Abstract
A dye cassette fluoresces green (ca 520 nm) in the cytoplasm, endoplasmic reticulum (ER), and lysosomes, but red in mitochondria, ie it illustrates ‘organelle-specific energy transfer’. This phenomenon may open new horizons in intracellular imaging.
Keywords: Fluorescent probes, Cell imaging, Energy transfer, Organelle selective, Confocal microscopy
Visualization of intracellular organelles is based on two approaches. Some organelles have distinctive shapes/cellular locations and are easily recognizable. Others are made conspicuous using fluorescent dyes known to target specific organelles, eg LysoTracker, ER-Tracker, MitoTracker, TubulinTracker.1-3 Generally, these dyes absorb in one wavelength range and emit in another; there are limited cases where the fluorescence emission wavelength of a dye changes significantly with its intracellular environment. Probes with fluorescence emission wavelengths that do vary with cellular location, however, could be valuable when monitoring dynamic organelle-specific functions.
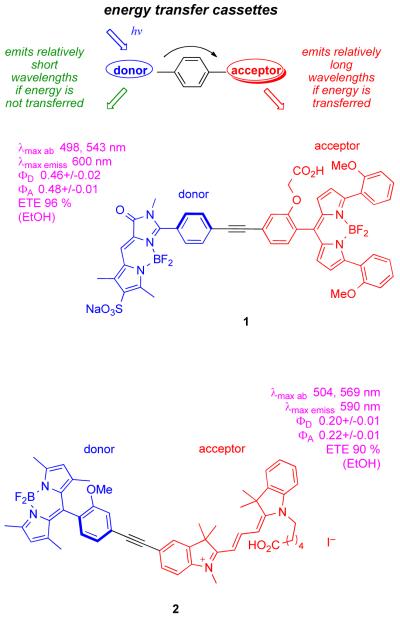
Our group has an ongoing interest in “energy transfer cassettes”. Briefly, these are molecules with donor components that absorb strongly at a convenient wavelength (eg 488 nm), and acceptor fragments that emit it efficiently at significantly longer wavelengths (Figure 1).4-7 Until now the objectives for designing these cellular imaging probes were based solely on predictable photophysical phenomena, eg minimization of the impact of back-scattering from the excitation source, possibilities for multiplexing. However, in the work described here an unexpected issue was encountered: the proportions of donor/acceptor emissions can be dependent on the cellular environment causing the cassettes to change color. This is described in the context of cassette 1 that does not display this phenomenon, and 2 that does.
Figure 1.
Concept of energy transfer cassettes, and probes 1 and 2.
Cassettes 1 and 2 were prepared via Sonogashira coupling BODIPY or cyanine-derived fragments as described in the supporting material. Salient features of their photophysical properties are indicated in Figure 1. The cassettes each have two distinct absorbance maxima corresponding to their donor and acceptor fragments. We have defined the ratio of the fluorescence quantum yields of cassettes when excited at the donor to that when excited at the acceptor as the energy transfer efficiency (ETE).7 The overall quantum yield for 2 is lower than for cassette 1, but both are bright fluorescent dyes. When excited at 488 nm, they glow red corresponding to fluorescence emission from the acceptor part.
After treatment of Clone 9 rat-liver cells with cassette 1 for 30 min the probe permeated inside and accumulated in the lysosomes and ER (Figure 2a; colocalization with LysoTracker® Green DND-26, and with ER-Tracker™ Blue-White DPX, respectively, see supporting). As in EtOH, no fluorescence was observed from the donor part of this cassette upon excitation at 488 nm, ie there was no fluorescent signal in the green channel. However, strong fluorescence was observed in the red channel indicating efficient energy transfer in both the lysosomes and in the ER; thus cassette 1 was largely uneffected by its cellular environment.
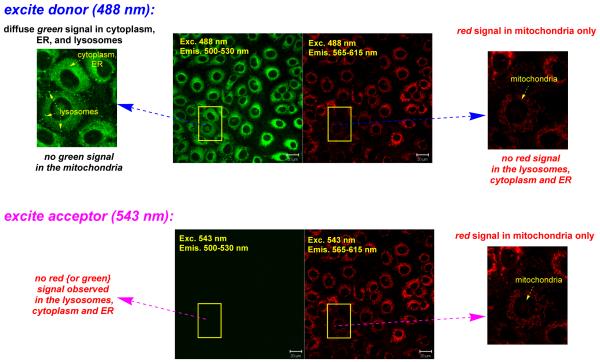
Figure 2.
Cellular uptake of cassettes 1 and 2 in Clone 9 cells. Part a shows that cassette 1 accumulates in the lysosomes and the ER, and perfect energy transfer is observed. Part b shows that cassette 2 labels the lysosomes, cytoplasm, ER and mitochondria, and the extent of energy transfer is organelle specific. Throughout the cassettes were excited at 488 nm ie donor part. Fluorescence images for the donor and acceptor emissions were collected with Band Pass (BP) 500-530 and BP 565-615 emission filters, respectively
Cassette 2 permeated Clone 9 cells and accumulated in the mitochondria, lysosomes and cytoplasm. The wavelength of the fluorescence observed was organelle specific (excitation at 488 nm, throughout). Thus, red emission (fluorescence from the acceptor, indicative of good energy transfer) was observed in the mitochondria. Conversely, only green emission was observed in the cytoplasm and lysosomes (Figure 2b).
Two possibilities were envisaged to account for the fact that the fluorescence of the acceptor is not observed for cassette 2 in the cytoplasm, lysosomes and in the ER. The first is that the energy transfer from the donor to the acceptor became inefficient in the environment of those intracellular regions. The second hypothesis is that fluorescence from the acceptor becomes quenched in the cytoplasm, lysosomes and in the ER, but not in the mitochondria. These alternatives could be distinguished by exciting the localized cassettes at the acceptor absorption maximum; under these conditions the acceptor would be visible if the first hypothesis applied, but not in the second case. In the event, when 2 was excited directly at 543 nm, ie at the acceptor part, only red emission from the mitochondria could be observed (Figure 3, bottom). No red signal was seen in the ER, cytosol or the lysosomes, suggesting the fluorescence from the cyanine acceptor is quenched in these environments. However, the energy transfer cannot be complete otherwise residual fluorescence from the donor parts would not be seen in the intracellular regions that fluoresce green.
Figure 3.
Fluorescence of cassette 2 when excited at the donor part (488 nm, top row) and the acceptor part (543 nm, bottom). It emits from the donor in the cytoplasm, ER and lysosomes, and from the acceptor in the mitochondria when excited at 488 nm. Direct excitation at the acceptor part (543 nm, bottom row) proves the emission from the acceptor is quenched in the cytoplasm, ER and lysosomes.
In summary, cassette 1 targeted the ER and lysosomes, the energy transfer observed for this cassette was near-perfect in both these environments, ie it always fluoresced red. Conversely, cassette 2 targeted a different set of organelles and fluoresced with different colors: mitochondria (red), cytoplasm including the ER (green), and lysosomes (green).
Cassette 1 has a neutral BODIPY-based acceptor fragment, while 2 has a cationic cyanine; this structural difference is most likely the origin of acceptor fluorescence quenching in some organelles, but just for the cyanine-based cassette 2. Further, the fluorescence of cyanines tends to be influenced by membrane potential effects.8-13 Overall, these data lead to a general conclusion that may be obvious, but is not often considered: some organelle specific dyes may be widely distributed in cells but only seen in regions where their fluorescence is not quenched. In general, organelle-selective energy transfer such as observed here may open new horizons for intracellular imaging.
Long Abstract, if required.
Two new energy transfer cassettes, 1 and 2 were prepared. These strongly absorb at around 500 nm through the donor part. Energy transfer from the donor to the acceptor is environment dependant; if it is complete then all fluorescence will be emitted from the acceptors at 590 - 600nm(red), while no energy transfer gives green fluorescence at about 520nm. There is a continuum of possibilities (some red, some green) between these extremes. Both cassettes 1 and 2 permeate into clone 9 rat-liver cells, wherein 1 targets the lysosomes and the endoplasmic recticulum (ER) and fluoresces red. Cassette 2 targeted the mitochondria, lysosomes, ER, and cytoplasm, however it fluoresced red in the mitochondria and green in the other organelles. The origin of this effect was quenching of the acceptor fluoresence in the organelles in which the cassette fluoresced green. This type of organelle-dependent energy transfer opens possibilities for intracellular imaging.
Supplementary Material
ACKNOWLEDGMENT
We thank The National Institute of Health (GM087981) and The Robert A. Welch Foundation (A-1121) for the financial support for this work.
Footnotes
SUPPORTING INFORMATION. Experimental procedures for the preparation of cassettes 1 and 2, photophysical studies, and additional cell imaging experiments (particularly colocalization).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Molecular Probes, pH Indicators-Chapter 20. Invitrogen Corporation; 2006. http://probes.invitrogen.com. [Google Scholar]
- 2.Oakley BR, Xiang X. Mycol. Ser. 2008;26:513–525. [Google Scholar]
- 3.Zorov DB, Kobrinsky E, Juhaszova M, Sollott SJ. Circ. Res. 2004;95:239–252. doi: 10.1161/01.RES.0000137875.42385.8e. [DOI] [PubMed] [Google Scholar]
- 4.Jiao G-S, Thoresen Lars H, Burgess K. J. Am. Chem. Soc. 2003;125:14668–14669. doi: 10.1021/ja037193l. [DOI] [PubMed] [Google Scholar]
- 5.Burgess K. US Patent No. US2005/0032120 A1. The Texas A&M University System, USA; US: 2005. p. 7. [Google Scholar]
- 6.Burgess K, Patent U, editors. The Texas A&M University System: US; 2009. p. 24. [Google Scholar]
- 7.Wu L, Loudet A, Barhoumi R, Burghardt RC, Burgess K. J. Am. Chem. Soc. 2009;131:9156–9157. doi: 10.1021/ja9029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waggoner AS. Methods Enzymol. 1979;55:689–95. doi: 10.1016/0076-6879(79)55077-0. [DOI] [PubMed] [Google Scholar]
- 9.Benson RC, Kues HA. J. Chem. Eng. Data. 1977;22:379–83. [Google Scholar]
- 10.Johnson LV, Walsh ML, Bockus BJ, Chen LB. J. Cell Biol. 1981;88:526–35. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson HA, Seligmann BE, Chused TM. J. Cell. Physiol. 1985;125:61–71. doi: 10.1002/jcp.1041250109. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Bushnell WR, Brambl R. Plant Physiol. 1987;84:1385–90. doi: 10.1104/pp.84.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patsenker LD, Tatarets AL, Terpetschnig EA. In: Advanced Fluorescence Reporters in Chemistry and Biology I: Fundamentals and Molecular Design. Demchenko AP, editor. 2010. pp. 65–104. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.