (1999) Structure, mechanism, and regulation of mammalian adenylyl cyclase. J Biol Chem 274:7599–7602 [DOI] [PubMed] [Google Scholar]
- Iwami G, Kawabe J, Ebina T, Cannon PJ, Homcy CJ, Ishikawa Y. (1995) Regulation of adenylyl cyclase by protein kinase A. J Biol Chem 270:12481–12484 [DOI] [PubMed] [Google Scholar]
- Lai NC, Roth DM, Gao MH, Fine S, Head BP, Zhu J, McKirnan MD, Kwong C, Dalton N, Urasawa K, et al. (2000) Intracoronary delivery of adenovirus encoding adenylyl cyclase VI increases left ventricular function and cAMP-generating capacity. Circulation 102:2396–2401 [DOI] [PubMed] [Google Scholar]
- Lai NC, Roth DM, Gao MH, Tang T, Dalton N, Lai YY, Spellman M, Clopton P, Hammond HK. (2004) Intracoronary adenovirus encoding adenylyl cyclase VI increases left ventricular function in heart failure. Circulation 110:330–336 [DOI] [PubMed] [Google Scholar]
- Lai NC, Tang T, Gao MH, Saito M, Takahashi T, Roth DM, Hammond HK. (2008) Activation of cardiac adenylyl cyclase expression increases function of the failing ischemic heart in mice. J Am Coll Cardiol 51:1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TH, Lai HL, Kao YY, Sun CN, Hwang MJ, Chern Y. (2002) Protein kinase C inhibits type VI adenylyl cyclase by phosphorylating the regulatory N domain and two catalytic C1 and C2 domains. J Biol Chem 277:15721–15728 [DOI] [PubMed] [Google Scholar]
- Maeda T, Sepulveda J, Chen HH, Stewart AF. (2002) Alpha(1)-adrenergic activation of the cardiac ankyrin repeat protein gene in cardiac myocytes. Gene 297:1–9 [DOI] [PubMed] [Google Scholar]
- Merkus D, Brzezinska AK, Zhang C, Saito S, Chilian WM. (2005) Cardiac myocytes control release of endothelin-1 in coronary vasculature. Am J Physiol Heart Circ Physiol 288:H2088–H2092 [DOI] [PubMed] [Google Scholar]
- Patel HH, Head BP, Petersen HN, Niesman IR, Huang D, Gross GJ, Insel PA, Roth DM. (2006) Protection of adult rat cardiac myocytes from ischemic cell death: role of caveolar microdomains and delta-opioid receptors. Am J Physiol Heart Circ Physiol 291:H344–H350 [DOI] [PubMed] [Google Scholar]
- Pinson A, Schlüter KD, Zhou XJ, Schwartz P, Kessler-Icekson G, Piper HM. (1993) Alpha- and beta-adrenergic stimulation of protein synthesis in cultured adult ventricular cardiomyocytes. J Mol Cell Cardiol 25:477–490 [DOI] [PubMed] [Google Scholar]
- Post SR, Hilal-Dandan R, Urasawa K, Brunton LL, Insel PA. (1995) Quantification of signalling components and amplification in the beta-adrenergic-receptor-adenylate cyclase pathway in isolated adult rat ventricular myocytes. Biochem J 311:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DM, Bayat H, Drumm JD, Gao MH, Swaney JS, Ander A, Hammond HK. (2002) Adenylyl cyclase increases survival in cardiomyopathy. Circulation 105:1989–1994 [DOI] [PubMed] [Google Scholar]
- Roth DM, Gao MH, Lai NC, Drumm J, Dalton N, Zhou JY, Zhu J, Entrikin D, Hammond HK. (1999) Cardiac-directed adenylyl cyclase expression improves heart function in murine cardiomyopathy. Circulation 99:3099–3102 [DOI] [PubMed] [Google Scholar]
- Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS. (2001) Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol 189:257–265 [DOI] [PubMed] [Google Scholar]
- Simpson P. (1985) Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circ Res 56:884–894 [DOI] [PubMed] [Google Scholar]
- Smit MJ, Iyengar R. (1998) Mammalian adenylyl cyclases. Adv Second Messenger Phosphoprotein Res 32:1–21 [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG. (1996) Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol 36:461–480 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tang T, Lai NC, Roth DM, Rebolledo B, Saito M, Lew WY, Clopton P, Hammond HK. (2006) Increased cardiac adenylyl cyclase expression is associated with increased survival after myocardial infarction. Circulation 114:388–396 [DOI] [PubMed] [Google Scholar]
- Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JX, Roth DM, Hammond HK. (2008) Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation 117:61–69 [DOI] [PubMed] [Google Scholar]
- Tang T, Gao MH, Roth DM, Guo T, Hammond HK. (2004) Adenylyl cyclase type VI corrects cardiac sarcoplasmic reticulum calcium uptake defects in cardiomyopathy. Am J Physiol Heart Circ Physiol 287:H1906–H1912 [DOI] [PubMed] [Google Scholar]
- Tang T, Lai NC, Hammond HK, Roth DM, Yang Y, Guo T, Gao MH. (2010) Adenylyl cyclase 6 deletion reduces left ventricular hypertrophy, dilation, dysfunction, and fibrosis in pressure-overloaded female mice. J Am Coll Cardiol 55:1476–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WJ, Gilman AG. (1995) Construction of a soluble adenylyl cyclase activated by Gs alpha and forskolin. Science 268:1769–1772 [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Sprang SR. (1998) The structure, catalytic mechanism and regulation of adenylyl cyclase. Curr Opin Struct Biol 8:713–719 [DOI] [PubMed] [Google Scholar]
- Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. (1997) Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTPgammaS. Science 278:1907–1916 [DOI] [PubMed] [Google Scholar]
- Wang SC, Lin JT, Chern Y. (2009) Novel regulation of adenylyl cyclases by direct protein-protein interactions: insights from snapin and ric8a. Neurosignals 17:169–180 [DOI] [PubMed] [Google Scholar]
- Whisnant RE, Gilman AG, Dessauer CW. (1996) Interaction of the two cytosolic domains of mammalian adenylyl cyclase. Proc Natl Acad Sci USA 93:6621–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GC, Lai HL, Lin YW, Chu YT, Chern Y. (2001) N-glycosylation and residues Asn805 and Asn890 are involved in the functional properties of type VI adenylyl cyclase. J Biol Chem 276:35450–35457 [DOI] [PubMed] [Google Scholar]
- Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. (2007) Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 130:247–258 [DOI] [PubMed] [Google Scholar]
- Yan SZ, Hahn D, Huang ZH, Tang WJ. (1996) Two cytoplasmic domains of mammalian adenylyl cyclase form a Gs alpha- and forskolin-activated enzyme in vitro. J Biol Chem 271:10941–10945 [DOI] [PubMed] [Google Scholar]
- Zaugg M, Lucchinetti E, Spahn DR, Pasch T, Schaub MC. (2002) Volatile anesthetics mimic cardiac preconditioning by priming the activation of mitochondrial K(ATP) channels via multiple signaling pathways. Anesthesiology 97:4–14 [DOI] [PubMed] [Google Scholar]