Abstract
Human organic anion transporter 1 (hOAT1) plays a critical role in the body disposition of environmental toxins and clinically important drugs, including anti-HIV therapeutics, antitumor drugs, antibiotics, antihypertensives, and anti-inflammatories. We have demonstrated previously that hOAT1 forms homo-oligomers in cultured cells and in rat kidney. However, the functional consequence of such oligomerization has never been elucidated. In the current study, we used a novel approach by examining the effects of short hydrophobic peptides corresponding to transmembrane domains (TMDs) 1 to 12 of hOAT1 on the oligomerization and function of the transporter. We constructed expression vectors encoding short fusion peptides corresponding to TMDs 1 to 12 of hOAT1. These peptides were transfected into hOAT1-expressing COS-7 cells. Our results showed that among all 12 peptides examined, only the peptide corresponding to TMD 6 of hOAT1 significantly disrupted hOAT1 oligomerization demonstrated by cross-linking and coimmunoprecipitation experiments. The same peptide also caused a reduced expression of hOAT1 at the cell surface. As a result, hOAT1-mediated transport activity was compromised. Our data suggest that the peptide corresponding to TMD 6 of hOAT1 is a potent inhibitor of hOAT1 oligomerization and that oligomerization of hOAT1 is critical for the expression of the transporter at the cell surface and consequently for the proper function of the transporter.
Introduction
Organic anion transporter 1 (OAT1) is the prototypic member of a family of organic anion transporters responsible for the body disposition of clinically important anionic drugs including anti-HIV therapeutics, antitumor drugs, antibiotics, antihypertensives, and anti-inflammatories (You, 2002; Dantzler and Wright, 2003; Srimaroeng et al., 2008; Ahn and Nigam, 2009; VanWert et al., 2010).
Ten OAT isoforms (OAT1–10) have been cloned, and their expressions have been identified in distinct tissues and cell membranes (Lopez-Nieto et al., 1997; Sekine et al., 1997, 1998; Sweet et al., 1997; Wolff et al., 1997; Cihlar et al., 1999; Kusuhara et al., 1999; Lu et al., 1999; Cha et al., 2000; Enomoto et al., 2002; Jutabha et al., 2003; Ekaratanawong et al., 2004; Monte et al., 2004; Youngblood and Sweet, 2004; Shin et al., 2007; Bahn et al., 2008). In the kidney, OAT1 and OAT3 use a tertiary transport mechanism to move organic anions across the basolateral membrane into the proximal tubule cells for subsequent exit across the apical membrane into the urine for elimination. Through this tertiary transport mechanism, Na+/K+-ATPase maintains an inwardly directed (blood-to-cell) Na+ gradient. The Na+ gradient then drives a sodium dicarboxylate cotransporter, sustaining an outwardly directed dicarboxylate gradient that is used by a dicarboxylate/organic anion exchanger, namely OAT, to move the organic anion substrate into the cell. This cascade of events indirectly links organic anion transport to metabolic energy and the Na+ gradient, allowing the entry of a negatively charged substrate against both its chemical concentration gradient and the electrical potential of the cell.
All of the cloned OATs share several common structural features, including 12 TMDs; multiple glycosylation sites localized in the first extracellular loop between transmembrane domains 1 and 2; and multiple potential phosphorylation sites present in the intracellular loop between transmembrane domains 6 and 7 and in the carboxyl terminus.
We have demonstrated previously that human OAT1 (hOAT1) exists in the plasma membrane of cultured cells as a homo-oligomer (Hong et al., 2005), possibly dimer and trimer. In the current study, we investigated functional consequence of such oligomerization. TMDs have been demonstrated to play important roles in the oligomerization of many membrane proteins. As a result, the short hydrophobic peptides corresponding to these TMDs have been shown to act as specific inhibitors for the oligomerization of these proteins (Bennasroune et al., 2004, 2005). Therefore, in the current study, we designed short hydrophobic peptides corresponding to TMDs 1 to 12 of hOAT1 and examined the effects of these peptides on hOAT1 oligomerization and function.
Materials and Methods
[3H]p-Aminohippuric acid (PAH) was from PerkinElmer Life and Analytical Sciences (Waltham, MA). Membrane-impermeable biotinylation reagent NHS-SS-biotin [succinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate], cross-linking reagent BS3, and streptavidin-agarose beads were purchased from Pierce Chemical (Rockford, IL). Protein A-agarose beads were purchased from Invitrogen (Carlsbad, CA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Construction of Expression Vectors for Short TMs.
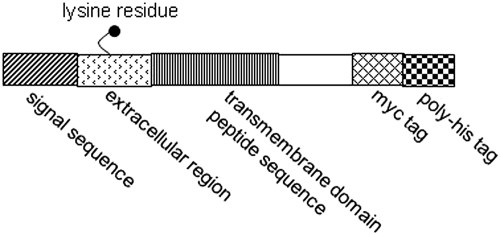
pSecTag 2B vector (Invitrogen) is a mammalian expression vector, which contains a cytomegalovirus promoter for high-level constitutive expression and a T7 priming site followed by a murine Ig k leader sequence, a multiple cloning site, and two tag sequences [myc and (poly)histidine]. The minigenes encoding the short TMD sequence peptides of hOAT1 were constructed by ligation of synthetic oligonucleotides by using the EcoRV and HindIII restriction sites. The final sequence comprised the signal peptide and an adjacent extracellular sequence joined to a sequence incorporating the entire putative TMD and C-terminal intracellular tag sequences (Myc and polyHis) (Fig. 1). Plasmids were checked by sequencing using the dideoxy chain termination method.
Fig. 1.
Schematic diagram of minigenes used to encode the TMD sequences. Each box represents a section of the constructions made within the Invitrogen pSecTag 2B plasmid.
Cell Culture.
Parental COS-7 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin/streptomycin (100 U/ml), and glucose (100 mg/ml) in a 5% CO2 atmosphere at 37°C. COS-7 cells stably expressing hOAT1-myc (Hong et al., 2005) were maintained in the same medium containing 0.2 mg/ml G418 (Geneticin; Invitrogen). The myc epitope was tagged to the carboxyl terminus of hOAT1 to facilitate the immunodetection of hOAT1.
Transfection of Plasmids.
In brief, ∼106 COS-7 cells were plated in six wells. Individual TMD plasmid (4 μg) was transfected into the cells using Lipofectamine 2000 (Invitrogen) as suggested by the manufacturer. Twenty-four hours after transfection, cells were ready for further experiments.
Transport Measurement.
Cells were plated in 48-well plates. For each well, uptake solution was added. The uptake solution consisted of phosphate-buffered saline/Ca2+/Mg2+ (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, 1 mM CaCl2, and 1 mM MgCl2, pH 7.4) and [3H]PAH. At the times indicated, uptake was stopped by aspirating off the uptake solution, and the wells were rapidly washed with ice-cold PBS. The cells were then solubilized in 0.2 N NaOH, neutralized in 0.2 N HCl, and separated into aliquots for liquid scintillation counting. The uptake count was standardized by the amount of protein in each well. Values are means ± S.E. (n = 3).
Cell Surface Biotinylation.
Cell surface expression levels of hOAT1 were examined using the membrane-impermeant biotinylation reagent Sulfo-NHS-SS-biotin. The cells were seeded onto six-well plates at 8 × 105 cells/well. After 24 h, the medium was removed, and the cells were washed twice with 3 ml of ice-cold PBS, pH 8.0. The plates were kept on ice, and all solutions were kept ice-cold for the rest of the procedure. Each well of cells was incubated with 1 ml of Sulfo-NHS-SS-biotin (0.5 mg/ml in PBS) in two successive 20-min incubations on ice with very gentle shaking. The reagent was freshly prepared for incubation. After biotinylation, each well was briefly rinsed with 3 ml of PBS containing 100 mM glycine and then incubated with the same solution for 20 min on ice to ensure complete quenching of the unreacted sulfo-NHS-SS-biotin. The cells were then dissolved on ice for 1 h in 400 μl of lysis buffer (10 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100, and 1/100 protease inhibitor mixture, pH 7.4). The unlysed cells were removed by centrifugation at 13,000 rpm at 4°C. Streptavidin-agarose beads were then added to the supernatant to isolate cell membrane protein. hOAT1 was detected in the pool of surface proteins by electrophoresis and immunoblotting using an anti-myc antibody (1:500; Mount Sinai Medical Center, New York, NY).
Purification of Crude Membrane Proteins.
COS-7 cells were homogenized in isolation buffer (250 mM sucrose, 10 mM triethanolamine, with 1/100 protease inhibitor mixture) and centrifuged at 1000g for 10 min at 4°C. The supernatant was then centrifuged at 17,000g for 20 min at 4°C. The pellet was resuspended in isolation buffer. Protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA). The protein solutions were stored at −80°C before further use.
Chemical Cross-Linking.
Membrane proteins were diluted to 1 mg/ml with cross-linking buffer (150 mM NaCl, 100 mM HEPES, 5 mM dithiothreitol, 5 mM EDTA, with 1/100 protease inhibitor mixture) and then incubated with 1 to 5 mM cross-linking reagent BS3 with end-over-end mixing for 30 min at room temperature. The cross-linking reaction was then stopped by incubation with 100 mM Tris/HCl, pH 7.5, for 15 min at room temperature. Appropriate volume (∼50 μl) of Laemmli buffer was added to denature the protein at 50°C for 30 min, followed by electrophoresis and immunoblotting.
Coimmunoprecipitation.
hOAT1-Flag was transfected into COS-7 cells stably expressing hOAT1-myc. The cells were lysed in immunoprecipitation buffer (10 mM Tris-HCl, pH 7.5, 10 mM NaCl, 0.5∼1% Triton X-100, 2 mM EDTA, 10% glycerol, and 1/100 protease inhibitor mixture). Cell lysates were precleaned with protein A-agarose beads to reduce nonspecific binding. The precleaned cell lysates were incubated with anti-Flag antibody (1:100) for 1.5 h at room temperature. Protein A-agarose beads were then added and mixed with end-over-end rotating at 4°C overnight. Proteins bound to the protein A-agarose beads were eluted with Laemmli buffer containing β-mercapto-ethanol and analyzed by immunoblotting with horseradish peroxidase-conjugated anti-myc antibody (1:500; Sigma-Aldrich).
Electrophoresis and Immunoblotting.
Protein samples (100 μg) were resolved on 7.5% SDS-polyacrylamide gel electrophoresis minigels and electroblotted onto polyvinylidene difluoride membranes. The blots were blocked for 1 h with 5% nonfat dry milk in PBS-0.05% Tween 20, washed, and incubated overnight at 4°C with primary antibody. The membranes were washed and then incubated with appropriate secondary antibody conjugated to horseradish peroxidase (1:5000), and signals were detected using a SuperSignal West Dura extended duration substrate kit (Pierce Chemical).
Data Analysis.
Statistical analysis was conducted using Student's paired t test for comparing two treatments. A one-way analysis of variance followed by a Dunnett's post hoc test was used for comparing among more than two treatments. A p value <0.05 was considered significant.
Results
Expression of Short Hydrophobic Peptides.
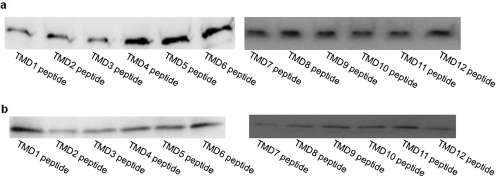
To test the potentially inhibitory effects of short hydrophobic peptides on oligomerization and function of hOAT1, we took advantage of the characteristics of the pSecTag 2B expression plasmid. In this plasmid, we constructed minigenes comprising a sequence signal at the N terminus, which should direct the protein to the cell surface, a short extracellular fragment, the sequences corresponding to TMDs 1 to 12 of hOAT1, a basic short stop-sequence to anchor the peptide in the proper orientation, and two C-terminal intracellular tags (myc and polyHis) (Fig. 1). The plasmids containing these minigenes were transfected into COS-7 cells separately. Transfected cells were lysed, followed by immunoblotting with anti-myc antibody. As shown in Fig. 2a, these short hydrophobic peptides were efficiently expressed in COS-7 cells.
Fig. 2.
Expression of short peptides. a, total expression of short peptides. plasmids (4 μg) encoding short peptides corresponding to TMDs 1–12 of hOAT1 were transfected into COS-7 cells as described under Materials and Methods. Twenty-four hours after transfection, cells were lysed followed by immunoblotting with anti-myc antibody (1:500). b, cell surface expression of short peptides. 4 μg of plasmids encoding peptides corresponding to TMDs 1 to 12 of hOAT1 were transfected into COS-7 cells. Twenty-four hours after transfection, surface expression of these peptides was analyzed by cell surface biotinylation approach as described under Materials and Methods.
Surface Expression of Short Hydrophobic Peptides.
To determine whether these short hydrophobic peptides can be inserted into plasma membrane, we took advantage of a single lysine residue in the short extracellular fragment of the minigene, which can be labeled by cell-impermeable biotinylation reagent NHS-SS-biotin and therefore allows the determination of surface expression of these peptides. The plasmids containing these minigenes were transfected into COS-7 cells separately. Transfected cells were biotinylated with NHS-SS-biotin, followed by immunoblotting with anti-myc antibody. As shown in Fig. 2b, these peptides were indeed expressed at the cell membrane.
Effects of Short Hydrophobic Peptides on hOAT1 Oligomerization.
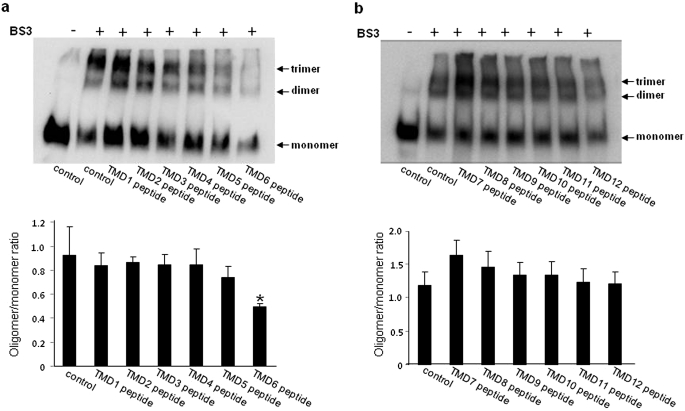
We demonstrated previously (Hong et al., 2005) that chemical cross-linking of intact membrane proteins from hOAT1-expressing cells converted quantitatively hOAT1 monomer to putative dimer and trimer, indicating that hOAT1 is present in the membrane as multimeric complexes. To determine the effects of these short hydrophobic peptides on hOAT1 oligomerization, cross-linking experiments were performed with membrane proteins isolated from hOAT1-myc-expressing cells transfected with or without these peptides. A shown in Fig. 3, the degree of hOAT1 oligomerization (ratios of oligomer/monomer, oligomer = the sum of dimmer + trimer) is similar between control cells and cells transfected with peptides corresponding to TMDs 1 to 5 and 7 to 12 of hOAT1, whereas the degree of hOAT1 oligomerization was significantly decreased in cells transfected with peptide corresponding to TMD 6 (TMD6 peptide) of hOAT1, suggesting that TMD6 peptide is a potent inhibitor for hOAT1 oligomerization. Because of the significant role of TMD6 peptide in hOAT1 oligomerization, additional studies were focused on this peptide.
Fig. 3.
Cross-linking analyses of the effect of short peptides on oligomerization of hOAT1. a and b, top, 4 μg of plasmids encoding peptides corresponding to TMDs 1 to 6 (a) and TMD 7 to 12 (b) of hOAT1 were transfected into COS-7 cells. Twenty-four hours after transfection, membrane proteins were collected and then cross-linked with a cross-linker BS3. After 30 min of the reaction, the cross-linking was stopped by addition of 1 M Tris-HCl, pH 7.5. The cross-linked proteins were separated on SDS-polyacrylamide gel electrophoresis and then subjected to immunoblotting with anti-myc antibody. The apparent molecular masses of hOAT1 oligomers were estimated based on the linear regression of the molecular mass markers used. a and b, bottom, densitometry analyses of results from that shown at the top as well as two other experiments (Supplemental Fig. 1). Asterisks indicate values significantly different (p < 0.05) from that of control.
The effect of TMD6 peptide on hOAT1 oligomerization was further analyzed through coimmunoprecipitation experiments. Flag-tagged hOAT1 was cotransfected with TMD6 peptide into hOAT1-myc-expressing cells. Flag-tagged hOAT1 was immunoprecipitated with anti-Flag antibody followed by immunoblotting with anti-FLAG antibody (to detect FLAG-tagged hOAT1) or anti-Myc antibody (to detect Myc-tagged hOAT1). As shown in Fig. 4, although the amount of Flag-tagged hOAT1 immunoprecipitated was similar in the presence and absence of TMD6 peptide (Fig. 4a), an indication of similar immunoprecipitation efficiency by anti-FLAG antibody in both situations, the amount of myc-tagged hOAT1, which coimmunoprecipitated with Flag-tagged hOAT1, was much smaller in the presence of TMD6 peptide than in the absence of the peptide (Fig. 4b), suggesting that the association between Flag-tagged hOAT1 and myc-tagged hOAT1 was weakened in the presence of TMD6 peptide.
Fig. 4.
Effect of TMD6 peptide on the association of hOAT1-FLAG with hOAT1-myc. Top, Flag-tagged hOAT1 and TMD6 peptide were cotransfected into COS-7 cells stably expressing hOAT1-myc. Twenty-four hours after transfection, cells were lysed and subjected to immunoprecipitation with anti-Flag antibody, followed by immunoblotting with anti-FLAG (a) or anti-myc (b) antibodies. Bottom, densitometry analysis of results from that shown in the top as well as two other experiments. Asterisks indicate values significantly different (p < 0.05) from that of control.
Effects of TM6 Peptide on hOAT1 Function.
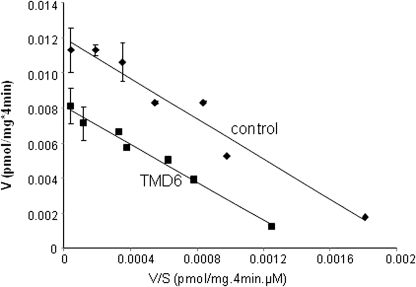
Our data above showed that TMD6 peptide disrupted hOAT1 oligomerization. To determine the functional consequence of hOAT1 oligomerization, hOAT1-mediated uptake of [3H]PAH, a prototypical OAT1 substrate, was measured in cells transfected with or without TMD6 peptide. As shown in Fig. 5, hOAT1 transport activity was much reduced in the presence of TMD6 peptide compared with that of the control. An Eadie-Hofstee plot analysis showed that the reduced transport activity resulted from a decrease in the maximum transport velocity Vmax (0.012 ± 0.001 pmol · μg−1 · min−1 in control cells and 0.008 ± 0.001 pmol · μg−1 · min−1 in TMD6 peptide-transfected cells) without significant change in the affinity (Km) for the substrate (5.71 ± 0.21 μM in control cells, and 5.44 ± 0.26 μM in TMD6 peptide-transfected cells).
Fig. 5.
Effect of TMD6 peptide on hOAT1-mediated transport. TMD 6 peptide (0.8 μg) was transfected into COS-7 cells stably expressing hOAT1-myc. Twenty-four hours after transfection, initial uptake (4 min) of [3H]PAH was measured at 8 to 800 μM PAH. The data represent uptake into hOAT1-expressing cells minus uptake into mock cells (parental COS-7 cells). Values are means ± S.E. (n = 3). V, velocity; S, substrate concentration. The experiments were repeated twice. The result from one representative experiment is shown here.
Effects of TMD6 Peptide on hOAT1 Expression.
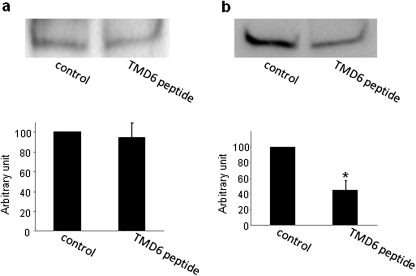
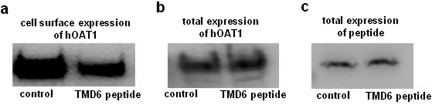
A decreased Vmax could be affected by either a decreased number of the transporter at the cell surface or a decreased transporter turnover number. Experiments that differentiate between these possibilities were conducted by measuring transporter expression both at the cell surface and in the total cell lysate through cell surface biotinylation approach. Our result showed that transfection of TMD6 peptide into the cells resulted in a decreased expression of hOAT1 at the cell surface (Fig. 6a) without affecting the total cell expression of the transporter (Fig. 6b).
Fig. 6.
Effect of TMD6 peptide on the expression of hOAT1. Plasmid encoding TMD6 peptide (4 μg) was transfected into COS-7 cells stably expressing hOAT1-myc as described above. Twenty-four hours after transfection, the surface expression of hOAT1 (a), the total expression of hOAT1 (b), and the total expression of peptides (c), were determined by immunoblotting.
Discussion
We demonstrated previously that hOAT1 forms oligomer both in cultured cells and in rat kidney (Hong et al., 2005). However, the functional consequence of such oligomerization and the structural segments of hOAT1 involved in such oligomerization have never been elucidated. In the current study, we investigated the effects of short hydrophobic peptides on the oligomerization and function of hOAT1. The short hydrophobic peptides corresponding to TMDs of several receptors have been used to demonstrate the role of interaction between transmembrane domains in receptor oligomerization and function. It has been shown that the introduction of plasmid-encoded short peptide with homologous sequence corresponding to TMD of the receptor can act as competitor and interfere with receptor oligomerization and function (Bennasroune et al., 2004, 2005). Such effects are highly selective because one homologous transmembrane domain sequence is only inhibitory for its “parental and cognate” receptor but not for unrelated receptor. We used such an approach in our current study.
Our cell surface biotinylation experiments showed (Fig. 2) that all of the 12 exogenous peptides were efficiently inserted into the plasma membrane of the cells. We demonstrated previously (Hong et al., 2005) that chemical cross-linking of intact membrane proteins from hOAT1-expressing cells converted quantitatively hOAT1 monomer to putative dimer and trimer, indicating that hOAT1 is present in the membrane as multimeric complexes. To determine the effects of these short hydrophobic peptides on hOAT1 oligomerization, cross-linking experiments were performed with membrane proteins isolated from hOAT1-expressing cells transfected with or without these peptides. Among all of the peptides examined, only the peptide corresponding to TMD 6 (TMD6 peptide) of hOAT1 significantly reduced the amount of oligomer formed (Fig. 3), suggesting that TM6 peptide is a potent inhibitor for hOAT1 oligomerization. It is important to note that the lower ratios of oligomer/monomer in sample containing TMD6 peptide do not seem to result from a reduced cross-linking efficiency because of a lower amount of hOAT1 in the sample. For example, the amount of hOAT1 containing TMD1 peptide is higher than that containing TMD5 peptide (Fig. 3a). Yet both situations had cross-linking efficiencies similar to those of the control.
The observation that TMD6 peptide was a potent inhibitor for hOAT1 oligomerization was further reinforced through coimmunoprecipitation study (Fig. 4). The association between Flag-tagged hOAT1 and myc-tagged hOAT1 was much weaker in the presence of TMD6 peptide compared with control. Therefore, TMD6 peptide can serve as a potent inhibitor for hOAT1 oligomerization. The moderate inhibition of hOAT1 oligomerization by TMD6 peptide may result from the possibility that TMDs may not be the only driving force for hOAT1 oligomerization. Addressing this interesting question will be the focus of our future study.
Our result also indicated that TMD 6 of hOAT1 may contribute to the oligomerization of the transporter. If this is indeed the case, it seems that a nonclassic oligomerization motif is involved because the classic oligomerization motives such as GXXXG (Russ and Engelman, 2000; Eilers et al., 2002), and α-helical coiled coil, a heptad repeat pattern of primarily apolar residues (Burkhard et al., 2001), are not present in this TMD. A similar situation was encountered in the study of an ATP-binding cassette transporter ABCG2 (Polgar et al., 2004). In their study, the removal of GXXXG motif in TMD 1 by site-directed mutagenesis did not show any effect on the oligomerization of ABCG2, which suggests that GXXXG motif in TMD 1 does not play an essential role in ABCG2 oligomerization.
Transfection of TMD6 peptide induced a ∼50% inhibition of uptake of PAH mediated by hOAT1. Our kinetic analysis showed that the reduced transport activity was contributed by a reduced maximum transport velocity (Vmax) without affecting the binding affinity (Km) for the substrates (Fig. 5). Vmax can be affected by either the number of the transporter at the cell surface or the transporter turnover number. To differentiate between these possibilities, we determined the effect of this peptide on hOAT1 expression both at the cell surface and in the total cell lysates. Our results showed that transfection of TMD6 peptide resulted in a reduced cell surface expression of hOAT1 without affecting its total cell expression (Fig. 6).
Oligomerization plays critical role in various aspects of transporter function. Several subunits in the oligomer may be required to form a single translocation pathway for the substrate to move across membrane (MacKinnon, 1991). Oligomerization is also believed to play a role in membrane trafficking of the transporters. After synthesis in the endoplasmic reticulum (ER), proteins undergo a strict process of quality control. Newly synthesized transporters may contain retention signal and are thereby retained in the ER. Oligomerization may shield/hide such retention signal and therefore is essential for the egress of the transporters from ER for subsequent targeting to the plasma membrane (Scholze et al., 2002; Ellgaard and Helenius, 2003; Veenhoff et al., 2002). Our future studies will explore the sites at which hOAT1 forms oligomers. In conclusion, our study provides the first demonstration that 1) the short hydrophobic peptide approach not only can be used for the study of the oligomerization of single TMD-containing receptors but also can be used for the study of the oligomerization of multiple TMD-containing transporters such as hOAT1; 2) peptide corresponding to TMD 6 of hOAT1 can serve as a potent inhibitor for hOAT1 oligomerization, and 3) oligomerization of hOAT1 is critical for the expression of the transporter at the cell surface.
Supplementary Material
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01-DK60034]; and the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM079123].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.070185.
ABBREVIATIONS:
- OAT1
- organic anion transporter 1
- TMD
- transmembrane domain
- PAH
- p-aminohippuric acid
- PBS
- phosphate-buffered saline
- ER
- endoplasmic reticulum.
Authorship Contributions
Participated in research design: Duan and You.
Conducted experiments: Duan and Li.
Performed data analysis: Duan, Li, and You.
Wrote or contributed to the writing of the manuscript: Duan and You.
References
- Ahn SY, Nigam SK. (2009) Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis. Mol Pharmacol 76:481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn A, Hagos Y, Reuter S, Balen D, Brzica H, Krick W, Burckhardt BC, Sabolic I, Burckhardt G. (2008) Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J Biol Chem 283:16332–16341 [DOI] [PubMed] [Google Scholar]
- Bennasroune A, Fickova M, Gardin A, Dirrig-Grosch S, Aunis D, Crémel G, Hubert P. (2004) Transmembrane peptides as inhibitors of ErbB receptor signaling. Mol Biol Cell 15:3464–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennasroune A, Gardin A, Auzan C, Clauser E, Dirrig-Grosch S, Meira M, Appert-Collin A, Aunis D, Crémel G, Hubert P. (2005) Inhibition by transmembrane peptides of chimeric insulin receptors. Cell Mol Life Sci 62:2124–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard P, Stetefeld J, Strelkov SV. (2001) Coiled coils: a highly versatile protein folding motif. Trends Cell Biol 11:82–88 [DOI] [PubMed] [Google Scholar]
- Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. (2000) Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem 275:4507–4512 [DOI] [PubMed] [Google Scholar]
- Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. (1999) The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol 56:570–580 [DOI] [PubMed] [Google Scholar]
- Dantzler WH, Wright SH. (2003) The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules. Biochim Biophys Acta 1618:185–193 [DOI] [PubMed] [Google Scholar]
- Eilers M, Patel AB, Liu W, Smith SO. (2002) Comparison of helix interactions in membrane and soluble alpha-bundle proteins. Biophys J 82:2720–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekaratanawong S, Anzai N, Jutabha P, Miyazaki H, Noshiro R, Takeda M, Kanai Y, Sophasan S, Endou H. (2004) Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J Pharmacol Sci 94:297–304 [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4:181–191 [DOI] [PubMed] [Google Scholar]
- Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, et al. (2002) Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 417:447–452 [DOI] [PubMed] [Google Scholar]
- Hong M, Xu W, Yoshida T, Tanaka K, Wolff DJ, Zhou F, Inouye M, You G. (2005) Human organic anion transporter hOAT1 forms homooligomers. J Biol Chem 280:32285–32290 [DOI] [PubMed] [Google Scholar]
- Jutabha P, Kanai Y, Hosoyamada M, Chairoungdua A, Kim DK, Iribe Y, Babu E, Kim JY, Anzai N, Chatsudthipong V, et al. (2003) Identification of a novel voltage-driven organic anion transporter present at apical membrane of renal proximal tubule. J Biol Chem 278:27930–27938 [DOI] [PubMed] [Google Scholar]
- Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, Sugiyama Y, Kanai Y, Endou H. (1999) Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J Biol Chem 274:13675–13680 [DOI] [PubMed] [Google Scholar]
- Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR, Nigam SK. (1997) Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. J Biol Chem 272:6471–6478 [DOI] [PubMed] [Google Scholar]
- Lu R, Chan BS, Schuster VL. (1999) Cloning of the human kidney PAH transporter: narrow substrate specificity and regulation by protein kinase C. Am J Physiol 276:F295–F303 [DOI] [PubMed] [Google Scholar]
- MacKinnon R. (1991) Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature 350:232–235 [DOI] [PubMed] [Google Scholar]
- Monte JC, Nagle MA, Eraly SA, Nigam SK. (2004) Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochem Biophys Res Commun 323:429–436 [DOI] [PubMed] [Google Scholar]
- Polgar O, Robey RW, Morisaki K, Dean M, Michejda C, Sauna ZE, Ambudkar SV, Tarasova N, Bates SE. (2004) Mutational analysis of ABCG2: role of the GXXXG motif. Biochemistry 43:9448–9456 [DOI] [PubMed] [Google Scholar]
- Russ WP, Engelman DM. (2000) The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol 296:911–919 [DOI] [PubMed] [Google Scholar]
- Scholze P, Freissmuth M, Sitte HH. (2002) Mutations within an intramembrane leucine heptad repeat disrupt oligomer formation of the rat GABA transporter 1. J Biol Chem 277:43682–43690 [DOI] [PubMed] [Google Scholar]
- Sekine T, Cha SH, Tsuda M, Apiwattanakul N, Nakajima N, Kanai Y, Endou H. (1998) Identification of multispecific organic anion transporter 2 expressed predominantly in the liver. FEBS Lett 429:179–182 [DOI] [PubMed] [Google Scholar]
- Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. (1997) Expression cloning and characterization of a novel multispecific organic anion transporter. J Biol Chem 272:18526–18529 [DOI] [PubMed] [Google Scholar]
- Shin HJ, Anzai N, Enomoto A, He X, Kim do K, Endou H, Kanai Y. (2007) Novel liver-specific organic anion transporter OAT7 that operates the exchange of sulfate conjugates for short chain fatty acid butyrate. Hepatology 45:1046–1055 [DOI] [PubMed] [Google Scholar]
- Srimaroeng C, Perry JL, Pritchard JB. (2008) Physiology, structure, and regulation of the cloned organic anion transporters. Xenobiotica 38:889–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DH, Wolff NA, Pritchard JB. (1997) Expression cloning and characterization of ROAT1. The basolateral organic anion transporter in rat kidney. J Biol Chem 272:30088–30095 [DOI] [PubMed] [Google Scholar]
- VanWert AL, Gionfriddo MR, Sweet DH. (2010) Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology. Biopharm Drug Dispos 31:1–71 [DOI] [PubMed] [Google Scholar]
- Veenhoff LM, Heuberger EH, Poolman B. (2002) Quaternary structure and function of transport proteins. Trends Biochem Sci 27:242–249 [DOI] [PubMed] [Google Scholar]
- Wolff NA, Werner A, Burkhardt S, Burckhardt G. (1997) Expression cloning and characterization of a renal organic anion transporter from winter flounder. FEBS Lett 417:287–291 [DOI] [PubMed] [Google Scholar]
- You G. (2002) Structure, function, and regulation of renal organic anion transporters. Med Res Rev 22:602–616 [DOI] [PubMed] [Google Scholar]
- Youngblood GL, Sweet DH. (2004) Identification and functional assessment of the novel murine organic anion transporter Oat5 (Slc22a19) expressed in kidney. Am J Physiol Renal Physiol 287:F236–F244 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.