Abstract
Some human subjects report vestibular disturbances such as vertigo, apparent motion, and nausea around or within high strength MRI systems operating at 4 T to 9.4 T. These vestibular effects have been ascribed to the consequences of movement through the high magnetic field. We have previously found that exposure to magnetic fields above 7 T suppresses rearing, causes locomotor circling, and induces conditioned taste aversion (CTA) in rodents. The present experiments were designed to test the effects on rats of motion through the magnetic field of the 14.1 T superconducting magnet. In experiment 1, we compared the effects of multiple rapid insertions and removals from the center of the magnet to the effects of continuous exposure. Repeated traversal of the magnetic field gradient with only momentary exposure to 14.1 T was sufficient to suppress rearing and induce a significant CTA. Repeated insertion and removal from the magnet, however, did not have a greater effect than a single 30-min exposure on either acute locomotor behavior or CTA acquisition. Prolonged exposure was required to induce locomotor circling. In the second series of experiments, we controlled the rate of insertion and removal by means of an electric motor. Locomotor circling appeared to be dependent on the speed of insertion and removal, but the suppression of rearing and the acquisition of CTA were independent of speed of insertion and removal. In experiment 3, we inserted rats into the center of the magnet and then rotated them about their rostral-caudal axis during a 30-min 14.1 T exposure. Rotation within the magnet did not modulate the behavioral effects of exposure. We conclude that, in rats, movement through the steep gradient of a high magnetic field has some behavioral effects, but sustained exposure to the homogenous center of the field is required for the full behavioral consequences.
Keywords: gradient, magnetic resonance imaging, circling, rearing, conditioned taste aversion, vestibular
Introduction
In recent years ultra-high static magnetic fields (7 T and above) have been employed in magnetic resonance imaging (MRI). MRI machines with 7 T, 8 T and 9.4 T have been used in human studies, and 21 T MRI has been used in animal studies [1]. While increased field strength allows for enhanced resolution in MRI, exposure to the high static magnetic fields also has transient sensory effects. In particular, some human subjects report vestibular disturbances such as vertigo, apparent motion, and nausea around or within high strength MRI systems operating at 4 T [2], 7 T [3, 4], 8 T [5–7] or 9.4 T [4, 8].
The vestibular effects of high magnet fields have been ascribed to the consequences of movement through the field. In most cases subjects were reported to have experienced the most vertigo during movement into or out of the MRI, i.e. when traversing the steep gradients surrounding the magnet [3–5, 9]. When positioned at the center of the MRI in the homogenous magnetic field, vertigo or nausea could be induced by head movements [3]. Thus. current MRI safety guidelines encourage inserting patients slowly into the magnet, in order to avoid the induction of currents within conductive fluids or tissues of the body [10, 11].
Similar to human reports, we have found that rodents show symptoms of vestibular stimulation after exposure to static magnetic fields of 7 T or above [12]. Exposure while restrained within the center of either superconducting NMR magnets or a resistive magnet at fields of 7 T or above suppressed spontaneous rearing and induced locomotor circling [13–16]. The direction of circling was dependent on the orientation of the rat or mouse. When the animal’s head was facing the positive pole of the magnet (B+), it walked in counterclockwise circles; when facing the negative pole of the magnet (B−), the animal walked in clockwise circles [13, 17]. The experience of magnetic field exposure is apparently aversive, because rats would avoid climbing into a 14.1 T magnetic field [18]. Furthermore, high magnetic fields can induce conditioned taste aversion or avoidance (CTA), such that rats and mice will avoid novel taste solutions that have been paired with magnetic field exposure [14, 19]. Magnetic field exposure also activates vestibular and visceral nuclei of the brainstem as visualized with c-Fos immunohistochemistry [20, 21]. These effects are all consistent with acute vestibular perturbation or accompanying motion sickness. In fact, the effects of magnetic field exposure were abolished by chemical labyrinthectomy [18, 22], suggesting that the vestibular apparatus of the inner ear is the locus of magnetic field interaction.
While the rodent results parallel the subjective reports of vertigo in humans, they depend more on exposure to the homogenous center of the magnet than on motion through the field as reported by humans. The behavioral effects on rats (e.g. propensity to circle or magnitude of CTA) increased with the strength of the magnetic field and with the duration of exposure at the center of the magnet [13, 17]. Brief exposure to the peak magnetic field (i.e. rapidly inserting a rat into the bore of the 14.1 T magnet for a 1-min exposure) was not sufficient to induce circling, suppress rearing, or induce a CTA [13], suggesting that passage through the field gradient had little effect. As well, 30-min exposure to the homogeneous peak field at the center of the 14.1 T magnet was more effective than 30-min exposure to the sharp gradients on either side of the magnet [23]. In most experiments the rats and mice were tightly restrained in Plexiglas chambers with nose cones, so that only very small movements would have been possible during exposure. Thus, the effects of exposure were unlikely to be due to self-generated head movements within the homogenous field.
The present experiments were designed to test explicitly the effects of motion through the magnetic field of the 14.1 T superconducting magnet. If stimulation of the vestibular system depends, e.g., on the induction of an electric field across the inner ear by movement through gradient of the magnetic field, then the behavioral effects on rodents should be increased by repeated passage through the magnetic field, or by more rapid traversal of the magnetic field, or by movement within the homogenous central field.
In experiment 1, we compared the effects of multiple rapid insertions and removals from the center of the magnet to the effects of continuous exposure. In the second series of experiments, we controlled the rate of insertion and removal by means of an electric motor. In experiment 3, we inserted rats into the center of the magnet and then rotated them about their rostral-caudal axis during a 30-min exposure.
Materials and Methods
Animals
Adult female Sprague-Dawley rats (8–10 weeks old, 175–200 g; Charles River) were housed individually in polycarbonate cages in the temperature-controlled animal facility at the National High Magnetic Field Laboratory at The Florida State University. The light/dark cycle was 12:12 with lights on at 0700 hours. All conditioning trials were conducted during the light cycle. The rats had free access to pelleted Purina Rat Chow 5001 and deionized-distilled water except as specified otherwise. All procedures were approved by the Institutional Animal Care and Use Committee of Florida State University.
Magnet
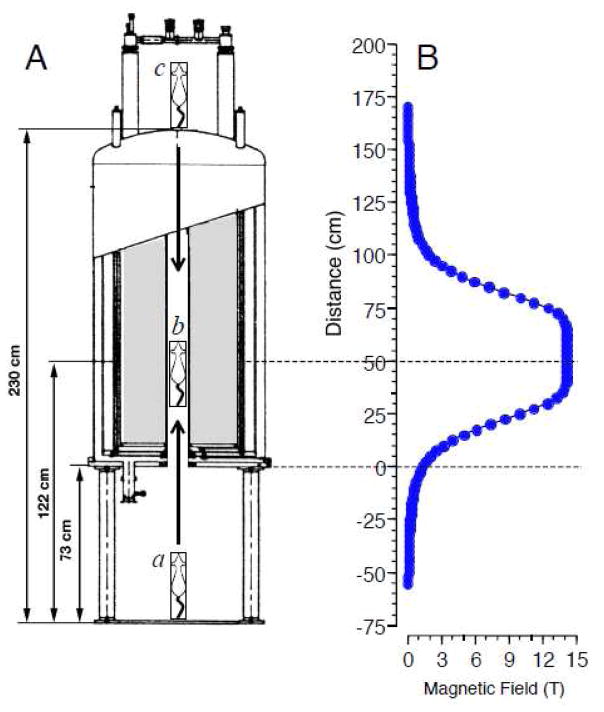
A 600 MHz Magnex Cryo magnet with an 89 mm bore and a fixed central field strength (B0) of 14.1 T was used (see Figure 1A). The magnet was located approximately 50 m from the animal facility. The magnetic field was orientated vertically so that the positive pole was at the top of the magnet. The lower opening of the magnet’s bore was 73 cm above the floor, and the bore extended for 157 cm; the center of the bore at 14.1 T was 50 cm from the lower opening of the bore. Shim magnets extending along the magnet’s bore for approximately ± 15 cm from the magnet core stabilized the magnetic field to give a central core field of uniform strength (see Figure 1B). The magnet was operated without radiofrequency pulses, so rats were exposed to a static magnetic field only.
Figure 1.
A. Schematic of insertion of restrained rat into the 14.1 T superconducting magnet. In most experiments, rats were raised head-first from the floor beneath the magnet (a) into the center of the magnet’s bore (b) either manually (Experiments 1 and 3) or using a variable speed motor (Experiments 2.1 and 2.3). In Experiment 2.2, rats were lowered tail-first from the top of the magnet (c) using the motor. In Experiment 3, rats were manually raised to the center of the magnet (b) and then rotated at 60 rpm around the vertical axis. B. Measured magnetic field strength along the vertical axis of the 14.1 T magnet. The lower opening of the magnet’s bore is at 0 cm. When positioned at 60 cm within the bore of the magnet (b), the head and body of the rat is exposed to a homogeneous 14.1 T magnetic field. On either side of the homogeneous field, the magnetic field drops off sharply with a peak gradient of ~50 T/m.
Exposure
Rats were placed in restraint tubes for sham- or magnet-exposure. The restraint tubes were 30 cm in length with an inside diameter of 5.6 cm and an outside diameter of 6.4 cm. A plug was inserted into the rostral end of the tube and held in position by nylon screws. The inside of this rostral plug was fabricated in a cone shape to accommodate the head of the rat. A 1-cm hole was bored in this plug at the apex of this cone to allow fresh breathing air. A second plug was inserted into the caudal end of the tube and could be adjusted to restrain the movement of the rat. A hole in the center of this plug accommodated the rat’s tail. When in the tube, the rat was almost completely immobilized.
Restrained rats were transported from the animal facility to the 14.1 T magnet in approximately 30 seconds. Rats exposed to the magnetic field were inserted 60 cm into the bore of the magnet for 30 min at 14.1 T (“magnet exposure”). Rats were inserted and removed manually (Experiments 1 and 3) or by using a reversible DC motor (Experiments 2.1–2.3). Manual insertion took 1–2 s to raise the rat into the center of the magnet. As controls for the effects of restraint, some rats were “sham-exposed” by placing them in the restraint tubes and inserting them into an opaque PVC pipe placed in the same room as the magnet but beyond the 500 μT line of the high magnetic field.
Behavioral Scoring
After 30-min sham-exposure or exposure within the bore of the magnet, the rostral plug of the restraining tube was removed and each rat was released into an open polycarbonate cage (37 cm wide by 47 cm long by 20 cm high) with chip bedding. The locomotor behavior of each rat was recorded on videotape for two minutes after release into the cage. (Most rats exhibited locomotor effects of the magnetic field for less than 1 minute; thus, 2 minutes of recording captured most of the phenomena of interest.) The rat was then returned to its home cage and ad libitum water was returned. The videotapes were scored later by an observer blind to the rats’ treatment. Instances of tight-circling behavior were quantified. Rats were scored as “circling” if they moved continuously around a full circle with diameter less than the length of the rat’s body. Partial circles or circles interrupted by stationary pauses were not counted. Rearing behavior (both forepaws off the floor of the cage and one or both forepaws on the side of the cage) was also scored at this time.
Conditioning
Eight days prior to the conditioning day, the rats were placed on a water restriction schedule under which they received daily water access in one drinking session, during which a water bottle was presented simultaneously with an empty bottle to accustom the rats to a 2-bottle choice. The first daily session was 3 h in length and the session times were diminished each day so that for two days before conditioning the rats received water access in a single 10-min session. On the conditioning day, rats were given access to 0.125% sodium saccharin solution (saccharin) for 10 min. Across all rats (n=134), mean conditioning day intake was 7.5 ± 0.3 g.
Immediately following saccharin access, rats were placed in restraint tubes for sham- or magnet-exposure for 30 min as described above. After 30-min exposure, the locomotor behavior of the rats was recorded for 2 min as above. Rats were then returned to their home cage and given ad libitum access to water overnight.
The strength of the CTA induced by the magnet was measured with daily 24-h, 2-bottle preference tests that were initiated the day after conditioning. (Note that this measures conditioned taste avoidance, without explicitly measuring orofacial rejection responses to intraoral infusions typical of strong aversions [24].) Two bottles were placed on the cages, one containing saccharin and the other distilled water. Fluid consumption was measured every 24 h and a preference score was calculated as the ratio of saccharin to total fluid consumption:
The preference tests were continued for up to 14 post-conditioning test days. The left/right position of saccharin and water bottles on the rats’ cages was reversed each day. Because saccharin access during the preference tests was not paired with any treatment, the preference tests constituted extinction trials. The CTA of an experimental group was considered extinguished when the average saccharin preference was not different from the average preference of sham-exposed rats. Short-term preference for saccharin measured during the first 24-h, 2-bottle test was analyzed as the magnitude of CTA; longer-term changes in preference across repeated 2-bottle tests were analyzed for extinction rate.
Statistics
Comparisons between groups on single-day data were analyzed with appropriate ANOVAs or t-tests (Statistica). Results collected over multiple 2-bottle preference test days were analyzed by 2-way ANOVA, with groups as one factor and test days as the second factor, which consisted of repeated sampling of the same subjects across test days. Post-hoc comparisons were made with the Newman-Keuls test. Data are presented as mean ± standard error of the mean.
Experiment 1. Repeated insertion into the magnet
If movement through the high magnetic field is responsible for the behavioral effects of magnetic field exposure, then repeated insertion into and removal from the magnet would have a greater effect than an uninterrupted constant exposure to the peak magnetic field.
To test this hypothesis, 3 groups of rats were tested. All groups were water-deprived and received 10-min access to saccharin prior to exposure. Rats in the first group (5x/0min, n=8) were placed in the restraint tube and then raised into the center of the 14.1 T magnet and immediately removed five times manually. Thus, they passed through the magnetic field 10 times (5 insertions and 5 removals) but only experienced the peak field momentarily. Rats in the second group (5x/30min, n=8) were placed in the restraint tube and raised to the center of the 14.1 T magnet, left for 6 min, then removed from the magnet; this procedure was repeated 5 times for each rat. Thus, rats in the 5x/30min group experienced 5 insertions and 5 removals, but were also exposed to the 14.1 T magnetic field for a total of 30 min. Rats in the third group (1x/30min, n=8) were placed in the restraint tube and raised to the center of the 14.1 T magnet, left for 30 min, and then removed from the magnet. Thus rats in the 1x/30min group experienced only one insertion and one removal, but they were exposed to the 14.1 T magnetic field continuously for 30 min.
As controls for handling without exposure to the magnetic field, additional rats (5x/sham, n=8) were restrained and placed in an opaque PVC tube outside the 500 μT line for 30 min; the sham-exposed rats were removed and returned to the opaque tube every 6 min during the 30-min sham exposure. Thus they experienced the same degree of handling as magnet-exposed rats in 5x/0min and 5x/30min groups, but without any exposure to the high magnetic field.
A final control group (1x/sham, n=8) was composed of rats that were restrained and placed in the opaque PVC tube outside the 500 μT line for 30 min, then removed. This group was restrained for 30 min but without repeated handling or magnet exposure.
Immediately after their final magnet or sham exposure, all rats were released into the open field test chamber, videotaped for 2 min, and then returned to their home cages. The next day 2-bottle 24-h preference tests were started to assess CTA acquisition.
Experiment 2. Speed of insertion and removal
Experiment 2.1 Slow vs. fast insertion
To test the effect of movement speed on the behavioral effects of a high magnetic field, rats were inserted into and removed from the center of the 14.1 T magnet at 3 different speeds as controlled by a reversible motor. Rats were water restricted as above. On conditioning day, each rat was given 10-min access to 0.125% saccharin and then placed in a restraint tube. The restraint tube was attached to a nylon cord which passed from the floor underneath the 14.1 T magnet, up through the vertical bore of the magnet, around a pulley fixed above the magnet, and then to a variable-speed reversible DC motor. The motor was calibrated so that the rats were raised from the floor, head first, into the center of the magnet at one of 3 speeds: 0.01 m/s (n= 7), 0.1 m/s (n=8), or 1.0 m/s (n=8). The distance traveled by the rat’s head from beneath the magnet to the center of the magnet was ~90 cm (see Figure 1Aa,b).
After 30-min exposure at the center of the magnet, the rats were lowered out of the magnet at the same speed as their insertion by reversing the motor. Locomotor activity was videotaped for 2 min after exposure. Two-bottle, 24-h preference tests were begun the next day. For statistical comparisons, results from magnet-exposed rats were compared to the 30-min sham-exposed group (1x/sham) of Experiment 1.
Experiment 2.2: Tail-first insertion
A fourth group of rats was included to test the effect of direction during insertion. Rats (n=8) were given 10-min access to saccharin, placed in the restraint tube, and lowered from the top of the 14. T magnet, tail first, to the center of the magnet (a distance of 1 m) at 1.0 m/s. Thus, the rats were inserted tail first into the magnet but oriented head-up while traversing the magnet’s bore and during exposure (see Figure 1Ac). After 30-min exposure, rats were raised out of the magnet at 1 m/s and locomotor activity was videotaped for 2 min. Two-bottle, 24-h preference tests were begun the next day.
Experiment 2.3: Post-exposure restraint
The variable speed at which rats were removed from the magnet raised a possible confound in observing the immediate effects of the magnetic field. Rats that were lowered slowly from the center of the magnet at 0.01 m/s remained restrained for 80–90 s longer than rats that were rapidly removed from the magnet at 1 m/s. Thus, locomotor effects would be assessed 80 s later in the slow group than in the fast group. This delay could bias the observed results, because circling is observed only within the first 2 min after magnet exposure.
To observe the effects of additional restraint time, two groups of rats (n=8/group) were given 10-min access to saccharin, placed in restraint tubes, and then rapidly raised into the center of the magnet. Both groups were exposed for 30 min to the 14.1 T magnetic field. After exposure, one group (fast/slow) was lowered slowly from the magnet over ~80 s and immediately released from restraint. The second group of rats was rapidly removed (over 1–2 s) from the magnet after exposure, but kept in the restraint tube for an additional 80 s before being released from restraint (fast/fast+restraint). Locomotor activity was videotaped for 2 min after release. Two-bottle, 24-h preference tests were begun the next day.
Experiment 3. Rotation within the magnet
The preceding experiments tested the effects of insertion at different speeds and direction into the center of the 14.1 T magnet, during which rats moved through steep magnetic field gradients. Once the rat is at rest within the static field at the center of the magnet, the homogeneous magnetic field should not produce a force or induce current in the animal. (Although torque might still be experienced by structures that are not aligned with the magnetic field). However, motion within the static magnetic field would generate relative variations in magnetic exposure that could induce currents or forces within the rat. In order to test the effects of motion during exposure to the static magnetic field, rats were inserted into the center of the magnet and then continuously rotated around the vertical axis for 30 min.
Rats were water-restricted as above, then given 10-min access to 0.125% saccharin. Rats were individually restrained, then inserted manually into the center of the 14.1 T magnet (magnet exposure) or placed in an opaque PVC tube (sham exposure). The restraint tube was positioned on top of a 30.5 cm long 2″ PVC pipe with a tee-pipe glued so that the stem of the “tee” hit the bottom of the magnet. A small bearing was attached to the stem of the “tee”, allowed the apparatus to be rotated within the bore of the magnet with a minimum of friction. On the bottom of the “tee”, a 12.7 cm pulley was attached. A fitting below this pulley was inserted in a glass bearing, allowing the entire apparatus to rotate freely. A reversible motor was placed outside the 500 μT line and was mounted on a stand with the shaft pointing upward with a 12.7 cm pulley. The pulley on the motor drove the pulley on the apparatus by a long belt, such that the apparatus was rotated at 60 rpm.
Half the magnet-exposed rats and half of the sham-exposed rats were rotated around the vertical axis (i.e. around the rats’ rostral-caudal axis) at 60 rpm for the entire 30 min of exposure (magnet+rotation group, n= 16; sham+rotation group, n = 16). Direction of rotation was counterbalanced within groups, such that half of the rats were rotated clockwise, and half were rotated counterclockwise. The remaining rats were left stationary during exposure (stationary-magnet group, n= 8; stationary-sham group, n= 8).
At the end of the 30-min exposure, rats were removed from the magnet or sham-magnet, released from restraint, and their locomotor activity was videotaped for 2 min. Two-bottle, 24-h preference tests were begun the next day.
Results
Experiment 1. Repeated insertion into the magnet
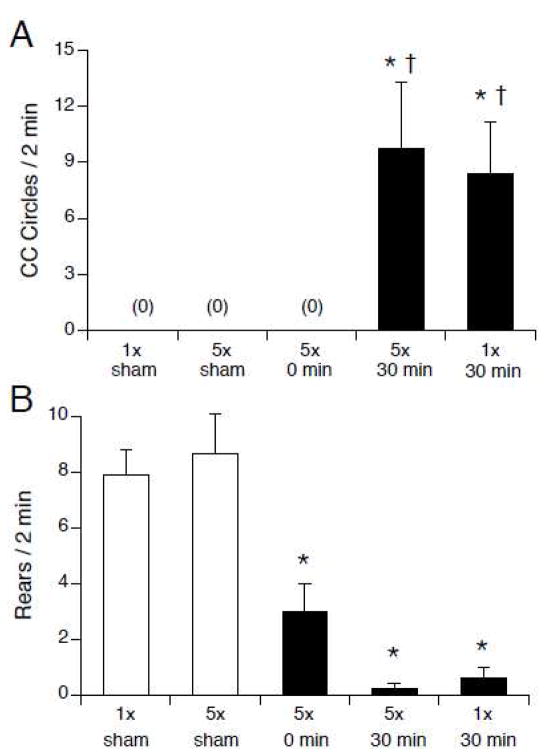
The acute locomotor effects of the 14.1 T magnetic field were determined by exposure to the magnetic field, but not by the number of insertions into the magnet (see Figure 2). Rats that were exposed to 14.1 T for 30 min (groups 5x/30min and 1x/30min) showed significant counterclockwise circling, while sham-exposed rats and 5x/0min rats that were inserted and removed five times rapidly from the magnet did not walk in circles (F(4,35) = 6.1, p < 0.001; see Figure 2A). Compared to sham-exposed rats, rearing was significantly suppressed in all magnet-exposed groups, including the 5x/0min rats that traversed the magnetic field repeatedly but were exposed for only a brief time (F(4,35) = 6.1, p < 0.001, see Figure 2B).
Figure 2.
Number of counterclockwise circles (A) and rears (B) observed immediately after single or repeated insertions into the 14.1 T magnet. Rats were either sham-exposed (white bars) or inserted into the magnet (black bars) once for 30 min, or rapidly inserted and removed from the magnet 5 times (5x/0min), or inserted and removed 5 times over the course of 30 min (5x/sham, 5x/30min). Circling was only observed after 30-min magnet exposure regardless of number of insertions. Rearing was suppressed in all magnet-exposed groups* p < 0.05 vs. 1x/sham; † p < 0.05 vs. 5x/0min.
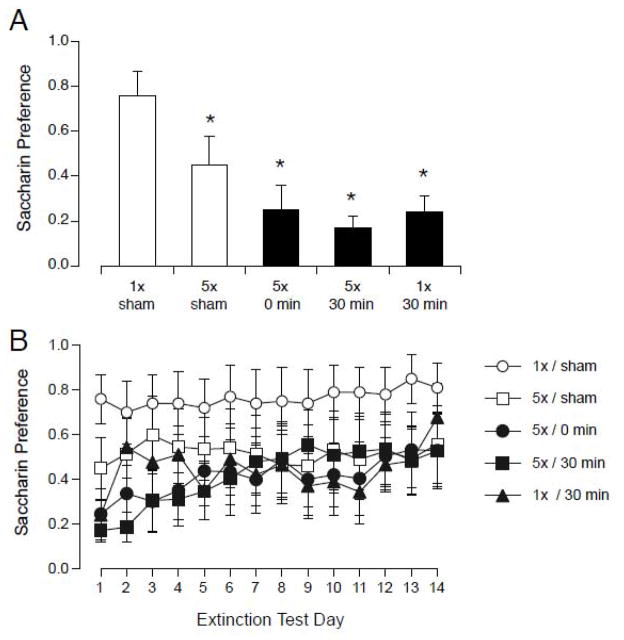
Both repeated handling and magnet exposure were sufficient to induce a significant CTA(see Figure 3). On the first day of 2-bottle testing, all groups showed a significantly lower preference for saccharin than the 1x/sham group (F(4,34) = 8.8, p < 0.0001; see Figure 3A). Because the 5x/sham group showed a significantly lower preference than the 1x/sham group, inserting and removing the rat repeatedly from the sham-magnet was apparently sufficient to induce a small CTA.
Figure 3.
Initial magnitude of CTA (A) and extinction (B) after pairing saccharin with sham-exposure (white) or exposure to 14.1 T magnetic field (black). Rats were sham-exposed (1x/sham) or magnet-exposed for 30 min (1x/30min), or rapidly inserted and removed from the magnet 5 times (5x/0min), or inserted and removed 5 times over the course of 30 min (5x/sham, 5x/30min). A. All magnet-exposed rats (black bars) and the 5x/sham group showed a significantly lower preference for saccharin on the first day of 2-bottle testing. B. Across all 14-days of extinction, there was a significant effect of day but not group. * p < 0.05 vs. 1x/sham.
Although some groups showed a significant CTA on the first day of 2-bottle testing, across the 14-days of extinction testing 2-way ANOVA found an effect of days ( F(13,52) = 3.11, p < 0.001), but no effect of group and no interaction. When magnet exposed animals were combined into a single group, then a significant effect of group (magnet- vs. sham-exposed; F(2,36)=3.76, p < 0.05) but not days was found. Although there was evidence for some extinction across days, there were no large differences between the magnet-exposed groups.
Thus, repeated traversal of the magnetic field gradient with only momentary exposure to the magnet’s core was sufficient to suppress rearing and induce a significant CTA. Repeated insertion and removal from the magnet, however, did not have a greater effect than a single prolonged exposure on either acute locomotor behavior or CTA acquisition. Prolonged exposure was required to induce locomotor circling.
These results are consistent with our earlier observation that a momentary insertion and removal from the 14.1 T magnet had no apparent effect, while a 1-min exposure to the 14.1 T magnetic field was sufficient to suppress rearing and induce CTA, but not sufficient to induce locomotor circling [13].
Experiment 2. Speed of insertion and removal
Experiment 2.1 Slow vs. fast insertion
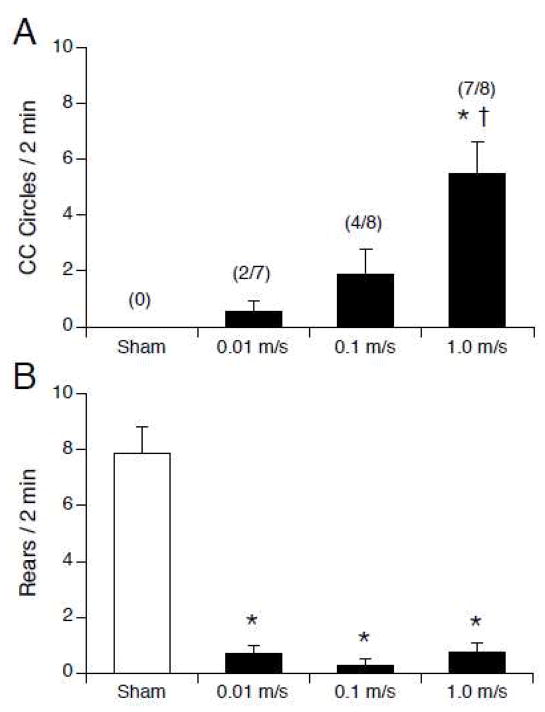
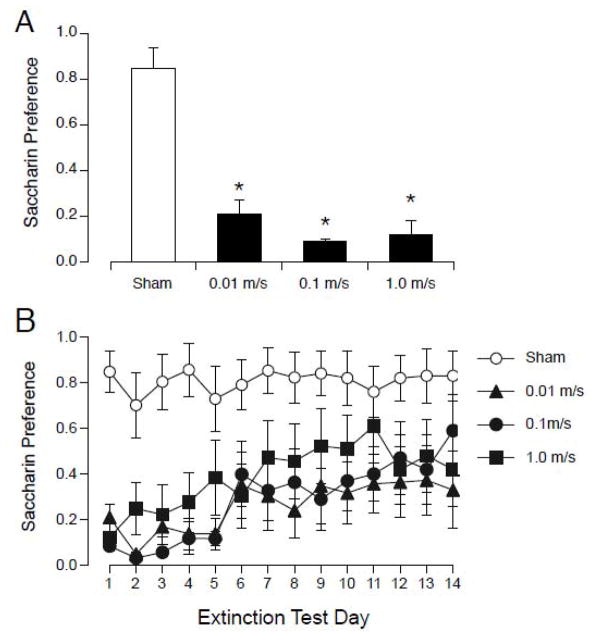
The induction of locomotor circling, but not suppression of rearing, was affected by the speed of insertion and removal from the 14.1 T magnet (see Figure 4). None of the sham-exposed rats circled. Magnetic field exposure induced counterclockwise circling in some rats, but the amount of circling was significantly greater in rats that were inserted and removed from the magnet at the highest speed (F(3,27) = 10.6, p< 0.0001; see Figure 4A). The number of rats that circled in each group also increased with speed of insertion and removal.
Figure 4.
Number of counterclockwise circles (A) and rears (B) observed after sham exposure or 30-min exposure to 14.1 T field. Rats were inserted and removed from the magnet at one of 3 speeds. A. Magnet exposure (black bars) induced counterclockwise locomotor circling; the number of circles increased as the speed of insertion and removal increased. The fraction of rats in each group circling is indicated in parentheses. B. Rearing was suppressed in all magnet-exposed rats regardless of insertion and removal speed.. * p < 0.05 vs sham; † p < 0.05 vs. 0.01 m/s and 0.1 m/s.
Compared to sham-exposed rats, all magnet-exposed rats showed a significant suppression of rearing (F(3,27) = 46.7, p < 0.0001; see Figure 4B). There was no difference in rearing among the groups inserted and removed at different speeds.
Magnet exposure caused acquisition of CTA regardless of the speed of insertion and removal (see Figure 5). On the first day of 2-bottle testing, all 3 magnet-exposed groups showed a low saccharin preference compared to the sham-group (F(3,27) = 33.8, p < 0.0001; see Figure 3A). Across the 14-days of extinction testing, 2-way ANOVA revealed an effect of group (F(3, 39) = 5.39, p < 0.005), days (F(13, 39) = 6.91, p < 0.001) and an interaction (F(39, 351) = 1.61, p < 0.05). There was no significant difference in saccharin preference among the magnet-exposed groups on any day of 2-bottle testing.
Figure 5.
Initial magnitude of CTA (A) and extinction (B) after pairing saccharin with sham-exposure or exposure to 14.1 T magnetic field with 3 different speeds of insertion and removal. A. On the first day of 2-bottle testing, all magnet-exposed groups (black bars) showed a significantly lower preference for saccharin compared to sham-exposed rats (white bar). B. Across 14 days of extinction testing, saccharin preference was not different among magnet-exposed groups. All magnet-exposed rats showed an initial CTA for saccharin that diminished over days.
Thus, the induction of locomotor circling appeared to be dependent on the speed of insertion and removal, but the suppression of rearing and the acquisition of CTA were independent of speed of insertion and removal.
Experiment 2.2: Tail-first insertion
Of the 8 rats that were lowered tail-first from the top of the magnet and removed head-first, 7 rats walked in counterclockwise circles (mean 5.0 ± 1.6) and only 1 rat reared once. On the first day of 2-bottle preference testing, the rats lowered tail-first showed a low preference for saccharin (0.35 ± 0.12) that was significantly lower than the sham group but not significantly different from rats raised head-first into the magnet (F(2,21)=15.2, p < 0.0001). Thus the induction and direction of circling was not dependent on the direction of insertion into the magnet, and a similar CTA was induced by the 30-min magnet exposure.
Experiment 2.3: Post-exposure restraint
In Experiment 2.1, rats that were removed slowly from the magnet showed significantly less locomotor circling than rats that were removed rapidly from the magnet. To determine if the lack of observed circling was due to the slow speed of removal or due to the delay before recording locomotor activity, rats were inserted rapidly into the magnet and then either removed slowly from the magnet (fast/slow group) or rapidly removed from the magnet and restrained for an additional 80 s prior to recording locomotor activity (fast/fast+restraint).
No rats in either group circled. One-way ANOVA on rearing showed a significant effect of magnet exposure (F(2,21) = 24.4, p < 0.0001), such that the magnet-exposed groups reared significantly less than sham-exposed rats (fast/slow 2.0 ± 0.5 rears/2 min; fast/fast+restraint: 2.1 ± 0.5 rears/2min; sham: 8.0 ± 1.0 rears/2 min).
Magnet exposure and speed of removal affected CTA acquisition. One-way ANOVA on the first day of 2-bottle testing showed a significant difference between groups (F(2,21) = 4.7, p < 0.05) such that the fast/fast+restraint group had a significantly lower preference than the sham group (sham 0.85± 0.08; fast/slow 0.61 ± 0.13; fast/fast 0.31 ± 0.14). Across 14-days of 2-bottle testing, there was no effect of group, an effect of days (F(2,13)= 4.27, p < 0.001) and an interaction (F(26, 273) = 1.60, p < 0.05). Preference in the fast/fast+restraint group was significantly lower than sham preferences for the first 6 days; preference in the fast/slow group was intermediate between sham and fast/fast+restraint groups.
Thus, rats removed slowly from the magnet over 80 s did not circle, but neither did rats removed rapidly from the magnet in 1–2 s but restrained for an additional 80 seconds. Therefore, the absence of circling in the rats removed slowly from the magnet could be accounted for entirely by the additional restraint time prior to recording their post-exposure locomotor activity. This is consistent with our previous observations that magnet-induced circling decays away within 2–3 minutes after magnet exposure [15]. (Rearing, however, was suppressed in both groups, which is consistent with the prior observation that suppression of rearing persists for ~10 min after exposure [16].)
Experiment 3. Rotation within the magnet
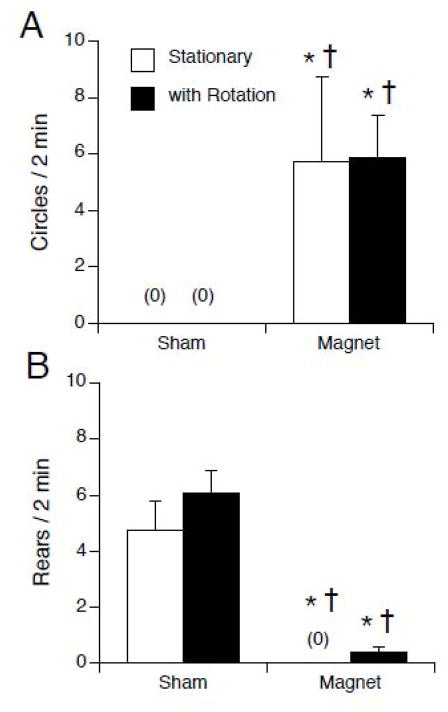
To test the effects of motion within the static magnetic field, rats were exposed for 30 min to 14.1 T or sham exposed. During exposure, rats were either stationary or rotated at 60 rpm around their rostral caudal axis. No difference was seen between rats rotated clockwise vs. counterclockwise during either sham exposure or magnet exposure, so the data was combined for each condition. Rotation of rats during sham exposure had no effect on locomotor behavior or CTA compared to stationary sham exposure. Magnet exposure induced locomotor circling and suppressed rearing compared to sham-exposure, but rotation during exposure did not modulate this effect (see Figure 6). Two-way ANOVA of circling showed an effect of magnet exposure (F(1, 44 ) = 15.00, p < 0.001) but no effect of rotation and no interaction (see Figure 6A). Likewise, 2- way ANOVA of rearing showed an effect of magnet (F(1,44) = 57.02, p < 0.001) but no effect of rotation and no interaction (see Figure 6A). By post hoc tests, both magnet-exposed groups were different from the sham-exposed groups for both behaviors.
Figure 6.
Locomotor circling (A) and rearing (B) after sham or magnet exposure while stationary (white bars) or rotating at 60 rpm during exposure (black bars). Magnetic field exposure induced locomotor circling and suppressed rearing; rotation during exposure had no apparent effect. * p < 0.05 vs. sham-stationary; † p < 0.05 vs. sham with rotation.
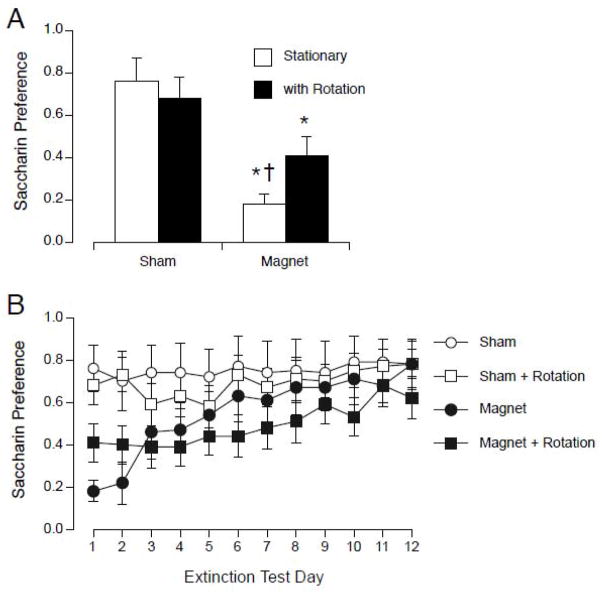
On the first day of 2-bottle preference testing, rotation alone did not induce CTA, and may have attenuated CTA induced by magnet exposure. Two-way ANOVA of the first day of preference testing showed an effect of magnet exposure (F(1,44) = 17.90, p < 0.001) but no effect of rotation and no interaction (see Figure 7A). Across 12 days of 2-bottle extinction testing, two-way ANOVA with groups and days as factors revealed no effect of group, an effect of days (F(11,33) = 8.08, p < 0.001) and an interaction (F(33,484) = 2.06, p < 0.005; see Figure 7B). Post-hoc tests revealed a few differences in extinction between the stationary-magnet and rotated-magnet groups: the stationary-magnet group was different from the sham groups on only days 1 and 2, while the rotation-magnet group was different the sham-groups on days 1, 3,4, and 6. However, on all days the saccharin preference of the rotated-magnet group was not different from that of the stationary-magnet group, nor was the rotated-sham group different from the stationary-sham group.
Figure 7.
A. Initial magnitude of CTA on the first day of 2 bottle testing after sham or magnet exposure while stationary (white bars) or rotating at 60 rpm during exposure (black bars). Both magnet-exposed groups showed a significantly lower preference for saccharin on the first day. Rotation alone did not induce a CTA. The group rotated during magnet exposure was not different from the group rotated during sham-exposure. * p < 0.05 vs. sham-stationary; † p < 0.05 vs. sham with rotation. (B) CTA Extinction across 12 days of 2-bottle preference testing. The sham-rotation group was not different from the sham-stationary group on any day, and the magnet-rotated group was not different from the magnet-stationary group on any day.
Discussion
If movement through the steep gradient of the magnetic field was responsible for behavioral effects, then we hypothesized that 1) repeated movement in and out of the magnet would have a larger effect than a single continuous exposure and 2) the magnitude of behavioral effects would be proportional to speed of movement during insertion and removal. Our results provide only partial support for these hypotheses. Rather, they suggest that prolonged exposure at the center of the 14.1 T magnet induces behavioral effects that are largely independent of movement through the field gradients.
Repeated insertion and removal
In Experiment 1, repeated insertion and removal from the magnet (with only momentary exposure to the magnet’s core) did not induce locomotor circling, while 30-min exposure to the center of the magnet did induce circling. Repeated insertion and removal was sufficient to suppress spontaneous rearing in rats. This is consistent with our previous findings that suppression of rearing is the most sensitive measure of an effect of the magnetic fields [13, 17], and may be indicative of a postural reflexive response to even mild vestibular perturbation [16].
Repeated insertion and removal also produced the same magnitude of CTA as a continuous 30-min exposure. However, the combination of repeated insertions and 30-min exposure (in group 5x/30 min) did not produce a greater CTA than either treatment alone. Also, the repeated manipulation of the rats may have contributed to CTA acquisition because the 5x/sham control group also showed a significantly decreased saccharin preference.
We can conclude that repeated passage through the field gradient does have some effect on rats (apparent as suppression of rearing), but that prolonged exposure to the center of the magnet is required for locomotor circling, the most obvious sign of vestibular perturbation. We have previously found that a single rapid insertion and removal of rats with only instantaneous exposure to 14.1 T was without behavioral effect [13]. As little as 1-min exposure to the center of the magnet was sufficient to cause some suppression of rearing and induce a CTA; the magnitude of behavioral effects increased as 14.1 T exposure time increased (i.e. from 1 min to 30 min) [13]. Also, the magnitude of effects was dependent on position within the magnet during exposure, with the largest effects found when the rat’s head was located in the homogenous center of the magnet (regardless of passage through the field gradient or center) [23]. Therefore, prolonged exposure to the static homogenous magnetic field must have an effect on rats in addition to any effect of movement through the field gradients.
Sham-exposed rats that underwent repeated handling (i.e. inserted and removed 5 times from the PVC “sham-magnet”) also showed a CTA. This may have been due to vestibular perturbation or other stressors associated with the repeated handling. Although the sham-magnet was located some distance from the 14 T magnet, the sham-exposed rats may have been manipulated within the fringe magnetic field (i.e. ≤ 500 μT) that is considerably higher than the background geomagnetic field. It has been shown that movement within such fields can cause a number of behavioral and physiological effects [25], which may have contributed to the response of sham-manipulated rats.
Speed of insertion and removal
Passage through a space-varying magnetic field (dB/dz) is equivalent to exposure to a time-varying field (dB/dt), which induces an electric field. Therefore subjects will be exposed to relatively strong electric fields as they pass through the field gradients during insertion into the magnet. Furthermore, the strength of the field will be proportional to the speed of movement through the gradient. In Experiment 2.1 we found that insertion of rats into the 14.1 T magnet at three different speeds produced equivalent suppression of rearing and equivalent CTA. The induction of locomotor circling was apparently proportional to the rate of insertion and removal, suggesting that vestibular perturbation was correlated with dB/dt. This is consistent with the self-report of humans that the illusion of motion is increased with faster insertion into MRI machines [3].
The interpretation is complicated, however, by the observation that magnet-induced locomotor circling decays within 1–2 min. Because the slowest speed of removal (0.01 m/s) required that rats be restrained for 80 s longer than rats removed at the highest speed (1.0 m/s), the diminished circling could be accounted for by restraint time alone, independent of removal speed (Experiment 2.2). Thus, we conclude that speed of passage through the field gradients does not contribute to the magnitude of behavioral effects, although faster removal allows for more prompt observation of acute transient effects. (Because we employed a large 14.1 T magnet for these experiments, it is also possible that maximal effects were achieved even at the lowest speed, so that no effect of varying speed was observed.)
Rotation within the magnet
Motion within a homogenous static field effectively exposes the subject to a changing magnetic field and thus generation of an electric field. Thus, humans report vertigo and nausea when moving their heads within the core of a 7 T magnet, either by nodding or by axial rotation [3]. To approximate this effect, in Experiment 3 rats were rotated at 60 rpm around their rostral/caudal axis while positioned in the center of the 14.1 T magnet. We found that rotation had little or no modulation of locomotor circling, rearing, or CTA acquisition.
However, orientation within the magnet and the axis of rotation may be important. Because of the narrow bore of the magnet, rats could only be rotated around one axis (i.e. roll). It is possible that rotation around either of the other axes (i.e. pitch or yaw) might amplify the effects of magnetic field exposure. We know that static orientation within the core of the magnet is important. Rats oriented with their head towards B+ circle counterclockwise, while rats oriented towards B− circle clockwise. Furthermore, when rats [17] or mice (unpublished data) are positioned perpendicular to the field (i.e. dorsal side towards B+), then magnetic field exposure has little or no behavioral effect. Therefore, it seems likely that rotating animals from a position of “rostral towards B+” to “rostral towards B−” would constitute a changing vestibular stimulus with more pronounced behavioral effects.
It should be noted that biological effects have been observed in rodents exposed to rotating magnetic fields of much lower strength than the 14.1 T magnetic field employed in the current study. For example, exposure to 200 μT to 9000 μT fields generated by rotating permanent magnets at 0.5 Hz have effects on development [26], open-field behavior [26–28], and analgesic responses [25].
As we have observed previously [12], continuous on-center vertical rotation around the rostral-caudal axis did not induce CTA in sham-exposed rats. This is consistent with minimal vestibular perturbation: continuous rotation causes minimal stimulation of the semicircular canals, and on-center rotation causes minimal stimulation of the otolith organs. Conversely, head motion during on-center rotation [29], sinusoidal rotation [30], off-center rotation [12], off-vertical rotation [31] or compound action around multiple axes [32] have been reported to produce greater motion sickness in humans or larger CTA in animals.
Mechanisms of gradient detection
Our results demonstrate that rats are influenced by exposure to or passage through the field gradient of the 14.1 T magnet. This is consistent with our earlier finding that rats can consciously detect the presence of the magnetic field gradient [18]. Rats that were trained to climb a plastic mesh ladder refuse to climb through the bore of the 14.1 T magnet; the rats appear to detect the gradient, because they reverse their movement when they reach a field strength of 1 – 2 T in the gradient prior to reaching the magnet’s bore.
Because their movement through the space-varying magnetic field (dB/dz) causes the rats to experience a time-varying magnetic field (dB/dt), the rats experience an electric field that might be sufficient to directly stimulate conductive tissues. At the maximum gradient of the 14.1 T magnet, rats are exposed to 50 T/m; movement at 1 m/s would therefore result in exposure to 50 T/s. At these field strengths, current densities greater than 1 A/m2 are predicted by computational models of the human body moving in MRI machines [33]. The threshold for alteration of synaptic activity in brain has been calculated at 1.45 T/s, and the threshold for stimulation of large nerves at 37.5 T/s [34]. Thus, there is the potential for electrical stimulation of the vestibular apparatus during the rats’ insertion and removal from the 14.1 T magnet [3].
Magnetohydrodynamic forces might also be induced in conductive fluids, such as the endolymph of the semicircular canals in the inner ear [3, 35, 36]. Movement of the endolymph through the field could generate a force on the endolymph, leading to pressure on the cupula and the transduction of apparent head rotation. It has also been suggested that magnetic force could be exerted on the otoconia of the utricle and saccule (see below) [3].
Mechanisms of static field detection
In addition to gradient effects, our results also suggest that exposure to the homogenous static field at the center of the 14.1 T magnet also affects the vestibular system, resulting in acute locomotor circling.
This is consistent with our earlier study demonstrating that exposure of the rat’s head to the central homogenous 14.1 T field was more effective than gradient exposure in producing locomotor circling, suppression of rearing, and acquisition of CTA [23]. We have recently begun to observe directly the effect of the magnetic field on rats by videotaping the head movements of rats while in the center of the 14.1 T magnet. After being placed in the center of the 14.1 T magnet, rats immediately tilt their heads to their right side (clockwise), and they maintain this posture with minimal head movement throughout the 30-min exposure to the homogeneous field. Thus, rats may experience a vestibular stimulus which elicits a rightward vestibular reflexive response during magnetic field exposure, and the counterclockwise circling after exposure may reflect a contralateral, leftward compensatory response when the magnetic stimulus is removed. The reasons for an asymmetrical and directional effect of the homogenous magnetic field are unknown.
Because exposure to the homogeneous and static magnetic field without motion results in exposure to a non-varying field (i.e. dB/dz = 0 and dB/dt = 0), there should be no electrical field stimulation nor any translational force experienced by the rats. However, torque might still be applied to magnetically susceptible material that is not aligned with the magnetic field. In fact, the orientation of the animal within the magnetic field is critical (see above). The susceptibility of animals exposed with their heads parallel to the magnetic field suggests that some component of the vestibular apparatus of the inner ear is not aligned with the field in this orientation.
A candidate structure within the inner ear are the otoconia crystals of the utricle and saccule. Preliminary experiments from our laboratory have found that otoconia-deficient head-tilt and tilted-head mutant mice do not appear to respond to high magnetic field exposure, suggesting that the normal otolith function is critical. The effects of a magnetic field on otoconia have been modeled based on the small magnetic susceptibility of calcium carbonate which is nonetheless greater than the susceptibility of surrounding water or tissue [3]. It has been estimated that the field gradient in the vicinity of a 7 T MRI would be sufficient to exert a perceptible force on the otoconia, i.e. to induce a subjective sense of linear acceleration [3], in line with human reports. This effect on otoconia, however, depends on a gradient and would not be present in the homogenous center of the magnet. Torque might be exerted in the center of the magnet if there is an anisotropic susceptibility to the otoconia (or to other macromolecules in the otolith organs). For an anisotropic effect, macromolecules would have differing susceptibilities along different axes; the homogenous magnetic field would induce torque to align the axis of greatest susceptibility with the field [35]. Anisotropy of magnetic susceptibility is found in calcium carbonate crystals [37], but it is unknown if there is significant anisotropy or alignment of the otoconia in vivo.
Research Highlights.
Moving rats in and out of a 14.1 T magnet reduced rearing and induced taste aversion.
Prolonged (motionless) exposure to 14.1 T was required for locomotor circling.
Speed of removal was correlated with amount of circling, but not with taste aversion.
Continuous rotation within the magnet did not amplify the effects of exposure.
Motion and static field exposure both contribute to effects of a high magnetic field.
Acknowledgments
Supported by National Institute on Deafness and Other Communication Disorders Grants RO1DC4607. We thank Drs. Timothy Cross and Zhehong Gan of the United States National Magnetic Field Laboratory for providing access to the magnet.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fu R, Brey WW, Shetty K, Gor’kov P, Saha S, Long JR, Grant SC, Chekmenev EY, Hu J, Gan Z, Sharma M, Zhang F, Logan TM, Brüschweller R, Edison A, Blue A, Dixon IR, Markiewicz WD, Cross TA. Ultra-wide bore 900 MHz high-resolution NMR at the National High Magnetic Field Laboratory. J Magn Reson. 2005;177:1–8. doi: 10.1016/j.jmr.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Schenck JF, Dumoulin CL, Redington RW, Kressel HY, Elliot RT, McDougall IL. Human exposure to 4.0-Tesla magnetic fields in a whole body scanner. Med Phys. 1992;19:1089–1098. doi: 10.1118/1.596827. [DOI] [PubMed] [Google Scholar]
- 3.Glover PM, Cavin I, Qian W, Bowtell R, Gowland PA. Magnetic-field-induced vertigo: a theoretical and experimental investigation. Bioelectromagnetics. 2007:28. doi: 10.1002/bem.20316. [DOI] [PubMed] [Google Scholar]
- 4.Theysohn JM, Maderwald S, Kraff O, Moenninghoff C, Ladd ME, Ladd SC. Subjective acceptance of 7 Tesla MRI for human imaging. MAGMA. 2008;21:63–72. doi: 10.1007/s10334-007-0095-x. [DOI] [PubMed] [Google Scholar]
- 5.Kangarlu A, Burgess RE, Zhu H, Nakayama T, Hamlin RL, Abduljalil AM, Robitaille PML. Cognitive, cardiac, and physiological safety studies in ultra high field magnetic resonance imaging. Magn Reson Imag. 1999;17:1407–1416. doi: 10.1016/s0730-725x(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 6.Chakeres DW, Kangarlu A, Boudoulas H, Young DC. Effect of static magnetic field exposure of up to 8 Tesla on sequential human vital sign measurements. J Magn Reson Imaging. 2003:18. doi: 10.1002/jmri.10367. [DOI] [PubMed] [Google Scholar]
- 7.Yang M, Christoforidis G, Abduljali A, Beversdorf D. Vital signs investigation in subjects undergoing MR imaging at 8T. AJNR Am J Neuroradiol. 2006;27:922–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Patel M, Williamsom RA, Dorevitch S, Buchanan S. Pilot study investigating the effect of the static magnetic field from a 9.4-T MRI on the vestibular system. J Occup Environ Med. 2008;50:576–583. doi: 10.1097/JOM.0b013e318162f5d6. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson IC, Sonstegaard R, Pliskin NH, Thulborn KR. Vital signs and cognitive function are not affected by 23-sodium and 17-oxygen magnetic resonance imaging of the human brain at 9.4 T. J Magn Reson Imaging. 2010;32:82–7. doi: 10.1002/jmri.22221. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Environmental Health Criteria. Vol. 232. Geneva: 2006. Static Fields. [Google Scholar]
- 11.International Commission on Non-Ionizing Radiation Protection. Guidelines on limits of exposure to static magnetic fields. Health Phys. 2009;96:504–14. doi: 10.1097/01.HP.0000343164.27920.4a. [DOI] [PubMed] [Google Scholar]
- 12.Houpt TA, Smith JC. Conditioned taste aversion induced by exposure to high-strength static magnetic fields. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; NY: 2009. pp. 422–441. [Google Scholar]
- 13.Houpt TA, Pittman DM, Barranco JM, Brooks EH, Smith JC. Behavioral effects of high strength magnetic fields on rats. J Neurosci. 2003;23:1498–505. doi: 10.1523/JNEUROSCI.23-04-01498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockwood DR, Kwon BS, Smith JC, Houpt TA. Behavioral effects of high strength static magnetic fields on restrained and unrestrained mice. Physiol Behav. 2003;78:635–40. doi: 10.1016/s0031-9384(03)00040-4. [DOI] [PubMed] [Google Scholar]
- 15.Houpt TA, Houpt CE. Circular swimming in mice after exposure to a high magnetic field. Physiol Behav. 2010;100:284–90. doi: 10.1016/j.physbeh.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houpt TA, Cassell JA, Riccardi C, Kwon BS, Smith JC. Suppression of drinking by exposure to a high-strength static magnetic field. Physiol Behav. 2007;90:59–65. doi: 10.1016/j.physbeh.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Houpt TA, Pittman DW, Riccardi C, Cassell JA, Lockwood DR, Barranco JM, Kwon BS, Smith JC. Behavioral effects on rats of high strength magnetic fields generated by a resistive electromagnet. Physiol Behav. 2005;86:379–89. doi: 10.1016/j.physbeh.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Houpt TA, Cassell JA, Riccardi C, DenBleyker MD, Hood A, Smith JC. Rats avoid high magnetic fields: dependence on an intact vestibular system. Physiol Behav. 2007;92:741–7. doi: 10.1016/j.physbeh.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nolte CM, Pittman DW, Kalevitch B, Henderson R, Smith JC. Magnetic field conditioned taste aversion in rats. Physiol Behav. 1998;63:683–688. doi: 10.1016/s0031-9384(97)00526-x. [DOI] [PubMed] [Google Scholar]
- 20.Snyder D, Jahng JW, Smith JC, Houpt TA. c-Fos induction in visceral and vestibular nuclei of the rat brainstem by a 9.4 T magnetic field. NeuroReport. 2000;11:1681–5. doi: 10.1097/00001756-200008210-00015. [DOI] [PubMed] [Google Scholar]
- 21.Cason A, Kwon BS, Smith JC, Houpt TA. c-Fos induction by a 14T magnetic field in visceral and vestibular relays of the female rat brainstem is modulated by estradiol. Brain Res. 2010;1347:48–57. doi: 10.1016/j.brainres.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cason AM, Kwon BS, Smith JC, Houpt TA. Labyrinthectomy abolishes the behavioral and neural response of rats to a high-strength static magnetic field. Physiol Behav. 2009;97:36–43. doi: 10.1016/j.physbeh.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Houpt TA, Cassell JA, Cason AM, Reidell A, Golden GJ, Riccardi C, Smith JC. Evidence for a cephalic site of action of high magnetic fields on the behavioral responses of rats. Physiol Behav. 2007;92:665–74. doi: 10.1016/j.physbeh.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learn Behav. 2003;31:165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- 25.Del Seppia C, Ghione S, Luschi P, Ossenkopp KP, Choleris E, Kavaliers M. Pain perception and electromagnetic fields. Neurosci Biobehav Rev. 2007;31:619–42. doi: 10.1016/j.neubiorev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Ossenkopp KP. Maturation and open-field behavior in rats exposed prenatally to an ELF low-intensity rotating magnetic field. Psychol Rep. 1972;30:371–374. doi: 10.2466/pr0.1972.30.2.371. [DOI] [PubMed] [Google Scholar]
- 27.Ossenkopp KP, Ossenkopp MD. Geophysical variables and behavior: XI. open-field behaviors in young rats exposed to an ELF rotating magnetic field. Psychol Rep. 1983;52:343–349. doi: 10.2466/pr0.1983.52.2.343. [DOI] [PubMed] [Google Scholar]
- 28.Persinger MA, Persinger MA, Ossenkopp KP, Glavin GB. Behavioral changes in adult rats exposed to ELF magnetic fields. Int J Biometeor. 1972;16:155–162. doi: 10.1007/BF01810286. [DOI] [PubMed] [Google Scholar]
- 29.Money KE. Motion sickness. Physiol Rev. 1970;50:1–39. doi: 10.1152/physrev.1970.50.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Cordick N, Parker LA, Ossenkopp KP. Rotation-induced conditioned rejection in the taste reactivity test. NeuroReport. 1999;10:1557–1559. doi: 10.1097/00001756-199905140-00030. [DOI] [PubMed] [Google Scholar]
- 31.Fox RA, Lauber AH, Daunton NG, Phillips M, Diaz L. Off-vertical rotation produces conditioned taste aversion and suppressed drinking in mice. Aviat Space Environ Med. 1984;55:632–5. [PubMed] [Google Scholar]
- 32.Fox RA, Daunton NG. Conditioned feeding suppression in rats produced by cross-coupled and simple motions. Aviat Space Environ Med. 1982;53:218–220. [PubMed] [Google Scholar]
- 33.Crozier S, Liu F. Numerical evaluation of the fields induced by body motion in or near high-field MRI scanners. Prog Biophys Molec Biol. 2005;87:267–278. doi: 10.1016/j.pbiomolbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 34.IEEE, C95.6: Standard for safety levels with respect to human exposure to electomagnetic fields, 0–3 kHz. New York: IEEE; 2002. [Google Scholar]
- 35.Schenck JF. Physical interactions of static magnetic fields with living tissues. Prog Biophys Molec Biol. 2005;87:185–204. doi: 10.1016/j.pbiomolbio.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Schenck JF. Health and physiological effects of human exposure to whole-body four-tesla magnetic fields during MRI. Ann NY Acad Sci. 1992;649:285–301. doi: 10.1111/j.1749-6632.1992.tb49617.x. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt V, Hirt AM, Hametner K, Gunther D. Magnetic anisotropy of carbonate minerals at room temperature and 77 K. Amer Mineralogist. 2007;92:1673–1684. [Google Scholar]