Abstract
Human chorionic gonadotropin (hCG) is crucial for successful pregnancy. Its many functions include angiogenesis and immune regulation. Despite years of research, the etiology of preeclampsia remains unknown. Marked by insufficient trophoblast invasion and poor spiral artery remodeling, preeclampsia has also been linked to immune dysregulation. Here we discuss the roles of hCG in the context of endovascular cross-talk between trophoblasts and endothelial cells and immune tolerance. We propose that functional and glycosylation modifications of hCG may contribute to the pathogenesis of preeclampsia.
Keywords: Uterine natural killer cells, human chorionic gonadotropin, preeclampsia, T regulatory cells, angiogenesis
1. Introduction
Pregnancy is a dynamic process characterized by immune tolerance, angiogenesis and hormonal regulation. Human chorionic gonadotropin (hCG) can be detected on the first day of implantation; its levels peak around gestational week 12 and diminish to low levels during the remainder of pregnancy [1]. hCG has many important functions in pregnancy, including the promotion of progesterone production, implantation and decidualization, angiogenesis, cytotrophoblast differentiation, and immune cell regulation (reviewed in [2]). With these myriad functions in mind, hCG dysregulation could lead to adverse pregnancy outcomes. Preeclampsia is a condition marked by insufficient trophoblast invasion and maternal spiral artery remodeling [3]. Recent studies have established a link between preeclampsia and immune cell dysregulation, including reduced numbers of uterine and circulating regulatory T cells (Tregs) and natural killer (uNK) cells [4,5]. It is thus possible that alterations in hCG production or function could contribute to the development of preeclampsia.
2. hCG Variants
hCG is composed of α- and β-subunits each consisting of a protein backbone with N-linked and O-linked oligosaccharides. It is now believed that there are four distinct variants: hCG, hyperglycosylated hCG (HhCG), the free β-subunit, and pituitary hCG [2]. Pituitary hCG is an intriguing phenomenon and is a subject of extensive debate in menopausal women. Low levels of hCG are detected during the preovulatory surge of luteinizing hormone (LH) [6]. These hCG forms can be further modified by partial degradation of the hCG molecule, nicking of the intact β-subunit, or variation of the attached oligosaccharides [7]. Variations in the number and type of these sugar branches result in significant differences in the molecule with important clinical implications. These variants play different roles in both normal and abnormal pregnancy [8]. HhCG, which has complex β-subunit N- and O-linked oligosaccharides structural alterations, is produced early during pregnancy; it does not have high affinity to LH/hCG receptors, yet promotes invasion and growth of cytotrophoblasts by interacting with transforming growth factor (TGF) β receptors [2, 9–13]. After the first three to four weeks of pregnancy, the levels of HhCG become very low and hCG is the major form [2]. Recent studies have reported additional variants with distinct sialylated oligosaccharides of the Lewis type pattern on hCG isolated from serum of pregnant women or choriocarcinoma cell lines. Differential expression of such carbohydrates is associated with inhibition of E-selectin-mediated homing of leukocytes and may contribute to early pregnancy loss through poor placental-immune interactions [14–16]. It is apparent that in the time between placentation and parturition, a dynamic structural conversion of one form of hCG to alternate forms of hCG is choreographed. This suggests that impairment or alterations in hCG glycosylation patterns may affect its signaling and biological activities.
3. hCG, angiogenesis and immune tolerance
The maternal-fetal interface is replete with immune cells which cross-talk with hormonal, endocrine, and angiogenic regulators to program a normal pregnancy outcome. Among immune cell types, regulatory T cells (Tregs), a specialized CD4 T cell subset phenotyped as CD4+/CD25+/Foxp3+, play an important role in protecting the fetus by dampening harmful inflammatory immune responses at the maternal-fetal interface. It has been shown in humans [17] that Treg numbers increase very early in pregnancy, peak during the early second trimester and then begin to decline until they reach pre-pregnancy levels. Tregs have also been shown to be crucial in immune tolerance of the fetus in the mouse pregnancy model [18] and also follow a gestational age-dependent presence in the uterus. Animal studies further indicate that tolerance to paternal antigens may be initiated during mating when seminal fluid and components of semen have been shown to trigger expansion of the Treg cell population [19]. Further, it has been shown that Tregs migrate toward areas of hCG production [20], indicating that in normal pregnancy, these cells may be attracted to hCG produced by trophoblasts at the maternal-fetal interface ensuring immune tolerance of the fetus. However, if hCG undergoes dysregulation during pregnancy, its control over immune tolerance pathways may be impaired.
Interleukin-10 (IL-10) and the tryptophan-metabolizing enzyme indoleamine 2,3-dioxygenase (IDO) are two important immune regulators. Levels of IL-10, a key immunosuppressant, increase in early pregnancy and remain elevated until the onset of labor [21], possibly regulating maternal immunity and allowing acceptance of the fetal allograft. As shown by our studies, IL-10 can regulate uNK cell maintenance and control their cytotoxic functions in response to pro-inflammatory challenges during pregnancy [22,23]. Further, decidual Tregs can inhibit immune stimulation of T cells through IL-10 production [24]. The temporal expression of IDO regulates the Tregs and prevents them from being converted to pro-inflammatory Th17 (T helper 17) cells [25]. hCG is able to stimulate IL-10 production in bone marrow derived dendritic cells (BMDC) from mice [26]. This same study found that treatment of BMDC with hCG and interferon gamma (IFN-γ) increased IDO mRNA production and enzyme activity, raising the question of whether hCG can stimulate IL-10 production and IDO activity in trophoblasts as well. Our unpublished results suggest that hCG can rescue pregnancy in IL-10−/− mice by subverting production of anti-angiogenic factors and by replenishing uterine immune cells.
It is noteworthy that hCG is now considered as an angiogenic factor [27,28] and thus may regulate an endovascular cross-talk between trophoblasts, endothelial cells, and immune cells represented by uNK cells. These specialized cells have been shown to play an important role in spiral artery remodeling and trophoblast invasion at least in animal studies [29,30]. We have recently demonstrated that vascular endothelial growth factor C (VEGF C) production by uNK cells is responsible for their non-cytotoxic activity, and that VEGF C producing uNK cells support endovascular processes in vitro [31]. It is possible that the tolerogenic phenotype of uterine NK cells during early decidualization is be influenced by hCG through stimulation of the quiescent angiogenic machinery. Recent studies indicate that the uNK cells are indeed influenced by hCG. Kane et al showed that hCG induces proliferation of human uNK cells, by interacting through the mannose receptor rather than the LH/hCG receptor [32]. Importantly, deglycosylated hCG was not able to bind to mannose receptors on uNK cells, again emphasizing the importance of carbohydrate patterns in the function of hCG.
4. hCG and preeclampsia
Preeclampsia, diagnosed by hypertension and proteinuria after 20 weeks of gestation, affects 5–10% of all pregnancies and remains a leading cause of maternal and fetal morbidity and mortality. Although based on clinical presentation, preeclampsia is considered as a late pregnancy disorder, but the molecular events leading to its onset seem to occur early in pregnancy. Portrayed as a two stage disorder, maternal symptoms of preeclampsia are considered to be consequences of pre-clinical placental pathology associated with poor placental perfusion, inflammation, ischemia/hypoxia, and trophoblast damage [33]. Despite the pro-angiogenic role of hCG, little is known about the endovascular interactions of trophoblasts and endothelial cells and its subsequent effects on spiral arteries especially in the presence of different forms of hCG. Recently we showed that injection of preeclampsia serum in pregnant IL-10−/− mice results in hypertension and proteinuria [34]. Importantly, the treatment also led to a perturbed immune cell population at the maternal-fetal interface. Interestingly, we found higher hCG levels in preeclampsia serum at term as compared to normal pregnancy serum [35]. It is possible that altered glycosylation patterns and/or presence of sialyl Lewis antigens on hCG in preeclampsia influences the recruitment and/or expansion of tolerance-imparting immune cell populations. Several studies have reported a decrease in Treg cell population both in the circulation and in placental bed sections in preeclamptic women as compared to those with normal pregnancy [3,36–38]. Since IL-10 and hCG are central to normal pregnancy outcome, it is tempting to speculate that deficiency in these molecules may predispose to severe preeclampsia pathology. Animal studies from our lab suggest that IL-10 deficient mice are more sensitive to serum- and hypoxia-induced onset of preeclampsia-like features, implying that IL-10 is likely to play a protective role against preeclampsia [34,39,40]. Preeclampsia serum [35] and 9.5% oxygen also affect uterine Treg numbers (unpublished observations). Thus given the functional associations co-regulated by hCG, IL-10 and Treg migration, it is possible that dysregulated hCG can have similar effects on uterine Tregs and contribute to preeclampsia (Fig. 1).
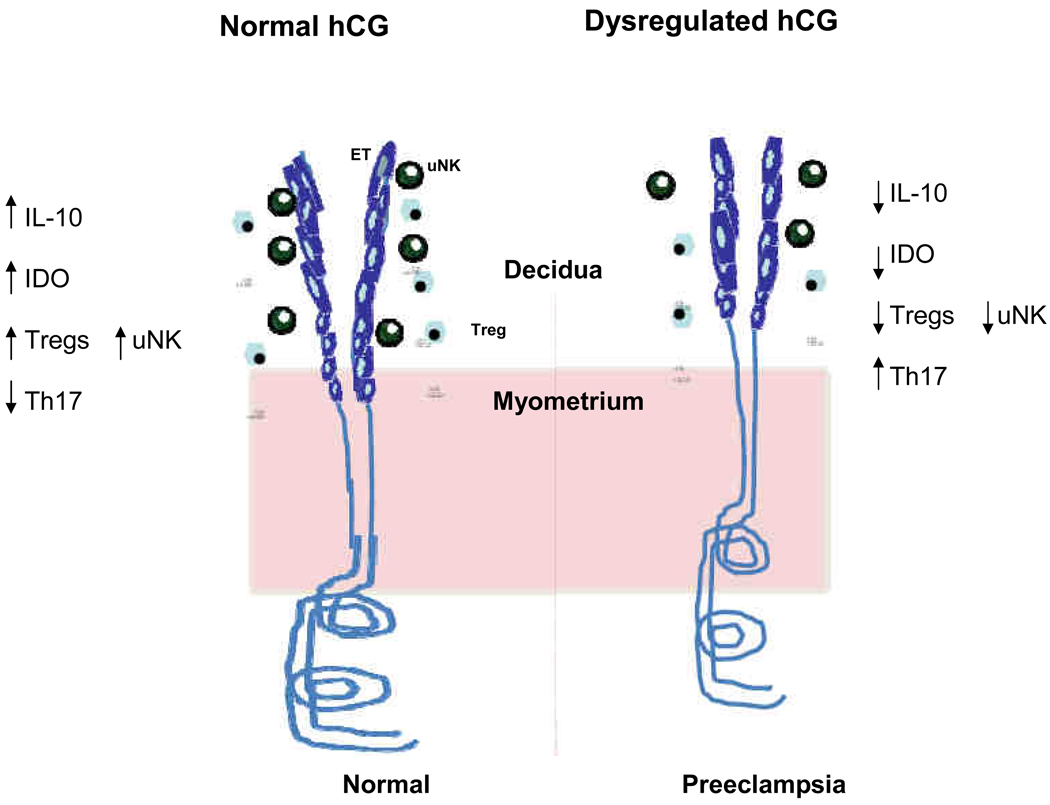
Fig. 1.
Dysregulation of hCG may lead to preeclampsia. In normal pregnancy, functional hCG, optimum expression of IL-10, and the temporal appearance of uNK and Tregs contribute to immune tolerance and angiogenesis. Dysregulated hCG due to altered structure/glycosylation may influence expression of IL-10, IDO and appearance of Tregs and uNK cells resulting in pregnancy complications such as preeclampsia. hCG: human chorionic gonadotropin, IL-10: interleukin-10, IDO: indoleamine 2,3-dioxygenase, uNK : uterine natural killer cells, Treg: regulatory T cells, ET: endovascular trophoblasts.
5. Conclusions
Preeclampsia is a disease of poorly understood etiology. Research continues to identify factors that contribute to its onset as a heterogeneous disease. hCG appears to be involved in many aspects of angiogenesis and immune tolerance (Fig. 2), prompting us to suggest that dysregulation of hCG could lead to pregnancy complications such as preeclampsia. This dysregulation could be either in the form of altered levels of hCG or modifications of the oligosaccharide side chains which have been proven to be vital in the function of hCG. Nevertheless, ramification of dysfunctional hCG that may occur in preeclampsia is likely to affect immune tolerance and angiogenesis, two vital features of successful pregnancy outcomes and needs further research. If it is indeed demonstrated that hCG plays a role in preeclampsia, it could prove to be a useful target for therapeutic intervention.
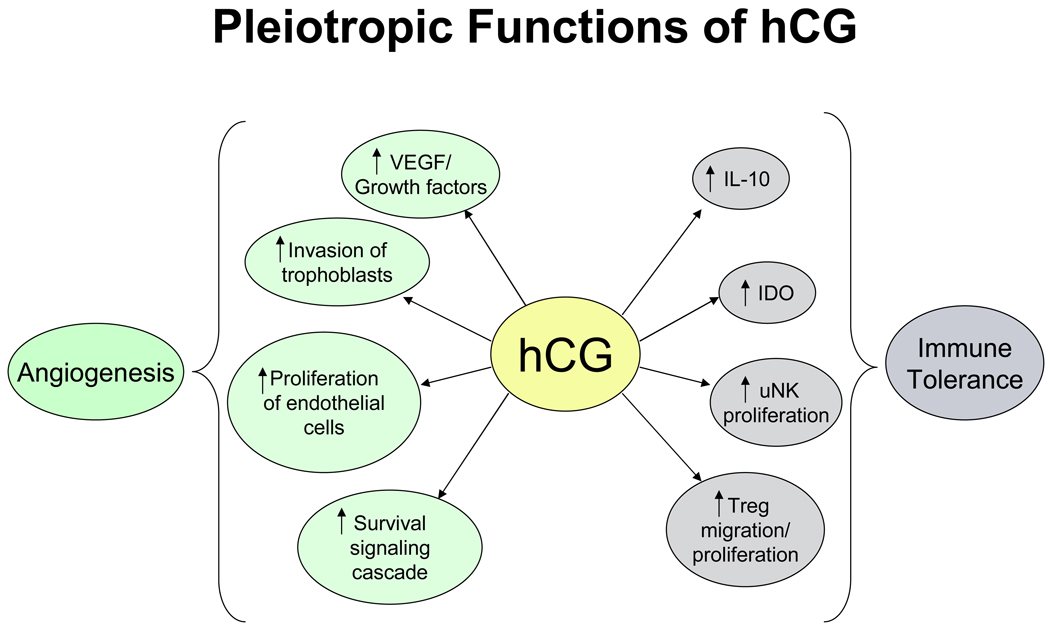
Fig. 2.
Angiogenic and immune tolerance function of hCG. hCG has many angiogenic functions such as stimulation of VEGF production, trophoblast invasion and proliferation of endothelial cells. By stimulating IL-10 and IDO expression, promoting uNK cell proliferation and Treg cell migration, hCG may play a role in immune tolerance at the maternal fetal interface. VEGF: vascular endothelial growth factor, hCG: human chorionic gonadotropin, IL-10: interleukin-10, IDO: indoleamine 2,3-dioxygenase, uNK: uterine natural killer cells, Treg: regulatory T cells.
Acknowledgements
This work was supported in part by NIH P20RR018728 and by the Rhode Island Research Alliance Collaborative Research Award 2009–28. We thank the members the Sharma lab for their critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cole LA. New discoveries on the biology and detection of human chorionic gonadotropin. Reprod Biol Endocrinol. 2009;7:8. doi: 10.1186/1477-7827-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8:102. doi: 10.1186/1477-7827-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 4.Toldi G, Svec P, Vasarhelyi B, Meszaros G, Rigo J, Tulassay T, Treszi A. Decreased number of FoxP3+ regulatory T cells in preeclampsia. Acta Obstet Gynecol Scand. 2008;87:1229–1233. doi: 10.1080/00016340802389470. [DOI] [PubMed] [Google Scholar]
- 5.Williams PJ, Bulmer JN, Searle RF, Innes BA, Robson SC. Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: a comparison with late normal pregnancy. Reproduction. 2009;138:177–184. doi: 10.1530/REP-09-0007. [DOI] [PubMed] [Google Scholar]
- 6.Cole LA, Sasaki Y, Muller CY. Normal production of human chorionic gonadotropin in menopause. N Engl J Med. 2007;356:1184–1186. doi: 10.1056/NEJMc066500. [DOI] [PubMed] [Google Scholar]
- 7.Stenman UH, Tiitinen A, Alfthan H, Valmu L. The classification, functions and clinical use of different isoforms of HCG. Hum Reprod Update. 2006;12:769–784. doi: 10.1093/humupd/dml029. [DOI] [PubMed] [Google Scholar]
- 8.de Medeiros SF, Norman RJ. Human choriogonadotropin protein core and sugar branches heterogeneity: basic and clinical insights. Hum Reprod Update. 2009;15:69–95. doi: 10.1093/humupd/dmn036. [DOI] [PubMed] [Google Scholar]
- 9.Cole LA, Kardana A, Andrade-Gordon P, Gawinowicz MA, Morris JC, Bergert ER, O'Connor J, Birken S. The Heterogeneity of Human Chorionic Gonadotropin (hCG). III. The Occurrence and Biological and Immunological Activities of Nicked hCG. Endocrinology. 1991;129:1559–1567. doi: 10.1210/endo-129-3-1559. [DOI] [PubMed] [Google Scholar]
- 10.Butler SA, Cole LA, Chard T, Iles RK. Dissociation of human chorionic gonadotropin into its free subunits is dependent on naturally occurring molecular structural variation, sample matrix and storage conditions. Ann Clin Biochem. 1998;35(Pt 6):754–760. doi: 10.1177/000456329803500608. [DOI] [PubMed] [Google Scholar]
- 11.Kovalevskaya G, Kakuma T, Schlatterer J, O'Connor JF. Hyperglycosylated HCG expression in pregnancy: cellular origin and clinical applications. Mol Cell Endocrinol. 2007;260–262:237–243. doi: 10.1016/j.mce.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Elliott MM, Kardana A, Lustbader JW, Cole LA. Carbohydrate and peptide structure of the alpha- and beta-subunits of human chorionic gonadotropin from normal and aberrant pregnancy and choriocarcinoma. Endocrine. 1997;7:15–32. doi: 10.1007/BF02778058. [DOI] [PubMed] [Google Scholar]
- 13.Cole LA. Hyperglycosylated hCG. Placenta. 2007;28:977–986. doi: 10.1016/j.placenta.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Jeschke U, Stahn R, Goletz C, Wang X, Briese V, Friese K. hCG in trophoblast tumour cells of the cell line Jeg3 and hCG isolated from amniotic fluid and serum of pregnant women carry oligosaccharides of the sialyl Lewis X and sialyl Lewis a type. Anticancer Res. 2003;23(2A):1087–1092. [PubMed] [Google Scholar]
- 15.Jeschke U, Toth B, Scholz C, Friese K, Makrigiannakis A. Glycoprotein and carbohydrate binding protein expression in the placenta in early pregnancy loss. J Reprod Immunol. 2010;85:99–105. doi: 10.1016/j.jri.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Stahn R, Goletz S, Stahn R, Wilmanowski R, Wang X, Briese V, Friese K, Jeschke U. Human chorionic gonadotropin (hCG) as inhibitior of E-selectin-mediated cell adhesion. Anticancer Res. 2005;25:1811–1816. [PubMed] [Google Scholar]
- 17.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5(3):266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 19.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlström AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80:1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schumacher A, Brachwitz N, Sohr S, Engeland K, Langwisch S, Dolaptchieva M, Alexander T, Taran A, Malfertheiner SF, Costa SD, Zimmermann G, Nitschke C, Volk HD, Alexander H, Gunzer M, Zenclussen AC. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol. 2009;182:5488–5497. doi: 10.4049/jimmunol.0803177. [DOI] [PubMed] [Google Scholar]
- 21.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol. 2000;164:5721–5728. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- 22.Thaxton JE, Romero R, Sharma S. TLR9 activation coupled to IL-10 deficiency induces adverse pregnancy outcomes. J Immunol. 2009;183(2):1144–1154. doi: 10.4049/jimmunol.0900788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy SP, Hanna NN, Fast LD, Shaw SK, Berg G, Padbury JF, Romero R, Sharma S. Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am J Obstet Gynecol. 2009;200(3) doi: 10.1016/j.ajog.2008.10.043. 308.e1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 25.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan H, Versnel MA, Cheung WY, Leenen PJ, Khan NA, Benner R, Kiekens RC. Chorionic gonadotropin can enhance innate immunity by stimulating macrophage function. J Leukoc Biol. 2007;82:926–933. doi: 10.1189/jlb.0207092. [DOI] [PubMed] [Google Scholar]
- 27.Berndt S, Perrier d'Hauterive S, Blacher S, Péqueux C, Lorquet S, Munaut C, Applanat M, Hervé MA, Lamandé N, Corvol P, van den Brûle F, Frankenne F, Poutanen M, Huhtaniemi I, Geenen V, Noël A, Foidart JM. Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB J. 2006;20(14):2630–2632. doi: 10.1096/fj.06-5885fje. [DOI] [PubMed] [Google Scholar]
- 28.Herr F, Baal N, Reisinger K, Lorenz A, McKinnon T, Preissner KT, Zygmunt M. HCG in the regulation of placental angiogenesis. Results of an in vitro study. Placenta. 2007 Apr;28 Suppl A:S85–S93. doi: 10.1016/j.placenta.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Croy BA, Esadeg S, Chantakru S, van den Heuvel M, Paffaro VA, He H, Black GP, Ashkar AA, Kiso Y, Zhang J. Update on pathways regulating the activation of uterine Natural Killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. J Reprod Immunol. 2003;59:175–191. doi: 10.1016/s0165-0378(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 30.Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63:434–444. doi: 10.1111/j.1600-0897.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 31.Kalkunte SS, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S. Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J Immunol. 2009;182:4085–4092. doi: 10.4049/jimmunol.0803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kane N, Kelly R, Saunders PT, Critchley HO. Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology. 2009;150:2882–2888. doi: 10.1210/en.2008-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts JM, Hubel CA. Is oxidative stress the link in the two–stage model of preeclampsia. Lancet. 1999;354:788–789. doi: 10.1016/S0140-6736(99)80002-6. [DOI] [PubMed] [Google Scholar]
- 34.Kalkunte S, Boij R, Norris W, Friedman J, Lai Z, Kurtis J, Lim KH, Padbury JF, Matthiesen L, Sharma S. Sera from preeclampsia patients elicit symptoms of human disease in mice and provide a basis for an in vitro predictive assay. Am J Pathol. 2010;177(5):2387–2398. doi: 10.2353/ajpath.2010.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalkunte S, Nevers T, Norris W, Banerjee P, Fazleabas A, Kuhn C, Jeschke U, Sharma S. Presence of non-functional hCG in preeclampsia and rescue of normal pregnancy by recombinant hCG. Placenta. 2010;31:A126. [Google Scholar]
- 36.Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, Shiozaki A, Rolinski J, Saito S. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre-eclampsia. Clin Exp Immunol. 2007;149:139–145. doi: 10.1111/j.1365-2249.2007.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, Nanan R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 38.Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ, Erwich JJ. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy. 2009;28:300–311. doi: 10.1080/10641950802601237. [DOI] [PubMed] [Google Scholar]
- 39.Lai Z, Kalkunte S, Sharma S. Pregnancy-specific effects of hypoxia: a mouse model for preeclampsia. Am J Reprod Immunol. 2009;61:398. [Google Scholar]
- 40.Lai Z, Kalkunte S, Sharma S. A critical role of IL-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011 doi: 10.1161/HYPERTENSIONAHA.110.163329. doi: 10.1161/HYPERTENSIONAHA.110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]