Abstract
Type 2 diabetes mellitus (T2DM), reaching epidemic proportions in humans, has emerged as a disease in aging captive populations of adult chimpanzees; however, little information is available regarding T2DM in chimpanzees. Our goals were to: (1) distinguish between normal, healthy chimpanzees and those with early (prediabetes) or advanced diabetes; (2) establish and compare the fasting (16 h) blood glucose reference range for chimpanzees at our facility with published reference ranges; and (3) establish hemoglobin A1c (HbA1c) reference intervals for healthy, nondiabetic chimpanzees and define threshold values for prediabetes and diabetes. If reliable, our reference ranges for FBG and HbA1c could become clinical tools for screening animals at risk and for monitoring therapeutic progress. The overall incidence of T2DM in our colony of 260 chimpanzees is 0.8% but is increased to 3.7% in animals older than 30 y (geriatric). For our defined reference intervals, chimpanzees with FBG or HbA1c levels up to the 85th percentile (glucose, less than or equal to 105 mg/dL; HbA1c, less than or equal to 5.0%) were considered healthy; those whose values lay between the 86th and 95th percentiles (glucose, 106 to 119 mg/dL; HbA1c, 5.1% to 5.2%) were possibly prediabetic, and animals whose values exceeded the 95th percentile (glucose, greater than or equal to 120 mg/dL; HbA1c, greater than 5.3%) were identified as potentially having diabetes. We found that our FBG range was comparable to other published results, with a positive correlation between HbA1c and glucose. Furthermore, the negligible HbA1c response to acute stress or recent food consumption suggests that HbA1c is highly useful for evaluating glycemic control during treatment of diabetic chimpanzees and is more informative concerning overall glucose control than are FBG levels alone.
Abbreviations: FBG, fasting blood glucose; HbA1c, hemoglobin A1c; T2DM, type 2 diabetes mellitus
The American Diabetes Association classifies clinical diabetes into several general subclasses: type 1, which primarily is caused by autoimmune destruction of pancreatic β cells, resulting in insulin deficiency and onset of typical symptoms of diabetes, such as polyuria, polydipsia, and weight loss; type 2 diabetes mellitus (T2DM) characterized by insulin resistance and relative insulin deficiency; gestational diabetes; and ‘other’ specific types associated with identifiable clinical conditions or syndromes.2 T2DM has most commonly been associated with advancing age and obesity,43 with its onset typically during middle-age. The development of T2DM is associated with excess adiposity or obesity but shows few symptoms in its earliest stages. In T2DM, functional failure of β cells and insulin resistance contribute to the hyperglycemic state and can lead to many complications; therefore, the overall therapeutic goal is to reduce hyperglycemia.32,36
Experimentally induced forms of insulin deficiency have led to a number of animal models of diabetes, with research primarily focused on rodents.50 Most notably, numerous primate species have been reported to develop spontaneous T2DM.12,15,16,33,34 The most studied are the macaque monkeys,4,5,18-21,61 as well as several other nonhuman primates including baboons and mandrills.10,44,52 Moreover, most macaque monkeys and baboons exhibit the same clinical characteristics as humans, including insulin resistance and a prolonged prediabetic phase of impaired β-cell function. Islet cell amyloid formation with partial β-cell loss is present in severe (advanced) diabetes in these animals.8,41,63 Furthermore, a long-term prediabetic state exists in some overweight rhesus, as demarcated by a surge in insulin levels and enhanced β-cell responsiveness. When this insulin surge begins to decline, hyperglycemia and overt T2DM are observed.18,19
According to long-term longitudinal studies of adult rhesus monkeys before and during the development of T2DM, fasting blood glucose (FBG) levels of greater than 80 mg/dL and hemoglobin A1c (HbA1c) concentrations exceeding 4.7% appear to be diagnostic of early or preT2DM in rhesus monkeys, and an FBG greater than 100 mg/dL and HbA1c greater than 5.0% are diagnostic of overt diabetes.19
Reports of diabetes in chimpanzees include a case of insulin-treated type 1 diabetes62 and several cases reported as T2DM.47,48 Prior diagnoses were based on persistent fasting hyperglycemia and the concurrent presence of glucosuria, as well as age of onset and onset of clinical symptoms.62 Little information regarding treatment options for diabetes in great apes is available, although most methods mimic those used in humans and other nonhuman primate species.16 These measures include dietary caloric restriction with forced reduction in allowed calories to produce forced weight reduction and treatment with oral glucose-lowering medications to enhance insulin output or sensitivity.16,22,63 Ultimately, daily insulin treatment is required to control T2DM in advanced cases. Monitoring of diabetes control in great apes is difficult and has traditionally been performed by routine measurement of serial fasting or random blood glucose tests.16 Isolation of chimpanzees for urine pan collection is possible. Positive reinforcement training for collection of urine samples is successful but takes time.
Prior to 2010, the recommended diagnostic criteria for diabetes in humans were based principally on FBG levels32 and were supplemented by findings of glucosuria, impaired response to glucose-tolerance testing, and hemoglobin A1c levels.3,40,43,45 The January 2010 Clinical Practice Recommendations of the American Diabetes Association elevated the importance of the HbA1c test to a diagnostic test for diabetes.2 In addition, a recent publication suggests that HbA1c may be superior to FBG as a diagnostic test for diabetes.49
Technical approaches aimed at establishing comparability of human HbA1c assays across laboratories now provide significant consistency and reliability to the HbA1c test.32 Hemoglobin A1c testing can provide reliable quantitative measurement of the level of glycated hemoglobin (HbA1c) in capillary or venous whole-blood samples.45 Protein glycation, or the nonenzymatic bonding of sugar groups to proteins such as hemoglobin, is a naturally occurring biologic process that increases under conditions of elevated glucose. Therefore, the HbA1c concentration reflects the ambient or average glucose levels over a 2- to 3-mo period, due to its dependence on slow RBC turnover.58 Therefore, the high levels of blood glucose characteristic of diabetes result in hyperglycation of hemoglobin A1c.1,43 In humans, the correlation between glycated hemoglobin A1c and blood glucose levels makes monitoring HbA1c levels useful for determination of long-term control of blood glucose.32
Because chimpanzees are our closest phylogenetic relative (greater than 99% DNA sequence identity),54 defining an HbA1c reference range for chimpanzees by using a standardized human point-of-care HbA1c test seems plausible. HbA1c values have been established definitively for rhesus macaques19 and defined in very broad terms for most nonhuman primates.12 The goal of the current study was to compare FBG values and HbA1c levels in healthy chimpanzees and those with T2DM, to define reference intervals useful for clinical guidelines and decision-making.
Materials and Methods
The chimpanzee (Pan troglodytes) population sampled at our facility (Alamogordo Primate Facility, Holloman Air Force Base, NM) consisted of 81 healthy animals (male, 32; female, 49) and 3 diabetic female chimps. Of these, 53 (male, 31; female, 22) were older than 30 y. Ages ranged from 6 to 52 y, with an average of 23.9 y. This colony was maintained in same-sex group housing, in compliance with the NIH's breeding moratorium. Healthy animals were fed a commercial primate diet (Lab Diet Monkey Jumbo 5LR2; Purina, St Louis, MO). Diabetic animals were fed a high-fiber commercial primate diet (Laboratory Fiber-Plus Monkey Diet Jumbo, 5050; Purina). All were maintained in compatible social groups of as many as 12 chimpanzees in indoor–outdoor enclosures. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals.31 The facility and its program are fully AAALAC-accredited, and all procedures were approved by our facility's IACUC.
Blood samples for measurement of FBG and HbA1c were collected by venipuncture from the 81 healthy chimpanzees during routine physical examinations performed on sedated animals (3.0 to 5.0 mg/kg IM; Telazol, Fort Dodge Animal Health, Fort Dodge, IA) that had been fasted for 16 h. FBG and HbA1c values were measured in whole blood. Health assessments were made at the time of the blood draw. Healthy chimpanzees were defined as those not on medications and with no previous disease diagnosis (that is, cardiovascular disease, renal disease, and others).
Diabetic animals had been diagnosed previously based on persistent elevated FBG levels, presence of glucose in the urine, and clinical response to oral glucose-lowering medications. Blood sampling of the 3 chimpanzees with T2DM was performed either by venipuncture of sedated animals (Telazol, 3.0 to 5.0 mg/kg IM) or by cooperative heel stick performed cageside by using standard lancet technique.
Blood glucose was analyzed automatically (Vetscan VS2 analyzer with Comprehensive Diagnostic Profile, Abaxis, Union City, CA) by using a modified hexokinase method. To reduce preanalytical variability, each rotor in the analyzer has built-in control standards that must pass quality-control measures, or the entire run is failed.
HbA1c was determined by using a commercial kit (A1cNow, Bayer Healthcare, Sunnyvale, CA) and was reported as a percentage (ratio of glycated hemoglobin A1c to total hemoglobin). The kit used is a point-of care test that uses both immunoassay and chemical technology to measure HbA1c and total hemoglobin. The preanalytical variability in the HbA1c assay was controlled by the use of blood samples having known HbA1c concentrations, as determined by a laboratory certified through the National Glycohemoglobin Standardization Program and following program-established reference methods.39
Statistical methods.
All statistical analyses were performed by using SyStat (version 11.0, SyStat Software, Richmond, CA). HbA1c and glucose levels both underwent ANOVA, with statistical significance determined by omnibus F tests.51 Shapiro–Wilks goodness-of-fit tests were used to test the assumption of normal (Gaussian) distribution. Data not normally distributed were transformed by using the power family of transformations:
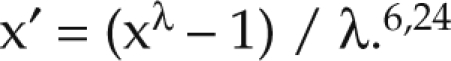 |
For FBG and HbA1c levels, the λ parameter was estimated by maximum likelihood at 0, resulting in a natural log transformation.24 Outliers were identified and eliminated by using the 1.5 interquartile range method.27,57
In human populations, age and sex are fundamental determinants of the distribution of health and illness.13 Because similar effects have been observed in chimpanzees,26,59 all statistical models initially included age and sex as covariates. Weight also was included as a covariate, because the link between obesity and T2DM in chimpanzees has already been established.60 Two decision rules were used for partitioning: 1) greater than or equal to 10% reduction of subgroup standard deviations after partitioning; and 2) a z* statistic that exceeded the critical value of 5.0.23,24,27 Reference intervals and percentiles of the normalized distribution were generated by using the nonparametric Harrell–Davis bootstrap method.25,29,64
Results
Determination of glucose reference range for chimpanzees.
Neither age (F1, 76 = 0.012, P = 0.341), sex (F1, 76 = 3.57, P = 0.063), nor body weight (F1, 76 = 0.081, P = 0.672) were significant predictors of fasting whole-blood glucose levels in healthy adult chimpanzees. The median level of fasting (16 h) glucose concentration among healthy chimpanzees was 88 mg/dL. We defined values up to the 85th percentile of the distribution (105 mg/dL) as representing healthy animals. Prediabetic chimpanzees were defined as having glucose levels between the 86th and 95th percentiles (106 to 119 mg/dL). Potentially diabetic chimpanzees were defined as those whose FBG were greater than or equal to the 95th percentile (120 mg/dL).
Determination of HbA1c reference range for chimpanzees.
Neither age (F1, 76 = 2.29, P = 0.135), body weight (F1, 76 = 0.81, P = 0.370), nor sex (F1, 76 = 2.69, P = 0.105) were significant predictors of HbA1c levels in healthy chimpanzees. The median level of HbA1c was 4.8%. The reference interval for healthy chimpanzees was defined as less than the 85th percentile (less than or equal to 5.0%). The interval of the 86th to 94th percentiles was defined as indicating prediabetic chimpanzees (5.1% to 5.2%). Animals with 5.3% HbA1c or more were classified as potentially diabetic.
Association between glucose and HbA1c.
The statistical relationship between HbA1c and glucose was analyzed by correlation and regression analyses. The Pearson correlation coefficient indicated that the relationship between these 2 biochemical markers was strongly linear (r = 0.462, P < 0.000). Linear regression11 further revealed that neither age (t = −0.267, P = 0.791) nor body weight (t = −0.166, P = 0.869) were significantly associated with observed glucose levels. Expected glucose levels inferred from HbA1c levels were strongly associated with the observed glucose concentration (t = 4.61, P < 0.000), and the model explained 21.4% of the variation.
Discussion
The FBG intervals defined here for chimpanzees corresponded to accepted reference values in healthy, prediabetic, and diabetic humans2 as well as to chimpanzee reference ranges established at other facilities.17,26,28,30,53,59 HbA1c reference values for healthy, prediabetic, and diabetic chimpanzee levels differed from human levels by approximately 1%, but the FBG value used for diagnosis of diabetes in humans is slightly higher (126 mg/dL) than that for chimpanzees (Table 1).2 Despite their close phylogenetic relationship to humans, chimpanzees have many differences in genes, gene expression patterns, and copy number variation that potentially could affect glucose metabolism.42 Compared with those for chimpanzees, the values for HbA1c and FBG are slightly lower in rhesus macaques, for which HbA1c greater than 5% and FBG exceeding 100 mg/dL are identified as indicative of overt diabetes.19 Therefore, HbA1c values show some species-specific variation.
Table 1.
Reference values for normal, glucose-impaired (prediabetic), and diabetic humans, chimpanzees, and rhesus macaques
| Fasting blood glucose (mg/dL) | HbA1c (%) | ||
| Humans | |||
| High healthy | 70–99 | ≤ 5.6 | |
| Glucose impaired | 100–125 | 5.7–6.4 | |
| Diabetic | ≥126 | ≥6.5 | |
| Chimpanzees | |||
| High healthy | ≤105 | ≤5.0 | |
| Glucose impaired | 106–119 | 5.1–5.2 | |
| Diabetic | ≥120 | ≥5.3 | |
| Rhesus macaques | |||
| High healthy | <80 | <4.7 | |
| Glucose impaired | 81–100 | 4.7–5.0 | |
| Diabetic | >100 | >5.0 |
Values for humans are from reference 2; those for chimpanzees and rhesus are from reference 19.
The FBG (16 h) reference range mean of 88 mg/dL that we determined for chimpanzees was comparable to similar reports in the literature, in which the reported mean ranged from 92 to 99 mg/dL (Table 2). Although we found no significant difference in values between sexes, another study did,26 based on larger numbers of animals sampled over a 5-y period. Some variation was seen due to age, with most occurring in the youngest age groups; however, the variation was closely related from year to year within individual chimpanzees.26 In rhesus monkeys, changes in FBG and HbA1c values over time have been demonstrated to be associated with disease rather than age.55
Table 2.
Mean fasting blood glucose values in sedated chimpanzees at various facilities
| Age of animals (y) | No. of animals sampled | Fasting blood glucose (mg/dL) | Sex-associated difference? | |
| Facility 1 | >8 | 550 | 99 | Not reported |
| Facility 2 | 6–10 | 49 | 88 | Not reported |
| >10 | 76 | 85 | Not reported | |
| Facility 3 | 4–6.9 | 29 | 88 | None |
| 7–9.9 | 27 | 87 | None | |
| >10 | 37 | 84 | None | |
| Facility 4 | 4–26 | 25 | 82 | None |
| Facility 5 | 0–50 | 252 | 93.6 | Yes |
| Our facility | 6–52 | 81 | 88 | None |
Data for facility 1 obtained from reference 53; facility 2, reference 30; facility 3, reference 28; facility 4, reference 17; and facility 5, reference 26.
Once our reference ranges were determined, we were able to better evaluate glycemic control in the 3 T2DM cases (Table 3). We had presumed that an HbA1c value of 7% or less was an indicator of diabetic control. This assumption was based on the recommendations of the American Diabetes Association for humans diagnosed with T2DM.2 We are now making therapeutic adjustments to maintain HbA1c levels below 6% in these chimpanzees. Over the last several years, these 3 cases have all responded fairly well to Glucophage (metformin; Bristol-Myers Squibb, Princeton, NJ), with the consistent trend of requiring higher dosages to provide euglycemia (Table 3). Metformin acts by increasing the sensitivity of the liver, muscle, fat, and other tissues to the uptake and effects of insulin, thereby lowering blood glucose levels. We have added glipizide, a sulfonylurea-class hypoglycemic drug, to further decrease blood glucose levels in chimpanzee CB0090 (Table 3). Glipizide's methods of action are not entirely understood; however, in general, it stimulates insulin release from the pancreas and reduces hepatic glucose release. For chimpanzee 778, tighter glycemic control is needed, given that her HbA1c exceeds 6.0%. Because all 3 of these chimpanzees are geriatric, other disease processes are likely, and the use of medications such as metformin and glipizide must be balanced with declining renal function and other needed medications. Weight loss can be difficult to maintain in socially housed groups of chimpanzees, particularly when the animals are uncooperative toward increasing their exercise levels; therefore, achieving optimal therapeutic goals is challenging.
Table 3.
Three cases of T2DM in chimpanzees with clinical history of mean fasting blood glucose and hemoglobin A1c values
| Animal | Description | Year | Weight (kg) | Mean fasting blood glucose (mg/dL) | Mean HbA1c (%) | Notes |
| CB0090 | Adult female; born 1 Jan 1971; HIV (+) | 2001 | 58 | 360 | not tested | T2DM diagnosed; began glucophage 850 mg twice daily; started high-fiber diet. |
| 2002 | 48.5 | 127 | not tested | Glucophage increased to 1063 mg twice daily. | ||
| 2003 | 62 | 132 | not tested | Renal cyst found on ultrasound exam. | ||
| 2004 | 62 | 342 | 10.2 | Systemic hypertension diagnosed. | ||
| 2005 | 65 | 135 | Glucophage increased to 2000 mg daily. Began glipizide 5 mg daily. | |||
| 2006 | 65.5 | 122 | 5.3 | |||
| 2007 | 66 | 106 | 5.5 | |||
| 2008 | 61 | 121 | 5.6 | |||
| 2009 | 61 | 141 | 4.6 | Slight anemia: A1c perhaps decreased artifactually due to decreased RBC. | ||
| 2010 | 58 | 109 | 4.7 | |||
| 836 | Adult female; born 1 Jan 1971; Hepatitis C (+) | 2003 | not tested | 148 | ||
| 2004 | not tested | 155 | 5.9 | |||
| 2005 | 51.5 | 144 | 6.3 | T2DM diagnosed; began glucophage 850 mg twice daily. | ||
| 2006 | 47 | 107 | 6.0 | |||
| 2007 | 46.5 | 112 | 5.6 | |||
| 2008 | 50 | not tested | 7.3 | Began high-fiber diet. | ||
| 2009 | 47.5 | 140 | 6.4 | |||
| 2010 | 45 | 110 | 5.6 | |||
| 778 | Adult female; born 23 July 1972 | 2005 | not tested | not tested | not tested | T2DM diagnosed; began glucophage 850 mg twice daily. |
| 2006 | 63.5 | 118 | 5.7 | |||
| 2007 | 64 | 110 | 5.6 | |||
| 2008 | 64.5 | 116 | 6.2 | Began high-fiber diet. | ||
| 2009 | 65.5 | 139 | 8.7 | |||
| 2010 | 66.5 | 118 | 6.6 | Better control needed—evaluate at next scheduled exam. |
HbA1c testing has some potential drawbacks. Disease conditions that affect RBC or hemoglobin will affect results. Variance in human HbA1c values is influenced by high versus low glycators,9,35,46 multiple glycated residues on α or βchains,38 differential glycation by red cell age,9,14,56 individual variation in the rate of glycation or the A1c set point,35,37,65 and different ratios of hemoglobin fractions.7 Some of these factors are likely to influence HbA1c results in chimpanzees as well.
A limitation of our study was the use of only a single sampling point per animal for determination of the ranges of FBG and HbA1c levels in healthy chimpanzees. Future clinical investigations will include continued FBG and HbA1c sampling of the colony. The use of larger sample sizes and addition of phenotypic parameters should allow further evaluation of the effects of abdominal circumference and body condition score on FBG and HbA1c levels. Collection and testing of urine for glucose will be performed during future examinations as well. In addition, measurement of insulin levels could be of great benefit but was not performed at the time of sampling in the current study. Whether HbA1c testing in chimpanzees will lead to earlier identification of impaired glucose tolerance (prediabetes) will require collection of more extensive longitudinal data.
Because HbA1c can be used reliably to predict fasting glucose over the preceding 90 to 120 d, daily or weekly heel-sticks of chimpanzees requiring monitoring (prediabetic animals or those on oral antidiabetic medications) potentially could be abandoned in favor of bimonthly or monthly monitoring of HbA1c during the period leading up to overt diabetes. Given the increasing prevalence of obesity among aging chimpanzees, such monitoring will become increasingly important to clinical veterinarians. A general association between obesity and increasing blood glucose values has been demonstrated in chimpanzees60 but was not observed in our study, perhaps due to the small numbers of confirmed cases (n = 3).
The advantages of the HbA1c test for monitoring include the ability to test cageside, reduced frequency for monitoring when compared with monitoring glucose levels alone, the convenience and animal welfare benefit of not requiring overnight fasting, and lack of acute environmental effects (stress or food-related) on values. In addition, HbA1c may better identify animals that are at risk of developing T2DM, even while the annual FBG value is within the normal range, because HbA1c is elevated due to increased postprandial glucose even while FBG remains normal. All of these factors make HbA1c testing particularly useful for consideration in screening captive chimpanzee populations and managing diabetic chimpanzees. Data from the 3 diabetic chimpanzees demonstrated the usefulness of HbA1c in the identification and monitoring of diabetic or prediabetic chimpanzees, in that we were better able to define the need to change treatment when HbA1c was added to our program.
In the current study, we define reliable and clinically useful reference intervals for FBG and HvA1c concentrations for use in glycemic control of captive chimpanzees with T2DM. HbA1c appears to offer additional advantages for clinical assessment over monitoring of FBG levels alone. These reference intervals can be used as aids in screening for and management of T2DM in captive chimpanzees.
Acknowledgments
We thank Stephen Curtis, Michael L Lammey, and Debbie Hernandez with assistance in the data collection phase of this project. Special thanks also to Tony Zavaskis and Rudy Hernandez for data entry and to Elaine Videan for revision guidance. This work was funded by National Center for Research Resources, NIH, no. N02-RR-1-209.
References
- 1.Ahmed N, Thornalley PJ. 2007. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab 9:233–245 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association 2010. Standards of medical care in diabetes, 2010. Diabetes Care 33 Suppl 1:S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett CM, Guo M, Dharmage SC. 2007. HbA1c as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med 24:333–343 [DOI] [PubMed] [Google Scholar]
- 4.Bodkin NL. 2000. The rhesus monkey (Macaca mulatta): a unique and valuable model for the study of spontaneous diabetes mellitus and associated conditions, p 309–325 In: Sima AF, Shafrir E. Animal models in diabetes: a primer. Oxford (UK): Taylor and Francis [Google Scholar]
- 5.Bodkin NL, Metzger BL, Hansen BC. 1989. Hepatic glucose production and insulin sensitivity preceding diabetes in monkeys. Am J Physiol 256: E676–E681 [DOI] [PubMed] [Google Scholar]
- 6.Box G, Cox D. 1964. An analysis of transformations. J R Stat Soc Series B Stat Methodol 26:211–252 [Google Scholar]
- 7.Bunn HF, Haney DN, Kamin S, Gabbey KH, Gallop PM. 1976. The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J Clin Invest 57:1652–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cefalu WT. 2006. Animal models of type 2 diabetes: clinical presentation and pathophysiological relevance to the human condition. ILAR J 47:186–198 [DOI] [PubMed] [Google Scholar]
- 9.Cohen RM. 2007. A1c: does one size fit all? Diabetes Care 30:2756–2758 [DOI] [PubMed] [Google Scholar]
- 10.Cromeens DM, Stephens LC. 1985. Insular amyloidosis and diabetes mellitus in a crab-eating macaque (Macaca fasicularis). Lab Anim Sci 35:642–645 [PubMed] [Google Scholar]
- 11.Draper N, Smith H. 1981. Applied regression analysis, 2nd ed New York (NY): Wiley [Google Scholar]
- 12.Dutton CJ, Parvin CA, Gronowski AM. 2003. Measurement of glycated hemoglobin percentages for use in the diagnosis and monitoring of diabetes mellitus in nonhuman primates. Am J Vet Res 64:562–568 [DOI] [PubMed] [Google Scholar]
- 13.Friis RH, Sellers TA. 2009. Epidemiology for public health practice, 4th ed Sudbury (MA): Jones and Bartlett [Google Scholar]
- 14.Fitzgibbons JF, Koler RD, Jones RT. 1976. Red cell age-related changes of hemoglobins A1a+b and A1c in normal diabetic subjects. J Clin Invest 58:820–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilardi KVK, Valverde CR. 1995. Glucose control with glipizide therapy in a diabetic dusky titi monkey (Callicebus moloch). J Zoo Wildl Med 26:82–86 [Google Scholar]
- 16.Gresl TA, Baum ST, Kemnitz JW. 2000. Glucose regulation in captive Pongo pygmaeus abeli, P. p. pygmaeus, and P. p. abeli × P. p. pygmaeus organutans. Zoo Biol 19:193–208 [Google Scholar]
- 17.Hainsey BM, Hubbard GB, Leland MM, Brasky KM. 1993. Clinical parameters of the normal baboons (Papio species) and chimpanzees (Pan troglodytes). Lab Anim Sci 43:236–243 [PubMed] [Google Scholar]
- 18.Hansen BC. 2003. Primate animal models of type 2 diabetes mellitus. In: LeRoith D, Taylor SI, Olefsky JM. Diabetes mellitus: a fundamental and clinical text, 3rd ed Philadelphia (PA): Lippincott Williams and Wilkins [Google Scholar]
- 19.Hansen BC. 2010. The evolution of diabetes in nonhuman primates: comparative physiology implications for human type 2 diabetes mellitus (T2DM). FASEB J 24:1055 [Google Scholar]
- 20.Hansen BC, Bodkin NL. 1986. Heterogeneity of insulin responses: phases leading to type 2 (noninsulin-dependent) diabetes mellitus in the rhesus monkey. Diabetologia 29:713–719 [DOI] [PubMed] [Google Scholar]
- 21.Hansen BC, Bodkin NL. 1990. β-cell hyperresponsiveness: earliest event in development of diabetes in monkeys. Am J Physiol 259:R612–R617 [DOI] [PubMed] [Google Scholar]
- 22.Hansen BC, Bodkin NL. 1993. Primary prevention of diabetes mellitus by prevention of obesity in monkeys. Diabetes 42:1809–1814 [DOI] [PubMed] [Google Scholar]
- 23.Harris EK, Boyd JC. 1990. On dividing reference data into subgroups to produce separate reference intervals. Clin Chem 36:265–270 [PubMed] [Google Scholar]
- 24.Harris EK, Boyd JC. 1995. Statistical basis of reference values in laboratory medicine. New York (NY): Marcel Dekker [Google Scholar]
- 25.Harrell FE, Davis CE. 1982. A new distribution-free quantile estimator. Biometrika 69:635–640 [Google Scholar]
- 26.Herndon JG, Tigges J. 2001. Hematologic and blood biochemical variables of captive chimpanzees: cross-sectional and longitudinal analyses. Comp Med 51:60–69 [PubMed] [Google Scholar]
- 27.Horn PS, Pesce AJ. 2005. Reference intervals: a user's guide. Washington (DC): AACC Press [Google Scholar]
- 28.Howell S, Hoffman K, Bartel L, Schwandt M, Morris J, Fritz J. 2003. Normal hematologic and serum clinical chemistry values for captive chimpanzees (Pan troglodytes). Comp Med 53:413–423 [PubMed] [Google Scholar]
- 29.Hutson AD, Ernst MD. 2000. The exact bootstrap mean and variance of an L estimator. J R Stat Soc Series B Stat Methodol 62:89–94 [Google Scholar]
- 30.Ihrig M, Tassinary LG, Bernacky B, Keeling ME. 2001. Hematologic and serum biochemical reference intervals for the chimpanzee (Pan troglodytes) characterized by age and sex. Comp Med 51:30–37 [PubMed] [Google Scholar]
- 31.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 32.International Expert Committee 2009. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishizaka T, Sato T, Kato K, Ohba M, Kimotsuki T, Yasuda M. 2003. Subcutaneous continuous glucose monitoring and dose adjustment decreases glycosylated hemoglobin in spontaneously diabetic cynolmolgus monkeys. Contemp Top Lab Anim Sci 42:36–40 [PubMed] [Google Scholar]
- 34.Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, Rudel LL, Wagner JD. 2007. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring) 15:1666–1674 [DOI] [PubMed] [Google Scholar]
- 35.Kilpatrick ES, Maylor PW, Keevil BG. 1998. Biological variation of glycated hemoglobin. Implications for diabetes screening and monitoring. Diabetes Care 21:261–264 [DOI] [PubMed] [Google Scholar]
- 36.Masharani U. 2008. Diabetes mellitus and hypoglycemia, p 1032–1073 In: McPhee SJ, Papdakis MA, Tierney LM., Jr Lange current medical diagnosis and treatment, 47th ed New York (NY): McGraw–Hill [Google Scholar]
- 37.Meigs JB, Nathan DM, Cupples LA, Wilson PW, Singer DE. 1996. Tracking of glycated hemoglobin in the original cohort of the Framington Heart Study. J Clin Epidemiol 49:411–417 [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi T, Miyazaki A, Shimizu A, Yamaguchi A, Nishimura S. 2002. Assessment of the effect of hemoglobin variants on routine HbA1c measurements by electrospray ionization mass spectrometry. Clin Chim Acta 323:89–101 [DOI] [PubMed] [Google Scholar]
- 39.NGSP [Internet]. 2010. Background. [Cited 1 July 2010]. Available at: http://www.ngsp.org/bground.asp
- 40.Nichols GA, Hillier TA, Brown JB. 2008. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med 121: 519–524 [DOI] [PubMed] [Google Scholar]
- 41.O'Brien TD, Wagner JD, Litwak KN, Carlson CS, Cefalu WT, Jordan K, Johnson KH, Butler PC. 1996. Islet amyloid and islet amyloid polypeptide in cynomolgus macaques (Macaca fasicularis): an animal model of human noninsulin-dependent diabetes mellitus. Vet Pathol 33:479–485 [DOI] [PubMed] [Google Scholar]
- 42.Perry GH, Yang F, Marques-Bonet T, Murphy C, Fitzgerald T, Lee AS, Hyland C, Stone AC, Hurles ME, Tyler-Smith C, Eichler EE, Carter NP, Lee C, Redon R. 2008. Copy number variation and evolution in humans and chimpanzees. Genome Res 18:1698–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters AL, Davidson MB, Schriger DL, Hasselblad V. 1996. A clinical approach for the diagnosis of diabetes mellitus: an analysis using glycosylated hemoglobin levels. Meta-analysis Research Group on the Diagnosis of Diabetes Using Glycated Hemoglobin. J Am Med Assoc 276:1246–1252 [PubMed] [Google Scholar]
- 44.Pirarat N, Kesdangsakolwut SS, Chotiapisitkul S, Assarasakorn S. 2008. Spontaneous diabetes mellitus in captive Mandrillus sphinx monkeys: a case report. 2008. J Med Primatol 37:162–165 [DOI] [PubMed] [Google Scholar]
- 45.Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, Flegal KM, Eberhardt MS, Goldstein DE. 2000. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the US population. Diabetes Care 23:187–191 [DOI] [PubMed] [Google Scholar]
- 46.Rohlfing C, Weidmeyer HM, Little R, Grotz VL, Tennill A, England J, Madsen R, Goldstein D. 2002. Biological variation of hemoglobin. Clin Chem 48:1116–1118 [PubMed] [Google Scholar]
- 47.Rosenblum IY, Barbolt TA, Howard CF. 1981. Diabetes mellitus in the chimpanzee (Pan troglodytes). J Med Primatol 10:93–101 [DOI] [PubMed] [Google Scholar]
- 48.Rosenblum IY, Coulston F. 1983. Impaired renal function in diabetic chimpanzees (Pan troglodytes). Exp Mol Pathol 38:224–229 [DOI] [PubMed] [Google Scholar]
- 49.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. 2010. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaffir E. 2007. Animal models of diabetes, 2nd ed Boca Raton (FL): CRC Press [Google Scholar]
- 51.Snedecor GW, Cochran WG. 1967. Statistical methods, 6th ed Ames (IA): Iowa State University Press [Google Scholar]
- 52.Stokes WS. 1986. Spontaneous diabetes mellitus in a baboon (Papio cynocephalus anubis). Lab Anim Sci 36:529–533 [PubMed] [Google Scholar]
- 53.Stone GA, Johnson BK, Druilhet R, Garza PB, Gibbs CJ. 2000. Immunophenotyping of peripheral blood, ranges of serum chemistries and clinical hematology values of healthy chimpanzees (Pan troglodytes). J Med Primatol 29:324–329 [DOI] [PubMed] [Google Scholar]
- 54.The Chimpanzee Sequencing and Analysis Consortium 2005. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 437:69–87 [DOI] [PubMed] [Google Scholar]
- 55.Tingo XT, Gerzanich G, Hansen BC. 2004. Age-related changes in metabolic parameters of nonhuman primates. J Gerontol A Biol Sci Med Sci 59:1081–1088 [DOI] [PubMed] [Google Scholar]
- 56.Tahara Y, Shima K. 1995. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care 18:440–447 [DOI] [PubMed] [Google Scholar]
- 57.Tukey JW. 1977. Exploratory data analysis. New York (NY): Addison-Wesley [Google Scholar]
- 58.Ulrich P, Cerami A. 2001. Protein glycation, diabetes, and aging. Recent Prog Horm Res 56:1–21 [DOI] [PubMed] [Google Scholar]
- 59.Videan EN, Fritz J, Murphy J. 2008. Effects of aging on hematology and serum clinical chemistry in chimpanzees (Pan troglodytes). Am J Primatol 70:327–338 [DOI] [PubMed] [Google Scholar]
- 60.Videan EN, Fritz J, Murphy J. 2007. Development of guidelines for assessing obesity in captive chimpanzees (Pan troglodytes). Zoo Biol 26:93–104 [DOI] [PubMed] [Google Scholar]
- 61.Wagner JD, Carlson CS, O'Brien TD, Anthony MS, Bullock BC, Cefalu WT. 1996. Diabetes mellitus and islet amyloidosis in cynomolgus monkey. Lab Anim Sci 46:36–41 [PubMed] [Google Scholar]
- 62.Wagner JD, Schmidtke DW, Quinn CP, Fleming TF, Bernacky B, Heller A. 1998. Continuous amperometric monitoring of glucose in a brittle diabetic chimpanzee with a miniature subcutaneous electrode. Proc Natl Acad Sci USA 95:6379–6382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagner JE, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ, Jr, Kaplan JR. 2006. Old world nonhuman primate models of type 2 diabetes mellitus. ILAR J 47:259–271 [DOI] [PubMed] [Google Scholar]
- 64.Wessa [Internet]. 2006. Harrell–Davis quantile estimator (v1.0.8) in free statistics software (v1.1.20), Office for Research Development and Education. [Cited 18 December 2009]. Available at: http://www.wessa.net/rwasp_harrell_davies.wasp
- 65.Yudkin JS, Forrest RD, Jackson CA, Ryle AJ, Davie S, Gould BJ. 1990. Unexplained variability of glycated haemoglobin in nondiabetic subjects not related to glycaemia. Diabetologia 33:208–215 [DOI] [PubMed] [Google Scholar]


