Abstract
Inhibitor-1 (I-1) is phosphorylated on threonine residue 35 (Thr35) by the cAMP-dependent protein kinase (PKA), inducing the potent inhibition of the serine-threonine-specific protein phosphatase 1 (PP1). We now report that the formation of a signaling complex containing PKA and I-1 by the A-kinase anchoring protein 18 (AKAP18) facilitates this regulation in cells. AKAP18 directly bound I-1, and AKAP18/I-1 complexes were isolated from both rat heart extract and transfected heterologous cells. It is noteworthy that prevention of PKA binding to the AKAP18 scaffold decreased I-1 phosphorylation by 48% in cells. Moreover, the I-1 target PP1 was also associated with AKAP18 complexes. The cAMP-mediated inhibition of phosphatase activity was contingent on PKA binding to the scaffold. These observations reveal an additional level of complexity in PP1 regulation because of its association with AKAP18 multimolecular signaling complexes and suggest that targeting of AKAP18 complexes may be an alternative method to alter phosphatase activity and modulate specific substrate dephosphorylation.
Introduction
Protein phosphorylation is a key regulator of cellular physiology that affects essentially all biological processes. Despite our vast knowledge of the consequences of protein phosphorylation, the molecular mechanisms that confer specificity to the enzymes controlling this post-translational modification are not well understood, especially for the handful of phosphatases that catalyze the dephosphorylation of protein substrates (Cohen, 2002; Virshup and Shenolikar, 2009). Seminal investigations have demonstrated that the cell has evolved multiple strategies that control the location, activity, and substrate specificity of each phosphatase (Cohen, 2002; Virshup and Shenolikar, 2009). Current research is focused on the contribution of individual multimolecular signaling complexes to the specificity of intracellular signal transduction.
The first protein discovered to regulate phosphatase activity was the protein phosphatase inhibitor-1 (I-1) (Huang and Glinsmann, 1976). I-1 is a 19-kDa, heat-stable protein that was identified more than 30 years ago as a regulator of glycogen metabolism (Huang and Glinsmann, 1976). Although I-1 has no known intrinsic catalytic activity, its binding reduces the activity of protein phosphatase 1 (PP1). I-1 is highly expressed in the brain, where it plays a role in synaptic plasticity (Genoux et al., 2002; Mansuy and Shenolikar, 2006). Although I-1 is expressed at low levels in the adult myocardium, recent work suggests that regulation of PP1 by I-1 at the sarcoplasmic reticulum is required for normal cardiac function, as well as for the response of the heart to disease (Nguyen et al., 2007; Nicolaou et al., 2009a). I-1 itself is phosphorylated by PKA, protein kinase C, and cyclin-dependent kinase 5 (Huang and Glinsmann, 1976; Rodriguez et al., 2006; Sahin et al., 2006; Nguyen et al., 2007), and phosphorylation of I-1 at Thr35 by PKA induces the selective inhibition of PP1 (Huang and Glinsmann, 1976; Nicolaou et al., 2009a). This event is induced by β-adrenergic stimulation in cardiac myocytes and potentiates the phosphorylation of key PKA substrates involved in excitation-contraction coupling by preventing their PP1-mediated dephosphorylation (Nicolaou et al., 2009a). Because excessive PP1 activity resulting from a lack of I-1 function has been suggested to contribute to heart failure, a more complete understanding of the molecular regulation of this phosphatase may aid in the design of novel therapeutics to prevent or treat heart disease (Carr et al., 2002; Braz et al., 2004; El-Armouche et al., 2008; Nicolaou et al., 2009b).
The mechanisms conferring specificity on PKA phosphorylation have been of considerable interest in recent years, and research has shown that many PKA targets are colocalized with the kinase via the association with A-kinase anchoring proteins (AKAPs) (Carnegie et al., 2009; Scott and Pawson, 2009). These scaffold proteins function to enhance the kinetics and specificity of PKA phosphorylation by sequestering the kinase with its target substrates, allowing for spatiotemporal control of cAMP signaling. It is noteworthy that disruption of PKA binding to AKAPs in the heart significantly decreases the ability of the kinase to phosphorylate many key proteins such as troponin I, the ryanodine receptor, and phospholamban (Mauban et al., 2009).
Because I-1 is a target for PKA, we investigated whether an AKAP mediates this event. We discovered that I-1 binds the large isoforms of AKAP18 in the heart. AKAP18 potentiated the phosphorylation of I-1 by PKA, and disruption of PKA binding to the scaffold significantly attenuated Thr35 phosphorylation in HEK293 cells. Moreover, PP1 was also associated with AKAP18 complexes, and the PKA-dependent inhibition of phosphatase activity required PKA-AKAP18 binding. These data imply that AKAP18 orchestrates the PKA-catalyzed phosphorylation of I-1 in the myocyte, providing an additional level of regulation of PP1 activity at the sarcoplasmic reticulum, in which the large isoforms of the AKAP are enriched.
Materials and Methods
Antibodies.
The following antibodies were used for immunoblotting: rabbit polyclonal to inhibitor-1 (1:10,000; Abcam Inc., Cambridge, MA), goat polyclonal to AKAP18 (1:1000; received from Dr. John Scott, University of Washington, Seattle, WA), rabbit polyclonal to GFP (1:500; Invitrogen, Carlsbad, CA), rabbit polyclonal phosphospecific antibody against Thr35 of I-1 (1:1000; Cell Signaling Technology Inc., Danvers, MA), mouse monoclonal to PP1 catalytic subunit (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal to myc tag (1:1000; Santa Cruz Biotechnology), mouse monoclonal to PKA-RI subunit (1:5000), mouse monoclonal to PKA-RII subunit (1:5000), and mouse monoclonal to PKA catalytic subunit (1:5000; all from BD Bioscience, San Jose, CA). The following antibodies were used for immunoprecipitations: rabbit polyclonal to inhibitor-1 (2 μg; Abcam), mouse monoclonal to PP1 catalytic subunit (5 μg; Santa Cruz Biotechnology), mouse monoclonal to PP2A catalytic subunit (5 μg; Santa Cruz Biotechnology), goat polyclonal to AKAP18 (5 μl; received from Dr. John Scott, University of Washington), mouse monoclonal to myc tag (5 μg; Santa Cruz Biotechnology), and mouse monoclonal to PKA-RII subunit (2 μg; BD Biosciences).
Expression Constructs.
For I-1 constructs, human I-1 was polymerase chain reaction-amplified using a cDNA (supplied by Dr. Shirish Shenolikar, Duke University, Durham, NC) and subcloned into the EcoRI-Xho sites of pGEX-4T1 and pCDNA3. Full-length human AKAP18γ (supplied by Dr. John Scott, University of Washington) and the AKAP18γ deletion constructs were cloned into either the EcoRI-BamHI sites of pEGFP-N1 or the EcoRI-HindIII sites of pET32a.
cAMP Agarose Purification.
Rat heart extract was prepared by homogenization of a single rat heart (PelFreeze) in 10 ml of HSE lysis buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, and protease inhibitors). After centrifugation at 12,000g for 20 min at 4°C, 1 ml of supernatant (500 μg) was incubated overnight with 60 μl of Rp-cAMPs-agarose (Biolog, Bremen, Germany) in the presence and absence of 100 mM cAMP (Sigma-Aldrich, St. Louis, MO). The beads were washed twice with HSE lysis buffer followed by a high salt wash (20 mM HEPES, pH 7.4, 600 mM NaCl, and 5 mM EDTA). Bound proteins were analyzed by immunoblotting.
For cAMP agarose pull-downs from HEK293 cells, cells were transfected at 70% confluence in 60-mm dishes using calcium phosphate. Cells were harvested and lysed 24 h after transfection in 0.5 ml of HSE buffer containing protease inhibitors. Cell lysate was added to 60 μl of Rp-cAMPs-agarose, and bound proteins were isolated as detailed above.
Pull-Down Experiments.
Heart extract (1 ml) was prepared in HSE buffer as described above and incubated overnight with glutathione-resin preabsorbed with bacterially expressed fusion proteins. The beads were then washed as described, and bound proteins were identified by immunoblotting. Alternatively, for pull-down experiments using purified proteins, GST-fusion proteins were incubated with S-tagged purified AKAP18 fragments bound to S-protein agarose in HSE buffer lacking EDTA. After an overnight incubation, beads were washed three times in the HSE buffer, and associated proteins were identified by immunoblot.
Immunoprecipitation.
For immunoprecipitation of AKAP18 constructs from heterologous cells, HEK293 cells were transfected at 70% confluence using the calcium phosphate method. Twenty-four hours after transfection, cells were treated with 1 μM isoproterenol for 5 min. Cells were rinsed in phosphate-buffered saline and then harvested and lysed in 0.5 ml of HSE buffer containing protease inhibitors plus phosphatase inhibitors (10 μM sodium pyrophosphate and 50 μM sodium fluoride). Supernatants were incubated with 4 μg of antibody and 20 μl of protein G-agarose beads. Bound proteins were analyzed by immunoblotting.
For immunoprecipitations from rat heart, extract was prepared as described above. Proteins were immunoprecipitated from 1 ml of extract using specific antibodies bound to protein G-agarose. After extensive washing with HSE buffer, the associated proteins were determined by immunoblot.
Phosphatase Assay.
Phosphatase activity was measured as described previously using 32P-labeled histone as a substrate (Dodge-Kafka et al., 2010). Histone was radiolabeled in a reaction containing 250 mM MOPS, pH 7.4, 2.5 mM MgAc, 100 mM β-mercaptoethanol, purified PKA catalytic subunit, 1 mM ATP, 20 mM histone, and 1 mCi [γ-32P]ATP (6000 Ci/mmol). The reaction was terminated by the addition of 50% TCA and [32P]histone was purified from free radionucleotide by centrifugation. After repeated washing, the [32P]histone was suspended in 200 μl of PP2A buffer (25 mM Tris, pH 7.4, 1 mM dithiothreitol, and 10 mM MgCl2.
To measure phosphatase activity, immunoprecipitated complexes were washed twice in HSE buffer and again in PP2A buffer. The immunoprecipitates were incubated for 30 min at 30°C in 20 μl of PP2A buffer containing 100,000 cpm of [32P]histone in the presence and absence of inhibitors. Reactions were terminated by the addition of 100 μl of 20% TCA followed by 10 min of centrifugation. TCA supernatants containing released 32PO4 were measured using a scintillation counter.
PKA Activity Assay.
PKA activity was measured according to the method of Corbin and Reimann (1974). Immunoprecipitated complexes were washed twice in HSE buffer and once in PKA assay buffer (50 mM Tris, pH 7.5, and 5 mM MgCl2). The beads were then incubated in assay buffer containing 30 μM Kemptide (Promega, Madison, WI), 100 μM ATP, 5 μM [γ32]ATP, and 3 μM cAMP. After incubating the samples at 30°C for 30 min, the reaction mixture was spotted onto phosphocellulose strips and washed five times in 75 mM phosphoric acid and once in 95% ethanol. Filters were air-dried and counted.
Detection of I-1 Phosphorylation.
For I-1 phosphorylation experiments, 24 h after transfection, cells were stimulated with 1 μM isoproterenol for 5 min. Cells were rinsed in phosphate-buffered saline and scraped into 0.5 ml of HSE buffer containing protease and phosphatase inhibitors (10 mM sodium pyrophosphate and 50 mM sodium fluoride). After centrifugation for 10 min at 13,000g, the clarified supernatant was added to 1 ml of isopropanol, and total cellular protein was precipitated by incubation at −20°C for 2 h followed by centrifugation at 13,000g for 30 min. Precipitated proteins were resuspended in 200 μl of 2× SDS-polyacrylamide gel electrophoresis sample buffer (0.5 M Tris-HCl, pH 6.8, 1% glycerol, 10% SDS, and 0.5% β-mercaptoethanol) that was added to the cellular pellet. For immunoblot analysis using a phosphospecific antibody, 20 μl of each isolated sample was subjected to SDS-polyacrylamide gel electrophoresis.
For in vitro I-1 phosphorylation, GST-fusion proteins were incubated with cellular extracts isolated from transfected HEK293 cells as described above. After an overnight incubation and washing of the beads with HSE buffer lacking EDTA, the complex was incubated in 25 μl of PKA assay buffer (25 mM Tris, pH 7.4, 1 mM dithiothreitol, 10 mM MgCl2, 1 mM ATP, and 3 mM cAMP) for 30 min at 37°C. The bound proteins were then subjected to immunoblot analysis.
Results
AKAP18 Associates with Inhibitor-1 In Vivo.
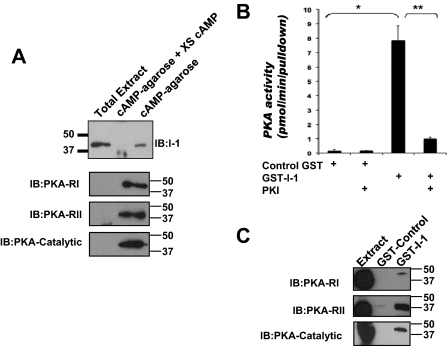
The formation of signaling complexes by AKAPs that include PKA and individual PKA substrates contributes to the efficiency and fidelity of cAMP signal transduction (Carnegie et al., 2009). Because I-1 is an important PKA target, we considered that I-1 might associate with anchored PKA in the heart. To test this idea, we isolated PKA-AKAP complexes from rat heart extract by affinity chromatography using Rp-cAMPs-agarose that binds the PKA regulatory subunits. This compound is a competitive inhibitor of PKA, so it does not stimulate activation of the kinase, thus leaving the holoenzyme intact. I-1 was consistently detected in the Rp-cAMPs pull-downs, but not under conditions in which excess cAMP was added to the extracts to block specific binding to the beads (Fig. 1A). As a control, the PKA regulatory subunit type I, type II, and the PKA catalytic subunit were found specifically in cAMP-agarose pull-downs. To confirm that the PKA holoenzyme containing the catalytic subunit associated with I-1, we used GST-tagged, purified I-1 to pull down PKA from rat heart extract. As shown in Fig. 1B, kinase activity was found specifically in GST-I-1 complexes, and not with GST alone. Kinase activity was specific for PKA, because the addition of the PKA-specific inhibitor PKI inhibited PKA activity in the pull-downs. Furthermore, PKA catalytic subunit as well as PKA regulatory type I and type II subunits were found in GST-I-1 pull-downs from rat heart extract. It is noteworthy that the percentage of PKA-RII was enriched 20-fold over RI, suggesting this AKAP is a primarily RII binding AKAP. In summary, these data show that I-1 associates with a PKA complex in the heart.
Fig. 1.
Inhibitor-1 associates with PKA in heart cells. A, rat heart extracts were incubated with Rp-cAMPs-agarose in the presence or absence of excess cAMP (50 mM). I-1 in whole extract (Ex, 2% of total) and the cAMP-associated fraction of I-1, PKA-RI, PKA-RII, and PKA-catalytic subunit were determined by immunoblotting; n = 3. B, rat heart extract was incubated with either GST-I-1 (3 μg) or control GST protein, and the bound proteins were assayed for PKA activity using Kemptide as a substrate; n = 3, *, p < 0.008; **, p < 0.01. C, PKA-RI, PKA-RII, and PKA-catalytic subunits present in GST control and GST-I-1 pull-downs were detected by immunoblot. Extract is 5% of the total protein used for each pull-down.
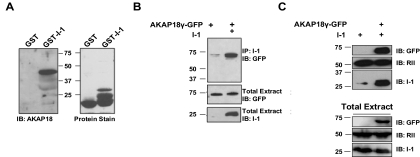
Next, we sought to identify which AKAP might mediate this interaction between PKA and inhibitor-1 in the heart. To do this, we isolated proteins in rat heart extracts that associated with I-1 using GST-tagged inhibitor-1 protein and then blotted for some highly characterized AKAPs found in the heart. As shown in Fig. 2A, a high-molecular-mass AKAP18 isoform was readily detected in GST-I-1 complexes, in addition to what are potentially AKAP18 isoforms with lower molecular mass that were less prevalent. As a control, I-1 did not associate with mAKAP, another muscle-enriched AKAP involved in the induction of cardiac hypertrophy (Supplementary Fig. 1) (Dodge-Kafka et al., 2010). The molecular mass of the intense, 45-kDa band implied that the major isoform pulled down by GST-I-1 was either AKAP18δ or AKAP18γ. It is noteworthy that this interaction was not observed using GST alone, demonstrating the specificity of the interaction. The association of AKAP18 with I-1 was confirmed using an I-1 antibody to immunoprecipitate the scaffold from HEK293 cells expressing GFP-tagged AKAP18γ and I-1 (Fig. 2B). In addition, complexes containing PKA were purified from the HEK293 cell extracts using Rp-cAMPs agarose. I-1 copurified with the agarose only when coexpressed with the anchoring protein, mimicking the results found with the endogenously expressed I-1 and AKAP18 in the heart (Fig. 2C). Together, these data suggest that AKAP18, I-1, and PKA form a multimolecular signaling complex in the heart and when expressed in heterologous HEK293 cells.
Fig. 2.
AKAP18 associates with I-1. A, isolated rat heart extract was incubated with either GST-I-1 or control GST-protein. AKAP18 was detected by Western blot (left) and total protein by Ponceau stain (right), n = 3. B, HEK293 cells expressing GFP-AKAP18γ in the presence and absence of I-1 were used for immunoprecipitations with an I-1 antibody, and AKAP18γ association was determined by immunoblot using a GFP antibody. C, HEK293 cells were transfected with inhibitor-1 in the presence and absence of GFP-tagged AKAP18γ. Isolated extracts were incubated with cAMP agarose, and immunoblotting demonstrated the association of I-1. Immunoblots of whole-cell extracts (2.5% of total) are shown as controls for both B and C; n = 3 for all.
I-1 Directly Binds a Long Isoform of AKAP18.
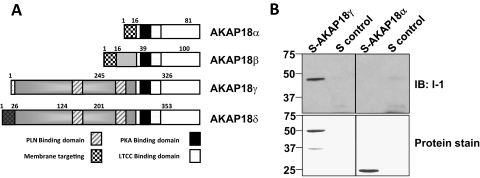
AKAP18γ is one of the larger protein isoforms encoded by the alternatively spliced AKAP18 gene (schematic shown in Fig. 3A). The smaller AKAP18α is N-terminally myristoylated and palmitoylated, allowing for attachment to the plasma membrane, in which it facilitates PKA phosphorylation of the L-type calcium channel (Fraser et al., 1998). In contrast, the large isoforms of AKAP18 are targeted to the sarcoplasmic reticulum, in which they form a signaling complex with phospholamban (Lygren et al., 2007). To define which isoform of AKAP18 is the primary target for I-1 binding, we performed pull-down assays using bacterially expressed GST-tagged I-1 with these two isoforms of AKAP18. GST-I-1 incubated with either bacterially expressed, S-tagged AKAP18α or AKAP18γ was subjected to S-tag pull-down assay. GST-I-1 was found in the S-protein agarose only in the presence of the larger isoform AKAP18γ. It is noteworthy that these data also demonstrate that I-1 and the scaffolding protein can bind directly in a purified system.
Fig. 3.
Direct binding of AKAP18γ and Inhibitor-1. A, schematic diagram depicting the known AKAP18 splice variants; PLN, phospholamban; LTCC, L-type calcium channel. B, GST-I-1 fusion protein (3 μg) was incubated with S-tagged AKAP18α, S-tagged AKAP18γ fusion protein (3 μg), or S-protein agarose alone. S-tagged pull-down assays were performed, and the association of GST-I-1 was determined by Western blot. Total protein was detected by Ponceau stain.
Expression of AKAP18 Enhances I-1 Phosphorylation on Threonine 35.
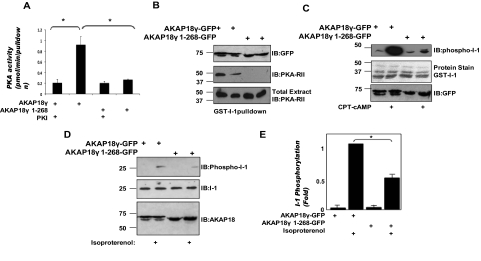
The role of an AKAP is to anchor the kinase in close proximity to its target to enhance PKA phosphorylation (Carnegie et al., 2009). Therefore, AKAP18 should enhance PKA-mediated phosphorylation of I-1 by assembling a signaling complex containing both I-1 and PKA. To test whether PKA binding to AKAP18 is important for I-1 phosphorylation, we first confirmed that a ternary complex consisting of AKAP18, PKA, and I-1 could be isolated from transfected HEK293 cells. Cell extracts were prepared from HEK293 cells expressing AKAP18γ or a C-terminal truncated AKAP18γ mutant lacking the PKA binding site (AKAP18γ 1–268). GST-I-1 was incubated with the isolated cell extracts, and PKA activity assays were performed on the pull-downs (Fig. 4A). Although I-1 bound both full-length AKAP18γ and AKAP18γ 1–268 (Fig. 4B, top), kinase activity was only detected with full-length AKAP18γ (Fig. 4A). Kinase activity was blocked by the addition of PKI, demonstrating the specificity of the reaction to measure PKA activity. In support of this finding, PKA-RII was not detected in GST-I-1 pull-downs from cells expressing AKAP18γ 1–268 (Fig. 4B, middle; GST-protein stain shown in Supplementary Fig. 2A). These data show that AKAP18γ can serve to couple PKA to I-1 in a heterologous system.
Fig. 4.
AKAP18 orchestrates I-1 phosphorylation by linking PKA with the inhibitor. A, GST-I-1 fusion protein (3 μg) was incubated with HEK293 cell lysate prepared from cells expressing AKAP18γ or AKAP18γ 1–268. PKA activity was determined in each GST pull-down in the presence and absence of 50 nM PKI. B, pull-downs were performed as described in A, and the association of the AKAP, as well as PKA-RII with GST-I-1, was demonstrated by immunoblot. C, GST-I-1 fusion protein (3 μg) was incubated with HEK293 cell lysate as prepared in A, and PKA associated with the GST pull-downs was activated by the addition of cAMP. I-1 phosphorylation was detected by Western blot using an antibody that specifically recognized the inhibitor when phosphorylated at Thr35 (top). Total I-1 in each pull-down is shown in the middle, whereas protein expression of the different AKAP18 constructs is shown at the bottom. D, HEK293 cells expressing I-1 and either full-length AKAP18γ or AKAP18γ 1–268 were stimulated with 1 μM isoproterenol for 5 min. Whole-cell lysates were analyzed by Western blot using antibodies against phospho-I-1 Thr35 (top), I-1 (middle), and GFP for the AKAP18 constructs (bottom). E, densitometric quantification of I-1 phosphorylation shown in C normalized to isoproterenol, AKAP18γ sample; n = 3 for each, *, p < 0.05.
Next, we determined whether the associated kinase could phosphorylate I-1 at Thr35. Consistent with the results of the PKA activity assays, cAMP-dependent phosphorylation of I-1 was greatly enhanced in GST-I-1 pull-downs containing full-length AKAP18γ compared with those containing the AKAP18γ PKA-binding mutant (Fig. 4C). To show that AKAP18 enhances the PKA phosphorylation of I-1 in response to G protein-coupled receptor stimulation, the AKAP18γ proteins were coexpressed with I-1 in HEK293 cells. In the absence of stimulation, no I-1 phosphorylation was detected using the phosphospecific antibody. However, β-adrenergic stimulation with 1 μM isoproterenol for 5 min rapidly enhanced I-1 phosphorylation when AKAP18γ was coexpressed (Fig. 4D) in a PKA-dependent manner (Supplementary Fig. 2B). It is noteworthy that this stimulated increase was significantly attenuated in cells transfected with I-1 and AKAP18γ lacking the PKA binding domain (42 ± 5.7% of wild type; Fig. 4E). Together, these data show that targeting of PKA to I-1 via the association with AKAP18 facilitates I-1 phosphorylation by the kinase.
AKAP18 Binds PP1 In Vivo.
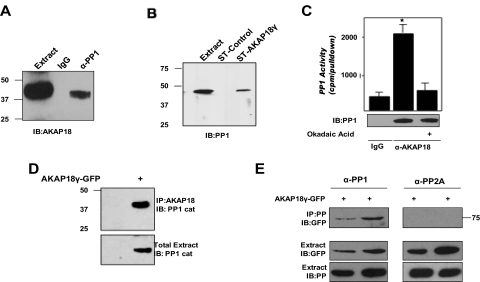
Because AKAPs often serve as scaffolds for multimolecular signaling complexes that include both kinase and phosphatases, we considered that the I-1 target PP1 might also be present in AKAP18γ complexes. To test this hypothesis, protein complexes were immunoprecipitated from rat heart extract using an antibody that recognizes the catalytic subunit of PP1 (Fig. 5A). A high-molecular-mass isoform of AKAP18 coprecipitated with the PP1 antibody, but not with IgG control, implying that PP1 and AKAP18γ and/or AKAP18δ associate in vivo. In reciprocal experiments, STREP-tagged AKAP18γ was used to pull down PP1 from rat heart extract (Fig. 5B). PP1 did not associate with STREP beads alone, demonstrating the specificity of this interaction. Furthermore, an okadaic acid-sensitive phosphatase activity was found in AKAP18 immunoprecipitates isolated from rat heart extract (Fig. 5C). Taken together, these data show that PP1 and AKAP18 form a complex in the rat heart.
Fig. 5.
AKAP18 is a PP1 anchoring protein. A, protein complexes were immunoprecipitated from rat heart extract with either an antibody specific to PP1 catalytic subunit or control IgG and detected using an AKAP18 antibody. Extract represents 2.5% of total input. B, rat heart extracts were incubated with either STREP-tagged (ST) AKAP18γ fusion protein or control STREP protein. PP1 in the pull-downs and total extract (2.5% of total input) were detected with an antibody to PP1 catalytic subunit. C, protein complexes were immunoprecipitated from rat heart extract with either an antibody specific to AKAP18 or control IgG and assayed for phosphatase activity in the presence or absence of okadaic acid (100 nM). The amount of PP1 in each group is demonstrated by Western blot analysis of the immunoprecipitate. D, protein complexes were immunoprecipitated from lysates prepared from control HEK293 cells or cells expressing GFP-tagged AKAP18γ using an AKAP18 antibody. Precipitated PP1 was detected using a catalytic subunit antibody. E, protein complexes were immunoprecipitated from lysates prepared from HEK293 cells expressing GFP-tagged AKAP18γ using either a PP1 or PP2A catalytic subunit antibody (designated as PP, protein phosphatase). AKAP18 in the immunoprecipitates and AKAP18, PP1, and PP2A in the total extract (2.5% of total) were detected by immunoblot; n = 3 for all.
Next, we confirmed that the PP1/AKAP18γ complexes could be reconstituted in transfected HEK293 cells. In support of our findings that PP1 and AKAP18 formed a complex in rat heart cell extract, PP1 also associated with AKAP18γ in transfected HEK293 cells (Fig. 5D). It is noteworthy that this association was lost when cells not expressing the anchoring protein were immunoprecipitated with the AKAP18 antibody, demonstrating the specificity of the interaction. It is noteworthy that we were unable to immunoprecipitate the scaffold with a PP2A catalytic subunit antibody (Fig. 5E), even though phosphatase activity was found in both PP2A and PP1 immunoprecipitations (Supplementary Fig. 2), suggesting that under these conditions, AKAP18γ selectively binds PP1. Taken together, our data demonstrate a complex consisting of AKAP18 and PP1 that can be isolated from both rat heart extract and from transfected HEK293 cells.
AKAP18 Is the Scaffold for a PKA, PP1, and I-1 Signaling Complex.
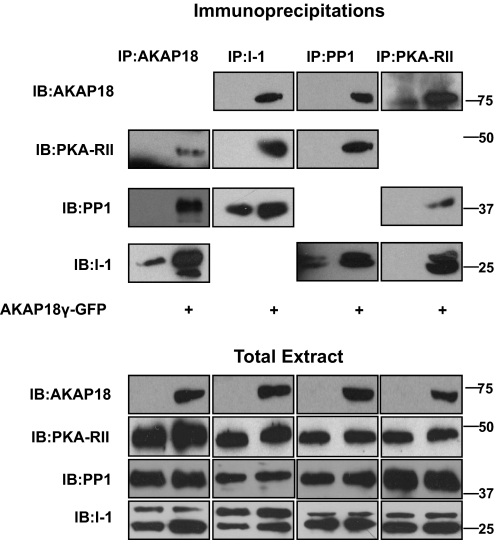
The data presented here suggest that I-1, PKA, and PP1 all associate with AKAP18 in both transfected cells and heart extract. Two implications of these data are that the anchoring protein may sequester these enzymes into the same signaling complex and that compartmentation is dependent on AKAP18. To test this hypothesis, we investigated the importance of AKAP18γ expression for formation of the complex. HEK293 cells were transfected with I-1 in the presence and absence of AKAP18γ, whereas endogenous PP1 catalytic and PKA regulatory subunits were used for these experiments. Each protein was immunoprecipitated, and the association of the other three proteins was determined by Western blot. As shown in Fig. 6, the expression of AKAP18γ was required for the association of the PKA-RII with I-1 and PP1, as well as the association of PP1 to both AKAP18γ and the PKA-RII. Furthermore, I-1 was only found in PKA-RII immunocomplexes upon expression of AKAP18γ. Although I-1 and PP1 can associate in the absence of AKAP18γ, coexpression of the anchoring protein significantly increased the association of the two proteins. Controls demonstrating the specificity of each immunoprecipitation are shown in Supplementary Fig. 4. The data presented in Fig. 6 effectively argue the importance of AKAP18 for linking PKA to I-1 and PP1.
Fig. 6.
AKAP18 sequesters PKA, PP1, and I-1 to a specific signaling complex. Protein complexes were immunoprecipitated from lysates prepared from HEK293 cells transfected with I-1 plus and minus GFP-AKAP18γ using antibodies to AKAP18, I-1, PP1, and PKA-RII. Proteins in the extracts (bottom) and immunoprecipitates (top) were detected with the respective antibodies. Total protein (5% of input) is shown below; n = 3 for each.
AKAP18 Orchestrates the PKA-Mediated Inhibition of PP1 Activity.
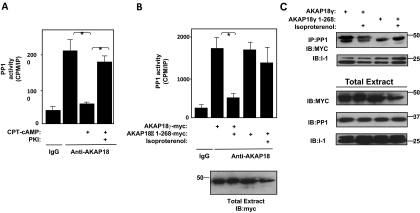
Phosphorylation of I-1 by PKA induces potent inhibition of PP1 activity (Huang and Glinsmann, 1976). Our data suggest that AKAP18-bound PKA facilitates this phosphorylation and, hence, should enhance the ability of I-1 to inhibit the phosphatase. To study this potential functional consequence of AKAP18 complex formation, we first isolated AKAP18 complexes from rat heart extract and determined the effect of cAMP on the associated endogenous PP1 activity. Activation of PKA in the complex by the addition of 3 μM cAMP inhibited PP1 activity (95 ± 4.67%) present in the AKAP18 immunoprecipitates (Fig. 7A). This inhibition was attributed to the activation of PKA, because inhibition was reversed by inclusion of the PKA inhibitor PKI (50 nM). These data demonstrate that the PKA-stimulated inhibition of AKAP18-bound PP1 is functional in native AKAP18 complexes isolated from rat heart.
Fig. 7.
PKA binding to AKAP18 is necessary for PKA-induced inhibition of PP1. A, protein complexes were immunoprecipitated from rat heart extract with either an antibody specific to AKAP18 or control IgG and assayed for phosphatase activity in the presence and absence of CPT-cAMP (3 μM) or PKI (50 nM) B, phosphatase activity associated with protein complexes immunoprecipitated using an antibody specific for myc-tag from lysates prepared from HEK293 cells expressing I-1 and either myc-tagged full-length AKAP18γ or AKAP18γ 1–268 was assayed. Endogenous PP1 was used. Cells were stimulated with 1 μM isoproterenol for 5 min as indicated. AKAP18 proteins in the total lysates were detected with a myc antibody; n = 3 for each, *, p < 0.05. C, cell lysates were prepared as in B. PP1 immunocomplexes were isolated, and association of AKAP18 and I-1 was determined by Western blot; n = 3 for each.
Showing the necessity of PKA anchoring to AKAP18 for PP1 inhibition extended these results. HEK293 cells were transfected with I-1 and either full-length AKAP18γ or the AKAP18γ PKA binding mutant. After stimulation with 1 μM isoproterenol for 5 min, complexes were isolated using an antibody that recognizes the anchoring protein, and the associated phosphatase activity was measured. As shown in Fig. 7B, PKA anchoring to the complex was required for effective inhibition of the associated phosphatase activity, because the cAMP-induced inhibition of PP1 was lost when AKAP18 could not bind PKA. This result is not due to a loss of complex formation, because similar amounts of AKAP18 and I-1 were found in PP1 immunocomplexes (Fig. 7C). Taken together, these data show that the binding of I-1, PP1, and PKA by AKAP18 permits the efficient regulation of PP1 activity by cAMP signaling in cells.
Discussion
In this report, we identify the first interaction between inhibitor-1 and an A-kinase anchoring protein, AKAP18. Functional AKAP18 complexes containing I-1, the target PP1, and PKA were isolated from both rat heart extract and transfected HEK293 cells. The functional significance of these interactions was demonstrated in two ways. First, complex formation facilitated the cellular phosphorylation of I-1, because disruption of PKA association with AKAP18 significantly decreased phosphorylation of I-1 at Thr35. Second, PKA binding to AKAP18 was required for isoproterenol-induced inhibition of the associated PP1 activity. This information establishes AKAP18 as a regulator of both I-1 and PP1 activity and advances our understanding of phosphatase regulation.
PKA phosphorylation of I-1 at Thr35 and the resulting inhibition of PP1 activity acts as a feed-forward mechanism to enhance PKA phosphorylation of downstream effectors that would otherwise be dephosphorylated by PP1 (Pathak et al., 2005; Nicolaou et al., 2009a). PKA anchoring to AKAP18 is now shown to play an integral role in this process, because disruption of PKA anchoring to AKAP18 prevented cAMP-mediated inhibition of the PP1 activity associated with the complex. As a result, in cells such as cardiac myocytes that express AKAP18γ and AKAP18δ, these complexes should be highly sensitive to cAMP signals.
Previous work has demonstrated the existence of multiprotein complexes containing I-1 and PP1. An I-1/PP1 complex mediated by interactions with the growth arrest and DNA damage protein 34 (GADD34) was found in active squirrel brains (Connor et al., 2001). GADD34 was shown to enhance the I-1-mediated inhibition of PP1 to regulate the phosphorylation of the transcription factor eIF-2α. Although GADD34 is found in the heart, the role of this complex in the regulation of PP1 in this tissue is unknown (Morton et al., 2006). An important difference between GADD34 and AKAP18 in the regulation of PP1 activity is that the AKAP also anchors PKA to the complex, enhancing I-1 phosphorylation. Thus, the ability of AKAP18 to sequester I-1 and PKA ensures spatial-temporal control of I-1 phosphorylation.
Several AKAPs have been implicated previously to bind to and regulate phosphatase activity. For example, the direct association between AKAP220 and PP1 inhibits phosphatase activity (Schillace et al., 2001). In addition, mAKAP binds to PP2A and mediates a cAMP-induced increase of phosphatase activity through PKA phosphorylation of the PP2A-B56δ subunit (Dodge-Kafka et al., 2010). In contrast to these other complexes, PP1 bound to AKAP18 is active, and PKA phosphorylation of the phosphatase's regulator results in its inhibition. To date, this is the first identification of an AKAP-mediated regulation of I-1 phosphorylation.
In the heart, I-1 activity is spatially restricted and selectively regulates PP1 activity at the sarcoplasmic reticulum, in which PP1 opposes phospholamban phosphorylation (Pathak et al., 2005; Nicolaou et al., 2009a). In contrast to the smaller isoforms of AKAP18 (α and β) that are targeted to the plasma membrane, the long isoforms (γ and δ) bind directly to phospholamban, facilitating PKA phosphorylation of phospholamban (Lygren et al., 2007). Our results imply that AKAP18 may facilitate the localization of I-1 to the sarcoplasmic reticulum, further promoting phospholamban phosphorylation. Future investigations in our laboratory will address how the intracellular targeting of I-1 by the long isoforms of AKAP18 affects the kinetics and sensitivity of phospholamban phosphorylation.
Supplementary Material
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants R01-HL82705, R01-HL075398].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.065425.
ABBREVIATIONS:
- I-1
- protein phosphatase 1 inhibitor-1
- PP1
- protein phosphatase 1
- PKA
- cAMP-dependent protein kinase
- AKAP
- A-kinase anchoring protein
- TCA
- trichloroacetic acid
- PP2A
- protein phosphatase 2A
- GFP
- green fluorescent protein
- HEK
- human embryonic kidney
- MOPS
- 3-(N-morpholino)propanesulfonic acid
- GST
- glutathione transferase
- PKI
- protein kinase I
- STREP
- Trp-Ser-His-Pro-Gln-Phe-Glu-Lys
- GADD34
- growth arrest and DNA damage protein 34
- Rp-cAMPs
- adenosine-3′,5′-cyclic monophosphorothioate, Rp-isomer.
Authorship Contributions
Participated in research design: Singh, Redden, Kapiloff, and Dodge-Kafka.
Conducted experiments: Singh, Redden, and Dodge-Kafka.
Performed data analysis: Singh, Redden, Kapiloff, and Dodge-Kafka.
Wrote or contributed to the writing of the manuscript: Singh, Redden, Kapiloff, and Dodge-Kafka.
Other: Kapiloff and Dodge-Kafka acquired funding for the research.
References
- Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, et al. (2004) PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med 10:248–254 [DOI] [PubMed] [Google Scholar]
- Carnegie GK, Means CK, Scott JD. (2009) A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life 61:394–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, Breeden K, Jing SL, Allen PB, Greengard P, et al. (2002) Type 1 phosphatase, a negative regulator of cardiac function. Mol Cell Biol 22:4124–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PT. (2002) Protein phosphatase 1—targeted in many directions. J Cell Sci 115:241–256 [DOI] [PubMed] [Google Scholar]
- Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. (2001) Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol 21:6841–6850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JD, Reimann EM. (1974) Assay of cyclic AMP-dependent protein kinases. Methods Enzymol 38:287–290 [DOI] [PubMed] [Google Scholar]
- Dodge-Kafka KL, Bauman A, Mayer N, Henson E, Heredia L, Ahn J, McAvoy T, Nairn AC, Kapiloff MS. (2010) cAMP-stimulated protein phosphatase 2A activity associated with muscle A kinase-anchoring protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3. J Biol Chem 285:11078–11086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Armouche A, Wittköpper K, Degenhardt F, Weinberger F, Didié M, Melnychenko I, Grimm M, Peeck M, Zimmermann WH, Unsöld B, Hasenfuss G, Dobrev D, Eschenhagen T. W., Didie M, Meinychenko I, Grimm M, Peeck M, Zimmermann WH, Unsold B, Hasenfuss G, Dobrev D, Eschenhagen T. (2008) Phosphatase inhibitor-1-deficient mice are protected from catecholamine-induced arrhythmias and myocardial hypertrophy. Cardiovasc Res 80:396–406 [DOI] [PubMed] [Google Scholar]
- Fraser ID, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD. (1998) A novel lipid-anchored A-kinase anchoring protein facilitates cAMP-responsive membrane events. EMBO J 17:2261–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. (2002) Protein phosphatase 1 is a molecular constraint on learning and memory. Nature 418:970–975 [DOI] [PubMed] [Google Scholar]
- Huang FL, Glinsmann WH. (1976) Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. Eur J Biochem 70:419–426 [DOI] [PubMed] [Google Scholar]
- Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, Litzenberg J, Lorenz D, Wiesner B, Rosenthal W, Zaccolo M, Taskén K, Klussmann E. (2007) AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep 8:1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy IM, Shenolikar S. (2006) Protein serine/threonine phosphatases in neuronal plasticity and disorders of learning and memory. Trends Neurosci 29:679–686 [DOI] [PubMed] [Google Scholar]
- Mauban JR, O'Donnell M, Warrier S, Manni S, Bond M. (2009) AKAP-scaffolding proteins and regulation of cardiac physiology. Physiology 24:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton E, Macrae IM, McCabe C, Brown SM, White F. (2006) Identification of the growth arrest and DNA damage protein GADD34 in the normal human heart and demonstration of alterations in expression following myocardial ischaemia. Int J Cardiol 107:126–129 [DOI] [PubMed] [Google Scholar]
- Nguyen C, Nishi A, Kansy JW, Fernandez J, Hayashi K, Gillardon F, Hemmings HC, Jr, Nairn AC, Bibb JA. (2007) Regulation of protein phosphatase inhibitor-1 by cyclin-dependent kinase 5. J Biol Chem 282:16511–16520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou P, Hajjar RJ, Kranias EG. (2009a) Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J Mol Cell Cardiol 47:365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou P, Rodriguez P, Ren X, Zhou X, Qian J, Sadayappan S, Mitton B, Pathak A, Robbins J, Hajjar RJ, et al. (2009b) Inducible expression of active protein phosphatase-1 inhibitor-1 enhances basal cardiac function and protects against ischemia/reperfusion injury. Circ Res 104:1012–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak A, del Monte F, Zhao W, Schultz JE, Lorenz JN, Bodi I, Weiser D, Hahn H, Carr AN, Syed F, et al. (2005) Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase 1. Circ Res 96:756–766 [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Mitton B, Waggoner JR, Kranias EG. (2006) Identification of a novel phosphorylation site in protein phosphatase inhibitor-1 as a negative regulator of cardiac function. J Biol Chem 281:38599–38608 [DOI] [PubMed] [Google Scholar]
- Sahin B, Shu H, Fernandez J, El-Armouche A, Molkentin JD, Nairn AC, Bibb JA. (2006) Phosphorylation of protein phosphatase inhibitor-1 by protein kinase C. J Biol Chem 281:24322–24335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillace RV, Voltz JW, Sim AT, Shenolikar S, Scott JD. (2001) Multiple interactions within the AKAP220 signaling complex contribute to protein phosphatase 1 regulation. J Biol Chem 276:12128–12134 [DOI] [PubMed] [Google Scholar]
- Scott JD, Pawson T. (2009) Cell signaling in space and time: where proteins come together and when they're apart. Science 326:1220–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup DM, Shenolikar S. (2009) From promiscuity to precision: protein phosphatases get a makeover. Mol Cell 33:537–545 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.