Abstract
Postoperative pain management in laboratory animals relies heavily on a limited number of drug classes, such as opioids and nonsteroidal antiinflammatory drugs. Here we evaluated the effects of saline, tramadol, tramadol with gabapentin, and buprenorphine (n = 6 per group) in a rat model of incisional pain by examining thermal hyperalgesia and weight-bearing daily for 6 d after surgery. All drugs were administered preemptively and continued for 2 consecutive days after surgery. Rats treated with saline or with tramadol only showed thermal hyperalgesia on days 1 through 4 and 1 through 3 after surgery, respectively. In contrast, buprenorphine-treated rats showed no thermal hyperalgesia on days 1 and 2 after surgery, and rats given tramadol with gabapentin showed reduced thermal hyperalgesia on days 2 and 4. For tests of weight-bearing, rats treated with saline or with tramadol only showed significantly less ipsilateral weight-bearing on day 1 after surgery, whereas rats given either buprenorphine or tramadol with gabapentin showed no significant change in ipsilateral weight-bearing after surgery. These data suggest that tramadol alone provides insufficient analgesia in this model of incisional pain; buprenorphine and, to a lesser extent, tramadol with gabapentin provide relief of thermal hyperalgesia and normalize weight-bearing.
Abbreviation: GABA, γ-aminobutyric acid
Pain management poses considerable challenges within the field of veterinary medicine. Administration of many analgesic drugs may cause unwanted side effects, creating an inappropriate model for the researcher. Opioids and nonsteroidal antiinflammatory drugs both may interfere with immunologic studies and behavioral testing.11,40 Effective pain control improves the recovery process, helps animals to return to normal behaviors, and maintains the physiologic status of animals.25 Preemptive and multimodal analgesic approaches are recommended to obtain optimal pain control. When doubt exists as to whether an animal is in pain, analgesic drugs should be administered.
Postoperative pain is one of the most common types of pain in the laboratory animal setting. It is a complex multisystemic response, with hypersensitivity to many stimuli,25 including hyperalgesia to thermal and mechanical6,7,18,44 stimuli. Although many opiates blunt this sensitization, other classes of drugs (N-methyl-D-aspartate, antidepressants, and others) have various effects, depending on the modality of pain studied.33,45
Buprenorphine, a partial μ-opioid receptor agonist, is a commonly used postoperative analgesic drug for many laboratory animal species due to its prolonged plasma half-life.16 Prior studies showing decreased incidence of ceiling effects or side effects, such as respiratory depression,14 in addition to well-established analgesia, make buprenorphine an appropriate choice for alleviation of mild to moderate pain.31,37 Recent evidence suggests that the submaximal response to buprenorphine at high doses may be due to interaction with the non-μ class of opioid-receptor-like (ORL1) receptors which, when upregulated, may attenuate the analgesic effects of buprenorphine.27,28 These data, coupled with the resistance of buprenorphine to naloxone reversal,19 add to the complexity of its analgesic mechanism and subsequent potential side effects.
Tramadol, a centrally acting analgesic, is known to provide pain relief by means of its primary metabolite, O-desmethyl-tramadol (approximate 600-fold higher μ-opioid receptor affinity than that of tramadol and 10-fold lower than that of morphine) through interaction with opioid receptors and the blockade of serotonin.4,21,43 Tramadol also acts on the descending pain pathway through norepinephrine and seratonin uptake inhibition for antinociceptive effects at the level of the medulla through the α-adrenergic pathway. These early studies may explain in part tramadol's lack of complete naloxone-induced reversal and withdrawal.21 Gabapentin is an antiepileptic drug which is a structural analog of γ-aminobutyric acid (GABA). Despite the suggestive name, gabapentin lacks demonstrable interaction with either GABAA or GABAB receptors within the CNS. Like other anticonvulsant drugs, gabapentin has been studied for the additional potential benefit of analgesia in models of neuropathic pain.29 More recently, evidence has been collected supporting the potential efficacy of gabapentin in blocking other pain pathways, surgical and inflammatory pain being 2 such examples.9,10 Although the mechanism of action contributing to these analgesic effects has yet to be elucidated fully, several studies have documented the utility of gabapentin, especially when combined with morphine.15,30 In 1996, several authors proposed a novel mechanism of action, showing high affinity of gabapentin for the α2δ subunit of a centrally located voltage-dependent calcium channel.20 Other groups have examined further potential analgesic qualities of gabapentin and suggest its utility as an antihyperalgesic rather than an antinociceptive agent.17
The potential nonopioid analgesic action of both tramadol and gabapentin, coupled with gabapentin's lack of cross tolerance when administered chronically in combination with morphine15 and the lack of a withdrawal response for after discontinuation of tramadol,21 make these drugs ideal candidates for study in adjunctive therapies in analgesia. An additional benefit is that neither tramadol nor gabapentin is a federally controlled substance category, thereby enhancing their practicality in the laboratory setting. Our goal in the current study was to test the hypothesis that the analgesic effects of tramadol used alone or in combination with gabapentin in a rat model of incisional pain are similar to or better than those of buprenorphine alone.
Materials and Methods
Animal subjects.
Experiments were performed on adult (weight, 300 to 350 g) male Sprague–Dawley rats (Charles River, Wilmington, MA). Rats were housed in groups of 3 in appropriately sized static microisolation cages with hardwood bedding (Sani-Chips, PJ Murphy Forest Products, Montville, NJ) in a climate-controlled room on a 12:12-h dark:light cycle with ad libitum access to food (LabDiet 5001, Purina Mills International, St Louis, MO) and water purified by reverse osmosis. All experiments were reviewed and approved by the Administrative Panel for Laboratory Animal Care at Stanford University. All animals used herein were treated in accordance with the Guide for the Care and Use of Laboratory Animals.23 All rats were weighed once before surgery and then every other day after surgery for the duration of the study. At the end of the study, all rats were euthanized by carbon dioxide asphyxiation.
Drug administration.
Tramadol HCl (Sigma, St Louis, MO) was administered intraperitoneally in 0.9% saline. Gabapentin (McKesson, Concord, NC) and buprenorphine (Hospira, Lake Forest, IL) were administered subcutaneously in 0.9% sterile saline. Solutions of tramadol and gabapentin were made fresh daily by passing through a 0.44-μm filter after dissolution and before injection. All drugs were given at a total volume of 1 mL/kg. Dosing determination was based on current publications.13,24,26,38
Surgery.
For all rats, anesthesia was induced by using isoflurane in pure oxygen inside an induction chamber. Once unconscious, rats were removed and placed on a nonrebreathing anesthetic circuit with mask delivery of isoflurane in pure oxygen throughout the procedure. A subcutaneous injection of cefazolin (20 mg/kg) was given to each rat prior to incision. Surgery was carried out as described,6 with minor modifications. Briefly, after aseptic preparation and draping, a 1-cm longitudinal skin incision was made on the plantar surface of the left hind paw, starting 0.5 cm distal to the tibiotarsus and extending toward the digits. The plantaris muscle was elevated and incised longitudinally, leaving the insertion and origin intact. After hemostasis, the incision was closed with 2 interrupted horizontal mattress sutures of 5-0 nylon. The wound was covered with triple-antibiotic ointment (Vetropolycin, Dechra Veterinary Products, Overland, KS). All rats were allowed to recover from anesthesia and surgery for 1 d before behavioral testing. All incisions were checked daily, and any apparent wound infections or dehiscence excluded the animal from study.
Study design.
At the time of surgery, rats were assigned randomly to 1 of 4 groups (n = 6 per group). The saline control group received 1 mL/kg saline subcutaneously 30 min prior to skin incision, with additional 1-mL/kg doses every 12 h for 60 h. The rats in the buprenorphine group were given 0.05 mg/kg buprenorphine subcutaneously 30 min prior to skin incision, followed by 0.05 mg/kg SC every 12 h for 60 h. The tramadol-only animals received 10 mg/kg tramadol intraperitoneally 30 min prior to skin incision, followed by 10 mg/kg IP every 12 h for 60 h. The final group of rats received both tramadol and gabapentin: 10 mg/kg tramadol intraperitoneally plus 80 mg/kg gabapentin subcutaneously 30 min prior to skin incision, followed by 10 mg/kg IP every 12 h for 60 h for tramadol and 80 mg/kg SC every 24 h for 48 h for gabapentin.
Beginning 1 d after surgery, rats underwent daily behavioral testing examining mechanical and thermal hyperalgesia between 0900 and 1100 for 6 consecutive days.
Behavioral studies.
Withdrawal responses to heat stimuli.
Heat hyperalgesia was assessed by measuring paw withdrawal latencies to radiant heat generated by a lighted bulb as described previously.12 Briefly, rats were placed in a clear plastic chamber (23 × 13 × 13 cm) and allowed to acclimate for 15 min before testing. Heat stimuli were produced by a 50-W lightbulb focused on the plantar surface of the hindpaw. The intensity of the lamp was adjusted and maintained to produce stable withdrawal latencies of approximately 13 to 16 s. Withdrawal latencies were measured to the nearest 0.1 s by using a timer and a photocell that terminated the trial upon paw withdrawal. Each hindpaw received 4 stimuli, alternating between hindpaws, with a minimum of 1 min between trials. Withdrawal latency for each hindpaw was defined as the mean of the last 3 trials. A 20-s cutoff was imposed on the stimulus duration to prevent tissue damage. Heat hyperalgesia was defined as a significant (P < 0.05) decrease in withdrawal response latency.
Hindlimb weight-bearing test.
An incapacitance tester (IITC Life Science, Woodland Hills, CA) was used to determine hindpaw weight distribution. Rats were placed in an angled acrylic glass chamber and positioned so that each hindpaw rested on a separate force plate, to measure the weight-bearing of each hindpaw of minimally restrained rats. The floor of the test box consisted of 2 independently functioning force-plates side by side, each of which were mounted on levers and connected to a polygraph equipped with amplifiers. Each rat underwent 3 measurements, each representing an automated digital average weight over 10 s, with a 15-s rest in between measurements in the rat's home cage. At each time point, 3 trial measurements each of the affected and control hindpaws were averaged. Results are presented as the percentage of weight born on the ipsilateral paw compared with that of the sum of both paws:
 |
Data analysis.
Data were analyzed by using repeated-measures ANOVA with 2 factors (drug and time). The drug factor had 4 levels (3 degrees of freedom), and the time factor had 7 levels (6 degrees of freedom) in which time is repeated. This analysis was followed by post hoc Dunnett pairwise comparisons to determine the effects of each treatment on responses to heat stimuli and mechanical weight-bearing in comparison with those of the saline-treated group or baseline values (SPSS, IBM, Somers, NY). For all analyses, a P value of less than 0.05 was considered statistically significant. Data are presented as mean ± SEM.
Results
Attenuation of heat hyperalgesia after administration of tramadol–gabapentin or buprenorphine.
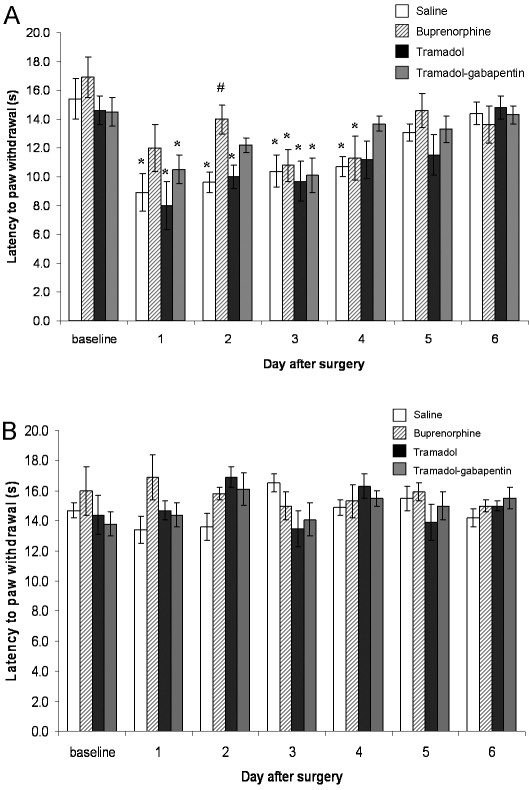
The administration of buprenorphine attenuated heat hyperalgesia in a rat model of incisional pain on days 1 and 2 after surgery, whereas tramadol–gabapentin attenuated heat hyperalgesia on day 2 only (Figure 1 A). The mean baseline paw withdrawal latency to heat did not differ between groups. Incision of the plantar aspect of the hindpaw significantly (P < 0.01) reduced the withdrawal latency in response to thermal stimulation (hyperalgesia) in rats in the saline group on day 1 after surgery (mean ± SEM, 8.9 ± 1.7 s) as compared with preoperative values (14.6 ± 1.0 s). This hyperalgesia lasted through day 4 after surgery (P < 0.01 for all values). Hyperalgesia was also present in the tramadol group from day 1 (8.0 ± 1.7 s) through day 3 (9.7 ± 1.4 s) after surgery relative to preoperative values. However, unlike the saline-treated rats, rats treated with tramadol only recovered from heat hyperalgesia beginning on day 4 (11.2 ± 1.3 s). When gabapentin was added to the tramadol treatment, hyperalgesia was detected only on days 1 (10.5 ± 1.0 s) and 3 (10.1 ± 1.2 s), as compared with the baseline value (14.5 ± 1.0 s). Like rats that received tramadol only, those given tramadol–gabapentin returned to baseline on day 4 after surgery (Figure 1 A). Rats given buprenorphine showed no significant change in withdrawal latency on days 1 (12.0 ± 1.6 s) and 2 (14.0 ± 1.0 s) after surgery, with significantly (P = 0.001) less heat hyperalgesia on day 2 as compared with the saline group (9.6 ± 0.7 s). After termination of buprenorphine treatment (that is, after day 2), thermal hyperalgesia returned to a level comparable to that of saline-treated rats. Withdrawal latencies for all groups returned to control values by days 5 and 6 after surgery. No significant differences were detected for withdrawal latency of the contralateral paw between groups at any time points (Figure 1 B).
Figure 1.
Effects of subcutaneous 1 mL/kg saline, 0.05 mg/kg subcutaneous buprenorphine, 10 mg/kg intraperitoneal tramadol, and 10 mg/kg intraperitoneal tramadol plus 80 mg/kg subcutaneous gabapentin on latency of paw withdrawal to radiant heat stimuli (s, mean ± SEM) induced by plantar incision on (A) ipsilateral and (B) contralateral hindpaws. Each group contained 6 rats. *, Significant (P < 0.05) difference compared with baseline value for group; #, significant (P < 0.05) difference compared with value for the saline group at the same time point.
Attenuation of ipsilateral mechanical weight-bearing deficits after administration of tramadol–gabapentin or buprenorphine.
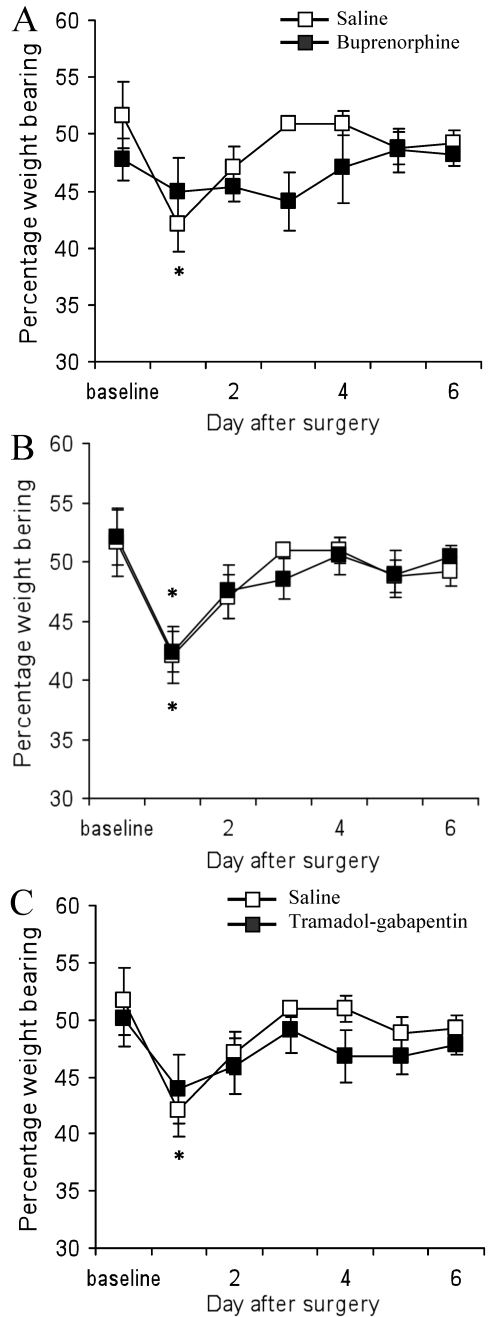
Administration of tramadol–gabapentin or buprenorphine attenuated ipsilateral mechanical weight-bearing deficits in the rat incisional pain model (Figure 2 A and C). Before plantar incision, mean weight-bearing values did not differ among groups. Saline-treated rats showed a decrease in the percentage weight-bearing on the ipsilateral paw after plantar incision as compared with baseline values (42.1% ± 5.9% and 51.6% ± 6.2%, respectively; P = 0.002) on day 1 after surgery; percentage weight-bearing returned to control values on days 2 through 6 after surgery. Similarly, rats given tramadol only showed a decrease in percentage ipsilateral weight-bearing on day 1 after surgery (42.4% ± 1.7%) as compared with the baseline value (52.0% ± 2.3%) and returned to control values on days 2 through 6. In contrast, the tramadol–gabapentin group showed no significant differences in percentage weight-bearing on day 1 (43.9% ± 3.0%) through day 6 (47.8 ± 0.9%) compared with the baseline value (50.0% ± 2.4%). Similarly, buprenorphine-treated rats showed no significant decrease in ipsilateral weight-bearing at any time point. In all groups, percentage weight-bearing returned to control values on days 2 through 6, regardless of treatment.
Figure 2.
Effects of (A) subcutaneous saline, 0.05 mg/kg subcutaneous buprenorphine, (B), 10 mg/kg intraperitoneal tramadol, and (C) 10 mg/kg intraperitoneal tramadol plus 80 mg/kg subcutaneous gabapentin on percentage ipsilateral weight-bearing (mean ± SEM) subsequent to paw incision. *, Significant (P < 0.05) difference compared with baseline value for group; #, significant (P < 0.05) difference compared with value for the saline group at the same time point.
Discussion
The present study demonstrates that (1) tramadol alone shows no effect on either heat hyperalgesia or mechanical weight bearing; (2) tramadol with gabapentin variably decreases heat hyperalgesia and weakly normalizes mechanical weight bearing; (3) buprenorphine decreases heat hyperalgesia and normalizes mechanical weight-bearing; (4) discontinuation of all drugs caused a return of heat hyperalgesia; and (5) all drugs used had no effect on contralateral hindpaw responses to thermal stimuli. Our results do not support the hypothesis that tramadol alone adequately relieves thermal hyperalgesia or normalizes weight-bearing. Pain evoked subsequent to surgical incision has been shown to evoke mechanical and heat hyperalgesia.6,32 The rat model of incisional pain caused ipsilateral hindlimb discomfort and subsequent redistribution of weight to the contralateral hindlimb. Hyperalgesia can manifest as early as 1.5 h22 and as much as 7 d32 after the incision is made. In our study, heat hyperalgesia lasted 4 d and decreased mechanical weight-bearing lasted 1 d in saline-treated rats.
Buprenorphine has long been used as a standard analgesic treatment in the laboratory setting. Key advantages include its long plasma half-life (6 to 12 h), effective control of mild to moderate pain, and multiple routes of administration.36 Alternatively, buprenorphine is a federally controlled drug, yields a marked ceiling effect, and is difficult to antagonize. In the current study, buprenorphine attenuated heat hyperalgesia on days 1 and 2 after surgery; however, when this drug was discontinued on day 3, heat hyperalgesia returned, possibly due to rebound hyperalgesia.13 Continued administration of buprenorphine on days 3 and 4 likely would prevent this phenomenon, suggesting that the duration of treatment is highly dependent on the research model used. Although extended administration of buprenorphine should be considered, concerns arise regarding side effects (decreased gastrointestinal motility, respiratory and cardiovascular depression, and immunomodulation) and, if used for an extended period, tolerance. All of these factors should be evaluated to balance animal health and welfare with research needs. In addition, buprenorphine normalized weight-bearing on day 1 after surgery, comparable to that from tramadol–gabapentin. Although there appear to have been demonstrable analgesic effects of both buprenorphine and tramadol–gabapentin on weight-bearing, the lack of significant differences between these groups compared with saline-treated controls may prompt questions regarding the specificity of this testing modality.
When added to perioperative analgesic regimens including narcotics or nonsteroidal antiinflammatory drugs, tramadol has been shown to decrease postoperative opioid consumption and prevent the need for rescue analgesia in humans35,41 and to decrease pain scores in animals.8 This drug has gained popularity because it is noncontrolled and elicits fewer adverse respiratory, gastrointestinal, and immunomodulatory effects than other opioids.40 In addition, tramadol has shown promise as a substitute for local anesthetics to control pain during minor surgeries in humans.1 The tramadol metabolite M1 and spinal serotonin subtype receptors 743 and 32 all play important roles in tramadol-induced analgesia. A strong body of evidence currently exists regarding the mitigating effects of opioids on both central and peripheral sensitization.7 Tramadol has shown to provide antihyperalgesia5 and can effectively and dose-dependently control heat hyperalgesia in chronic nerve constriction injury in rats.39 However, our results indicate that tramadol reversed neither heat hyperalgesia nor decreased ipsilateral mechanical weight-bearing. In contrast to our findings, other researchers24 have demonstrated reversal of mechanical hyperalgesia after using the same dose of tramadol and route of administration. Although heat hyperalgesia and decreased weight-bearing were comparable to those of the saline-treated group in our study, rats treated with tramadol showed an earlier return to baseline (that is, day 4). This effect may be due to the ability of tramadol to modulate the baseline activity of receptors or neurons, albeit not to a degree sufficient to convert the intense hyperalgesia elicited in the current study.
Both tramadol and gabapentin provide analgesia when given as sole agents: tramadol provides 20 min of analgesia when given intrathecally3 or systemically;5 and systemic administration of gabapentin reverses mechanical hyperalgesia better than tactile allodynia.42 Therefore, we decided to combine tramadol with gabapentin, expecting to see profound analgesia. However, combining these 2 compounds at the doses selected ameliorated heat hyperalgesia and weight-bearing deficits less effectively than did buprenorphine alone. Similar to the tramadol-treated group and compared with those that received saline, rats treated with tramadol–gabapentin demonstrated a faster return to baseline on day 4 after surgery. Given these data, the tramadol–gabapentin combination appeared to offer a benefit over tramadol alone. Because relatively little is known regarding tramadol and gabapentin, further investigation is needed. Although gabapentin was mildly effective when combined with tramadol for alleviation of hyperalgesia in this study, it may yield more promising results regarding synergistic antihyperalgesic properties with a more appropriate analgesic agent than tramadol. The doses we used in this study were not derived from novel dose–response curves but from previously published data.24,26,38
Due to inconsistent data among researchers, further study examining dosage, dosing interval, duration, and side effects may be warranted to determine whether current postoperative analgesic standards for rodents need to be reconsidered. We feel that more care should be taken to tailor the analgesic regimen to the study in question. Future investigations may test the effects of buprenorphine administered in combination with gabapentin as evidenced by the analgesia and earlier return to baseline levels exhibited in the tramadol–gabapentin rats subsequent to heat stimuli. Ideally, these investigations should include isobolgraphic analysis to determine whether synergy or antagonism play a role in drug interactions clinically.
Pre-, peri-, and postoperative pain management are ever evolving and require constant refinement. Interspecies differences pose special challenges, both in terms of drug pharmacology as well as the ability of researchers and veterinary clinicians to assess pain in nonhuman patients. Regardless of the pain-eliciting insult, multimodal and preemptive analgesia are still paramount to effective pain control. Although the present study did not illustrate a demonstrable analgesic effect with tramadol as a sole agent, its use in adjunctive therapy may still be justified. For a standard regimen, buprenorphine (a partial μ agonist) is effective in controlling postoperative pain. Our results support the use of buprenorphine to alleviate thermal hyperalgesia and normalize ipsilateral weight-bearing subsequent to foot pad incision. Tramadol with gabapentin had similar effects, whereas tramadol alone yielded no difference as compared with saline administration.
Acknowledgments
We thank the Stanford VSC caretaking staff, especially Sonny Amores, and the members of the Yeoman laboratory, especially Dr Michael Klukinov for his methodologic expertise. This study was supported by the Department of Comparative Medicine, Stanford School of Medicine.
References
- 1.Altunkaya H, Ozer Y, Kargi E, Ozkocak I, Hosnuter M, Demirel CB, Babuccu O. 2004. The postoperative analgesic effect of tramadol when used as subcutaneous local anesthetic. Anesth Analg 99:1461–1464 [DOI] [PubMed] [Google Scholar]
- 2.Arcioni R, della Rocca M, Romanò S, Romano R, Pietropaoli P, Gasparetto A. 2002. Ondansetron inhibits the analgesic effects of tramadol: a possible 5HT(3) spinal receptor involvement in acute pain in humans. Anesth Analg 94:1553–1557 [DOI] [PubMed] [Google Scholar]
- 3.Bernatzky G, Jurna I. 1986. Intrathecal injection of codeine, buprenorphine, tilidine, tramadol, and nefopam depresses the tail-flick response in rats. Eur J Pharmacol 120:75–80 [DOI] [PubMed] [Google Scholar]
- 4.Berrocoso E, De Benito MD, Mico JA. 2007. Role of serotonin 5HT1A and opioid receptors in the antiallodynic effect of tramadol in the chronic constriction injury model of neuropathic pain in rats. Psychopharmacology (Berl) 193:97–105 [DOI] [PubMed] [Google Scholar]
- 5.Bianchi M, Panerai AE. 1998. Antihyperalgeic effects of tramadol in the rat. Brain Res 797:163–166 [DOI] [PubMed] [Google Scholar]
- 6.Brennan TJ, Vandermeulen EP, Gebhart GF. 1996. Characterization of a rat model of incisional pain. Pain 64:493–501 [DOI] [PubMed] [Google Scholar]
- 7.Brennan TJ, Zahn PK, Pogatzki-Zahn EM. 2005. Mechanisms of incisional pain. Anesthesiol Clin North America 23:1–20 [DOI] [PubMed] [Google Scholar]
- 8.Brondani JT, Loureiro Luna SP, Beier SL, Minto BW, Padovani CR. 2009. Analgesic efficacy of perioperative use of vedaprofen, tramadol, or their combination in cats undergoing ovariohysterectomy. J Feline Med Surg 11:420–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buvanendran A, Kroin JS. 2009. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol 22:588–593 [DOI] [PubMed] [Google Scholar]
- 10.Chandran P, Pai M, Blomme EA, Hsieh GC, Decker MW, Honore P. 2009. Pharmacological modulation of movement-evoked pain in a rat model of osteoarthritis. Eur J Pharmacol 613:39–45 [DOI] [PubMed] [Google Scholar]
- 11.Chen YL, Law PY, Loh HH. 2006. Nuclear factor κβ signaling in opioid functions and receptor gene expression. J Neuroimmune Pharmacol 1:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheppudira BP. 2006. Characterization of hind paw licking and lifting to noxious radiant heat in the rat with and without chronic inflammation. J Neurosci Methods 155:122–125 [DOI] [PubMed] [Google Scholar]
- 13.Curtin LI, Grakowsky JA, Suarez M, Thompson AC, DiPirro JM, Martin LB, Kristal MB. 2009. Evaluation of buprenorphine in a postoperative pain model in rats. Comp Med 59:60–71 [PMC free article] [PubMed] [Google Scholar]
- 14.Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. 2005. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 94:825–834 [DOI] [PubMed] [Google Scholar]
- 15.De la O-Arciniega M, Diaz-Reval MI, Cortes-Arroyo AR, Dominguez-Ramirez AM, Lopez-Munoz FJ. 2009. Antinociceptive synergism of morphine and gabapentin in neuropathic pain induced by chronic constriction injury. Pharmacol Biochem Behav 92:457–464 [DOI] [PubMed] [Google Scholar]
- 16.Dobkin AB. 1977. Buprenorphine hydrochloride: determination of analgesic potency. Can Anaesth Soc J 24:186–193 [DOI] [PubMed] [Google Scholar]
- 17.Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. 1997. Gabapentin (Neurontin) and S-(+)-3-isobutylGABA represent a novel class of selective antihyperalgesic agents. Br J Pharmacol 121:1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Füredi R, Bölcskei K, Szolcsányi J, Petho G. 2010. Comparison of the peripheral mediator background of heat injury- and plantar incision-induced drop in the noxious heat threshold in the rat. Life Sci 86:244–250 [DOI] [PubMed] [Google Scholar]
- 19.Gal TJ. 1989. Naloxone reversal of buprenorphine-induced respiratory depression. Clin Pharmacol Ther 45:66–71 [DOI] [PubMed] [Google Scholar]
- 20.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. 1996. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel. J Biol Chem 271:5768–5776 [DOI] [PubMed] [Google Scholar]
- 21.Grond S, Sablotzki A. 2004. Clinical pharmacology of tramadol. Clin Pharmacokinet 43:879–923 [DOI] [PubMed] [Google Scholar]
- 22.Hayashida K, DeGoes S, Curry R, Eisenach JC. 2007. Gabapentin activates spinal noradrenergic activity in rats and humans and reduces hypersensitivity after surgery. Anesthesiology 106:557–562 [DOI] [PubMed] [Google Scholar]
- 23.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press [Google Scholar]
- 24.Kamerman P, Koller A, Loram L. 2007. Postoperative administration of the analgesic tramadol, but not the selective cyclooxygenase-2 inhibitor parexoxib, abolishes postoperative hyperalgesia in a new model of postoperative pain in rats. Pharmacology 80:244–248 [DOI] [PubMed] [Google Scholar]
- 25.Kehlet H, Holte K. 2001. Effect of postoperative analgesia on surgical outcome. Br J Anaesth 87:62–72 [DOI] [PubMed] [Google Scholar]
- 26.Liu YM, Zhu SM, Wang KR, Feng ZY, Chen QL. 2008. Effect of tramadol on immune responses and nociceptive thresholds in a rat model of incisional pain. J Zhejiang Univ Sci B 9:895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutfy K, Cowan A. 2004. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol 2:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, Evans CJ. 2003. Buprenorphine-induced antinociception is mediated by the µ-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci 23:10331–10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao J, Chen LL. 2000. Gabapentin in pain management. Anesth Analg 91:680–687 [DOI] [PubMed] [Google Scholar]
- 30.Meymandi MS, Sepehri G, Mobasher M. 2006. Gabapentin enhances the analgesic response to morphine in acute model of pain in male rats. Pharmacol Biochem Behav 85:185–189 [DOI] [PubMed] [Google Scholar]
- 31.Oifa S, Sydoruk T, White I, Ekstein MP, Marouani N, Chazan S, Skornick Y, Weinbroum AA. 2009. Effects of intravenous patient-controlled analgesia with buprenorphine and morphine alone and in combination during the first 12 postoperative hours: a randomized, double-blind, 4-arm trial in adults undergoing abdominal surgery. Clin Ther 31:527–541 [DOI] [PubMed] [Google Scholar]
- 32.Pogatzki EM, Raja SN. 2003. A mouse model of incisional pain. Anesthesiology 99:1023–1027 [DOI] [PubMed] [Google Scholar]
- 33.Pogatzki EM, Zahn PK, Brennan TJ. 2000. Effect of pretreatment with intrathecal excitatory amino acid receptor antagonists on the development of pain behavior caused by plantar incision. Anesthesiology 93:489–496 [DOI] [PubMed] [Google Scholar]
- 34.Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. 1992. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther 260:275–285 [PubMed] [Google Scholar]
- 35.Rahimi SY, Alleyne CH, Vernier E, Witcher MR, Vender JR. 2010. Postoperative pain management with tramadol after craniotomy: evaluation and cost analysis. J Neurosurg 112:268–272 [DOI] [PubMed] [Google Scholar]
- 36.Robertson SA, Lascelles BD, Taylor PM, Sear JW. 2005. PK–PD modeling of buprenorphine in cats: intravenous and transmucosal administration. J Vet Pharmacol Ther 28:453–460 [DOI] [PubMed] [Google Scholar]
- 37.Shih AC, Robertson S, Isaza N, Pablo L, Davies W. 2008. Comparison between effects of buprenorphine, carprofen, and buprenorphine with carprofen for canine ovariohysterectomy. Vet Anaesth Analg 35:69–79 [DOI] [PubMed] [Google Scholar]
- 38.Todorovic SM, Rastogi AJ, Jevtovic-Todorovic V. 2003. Potent analgesic effects of anticonvulsants on peripheral thermal nociception in rats. Br J Pharmacol 140:255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai YC, Sung YH, Chang PJ, Kang FC, Chu KS. 2000. Tramadol relieves thermal hyperalgesia in rats with chronic constriction injury of the sciatic nerve. Fundam Clin Pharmacol 14:335–340 [DOI] [PubMed] [Google Scholar]
- 40.Vallejo R, de Leon-Casasola O, Benyamin R. 2004. Opioid therapy and immunosuppression: a review. Am J Ther 11:354–365 [DOI] [PubMed] [Google Scholar]
- 41.Wang F, Shen X, Xu S, Liu Y. 2009. Preoperative tramadol combined with postoperative small-dose tramadol infusion after total abdominal hysterectomy: a double-blind, randomized, controlled trial. Pharmacol Rep 61:1198–1205 [DOI] [PubMed] [Google Scholar]
- 42.Whiteside GT, Harrison J, Boulet J, Mark L, Pearson M, Gottshall S, Walker K. 2004. Pharmacological characterization of a rat model of incisional pain. Br J Pharmacol 141:85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie H, Dong ZQ, Ma F, Bauer WR, Wang X, Wu GC. 2008. Involvement of serotonin 2A receptors in the analgesic effect of tramadol in monoarthritic rats. Brain Res 1210:76–83 [DOI] [PubMed] [Google Scholar]
- 44.Yanarates O, Dogrul A, Yildirim V, Sahin A, Sizlan A, Seyrek M, Akgül O, Kozak O, Kurt E, Aypar U. 2010. Spinal 5HT7 receptors play an important role in the antinociceptive and antihyperalgesic effects of tramadol and its metabolite, O-desmethyltramadol, via activation of descending serotonergic pathways. Anesthesiology 112:696–710 [DOI] [PubMed] [Google Scholar]
- 45.Zahn PK, Brennan TJ. 1998. Lack of effect of intrathecally administered N-methyl-D-aspartate receptor antagonists in a rat model for postoperative pain. Anesthesiology 88:143–156 [DOI] [PubMed] [Google Scholar]