Abstract
Murine ulcerative dermatitis (UD) is a common progressive condition of mice with a C57BL/6 background. Typically, mice present with scabs and crusts on the skin of the dorsal neck and ears, and are often severely pruritic. Animals tend to scratch the lesions, causing additional trauma to the already ulcerated and inflamed skin. Therapeutic intervention largely has been unsuccessful, in part due to the lack of a known cause for the disease. Though the exact etiology of UD has not been elucidated, substance P (SP) has recently been demonstrated as an important neuropeptide linked to the itch–scratch cycle. SP functions at the tachykinin neurokinin 1 (NK1) receptor. We hypothesized that inhibition of SP binding to the NK1 receptor would decrease the itch sensation, thus decreasing scratching behavior and subsequent skin trauma. The purpose of this study was to evaluate the effectiveness of an NK1 receptor antagonist, maropitant citrate, as a treatment for murine UD. Treatment with 1 mg/kg maropitant citrate significantly reduced the size of UD lesions in mice.
Abbreviations: NK1, neurokinin type 1; UD, ulcerative dermatitis
Idiopathic ulcerative dermatitis (UD) is a problematic condition commonly diagnosed in C57BL/6 mice and related strains.12,23 Characterized by intense pruritis, dermal ulceration, necrosis, degloving of skin segments, fibrosis, and resulting skin contracture, murine UD is a leading cause of morbidity in mice and can have a large economic impact on research colonies, given that the condition frequently becomes debilitating, requiring euthanasia.12,14
UD lesions are often single and occur most frequently at the nape of the neck and shoulders, but multifocal dermal ulcerations can develop anywhere on the body.14 Lesions are characterized by areas of skin ulceration, serocellular crusts, and alopecia.12,23 Microscopically, UD lesions exhibit profound inflammation with predominantly neutrophil, lymphocyte, macrophage, and mast cell infiltration.12,23 The epidermis adjacent to an ulcer may appear normal but is often hyperplastic with varying degrees of hyperkeratosis, acanthosis, or orthokeratosis. Chronic ulcers may have crusts covering a large bed of granulation tissue with large numbers of macrophages and lymphocytes. Severe pruritis induces mice to scratch the ulcerated skin, leading to secondary infections and further tissue damage to the area.12,23 In addition, immune stimulation may occur, resulting in lymphadenopathy and splenomegaly.12 Therefore, UD has the potential to confound not only dermatologic studies but also research involving the immune system.23 Concern for animal welfare in mice with UD often results in euthanasia before planned study endpoints, leading to loss of experimental data. At our institution, murine UD accounts for approximately 17% of rodent illness reports annually, illustrating the potential for tremendous animal welfare and economic impact.
The etiology of murine UD is not understood completely, but it is believed to be multifactorial, including genetic and environmental components.12,23 Associations with feeding a diet high in fat or vitamin A, ad libitum feeding, stress, and infection with Myobia musculi and Myocoptes musculinus have been implicated in the development of UD.3,9,12-14,19 In addition, mice deficient in inducible nitric oxide synthase have a high prevalence of UD, presumably due to delayed wound closure and increased susceptibility to bacterial infections.12
Attempts to treat UD by using topical and systemic antibiotics, corticosteroids, antihistamines, vitamin E, antiseptics such as chlorhexidine and 10% povidine iodine, EMLA cream (2.5% lidocaine and 2.5% prilocaine), calamine lotion, environmental manipulation, and dietary restriction have been limited in their success.7,12,14,20,22 Others report nail trimming decreases the severity of skin damage and increases the rate of healing in rodents with UD lesions.7,15,22 Though nail trimming may be effective, it may not be a feasible treatment option in a large research facility. Controlled studies are needed to determine the etiology of UD and explore novel pharmacologic agents as potential treatments.
In human cutaneous diseases, such as contact and atopic dermatitis, pruritis is the most common symptom. In both humans and mice, several endogenous substances contribute to the sensation of itching including histamine, cytokines, and various neuropeptides.1,10,16 Dermal swelling and inflammation induced by substance P, an endogenous neuropeptide, is indirectly mediated through the release of multiple compounds, including histamine, tumor necrosis factor-α, and prostaglandin E2, from mast cells.13,16,17 Substance P recently was shown to induce scratching behavior in ICR mice in the absence of mast cells, suggesting that this neuropeptide may have a direct role in the sensation of itch.1,17
Substance P exhibits a strong affinity specifically for the neurokinin type 1 (NK1) receptor.1,4,13 NK1 receptors are distributed throughout the CNS and peripherally near arteries and veins. In the periphery, NK1 receptors function to regulate local blood flow and vascular permeability through vasodilation and extravasation of plasma proteins.4 In the skin, NK1 receptors are present in the terminals of primary sensory neurons, as well as in macrophages, fibroblasts, endothelial cells, and keratinocytes.1,13
In one study, substance P induced scratching behavior in mice, whereas coadministration of NK1 receptor antagonists and substance P led to a profound decrease in this behavior.1 Other studies have shown a decrease in substance-P–positive nerves and mast cells within the dermis after administration of various NK1 receptor antagonists.10,13,16 Maropitant citrate (Cerenia, Pfizer Animal Health, New York, NY), a potent and highly selective novel synthetic NK1 receptor antagonist, was recently introduced to the veterinary market.2,21 Its safety and efficacy as an antiemetic at doses of 8 mg/kg or lower has been well established in dogs and cats.2,4-6,8,21 Reported use of maropitant citrate in rodents is limited to administration in gerbils in determining the ability of the drug to penetrate the CNS and inhibit the foot-tapping response, a model of fear and anxiety.4,24 In the current study, we evaluated the efficacy of maropitant citrate in the treatment of murine UD.
Materials and Methods
Animals.
A total of 90 mice (female, 46; male, 44) of C57BL/6 background strain, ranging in age from 14 wk to 27 mo, were enrolled in this study. The mice were obtained from investigators at the Yale University School of Medicine (New Haven, CT) and represented 62 different strains (Figure 1). All mice were free of epizootic diarrhea of infant mice virus, lymphocytic choriomeningitis virus, ectromelia virus, mouse hepatitis virus, Mycoplasma spp., and endo- and ectoparasites, based on testing of individual experimental animals or recent sentinel data. Experimental procedures were approved by the Yale University Institutional Animal Care and Use Committee and were in accordance with all federal policies and guidelines governing the use of vertebrate animals.
Figure 1.
Genotypes of mice used in the current study. KO, knockout.
Mice were singly housed in sterile, individually ventilated cages (Allentown Caging, Allentown, PA) with corncob bedding (1/8 in., catalog no. 7092, Harlan, South Easton, MA) or paper bedding (ALPHA-dri, Shepherd Specialty Papers, Milford, NJ) and nesting material (Nestlet, Ancare, Bellmore, NY) on a 12:12-h light:dark cycle. Autoclaved rodent chow (diet 2018, Harlan Teklad) and hyperchlorinated water by water bottle were offered ad libitum. Room temperature and relative humidity were maintained between 22.2 ± 1.1 °C (72 ± 2 °F) and 50% ± 10%, respectively.
Identification and enrollment of affected animals.
Mice with ulcerative skin lesions were identified as potential study candidates by husbandry technicians and veterinary technologists during routine daily health checks. A veterinarian confirmed the diagnosis of UD on the basis of a physical exam and characteristic appearance of lesions and determined the appropriateness of each mouse for enrollment in the study. Each mouse was assigned randomly to 1 of 6 treatment groups. Mice with deep ulcerative skin lesions or other obvious serious health issues and those deficient in inducible nitric oxide synthase were excluded. All experimental manipulations of mice occurred in a class II biosafety cabinet.
UD lesion scoring and treatment groups.
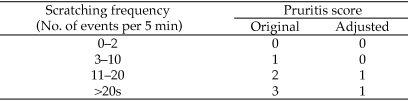
During a 5-min observation period, a veterinarian blinded to treatment counted and recorded the number of times individual mice scratched at their UD lesions. A baseline pruritis score of 0 to 3 was assigned to each animal based on the number of scratches observed (Figure 2). Mice subsequently were weighed and then briefly anesthetized in a bell jar containing 30% isoflurane in propylene glycol (v/v) on a gauze square placed beneath a small piece of metal screen.11 Animals had no direct contact with the anesthetic agent. For each mouse and UD lesion, representative photographs were taken. UD lesion location, shape, area measurements (in mm2, length × width), and character (moist or dry; mild [1], moderate [2], severe [3]) were recorded. Mild UD was defined as a single, small focal lesion or multiple pinpoint areas of superficial skin ulceration. Severe UD lesions were deeper and penetrated through both the epidermis and dermis, exposing underlying musculature. Moderate UD lesions were larger and deeper than mild lesions but not as extensive or deep as severe lesions. Intraperitoneal injection of maropitant at 1 or 5 mg/kg was administered once daily for 5 or 10 d. Control mice received an intraperitoneal injection of sterile saline for the same duration. Observations, photographs, lesion measurements, and scoring were recorded once weekly through the experimental endpoint at day 57. At the final time point, mice were euthanized by using carbon dioxide asphyxiation, and gross necropsies were performed. Percentage change in UD lesion size was calculated for each time point (days 8, 15, 22, 29, 36, 43, 50, and 57) by using the following formula: [1 – (UD lesion size at weekly time point / UD lesion size on day 1)] × 100.
Figure 2.
Pruritis scoring system.
Histology.
Representative sections of skin were taken from sites of current or previous UD lesions from 3 representative mice in each treatment group. Skin samples were immersion-fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin by routine methods.
Statistical analysis.
Statistical analysis of lesion size and pruritis score was performed by using the Generalized Estimating Equations model (SAS version 9.2, SAS Institute, Cary, NC) to account for the correlation between measures on different time points for each mouse. For both percentage of improvement in lesion size and pruritis score, the covariates included in both models were treatment assignment, time, and the interaction between treatment assignment and time. For pruritis score, the baseline pruritis score was included also. Repeated-measures analysis (SAS version 9.2, SAS Institute) was performed for body weight change, with the covariates treatment assignment, time, and interaction between treatment assignment and time. For all tests, statistical significance was defined as a P value less than 0.05.
Results
There were no statistically significant differences in UD lesion size or pruritis score between mice treated for 5 or 10 d. Therefore, the treatment groups were combined and analyzed as 3 groups (water, 1 mg/kg maropitant citrate, 5 mg/kg maropitant citrate).
UD lesion healing.
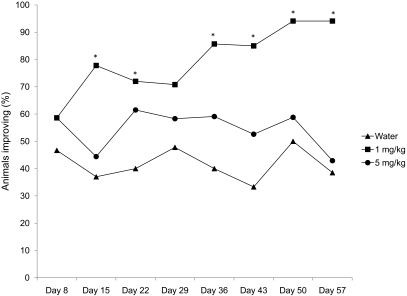
Figure 3 shows the percentage of animals per treatment group that showed 10% or greater reduction in lesion size (improvement) over the course of the study. The group treated with 1 mg/kg maropitant citrate had significantly (P < 0.01) more animals with at least 10% improvement in lesion size than did either the water-treated controls or the 5-mg/kg maropitant citrate-treated group. Mice that received 5 mg/kg maropitant citrate did not differ (P = 0.29) from the water-treated group. At day 15, treatment effects analysis revealed a significant (P < 0.01) difference between mice treated with 1 mg/kg maropitant citrate or water, and the odds of lesions improving at least 10% after 1 mg/kg maropitant citrate was 1.9 times that of water-treated mice. On day 57, the treatment-associated difference continued to demonstrate statistical significance, and the likelihood of lesions improving by at least 10% after 1 mg/kg maropitant citrate was 2.7 times that of water-treated mice.
Figure 3.
Percentage by treatment group of mice showing at least a 10% reduction in lesion size. The 1-mg/kg group improved significantly (*, P < 0.01) compared with the control group. No significant difference was observed between the 5-mg/kg and control groups.
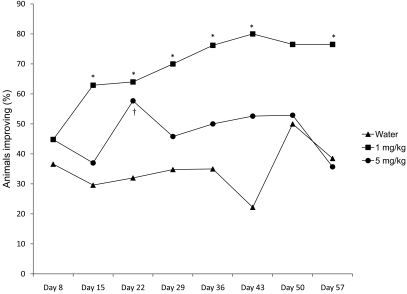
Figure 4 shows the percentage of mice per treatment group whose UD lesion size decreased at least 25% over the course of the study. The group given 1 mg/kg maropitant citrate had more animals with at least 25% improvement than did either the water-treated group (P < 0.01) or the 5 mg/kg group (P = 0.03). The 5 mg/kg maropitant citrate and water groups did not differ (P = 0.24). At day 15, treatment effects differed (P = 0.03) between the 1 mg/kg maropitant citrate and water-treated groups, and the odds of lesions improving 25% or more when treated with 1 mg/kg maropitant citrate was 1.25 times that of water-treated mice. On day 43, the treatment difference was still significant and the odds of at least 25% improvement was 2.6 times higher among mice treated with 1 mg/kg maropitant citrate than those mice treated with water.
Figure 4.
Percentages by treatment group of mice showing at least a 25% reduction in lesion size. The 1-mg/kg group improved significantly (*, P < 0.04) compared with the control group. The 5-mg/kg group was significantly different (†, P < 0.05) from the control group at day 22.
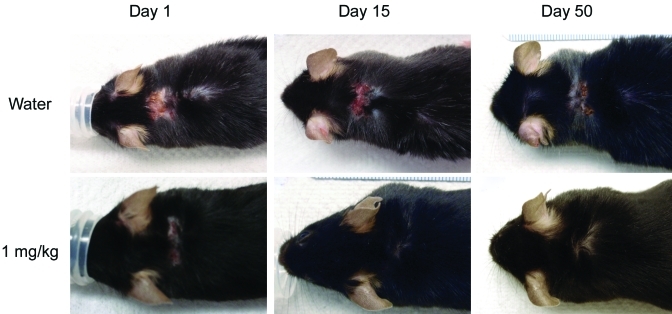
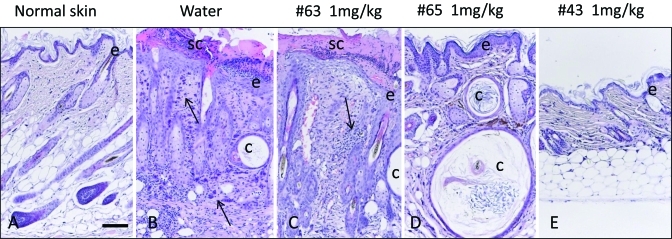
Figure 5 shows an example of the progression of healing of UD lesions by group. UD lesions fluctuated over time regardless of treatment group, and mice with small lesions tended to have improved healing. Lesions in mice treated with 1 mg/kg maropitant citrate tended to decrease in size sooner than those in mice treated with either water or 5 mg/kg maropitant citrate. Histologically, a similar spectrum of qualitative changes characteristic for UD was identified in both treated and untreated mice.12 These changes were characterized by epidermal hyperplasia, serocellular crusting, follicular cysts, dermal fibrosis, and dermal and hypodermal inflammatory infiltrates. The nature of the infiltrates varied from predominantly lymphohistiocytic and mast cell dominated to neutrophilic in ulcerated tissues. Lesions of varying severity often coexisted in the same animal. Figure 6 comprises representative images of histologic findings at day 57 from water-treated mice and 3 mice treated with 1 mg/kg maropitant citrate. UD lesions fluctuated over time, and lesion area tended to decrease within the first 4 wk after treatment, followed by lesion progression. In some mice UD lesions resolved completely, but occasionally new UD lesions developed at later time points in the study in different anatomic locations on the body. Clinical improvement was accompanied by reduction of dermal inflammation, epidermal hyperplasia, and crusting. Follicular cysts and dermal fibrosis were common.
Figure 5.
Typical progression of healing of UD lesions by treatment group.
Figure 6.
Representative histology from (A) normal, (B) water-treated (control), and (C) maropitant-treated (1 mg/kg) mice at 57 d. Changes include epidermal hyperplasia (e), serocellular crusting (sc), follicular cysts (c), and dermal inflammatory infiltrates (arrows). Hematoxylin and eosin stain; bar, 50 μm.
Pruritis score.
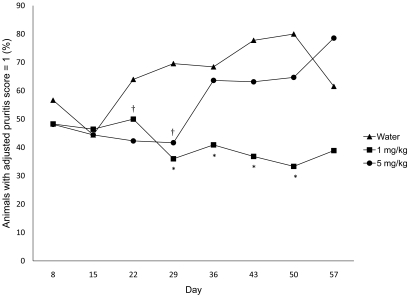
For statistical purposes we assigned an adjusted pruritis score (Figure 2), which was based on our original pruritis scoring system. Figure 7 shows the percentage of animals by treatment group that were assigned an adjusted pruritis score of 1. At days 29 to 50, mice treated with 1 mg/kg maropitant citrate were significantly (P < 0.01) less pruritic than were water-treated animals. Mice treated with 5 mg/kg maropitant citrate showed a significant (P < 0.01) decrease in pruritis compared with that in water-treated animals only on days 22 and 29.
Figure 7.
Percentages by treatment group of mice with an adjusted pruritis score of 1. On days 29 to 50, the group treated with 1 mg/kg maropitant was significantly (*, P < 0.01) less pruritic than was the control group. Mice treated with 5 mg/kg maropitant were significantly (†, P < 0.01) less pruritic on days 22 and 29, compared with the control group.
Body weight.
Changes in body weight were not significant (P = 0.71) among treatment groups (data not shown).
Bedding.
Subjectively, lesion healing in mice housed on paper bedding did not differ from that in animals maintained on corncob bedding. Both bedding substrates were noted to stick to moist UD lesions, but sticking of the paper bedding seemed less likely.
Discussion
Idiopathic UD is a common cause for euthanasia for humane reasons, resulting in loss of research data. The etiology of the disease has not been determined as yet, leaving veterinarians and investigators with no effective preventative measures and only palliative care as a treatment option. Studies suggest the involvement of substance P as contributing factor to the sensation of itch, but the exact mechanism by which substance P is involved in murine UD has yet to be discovered.1,9,10,13,16-18 Here we report the successful and novel use of maropitant citrate (Cerenia, Pfizer Animal Health) for the treatment of murine UD.
Maropitant citrate administered at 1 mg/kg IP for 5 to 10 d (as recommended for dogs by the manufacturer's package insert) significantly decreased pruritis, leading to a 10% decrease in lesion size in 78% of mice by day 15 in and 94% of animals by day 57. The same dose resulted in reductions of 25% or more in lesion size in 59% of mice by day 15 and 83% by day 43. In contrast, a 10% decrease in lesion size occurred in only 37% of water-treated animals by day 15 and in 39% by day 57. These findings are important because in some instances, even partial resolution of UD lesions may improve animal welfare and preclude premature euthanasia, thereby extending the research life of the animal and allowing mice to have continued participation in projects to the planned end point. Although some mice showed complete resolution of lesions, treatment with maropitant citrate did not prevent development of new UD lesions. Development of new lesions does not suggest failure of treatment, nor does it imply anything regarding the likelihood of success of subsequent treatment. For reasons currently unknown, the efficacy of treatment was consistently greater for the 1-mg/kg dose than the 5-mg/kg dose.
Improvement of UD was more likely in cases for which treatment was initiated when lesions were smaller than 100 mm2 than in those with larger lesions (data not shown). Despite small lesion size, some mice did not respond to treatment, resulting in continuation of the itch–scratch cycle, progression of the UD lesions, and euthanasia prior to day 57. Whether repeated treatment with maropitant citrate has a positive effect on the healing of UD in mice with similar lesions, allowing the animals to survive to the study endpoint, will be a focus of future studies.
Maropitant citrate is neither a steroid nor a nonsteroidal antiinflammatory drug and therefore may be a useful alternative to drugs currently used for UD treatment, particularly in immunology studies. However, maropitant citrate should be used with caution in mouse strains with a compromised blood–brain barrier, given that the drug has the potential to cross into the brain and bind NK1 receptors.2,5,6 NK1 receptors are also located in the skin, and it is unclear from the current study whether maropitant citrate acts at NK1 receptors in the brain, skin, or a combination of both. Further studies using immunohistochemical staining may help to clarify the issue.
Throughout the course of the study, a few mice died in both experimental and control groups. Results from gross necropsies lead us to suspect that all mice died of causes unrelated to treatment. The phenotypes of individual genetically engineered mice may have contributed to the death of some animals. Mice treated with maropitant citrate developed no outward signs of clinical illness or significant changes in body weight associated with drug administration. However, additional long-term studies should be performed to determine the drug's potential for effects on behavior, reproduction, and physiology of mice used in research.
In summary, UD-affected mice treated with maropitant citrate were significantly less pruritic than were control mice. Treatment is inexpensive and simple to perform with minimal training, but may be labor intensive due to the requirement for daily injections. Maropitant citrate appears to be effective at decreasing UD-related pruritis and lesion size in mice when dosed at 1 mg/kg IP for as long as 10 d. Inclusion of maropitant citrate as a treatment option for murine UD appears beneficial for improving animal welfare and prevention of premature euthanasia due to progressive UD lesions.
Acknowledgment
We thank the investigators at Yale University School of Medicine for providing the mice used in this study.
References
- 1.Andoh T, Nagasawa T, Satoh M, Kuraishi Y. 1998. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J Pharmacol Exp Ther 286:1140–1145 [PubMed] [Google Scholar]
- 2.Conder GA, Sedlacek HS, Boucher JF, Clemence RG. 2008. Efficacy and safety of maropitant, a selective neurokinin 1 receptor antagonist, in 2 randomized clinical trials for prevention of vomiting due to motion sickness in dogs. J Vet Pharmacol Ther 31:528–532 [DOI] [PubMed] [Google Scholar]
- 3.Dawson DV, Whitmore SP, Bresnahan JF. 1986. Genetic control of susceptibility to mite-associated ulcerative dermatitis. Lab Anim Sci 36:262–267 [PubMed] [Google Scholar]
- 4.de la Puente-Redondo V, Tingley FD, 3rd, Schneider RP, Hickman MA. 2007. The neurokinin 1 antagonist activity of maropitant, an antiemetic drug for dogs, in a gerbil model. J Vet Pharmacol Ther 30:281–287 [DOI] [PubMed] [Google Scholar]
- 5.de la Puente-Redondo VA, Siedek EM, Benchaoui HA, Tilt N, Rowan TG, Clemence RG. 2007. The antiemetic efficacy of maropitant (Cerenia) in the treatment of ongoing emesis caused by a wide range of underlying clinical aetiologies in canine patients in europe. J Small Anim Pract 48:93–98 [DOI] [PubMed] [Google Scholar]
- 6.de la Puente-Redondo VA, Tilt N, Rowan TG, Clemence RG. 2007. Efficacy of maropitant for treatment and prevention of emesis caused by intravenous infusion of cisplatin in dogs. Am J Vet Res 68:48–56 [DOI] [PubMed] [Google Scholar]
- 7.Fox JG, Niemi SM, Murphy JC, Quimby FW. 1977. Ulcerative dermatitis in the rat. Lab Anim Sci 27:671–678 [PubMed] [Google Scholar]
- 8.Hickman MA, Cox SR, Mahabir S, Miskell C, Lin J, Bunger A, McCall RB. 2008. Safety, pharmacokinetics, and use of the novel NK1 receptor antagonist maropitant (Cerenia) for the prevention of emesis and motion sickness in cats. J Vet Pharmacol Ther 31:220–229 [DOI] [PubMed] [Google Scholar]
- 9.Hosokawa C, Takeuchi S, Furue M. 2009. Severity scores, itch scores and plasma substance P levels in atopic dermatitis treated with standard topical therapy with oral olopatadine hydrochloride. J Dermatol 36:185–190 [DOI] [PubMed] [Google Scholar]
- 10.Inagaki N, Shiraishi N, Igeta K, Nagao M, Kim JF, Chikumoto T, Itoh T, Katoh H, Tanaka H, Nagai H. 2010. Depletion of substance P, a mechanism for inhibition of mouse scratching behavior by tacrolimus. Eur J Pharmacol 626:283–289 [DOI] [PubMed] [Google Scholar]
- 11.Itah R, Gitelman I, Davis C. 2004. A replacement for methoxyflurane (metofane) in open-circuit anaesthesia. Lab Anim 38:280–285 [DOI] [PubMed] [Google Scholar]
- 12.Kastenmayer RJ, Fain MA, Perdue KA. 2006. A retrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci 45:8–12 [PubMed] [Google Scholar]
- 13.Kawana S, Liang Z, Nagano M, Suzuki H. 2006. Role of substance P in stress-derived degranulation of dermal mast cells in mice. J Dermatol Sci 42:47–54 [DOI] [PubMed] [Google Scholar]
- 14.Lawson GW, Sato A, Fairbanks LA, Lawson PT. 2005. Vitamin E as a treatment for ulcerative dermatitis in C57BL/6 mice and strains with a C57BL/6 background. Contemp Top Lab Anim Sci 44:18–21 [PubMed] [Google Scholar]
- 15.Mufford T, Richardson L. 2009. Nail trims versus the previous standard of care for treatment of mice with ulcerative dermatitis. J Am Assoc Lab Anim Sci 48:546 [Google Scholar]
- 16.Ohmura T, Hayashi T, Satoh Y, Konomi A, Jung B, Satoh H. 2004. Involvement of substance P in scratching behaviour in an atopic dermatitis model. Eur J Pharmacol 491:191–194 [DOI] [PubMed] [Google Scholar]
- 17.Ohmura T, Tsunenari I, Hayashi T, Satoh Y, Konomi A, Nanri H, Kawachi M, Morikawa M, Kadota T, Satoh H. 2004. Role of substance P in an NC/Nga mouse model of atopic dermatitis-like disease. Int Arch Allergy Immunol 133:389–397 [DOI] [PubMed] [Google Scholar]
- 18.Paus R, Schmelz M, Biro T, Steinhoff M. 2006. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest 116:1174–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Percy DH, Barthold SW. 2007. Pathology of laboratory rodents and rabbits, 3rd ed Ames (IA): Blackwell Publishing [Google Scholar]
- 20.Perkins SN, Hursting SD, Phang JM, Haines DC. 1998. Calorie restriction reduces ulcerative dermatitis and infection-related mortality in p53-deficient and wildtype mice. J Invest Dermatol 111:292–296 [DOI] [PubMed] [Google Scholar]
- 21.Ramsey DS, Kincaid K, Watkins JA, Boucher JF, Conder GA, Eagleson JS, Clemence RG. 2008. Safety and efficacy of injectable and oral maropitant, a selective neurokinin 1 receptor antagonist, in a randomized clinical trial for treatment of vomiting in dogs. J Vet Pharmacol Ther 31:538–543 [DOI] [PubMed] [Google Scholar]
- 22.Seta S, Buttrey P. 2009. A simplified method for the treatment of mouse dermatitis. J Am Assoc Lab Anim Sci 48:608 [Google Scholar]
- 23.Sundberg JP. 1994. Handbook of mouse mutations with skin and hair abnormalities: animal models and biomedical tools. ,p: CRC Press [Google Scholar]
- 24.Sundqvist M, Kristensson E, Adolfsson R, Leffler A, Ahlstedt I, Engberg S, Drmota T, Sigfridsson K, Jussila R, de Verdier J, Noven A, Johansson A, Pahlman I, von Mentzer B, Lindstrom E. 2007. Senktide-induced gerbil foot tapping behaviour is blocked by selective tachykinin NK1 and NK3 receptor antagonists. Eur J Pharmacol 577:78–86 [DOI] [PubMed] [Google Scholar]