Abstract
Verapamil is a prototypical phenylalkylamine (PAA), and it was the first calcium channel blocker to be used clinically. It tonically blocks L-type channels in the inner pore with micromolar affinity, and its affinity increases at depolarized membrane potentials. In T-type calcium channels, verapamil blocks with micromolar affinity and has modestly increased affinity at depolarized potentials. We found that a related PAA, 4-desmethoxyverapamil (D888), is comparable with verapamil both in affinity and in state-dependence. Permanently charged verapamil was more effective intracellularly than neutral verapamil. Charged PAAs were able to access their binding site from both inside and outside the cell. Furthermore, membrane-impermeant [2-(trimethylammonium)ethyl]methanethiosulfonate was able to access the inner pore from outside of the cell. We examined a homology model of the T-type calcium channel to look for possible routes of drug entry. Mutation of L1825W produced a channel that was blocked significantly more slowly by charged verapamil from the outside, with an increase in apparent affinity when the drug was applied from the inside. Data suggest that T-type channels have a back pathway through which charged drugs can access the inner pore of the channel without passing through the plasma membrane.
Introduction
T-type calcium channels (T-type channels) are a subfamily of voltage-gated calcium channels that are activated at relatively hyperpolarized membrane potentials (Perez-Reyes, 2003). The three isoforms of T-type calcium channels (CaV3.1, -3.2, and -3.3) are distributed throughout the body, with identified roles in the nervous system, the vasculature, and the developing heart. The most widely used T-type channel blocker is mibefradil, which was clinically used as an antihypertensive for a brief period before being withdrawn because of fatal interactions with other drugs. Mibefradil was initially believed to be selective for T-type channels over other ion channel targets, but more recent data suggest that its selectivity is limited, and its antihypertensive effects are largely absent in animals with a conditional knockout of an isoform of the L-type channel, in whom T-type channels seem to be unaffected (Cribbs, 2006; Moosmang et al., 2006). More specific T-type channel blockers have been developed (Uebele et al., 2009), but these drugs are not well characterized and are not yet in use clinically. Many classes of drugs modulate or block these channels (Lacinová, 2004). Among these are the phenylalkylamines (PAAs), the best known of which, verapamil, has long been used clinically. Because of its fast pharmacodynamics, verapamil is currently used in hospital settings to terminate cardiac arrhythmias (Kato et al., 2004). Furthermore, the development of a sustained-release formulation of verapamil has resulted in verapamil's recognition as a tool for combination treatment of hypertension, particularly in patients with diabetes (Reynolds et al., 2005).
Verapamil blocks both L-type and T-type channels with higher affinity for depolarized channels than for resting channels. This property, known as state-dependence, was initially described for local anesthetics blocking voltage-gated sodium channels (Hille, 1977). Conformational changes that occur upon opening and inactivation are believed to produce higher-affinity binding sites than those available in closed channels. In L-type channels, the affinity of verapamil for depolarized channels is on the order of 10-fold higher than for closed channels (Johnson et al., 1996). In T-type channels, the state-dependence is less dramatic, with less than 2-fold more block of depolarized channels than closed channels in the presence of 10 μM verapamil (Freeze et al., 2006).
D888, also known as devapamil or desmethoxyverapamil, is a related PAA lacking one methoxy group. It has affinity in the tens of nanomolar for resting state block of L-type channels (Johnson et al., 1996), and exhibits a 5-fold increase in affinity for depolarized channels. Determinants of D888 binding have been located in the inner pore of L-type channels (Hockerman et al., 1995, 1997a). Mutation of three residues near the selectivity filter of L-type channels resulted in a 100-fold reduction in the affinity of D888 (Hockerman et al., 1995). It should be noted, however, that these mutations did not affect the affinity of verapamil (Johnson et al., 1996).
PAAs are tertiary amines and, therefore, can exist as both neutral and positively charged molecules. Their ability to deprotonate to a neutral form at physiological pH is believed to be critical for their ability to reach a binding site in the inner pore of ion channels. Several studies have attempted to determine the route by which PAAs access their binding site in L-type channels (Mannhold et al., 1978; Hescheler et al., 1982; Leblanc and Hume, 1989; Wegener and Nawrath, 1995; Berjukov et al., 1996). Permanently charged derivatives of several PAAs, including verapamil and D888, have been used to determine whether the binding site is accessible from the intracellular or extracellular side, or from both sides. The preponderance of evidence in L-type channels suggests that PAAs bind in the inner pore. They are presumed to pass through the plasma membrane in their uncharged state to block the channel from inside (Hockerman et al., 1997b).
Detailed knowledge of drug binding sites and access routes is absent for any T-type channel blockers. Therefore, in this study, we sought to use the well studied interactions between PAAs and L-type channels to extend our understanding of the closely related T-type channel. We examined the relative affinity of verapamil and D888 both for tonic block and for use-dependent block. We also examined the sidedness of PAA binding to T-type channels using permanently charged PAAs to begin to determine where and how PAAs bind to T-type channels.
Materials and Methods
Heterologous Expression.
The cDNA CaV3.1 217, encoding one of the most prevalent splice variants of CaV3.1, was kindly provided by M. C. Emerick and W. S. Agnew (The Johns Hopkins University School of Medicine, Baltimore, MD). It was subcloned into three different vectors: pcDNA3.1/Zeocin, which was stably expressed in HEK 293 cells; pDNA5/FRT, which was stably expressed in HEK 293/FLP cells; and pcDNA5/FRT/TO, which was stably expressed in Flp-In T-REx-293 cells (Invitrogen, Carlsbad, CA). Stable cell lines were created using either 200 μg/ml Zeocin for HEK 293 cells, or 100 μg/ml HygroGold for HEK 293/FLP cells and T-REx-293 cells. Cells were maintained in 100-mm Corning culture dishes (Corning Life Sciences, Lowell, MA) in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 1% l-glutamine, and either 100 μg/ml Zeocin (for HEK 293 cells) or 50 μg/ml HygroGold (for HEK 293/FLP; Invivogen, San Diego, CA), or 15 μg/ml blasticidin, and 50 μg/ml HygroGold (for T-REx-293 cells). CaV3.1 expression in T-REx-293 cells was induced approximately 17 h before use in electrophysiology experiments with 0.05 to 0.2 μg/ml tetracycline, depending on the desired channel density. The three cell lines were used interchangeably, because the currents produced were indistinguishable. The only difference between the cell lines was the amount of channel expression. Reagents were obtained from Invitrogen unless otherwise indicated.
Site-Directed Mutagenesis.
Point mutations were generated using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer's protocols. Silent restriction enzyme sites were also introduced to allow for identification of positive clones via qualitative restriction enzyme mapping. DNA sequencing was performed to confirm mapping results.
Solutions and Chemicals.
The bath solution used for most ionic current experiments contained 140 mM NaCl, 2 mM CaCl2, and 10 mM HEPES, titrated to pH 7.4 with NaOH. For experiments with L1825W channels, the bath solution contained 135 mM NaCl, 5 mM CaCl2, and 10 mM HEPES, titrated to pH 7.4. For experiments with Q1828A cells and some experiments with Q1828C cells, the bath solution contained 132 mM NaCl, 10 mM CaCl2, and 10 mM HEPES, titrated to pH 7.4 with NaOH. The pipette solution contained 130 mM NaCl, 5 mM magnesium-ATP, 1 mM CaCl2, 11 mM EGTA, and 10 mM HEPES, and was titrated to pH 7.4 with NaOH. The bath solution for experiments with Q1828C in sodium contained 140 mM NaCl, 1 mM EGTA, and 10 mM HEPES, titrated to pH 7.4 with NaOH. Verapamil hydrochloride (Sigma-Aldrich, St. Louis, MO) and D888 (Abbott Laboratories, Abbott Park, IL) were prepared as stocks in dimethyl sulfoxide. All PAAs were diluted to the desired concentration in bath or pipette solution daily. MTSET (Toronto Research Chemicals Inc., Toronto, ON, Canada) was diluted into bath solution and used within 60 min.
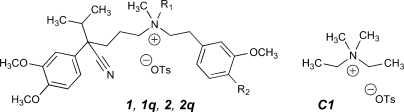
The synthesis of permanently charged PAAs (Fig. 1) is described in the Supplemental Data. To ensure that our results using permanently charged drugs were not due to contamination with neutral PAAs, we also tested quaternary drugs used previously in other studies. D575 was a kind gift of Abbott Laboratories, and qD888 was generously provided by Dr. Jörg Streissnig (University of Innsbruck, Innsbruck, Austria).
Fig. 1.
Channel-active agents studied. Shown are the following: verapamil, 1 (R1 = H, R2 = OCH3); N-methylverapamil, 1q (R1 = CH3, R2 = OCH3); devapamil, 2 (D888; R1 = R2 = H); N-methyldevapamil, 2q (R1 = CH3, R2 = H). OTs represents the p-toluenesulfonate counterion dosed with the synthetic compounds, which will exchange with buffer anions. C1 is a synthetic control substance, (diethyldimethyl)ammonium tosylate, which has in common with the active agents the quaternary ammonium center and the tosylate counterion.
Electrophysiological Recordings and Analysis.
For experiments with drugs in the bath solution, drugs were washed on during a train of pulses to −10 mV at a frequency of 0.2 Hz. Block was typically established over the course of 10 to 15 pulses. All recordings included in this study were made after the peak current elicited at −10 mV was stable for at least three pulses (see Supplemental Fig. 1 for example).
Recordings were made using an Axopatch 200B feedback amplifier (Molecular Devices, Sunnyvale, CA) with a Digidata 1321A digitizer and pClamp 8.1 data acquisition software (Molecular Devices). Patch pipettes were pulled with a P97 micropipette puller (Sutter Instruments, Novato, CA) from TW 150-4 borosilicate glass (World Precision Instruments, Sarasota, FL), and had resistances of 850 kΩ to 1.6 MΩ when filled with pipette solution. Recordings were made using whole-cell voltage clamp of trypsinized cells (0.25% Trypsin-EDTA; Sigma-Aldrich) 3 to 5 days after plating. Recordings were made at room temperature (20–26°C). Because the extent of state-dependent block was highly temperature-sensitive, a Sensortek TS-4 temperature controller was used to hold the bath temperature at 21 ± 1°C for most train protocols.
Data were filtered at 5 kHz by an eight-pole low-pass Bessel filter and digitized at 10 kHz. Currents were additionally filtered at 2 kHz offline. Currents were capacity-corrected using 8 to 16 subthreshold responses (voltage steps of 20 mV) and leak-corrected, based on linear leak resistance calculated at potentials negative to −80 mV or by linear interpolation between the current at the holding potential and 0 mV. The data were analyzed using locally written programs in MATLAB (The Math Works, Natick, MA) and Origin software (OriginLab Corp., Northampton, MA). Differences between parameters were assessed using Student's t test. Current-voltage relationships were fit using a modification of the Boltzmann equation:
where A1 is the amplitude, A2 the baseline, x the voltage, x0 the half-point of the relationship, and dx the slope factor in millivolts. A slope factor ≤−5 was considered to be evidence of adequate voltage control.
Results
The Affinity of Verapamil and D888 for Tonic Block of CaV3.1 Is Comparable.
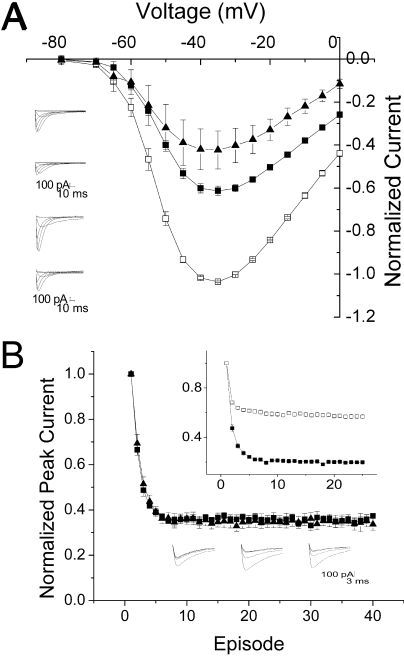
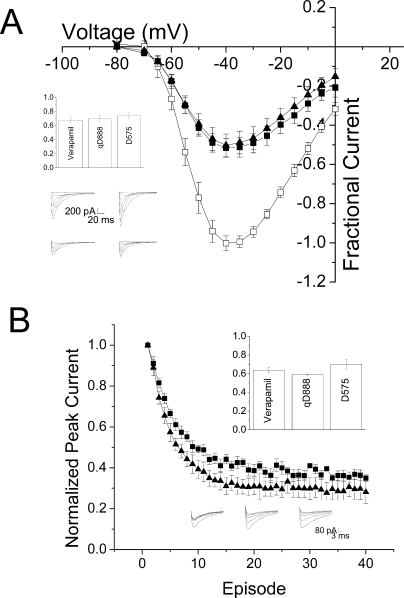
Verapamil produces tonic block (i.e., block at the resting potential), measured with infrequent depolarizations, in both T-type and L-type channels. In heterologous expression systems, the affinity of verapamil is similar, with an ED50 for L-type channels of ∼10 μM, versus an ED50 of ∼20 μM for T-type channels (Johnson et al., 1996; Freeze et al., 2006). D888 is a much higher-affinity ligand for L-type channels than verapamil, with an ED50 for closed channel block of ∼50 nM (Johnson et al., 1996). We examined the ability of verapamil and D888 to tonically block CaV3.1. Current-voltage relationships were measured using sequential depolarizations at 0.2 Hz, a frequency that allows channels to recover fully from inactivation between depolarizations. Using this protocol, the fraction of current blocked at −10 mV by 20 μM D888 was 59.6 ± 6%, comparable with 61.1 ± 0.8% for verapamil (Fig. 2A).
Fig. 2.
Block by verapamil and D888 is comparable. A, mean current-voltage relationship ± S.E.M. produced in the absence (□) and presence of 20 μM verapamil (■, n = 6) or D888 (▴, n = 3). Currents were normalized to peak current at −30 mV in the absence of drug. Inset, representative currents in the absence (top) and presence (bottom) of 20 μM verapamil (top) or D888 (bottom). B, mean peak current ± S.E.M. with the mean control current subtracted to separate the accumulation of inactivated channels from the effect of 20 μM verapamil (■, n = 4) or D888 (▴, n = 3). Bottom inset, representative currents produced by repetitive pulses from −110 to −10 mV in control (left) or 20 μM verapamil (middle) and D888 (right). Top inset, mean peak current ± S.E.M. elicited by repetitive pulses from −110 to −10 mV in control cells (□, n = 6) and presence of 20 μM verapamil (■, n = 4) or D888 (▴, n = 3). Currents are normalized to the first pulse in the train.
The Affinity of Verapamil and D888 for Use-Dependent Block Is also Similar.
PAAs have demonstrated substantially higher affinity for depolarized L-type channels, resulting in use-dependent block. In particular, D888 has 5-fold higher affinity for depolarized than for resting L-type channels (Johnson et al., 1996). In T-type channels, block by verapamil is modestly use-dependent (Freeze et al., 2006). We tested the use-dependence of D888 using a train of 25-ms depolarizing pulses to −10 mV with an interpulse interval of 100 ms. In control cells, accumulation of inactivated channels resulted in a 40% decrease in peak current over the first few pulses of the train, which stabilized as equilibrium was reached between channels entering and leaving the inactivated state (Fig. 2B, inset). The time course of this reduction was 0.8 ± 0.08 pulses. The addition of D888 or verapamil produced an additional reduction in peak current, representing the combination of inactivated and blocked channels. Use-dependent drug block was evaluated by subtracting the amplitude of control currents from those recorded in the presence of drug. D888 (20 μM) produced 63 ± 2% use-dependent block, compared with 59 ± 6% produced by the same concentration of verapamil. The time course of the subtracted peaks could be fit with a single exponential, yielding a τ of 1.3 ± 0.1 pulses, versus 1.5 ± 0.1 pulses for verapamil. Thus, use-dependent block by D888 could not be distinguished from verapamil either in extent or time course, and T-type channels, unlike L-type channels, are not sensitive to the absence of the second methoxy group on D888 (Fig. 1).
Verapamil Blocks Intracellularly at High Concentrations.
In the L-type channel, PAAs are believed to bind in the inner pore, and reach their binding site from the intracellular side (Hockerman et al., 1997b). If this is also the case for the T-type channel, then we would expect that verapamil included in the pipette would be able to reach its binding site and produce block of the T-type channel. One must, when carrying out this experiment, account for the diffusion of PAAs out of the pipette, into the cell, and across the cell membrane. For a charged drug, this might be expected to delay the appearance of block, but one would predict that complete block would eventually develop. For a tertiary or uncharged drug, the escape of the drug from the cell by diffusion across the bilayer would result in a lower concentration of drug near the channels and would be expected to produce a lower apparent affinity. Experiments with lidocaine, a tertiary drug for which binding determinants have been identified in the inner pore of voltage-gated sodium channels, indicate that approximately 10-fold more drug is needed in the pipette than in the bath to produce an equivalent effect for that drug and channel (Supplemental Fig. 2).
We, therefore, tested verapamil in the pipette at 10-fold greater than the ED50 for drug dissolved in the bath. Tonic block could not be evaluated in these experiments, because the drug has at least some access to the channels from the moment of break in. However, we could evaluate use-dependent block. At 200 μM, verapamil produced 18 ± 3% use-dependent block, much less than the 59 ± 6% produced by 20 μM verapamil applied extracellularly (Fig. 3). Successive trains run in the same cell did not demonstrate any further development of use-dependent block, even as long as 15 min after break in (Supplemental Fig. 3). Increasing the concentration of verapamil to 2 mM, 100-fold greater than the ED50 for drug dissolved in the bath, was required to achieve 50 ± 5% use-dependent block, which approached the level seen with 20 μM verapamil in the bath. At 200 μM verapamil, the time course of use-dependent block had a τ of 4.5 ± 0.4 pulses, significantly slower than for 20 μM extracellular verapamil (p < 0.01). At 2 mM, the concentration at which the extent of block resembled 20 μM extracellular verapamil, the time constant of block had a τ of 1.6 ± 0.5 pulses, similar to that for 20 μM extracellular verapamil. Although lidocaine and verapamil are both tertiary amines, verapamil is quite hydrophobic, with two aromatic rings and short aliphatic chains in the middle linker. These data, combined with the chemical differences between lidocaine and verapamil, strongly suggest that verapamil is able to escape across the membrane much more readily than lidocaine, resulting in a greater difference between apparent inside and outside affinity for verapamil than for lidocaine.
Fig. 3.
PAAs are effective intracellularly at high concentrations. Mean peak currents ± S.E.M. normalized to the first pulse of the train and with the mean control peaks subtracted. Verapamil (200 μM) (▴, n = 4) produced 20 ± 3% block by the 30th pulse, whereas 2 mM verapamil (■, n = 5) produced 69 ± 7% block. Inset, representative currents elicited by repetitive depolarizations from −110 to −10 mV with an interpulse interval of 100 ms in a control cell (left), a cell with 200 μM verapamil (middle), and a cell with 2 mM verapamil (right).
Permanently Charged Verapamil Blocks Intracellularly.
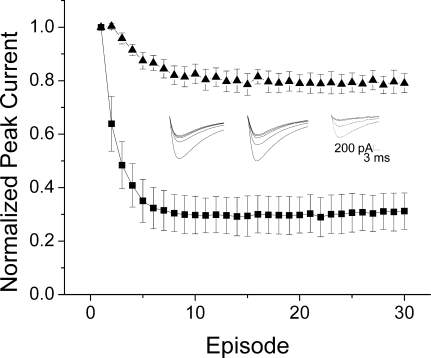
If the low affinity of verapamil when placed in the pipette is due to escape across the membrane, then we would expect a membrane-impermeant PAA to display higher apparent affinity than verapamil from the intracellular side, although perhaps with a slow onset. We assessed the effect of a quaternary, permanently charged analog of verapamil, D575, in the pipette. D575 (200 μM) in the pipette produced 63 ± 1% use-dependent block when stepped to −10 mV, significantly more than was produced by the same concentration of neutral verapamil (p < 0.001, Fig. 4). In addition, both the extent and the time course of block by D575 showed modest voltage dependence; depolarization to −30 mV produced 47 ± 1% block, whereas depolarization to +10 mV produced 75 ± 2% block. The voltage-dependence was most easily seen in the time course of use-dependent block, which became more rapid as the cells were depolarized farther (Fig. 4, inset). In contrast, neutral verapamil did not display voltage-dependence in these parameters (data not shown).
Fig. 4.
Permanently charged D575 produces block when included in the pipette. Mean peak currents ± S.E.M., normalized to the first pulse of the train and with mean control values subtracted. D575 (200 μM) produced 47 ± 1% block when stepped to −30 mV (■), 63 ± 1% block when stepped to −10 mV (□), and 75 ± 2% block when stepped to +10 mV ( ) (n = 8). Left inset, representative currents elicited by repetitive depolarizations from −110 to −10 mV with an interpulse interval of 100 ms in a cell recorded with 200 μM D575 included in the pipette. Right inset, τ values from exponential fits show voltage-dependence for D575 (■, n = 8).
) (n = 8). Left inset, representative currents elicited by repetitive depolarizations from −110 to −10 mV with an interpulse interval of 100 ms in a cell recorded with 200 μM D575 included in the pipette. Right inset, τ values from exponential fits show voltage-dependence for D575 (■, n = 8).
The ability of 200 μM D575 to produce significantly more block than 200 μM verapamil is consistent with the idea that neutral verapamil can escape from the cell more easily than the permanently charged D575. However, 10 times more D575 was still required on the inside to produce the level of block seen with 20 μM verapamil on the outside. This is unlikely to be a consequence of slow diffusion of the drug from the pipette. As was observed for the neutral verapamil, the amount of use-dependent block produced in successive trains did not increase over more than 15 min (Supplemental Fig. 3).
Charged PAAs also Block T-Type Channels from the Extracellular Surface.
D575, as well as other permanently charged PAAs, are believed to be unable to cross the plasma membrane. In the L-type channel, these drugs have been used to determine which side of the channel contains drug-binding sites. Although there are some technical differences between studies, overall, the data suggest that most permanently charged PAAs are effective against L-type channels when placed intracellularly but not extracellularly (Mannhold et al., 1978; Hescheler et al., 1982; Leblanc and Hume, 1989). The permanently charged quaternary analog of D888 (qD888) has been shown to block L-type channels from both inside and outside, but the characteristics of block differ depending on the route of application (Berjukov et al., 1996).
D575 and qD888 were applied to T-type channels from the bath. Surprisingly, both charged drugs produced robust block (Fig. 5). Tonic block and use-dependent block were both present. Most interestingly, block by the quaternary drugs was indistinguishable from block produced by neutral variants. The kinetics of extracellular block by charged drugs were 2- to 3-fold slower than was seen with neutral verapamil (4.4 ± 0.4 pulses for 20 μM D575 and 5.6 ± 1.3 pulses for 20 μM qD888 versus 1.5 ± 0.1 pulses for verapamil, p < 0.01). In addition, in contrast to the effects of intracellular application of the charged drugs, there was no evidence of voltage-dependence for use-dependent block with charged drugs in the bath over potentials ranging from −30 to +50 mV.
Fig. 5.
Charged PAAs block CaV3.1 from the outside. A, mean current-voltage relationship ± S.E.M. produced in the absence (□) and presence of 20 μM D575 (▴, n = 5) or 20 μM qD888 (■, n = 5). Currents were normalized to peak current at −30 mV in the absence of drug. Top inset, fractional block at −10 mV produced by 20 μM D575 or qD888 is not significantly different from 20 μM verapamil; n = 3 for verapamil. Bottom inset, representative currents in the absence (top) and presence (bottom) of D575 (left) and qD888 (right). B, both 20 μM D575 and 20 μM qD888 produced use-dependent block. Mean peak currents ± S.E.M., normalized to the first pulse of the train and with mean control values subtracted; n = 3 for D575, n = 4 for qD888. Top inset, fractional block at the 30th pulse is not significantly different from block produced by 20 μM verapamil. Bottom inset, representative currents in the absence (left) and presence of D575 (middle) and qD888 (right).
A Permanently Charged MTS Reagent Can also Enter T-Type Channels from the Bath.
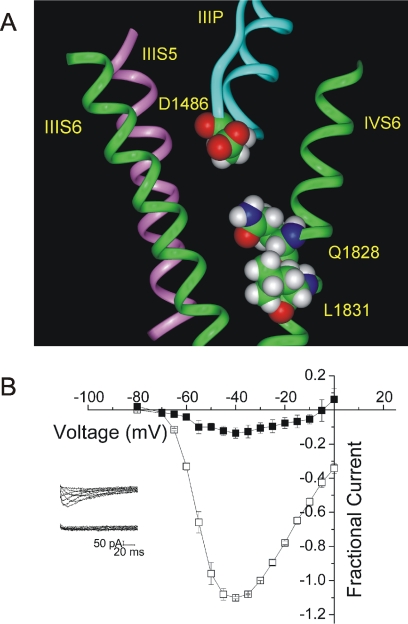
It is possible that the ability of charged PAAs to block T-type channels from the extracellular side is due to the presence of a second, extracellular binding site for PAAs. On the other hand, it is possible that the charged PAAs are able in some way to penetrate the channel and reach a binding site below the selectivity filter. To distinguish between these possibilities, we introduced cysteine residues at two positions in the inner pore of the channel, Gln1828 and Leu1831. When these residues are substituted into a homology model of the T-type channel based on the crystal structure of KcsA, they are predicted to face into the inner pore of the channel, below the selectivity filter (Fig. 6A) (Lipkind and Fozzard, 2003). We applied permanently charged MTSET, which is generally accepted not to cross the plasma membrane, to channels expressing each of these mutants. Application of MTSET to the outside of cells resulted in nearly complete block of inward current in both Q1828C channels (95 ± 3% block at −10 mV; Fig. 6B) and L1831C channels (86 ± 4% block at −20 mV) (Supplemental Fig. 4). Block by MTSET occurred within tens of seconds and was irreversible, whereas WT channels were not blocked over at least 7 min, suggesting that the effect of MTSET was not due to modification of an endogenous cysteine (data not shown). In addition, MTSET had no effect on channels with another small residue (alanine) substituted at this position, suggesting that changes at this residue did not distort the channel, exposing an endogenous cysteine (Q1828A; data not shown). In addition to blocking inward current, MTSET induced a shift in the apparent reversal potential when inward currents were carried by Ca2+, and outward currents were carried by Na+. This suggested that Ca2+ current was more completely blocked than Na+ current. To confirm this, we tested an MTS reagent in the absence of Ca2+. 2-aminoethyl methanethiosulfonate hydrochloride (200 μM) blocked inward Na+ currents by 36 ± 12%, significantly less than the 95% block of inward current when carried by Ca2+ (Supplemental Fig. 5). We interpret the sensitivity of MTS reagents to the species of permeant ion to suggest that the modification is in the permeation pathway. These data strongly support the idea that MTSET can modify the introduced cysteine residue in the inner pore of the T-type channel when applied extracellularly. Because MTSET would not be expected to pass either through the membrane or through the selectivity filter, we must suggest that a pathway exists for the entry of charged drugs into the inner pore of the T-type channel.
Fig. 6.
MTSET can access the inner pore of CaV3.1 from the bath. A, a view of half of the inner pore of the T-type channel (the interface between domain III-S6 and domain IV-S6). Two of the four S6 helices (domains III and IV) are shown as green ribbons. Domain III-S5 is shown as a pink ribbon, and the domain III P-loop is shown in blue. Gln1828 and Leu1831, shown as space-filling images, are predicted to project toward the reader and into the inner pore. B, mean current-voltage relationship ± S.E.M. produced in the absence (□) and presence (■) of 2 mM MTSET in Q1828C channels, with 2 mM Ca2+ in the bath. Currents were normalized to peak current at −30 mV in the absence of MTSET; n = 3. Inset, sample traces recorded in the absence (top) and presence (bottom) of 2 mM MTSET.
A Back Pathway for Drug Entry May Exist in T-Type Channels.
There is precedent for extracellular entry of charged drugs into ion channels. Quaternary derivatives of local anesthetics such as QX314 and QX222 (QX) can enter some, but not all, isoforms of the voltage-gated sodium channel from the extracellular side (Alpert et al., 1989; Qu et al., 1995). In particular, the cardiac sodium channel (NaV1.5) can be blocked by QX from the extracellular side. Mutational analysis has identified specific residues, predicted to be behind the selectivity filter, that are present in NaV1.5 but not in other sodium channels, which contribute to this accessibility (Qu et al., 1995; Sunami et al., 1997, 2000, 2001). In addition, in L-type channels, a permanently charged, quaternary form of amlodipine (a dihydropyridine), has been shown to enter L-type channels from the extracellular side (Heath et al., 1997).
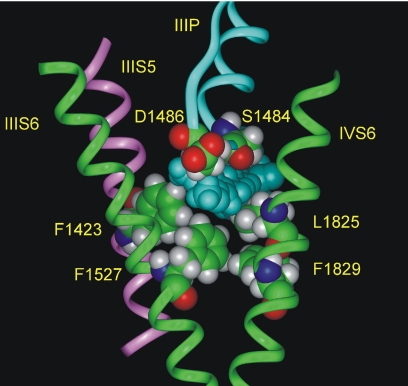
We examined a homology model of CaV3.1 to look for possible pathways by which charged drugs might enter CaV3.1 (Lipkind and Fozzard, 2003). The model suggests that a crevice may exist at the interface between S6 segments of two neighboring domains, behind the P-loop. Because PAAs, like dihydropyridines, predominantly interact with S6 segments of domains III and IV, we considered a possible pathway between the III-S6, III-S5, and IV-S6 segments (Fig. 7). This space is predicted to be large enough to accommodate verapamil. Because the selectivity filter in calcium channels is believed to sit close to the extracellular side of the membrane (Lipkind and Fozzard, 2001), this space would be close to the extracellular surface. It is possible that the charged PAAs might access the inner pore of the channel by passing through the thin, disordered region of membrane separating this crevice from the extracellular milieu.
Fig. 7.
An extracellular pathway for verapamil inside the inner pore of the T-type channel. Verapamil (shown in blue space-filling images) can be accommodated in the interface between domain III-S6, IV-S6 (shown by green ribbons), domain III-S5 (pink ribbon), and the domain III P-loop (blue ribbons). Some amino acid residues bordering this crevice are shown as space-filling images.
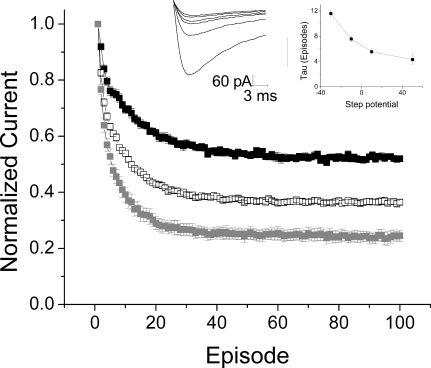
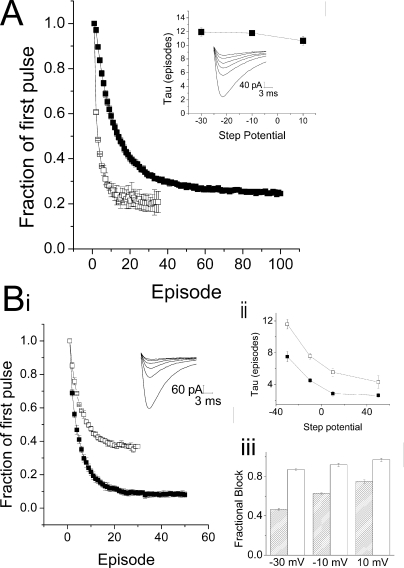
We sought to test this possibility by introducing bulky residues that would be predicted to protrude into the crevice and might interfere with the movement of drugs through this space. Substitution of an isoleucine for Ser1484 of the domain III P-loop, which would form the top of this cavity, had no effect on the ability of D575 to block these channels (20 μM; data not shown). However, substitution of a tryptophan, the amino acid with the largest indole side chain, for Leu1825 of domain IV-S6, which is hypothesized to form one of the side walls of the crevice, produced a channel in which the development of use-dependent block by 20 μM D575 was significantly slowed (Fig. 8A). The τ for onset of use-dependent block in L1825W channels from the outside was 11.4 ± 0.4 pulses versus 4.4 ± 0.4 pulses for WT channels (p < 0.001). In contrast, the time course of block of L1825W channels from the inside was significantly faster than the time course of block for WT channels for all voltages except for the most positive (p < 0.001). In further support of the idea that an access pathway was blocked by the tryptophan, application of D575 to L1825W channels from the inside produced more block than the same concentration in the WT, both at 200 and at 20 μM (Supplemental Fig. 6). This suggests that D575 may escape from WT channels, even though it cannot cross the membrane, by moving through the extracellular access pathway, thereby accounting for the less than complete block observed. Partially occluding this pathway by introducing a tryptophan restricts the movement of drug through the channel, resulting in increased apparent affinity for D575 when applied the inside. The Van der Waals volumes of leucine and tryptophan are 124 and 163 Å3, respectively (Creighton, 1993). Therefore, the tryptophan side chain may restrict the putative pathway for verapamil by approximately 40 Å3. The restriction introduced by the tryptophan also introduces an additional kinetic barrier to the entry of D575 from the extracellular side of the channel to the inner pore. The ability of alteration of one residue to affect both intracellular and extracellular block by D575 supports the interpretation that a pathway, rather than a binding site, is being perturbed.
Fig. 8.
Insertion of a tryptophan in the back pathway reduces drug entry and exit. A, outside, mean peak currents ± S.E.M., recorded with 20 μM D575 in the bath, in WT (□, n = 3) and L1825W channels (■, n = 6). Currents were normalized to the first pulse of the train and mean control values were subtracted. Insets, representative currents elicited by repetitive depolarizations from −110 to −10 mV with an interpulse interval of 100 ms in a cell expressing L1825W, recorded with 20 μM D575 in the bath. τ Values from exponential fits show no change with voltage. B, inside: i, mean peak currents ± S.E.M., recorded with 200 μM D575 included in the pipette, in WT (□, n = 3) and L1825W channels (■, n = 4). Currents were normalized to the first pulse of the train, and mean control values were subtracted. Inset, representative currents elicited by repetitive depolarizations from −110 to −10 mV with an interpulse interval of 100 ms in a cell expressing L1825W, recorded with 200 μM D575 in the pipette. ii, τ values from exponential fits show voltage-dependence for D575 in both WT (□) and L1825W channels (■). iii, comparing the extent of block obtained with 200 μM D575 in WT ( ) and L1825W channels (□) shows some voltage-dependence in each case, with much greater block obtained in L1825W channels at the same drug concentration.
) and L1825W channels (□) shows some voltage-dependence in each case, with much greater block obtained in L1825W channels at the same drug concentration.
Discussion
There is extensive literature regarding the sidedness of PAA block of L-type channels. Mannhold et al. (1978), established the idea that verapamil binds from the intracellular face of the channel by demonstrating, as part of a large structure-function study, that a permanently charged derivative of verapamil (referred to as H1 in their study) was not effective in preventing cardiac muscle contraction when applied in the bath (Mannhold et al., 1978). Thereafter, the PAA D600 was shown to be effective from both sides, whereas a permanently charged version, D890, was effective only when injected inside cardiac myocytes (Hescheler et al., 1982). Permanently charged D888 is unusual among PAAs, because it seems to block L-type channels from either side of the membrane (Berjukov et al., 1996). However, the effects of permanently charged D888 are not symmetrical—block from the inside is predominantly use-dependent, whereas block from the outside is primarily tonic, leading these investigators to suggest that L-type channels may have separate inside and outside binding sites. In general, most studies suggested that PAAs accessed their binding site from inside the cell.
Neutral PAAs, which can pass through the membrane, should be effective regardless of whether they are placed in the pipette or in the bath. However, block of L-type channels by neutral PAAs included in the pipette was not always observed (Leblanc and Hume, 1989). Neutral PAAs have been problematic in other channels as well. In KV1.3, for example, intracellular verapamil was thought to be ineffective, until it was tested in the inside-out patch configuration (Rauer and Grissmer, 1996). Concentrations of drug that have no effect in whole-cell patch clamp potently block potassium channels in this configuration, whereas permanently charged PAAs are active in both configurations. This strongly supports the idea that the physical properties of verapamil and its analogs allow them to diffuse so readily out of cells into the bath that the concentration at the channel is too low for block to be observed. This may explain why Hescheler et al. (1982) were able to see block with neutral D600, because they used a pressure injection rather than passive diffusion. In our hands, very large concentrations (two orders of magnitude higher than the ED50 for extracellular block) were required to achieve robust inhibition with neutral drug from the inside.
The major finding of this study of T-type channels is that PAAs produce block when applied either intracellularly or extracellularly. Both permanently charged verapamil and permanently charged D888 produced block from the extracellular side, with potencies indistinguishable from their neutral counterparts. When a large amino acid side chain was introduced into a potential extracellular access pathway, use-dependent block by charged drugs was significantly slowed, and block by intracellularly applied permanently charged drugs increased.
Because permanently charged drugs are unable to move freely across the membrane, a given concentration of charged drug would be expected to be equally effective from either side. This was not the case in T-type channels—permanently charged drugs were more effective when on the extracellular side. The passage of time did not increase the amount of use-dependent block or accelerate the time course, as one might expect if diffusion out of the pipette were the limiting factor (Supplemental Fig. 3). The ability of permanently charged drugs to block from the outside, as well as the difference in efficacy between charged drugs placed on the inside and the outside of the cell, is consistent with the existence of two routes for drug access to the binding site with differing kinetic characteristics. When charged drugs are placed on the outside, they move into the channel through the extracellular access pathway but can then exit either back out of the cell or through the pore to the inside of the cell. Charged drugs on the inside enter through the pore, and have the same two possible exit pathways. The greater effectiveness of charged drugs from the outside of the cell could indicate that the on-rate of drugs moving from the outside is faster than from the inside. On the other hand, a diffusional gradient between the outside of the cell and the binding site could slow exit and might allow for greater rebinding when drugs are present in the bath.
Our examination of the L1825W mutant provides support for the existence of an extracellular access pathway. Development of block by charged drugs on the outside was significantly slowed in the mutant, consistent with reduced movement of the drugs through the extracellular access pathway. Furthermore, low concentrations of charged drugs were more effective inside cells expressing L1825W channels than in cells expressing WT channels. This suggests that reducing the ability of drugs to move away from the binding site through the extracellular access pathway reduces the combined off-rate for drug binding. It is noteworthy that access of the permanently charged drug to its binding site is impeded, but not eliminated, by this single amino acid exchange. This may indicate that the extracellular access pathway in the T-type channel is affected by more amino acid residues than the pathway that exists for movement of QX314 through the cardiac sodium channel, which can be completely blocked (or completely recapitulated) by the substitution of a single amino acid (Qu et al., 1995).
The differing efficacy of charged drugs placed inside and outside the cell could also indicate the presence of two different binding sites with differing affinities for the drugs. However, support for the presence of an extracellular access pathway is provided by the ability of MTSET to rapidly and irreversibly block currents in two different mutant channels with a cysteine introduced into the inner pore of the channel. MTSET had no effect on WT channels or on channels with an alanine substitution instead of the cysteine substitution. This argues against modification of an endogenous cysteine revealed in the WT channel or through a distortion in the channel introduced by the mutation. Furthermore, the block created by MTS modification was sensitive to the species of permeant ion; Ca2+ current was blocked to a much higher extent than Na+ current, indicating that the residue modified was in the permeation pathway. Taken together, these data support the interpretation that MTSET is able to reach cysteines introduced into the inner pore of the T-type channel from the bath, just as permanently charged drugs can reach their binding site.
It is noteworthy that the time course of the development of use dependent block was voltage dependent when charged drugs were placed on the inside. This was not observed with neutral verapamil or when either neutral or charged drugs were placed in the bath. Movement of charged drugs into the channel was facilitated by increasing depolarization, likely because of interactions between the drug and the electric field. Charged drugs on the outside were not voltage dependent over a range of potentials from −30 mV to + 50 mV. This might indicate that when the charged drug travels through the extracellular access pathway to reach its binding site, the substantially lower dielectric of the hydrophobic pathway in contrast to the hydrophilic environment of the direct pathway through the inner pore might shield the drug from alterations in the field that result from channel depolarization.
Several models of PAA binding to L-type channels exist (Lipkind and Fozzard, 2003; Cheng et al., 2009). Whereas the models differ with respect to details of the conformation of PAAs in the pore, they agree in many respects. Both models depend, in part, on residues involved in the high-affinity D888 binding site present in L-type channels. However, a high-affinity site for D888 does not exist in T-type channels. Block by D888 is comparable with block by verapamil in extent, in state-dependence, and even in the time course for onset of use-dependent block. It is noteworthy that both models place part of PAAs in the domain III–IV interface, with the remainder of the molecule in the inner pore. Using a homology model (Lipkind and Fozzard, 2003) to predict the location of an extracellular access pathway that would reach the PAA binding site is, therefore, reasonable, without regard to the specifics of the modeled drug-channel interaction. We have used a model to predict the location of a pathway to the domain III–IV interface. Mutation of Leu1825, which is postulated to form the back wall of the pathway, to a tryptophan, with its voluminous side chain, rendered charged drugs less effective from the outside. Not only was the extent of use-dependent block reduced, but the time course of block from the outside was three times slower from the outside than the inside, in contrast to WT channels, in which the time course of block did not differ. These data suggest that a novel pathway for drug access may exist in T-type channels.
Supplementary Material
Acknowledgments
We thank Connie Mlecko, Dr. Jack Kyle, and Dr. Elena Nikitina for technical assistance.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants R01-HL65680, T32-HL007381].
Some of these data were presented in abstract form: Bergson P, Lipkind G, and Hanck D (2009) Verapamil block of T-type calcium channels. Soc Neurosci Abstr 35:518.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.069492.
ABBREVIATIONS:
- CaV3.1
- the T-type voltage-gated calcium channel isoform
- PAA
- phenylalkylamine
- MTSET
- [2-(trimethylammonium)ethyl] methanethiosulfonate
- WT
- wild type
- HEK
- human embryonic kidney
- D888
- 4-desmethoxyverapamil
- MTS
- methanethiosulfonate
- QX314
- 2-((2,6-dimethylphenyl)amino)-N,N,N-triethyl-2-oxoethanaminium
- QX222
- 2-((2,6-dimethylphenyl)amino)-N,N,N-trimethyl-2-oxoethanaminium.
Authorship Contributions
Participated in research design: Bergson, Lipkind, Duban, and Hanck.
Conducted experiments: Bergson, Lipkind, and Lee.
Contributed new reagents or analytic tools: Lee and Duban.
Performed data analysis: Bergson and Hanck.
Wrote or contributed to the writing of the manuscript: Bergson, Lipkind, Duban, and Hanck.
Other: Hanck acquired funding for this research.
References
- Alpert LA, Fozzard HA, Hanck DA, Makielski JC. (1989) Is there a second external lidocaine binding site on mammalian cardiac cells? Am J Physiol 257:H79–H84 [DOI] [PubMed] [Google Scholar]
- Berjukov S, Aczel S, Beyer B, Kimball SD, Dichtl M, Hering S, Striessnig J. (1996) Extra- and intracellular action of quaternary devapamil on muscle L-type Ca2+-channels. Br J Pharmacol 119:1197–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton TE. (1993) Proteins: Structure and Molecular Properties, WH Freeman and Co., New York [Google Scholar]
- Cribbs LL. (2006) T-type Ca2+ channels in vascular smooth muscle: multiple functions. Cell Calcium 40:221–230 [DOI] [PubMed] [Google Scholar]
- Cheng RC, Tikhonov DB, Zhorov BS. (2009) Structural model for phenylalkylamine binding to L-type calcium channels. J Biol Chem 284:28332–28342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze BS, McNulty MM, Hanck DA. (2006) State-dependent verapamil block of the cloned human Ca(v)3.1 T-type Ca2+ channel. Mol Pharmacol 70:718–726 [DOI] [PubMed] [Google Scholar]
- Heath B, Xia J, Kass RS. (1997) Molecular pharmacology of UK-118, 434–05, a permanently charged amlodipine analog. Int J Cardiol 62:S47–S54 [DOI] [PubMed] [Google Scholar]
- Hescheler J, Pelzer D, Trube G, Trautwein W. (1982) Does the organic calcium channel blocker D600 act from inside or outside on the cardiac cell membrane? Pflugers Arch 393:287–291 [DOI] [PubMed] [Google Scholar]
- Hille B. (1977) Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol 69:497–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockerman GH, Johnson BD, Abbott MR, Scheuer T, Catterall WA. (1997a) Molecular determinants of high affinity phenylalkylamine block of L-type calcium channels in transmembrane segment IIIS6 and the pore region of the alpha1 subunit. J Biol Chem 272:18759–18765 [DOI] [PubMed] [Google Scholar]
- Hockerman GH, Johnson BD, Scheuer T, Catterall WA. (1995) Molecular determinants of high affinity phenylalkylamine block of L-type calcium channels. J Biol Chem 270:22119–22122 [DOI] [PubMed] [Google Scholar]
- Hockerman GH, Peterson BZ, Johnson BD, Catterall WA. (1997b) Molecular determinants of drug binding and action on L-type calcium channels. Annu Rev Pharmacol Toxicol 37:361–396 [DOI] [PubMed] [Google Scholar]
- Johnson BD, Hockerman GH, Scheuer T, Catterall WA. (1996) Distinct effects of mutations in transmembrane segment IVS6 on block of L-type calcium channels by structurally similar phenylalkylamines. Mol Pharmacol 50:1388–1400 [PubMed] [Google Scholar]
- Kato M, Dote K, Sasaki S, Takemoto H, Habara S, Hasegawa D. (2004) Intracoronary verapamil rapidly terminates reperfusion tachyarrhythmias in acute myocardial infarction. Chest 126:702–708 [DOI] [PubMed] [Google Scholar]
- Lacinová L. (2004) Pharmacology of recombinant low-voltage activated calcium channels. Curr Drug Targets CNS Neurol Disord 3:105–111 [DOI] [PubMed] [Google Scholar]
- Leblanc N, Hume JR. (1989) D 600 block of L-type Ca2+ channel in vascular smooth muscle cells: comparison with permanently charged derivative, D 890. Am J Physiol 257:C689–C695 [DOI] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. (2001) Modeling of the outer vestibule and selectivity filter of the L-type Ca2+ channel. Biochemistry 40:6786–6794 [DOI] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. (2003) Molecular modeling of interactions of dihydropyridines and phenylalkylamines with the inner pore of the L-type Ca2+ channel. Mol Pharmacol 63:499–511 [DOI] [PubMed] [Google Scholar]
- Mannhold R, Steiner R, Haas W, Kaufmann R. (1978) Investigations on the structure-activity relationships of verapamil. Naunyn Schmiedebergs Arch Pharmacol 302:217–226 [DOI] [PubMed] [Google Scholar]
- Moosmang S, Haider N, Brüderl B, Welling A, Hofmann F. (2006) Antihypertensive effects of the putative T-type calcium channel antagonist mibefradil are mediated by the L-type calcium channel Cav1.2. Circ Res 98:105–110 [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. (2003) Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 83:117–161 [DOI] [PubMed] [Google Scholar]
- Qu Y, Rogers J, Tanada T, Scheuer T, Catterall WA. (1995) Molecular determinants of drug access to the receptor site for antiarrhythmic drugs in the cardiac Na+ channel. Proc Natl Acad Sci USA 92:11839–11843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauer H, Grissmer S. (1996) Evidence for an internal phenylalkylamine action on the voltage-gated potassium channel Kv1.3. Mol Pharmacol 50:1625–1634 [PubMed] [Google Scholar]
- Reynolds NA, Wagstaff AJ, Keam SJ. (2005) Trandolapril/verapamil sustained release: a review of its use in the treatment of essential hypertension. Drugs 65:1893–1914 [DOI] [PubMed] [Google Scholar]
- Sunami A, Dudley SC, Jr, Fozzard HA. (1997) Sodium channel selectivity filter regulates antiarrhythmic drug binding. Proc Natl Acad Sci USA 94:14126–14131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunami A, Glaaser IW, Fozzard HA. (2000) A critical residue for isoform difference in tetrodotoxin affinity is a molecular determinant of the external access path for local anesthetics in the cardiac sodium channel. Proc Natl Acad Sci USA 97:2326–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunami A, Glaaser IW, Fozzard HA. (2001) Structural and gating changes of the sodium channel induced by mutation of a residue in the upper third of IVS6, creating an external access path for local anesthetics. Mol Pharmacol 59:684–691 [DOI] [PubMed] [Google Scholar]
- Uebele VN, Nuss CE, Fox SV, Garson SL, Cristescu R, Doran SM, Kraus RL, Santarelli VP, Li Y, Barrow JC, et al. (2009) Positive allosteric interaction of structurally diverse T-type calcium channel antagonists. Cell Biochem Biophys 55:81–93 [DOI] [PubMed] [Google Scholar]
- Wegener JW, Nawrath H. (1995) Extracellular site of action of phenylalkylamines on L-type calcium current in rat ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol 352:322–330 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.