Abstract
Minimizing the pain or discomfort of research animals through refinement of surgical techniques is inherent in the humane use of animals in investigative studies. The current approach for intraperitoneal implantation of radiotelemetry devices in mice is a ventral midline incision. An optional surgical approach is a flank incision. We used multidimensional analysis to compare midline and flank approaches for implantation of radiotelemetry devices in regard to time of surgery, activity, temperature, food intake, gel intake, body weight, and vitality scores. A third group was used to evaluate the effects of buprenorphine in healthy mice. The study demonstrated positive benefits related to the flank approach, including quicker surgery times, improved activity levels, more stable temperature homeostasis, smaller losses in body weight, and quicker return to presurgical baseline levels of food intake. In addition, direct effects of buprenorphine included decreases in food intake and body weight, with the effects on body weight lasting approximately 8 d after treatment. Collectively, these results suggest that implantation of intraperitoneal radiotelemetry devices by using a flank approach is beneficial to mice.
As prey species, rodents attempt to minimize pain-associated behaviors in many circumstances.1,12 The tissue injury inherent with surgery will affect the amount of pain experienced by the animal, with the pain directly related to the extent of tissue damage and inflammation.12 Reducing postoperative pain accelerates recovery, improves the quality of data, and is beneficial to the animal.3 The assessment of postprocedural pain and distress is subjective and depends on evaluating a variety of measures.25 Looking at only a single indicator can be misleading. Assessing pain and discomfort by using a multifactoral, composite approach including data provided through implantable radiotelemetry devices and physical observation can allow detection of even subtle levels of pain or discomfort an animal may be experiencing.
Midline laparotomy can be associated with decreases in body weight, food intake, and water intake related to effects of the incision.3 In the absence of inflammation, a laparotomy causes moderate pain.12 One recent study evaluated the effectiveness of various analgesics after midline abdominal implantation of intraperitoneal telemetry devices by looking at specific metabolic, behavioral, and physical parameters as pain indicators.3 Based on appetite, water consumption, body weight, activity level, and internal body temperature, the results of the study clearly outlined the diminished pain response related to specific pharmacologic combinations.
Minor refinements in a surgical procedure to minimize the extent of surgical trauma can result in significant improvements in animal health and welfare.32 The surgical site of implantation has been reported to influence recovery, specifically with subcutaneous compared with laparotomy implantation.21 Recently, attention has been directed toward identifying ways to refine accepted surgical techniques, including telemetry implantation procedures, to reduce pain and distress of the patient.4,32 The effects of radiotelemetry devices on animal welfare depend on the technique used for implantation, skill and experience of the surgeon, administration of analgesia, and other factors related to the device itself.4 The current standard for implantation of intraperitoneal radiotelemetry devices in mice is by a midline abdominal approach.11 This approach is used in other species also, as evidenced in a recent publication looking at physiologic reference ranges in captive black-tailed prairie dogs.17 Animals are reported to tolerate implantation of radiotelemetry devices as long as high-quality techniques are used.19 Disadvantages to a midline approach include wound dehiscence or failure due to a perimedian approach into the rectus abdominus muscle, evisceration, prolonged recovery, and increased tissue damage due to the nature of the surgery.8,20
Outside the United States, the flank is a popular approach for accessing the abdomen to perform feline ovariohysterectomy.8 However, intraperitoneal implantation of radiotelemetry devices in mice by using a flank approach has not been reported until now. Several advantages support a flank approach. A flank incision allows for increased visualization of the surgical site after implantation, a decrease in gravitational stress directly on the surgical site, and a reduced potential for evisceration if wound dehiscence occurs.24 Other advantages include: a decrease in pain related to the stretching of midline abdominal musculature; less severe effects on physiology as evidenced through positive changes in food consumption, less change in body weight, improved activity levels during recovery, and better maintenance of internal body temperature; a shortened surgery time with an improved recovery; and a quicker return to use of the animal for the investigator. Observations from intraperitoneal radiotelemetry surgeries at our facility suggest a clear difference in the postsurgical recovery of mice implanted with a radiotelemetry device by a flank incision as compared with a midline incision.23
The current study focuses on the surgical approach for placement of intraperitoneal radiotelemetry devices in mice and compares postsurgical recovery between midline and flank surgical approaches by looking at multiple pain gauges of health including activity level, body temperature, food intake, gel intake, body weight, and vitality scores. In addition, differences in surgical times were compared between groups to identify benefits to animals. Finally, a third nonsurgical group, treated only with buprenorphine, was incorporated to establish a normal baseline for additional comparison of surgical groups with normal healthy mice.
Materials and Methods
Animals.
This study was approved by the Institutional Animal Care and Use Committee of the US Army Medical Research Institute of Infectious Diseases. All research was conducted in compliance with applicable federal laws and regulations relating to animals used in experimentation. The facility where this research was conducted is fully AAALAC-accredited.
A total of 45 CF1 (strain code, 023; age, 6 to 8 wk; mixed gender) mice were acquired through Charles River (Kingston, NY). The CF1 mouse, an albino outbred mouse strain, serves as a general multipurpose model for research.6 The 45 mice were received in the same shipment from the same supplier, acclimated, randomly separated into their respective groups, and then placed into individual cages 6 d before the study for further acclimation.
Equipment and environment.
The mice were individually housed in 2 ventilated racks (Allentown Caging Equipment Company, Allentown, NJ). Bedding material (Alpha-Dri, Shepherd Specialty Papers, Portage, MI) and cotton nest pads (Nestlets, Ancare Corporation, Bellmore, NY) were provided in each cage for enrichment and warmth. Providing nesting material allows a thermally challenged animal to better control its body temperature.25 Although group housing has been shown to have fewer negative effects than individual housing in mice receiving abdominal surgery, including higher basal body temperature and increased activity levels, study mice were housed individually because of the original experimental design and the limitation that the receivers used could detect only one radiotelemetry signal.28 Turning on and off telemetry devices to allow for group housing in a telemetry study was not logistically feasible.33 Two days prior to surgery, the cages were rearranged in the ventilated rack to allow for radiotelemetry.
Aqua-Jel (Lab Etc, Clayton, DE) was used as a source of hydration. Each 300-g pack of gel was cut into 10 squares (30 g each) daily, weighed, and placed in the wire feeding rack of the cage. After 24 h, the gel was weighed again and discarded and new gel supplied to the mice. Between 10 and 20 g of a standard pelleted rodent diet (7022, Harlan Laboratories, Indianapolis, IN) was provided to each mouse as well. A digital scale (model SG32001 Deltarange, Mettler Toledo, Columbus, OH) was used to measure body, food, and gel weights to the nearest 0.1 g. Mice were weighed in a tared restraint device (Plaslabs, Lansing, MI). Food and gel weights were measured in tared plastic feeding trays. Daily room temperatures ranged from a 1-d low of 70.4 °F (21.3 °C) to a 1-d high of 71.4 °F (21.9 °C). Daily humidity ranged from a 1-d low of 45% to a 1-d high of 57.8%. Light cycles were set at 12 h on (0600 to 1800) and 12 h off (1800 to 0600) for the entire study period. All treatments were provided and physical measurements were taken during the 0600-to-1800 lights-on period.
Radiotelemetry system.
Implantable radiotelemetry devices are widely used in research to track internal physiologic parameters in animal species including blood pressure, heart rate, blood flow, body temperature, activity indices, electrocardiogram and other biopotentials (electroencephalogram, electromyogram), and respiratory rate.4,17,19, 32 Recent publications provide an excellent description of radiotelemetry including a thorough description of the applications, advantages and disadvantages, potential complications, surgical approach, postsurgical pain management, and temporal recovery of mice after surgical implantation.4,7,16-19,21, 29, 32
The radiotelemetry device used was the PhysioTel TA-F20 Mouse Transmitter (Data Sciences International, St Paul, MN).10 Each of 30 receivers (PhysioTel RPC1, Data Sciences International) was hardwired by USB cable into 1 of 2 matrix systems linked to a Windows-based (Microsoft, Redmond, WA) personal computer.9 To prevent errant frequency readings caused by neighboring receivers, the receivers were spaced by using a staggered or checkerboard pattern, by alternating a control mouse cage without a receiver (or an empty cage slot) with a cage housing a randomly implanted mouse and receiver. The radiotelemetry data were collected by using ART (Data Sciences International) software. Transmitters were activated on day 0 of the study. Although it is recommended to activate radiotelemetry devices at least 24 h prior to collection of data, battery life was a concern. Further specifics on the radiotelemetry system are available at the DSI website and through the operator's manual for the radiotelemetry system.9
Experimental design.
Day 0 was defined as the day on which the radiotelemetry devices were implanted into the mice. On day 0, the 45 mice were randomly placed into 1 of 3 groups of 15 mice each, and arranged in the ventilated caging system as described. Randomization minimized the confounding effects of gender, body weight, estrus cycle, cage location within the ventilation racks, and numerous other variables within the study. One group received intraperitoneal radiotelemetry implantation through a flank abdominal approach; another group received the device through a midline abdominal approach. The remaining group received analgesic (buprenorphine) only.3,14 The surgical groups were compared directly with each other and with the buprenorphine control group. The surgical approach was determined randomly by the veterinary surgeon and blinded to the investigator to reduce bias. Food and gel both were placed in the elevated wire feeder. It has been reported that even accessing the water bottle immediately after surgery was hindered by a transmitter and pain associated with the surgical wound.16,21 Feeding the food and gel in the wire feeding trays forced the mice to stretch their abdomens. All experimental and control mice received buprenorphine on day 0.
For intraperitoneal radiotelemetry implantation, mice were anesthetized (0.15 mL per 25 g body weight) with a ketamine (9 mg/mL)–acepromazine (0.9 mg/mL)–xylazine (1.8 mg/mL) solution. All surgical mice received the same anesthetic regimen with the assumption that the duration of anesthesia was neutral between surgical groups. For analgesia, all mice received buprenorphine (0.3 mg/mL IM twice daily; approximately 0.009 mg per 25 g) for 48 h after surgery, from day 0 (study period 1) through day 2 (beginning of study period 2).
Surgical description of flank approach.
The flank approach passes through several layers, including the skin, fascia, terminal and transverse abdominal oblique muscles, and peritoneum.8 The right flank from the level of the spine to the midline abdomen was shaved. After aseptic preparation, each mouse was placed in left lateral recumbency. The surgical approach was through the right flank abdominal wall, in the region of the abdominis lateralis at a level just proximal to the curvature of the right stifle. A 1-cm incision was made through the skin on the right flank by using Metzenbaum surgical scissors. Blunt dissection with thumb forceps and hemostats was used to dissect fat and fascia and tease apart the abdominal oblique musculature, carefully exposing the abdominal peritoneum. The abdominal peritoneum was lifted away with forceps gently from the abdominal internal organs, and a small incision was made into the peritoneum using scissors. The hole was enlarged by using blunt dissection with hemostats. The radiotelemetry device was placed into the abdominal cavity by using forceps to manipulate the device. The peritoneum was closed by using a 4-0 braided, absorbable suture (Vicryl, Ethicon, Somerville, NJ) in a cruciate pattern. Cyanoacrylate tissue adhesive (Vetbond, 3M, St Paul, MN) was applied for skin closure. Mice were maintained on warmed gel packs during surgery and recovery until sternal and moving around in the cage.
Surgical description of midline abdominal approach.
The surgical procedure for midline intraperitoneal implantation of the TA-F20 device is described elsewhere.11,15 As is common with many surgical approaches, variation exists in the technique used based on surgeon preference. The main variation in the current study involves the use of cyanoacrylate tissue adhesive instead of suture for skin closure. Tissue adhesive has proven to be a successful means of closure at our institute. In brief, the ventral abdomen of each mouse was shaved caudally from the xyphoid region to the genitalia. After aseptic preparation, the animal was placed in dorsal recumbency. The surgical approach was through the ventral midline abdominal wall in the region of the umbilicus. A 1-cm incision was made into the ventral abdominal wall through the skin by using Metzenbaum surgical scissors. The subcutaneous tissue was incised with Metzenbaum scissors and dissected to the level of the peritoneal musculature. The linea alba was identified as a translucent line of connective tissue along the ventral midline of the external peritoneal musculature. Once identified, the peritoneal musculature was tented by using thumb forceps and an incision made into the linea alba by using a no. 11 blade. The incision was extended to approximately 1 cm in length by using Metzenbaum scissors, and the peritoneum was identified and incised with scissors. The radiotelemetry device was placed into the abdominal cavity. The peritoneum and linea alba were reapposed by using a simple interrupted suture pattern of 4-0 Vicryl (Ethicon). The skin was apposed completely by using forceps, and cyanoacrylate tissue adhesive (Vetbond, 3M) was applied for closure. Mice were maintained on warmed gel packs during surgery and recovery until sternal and moving around in the cage.
Surgery times.
The surgery times for both the flank approaches and midline abdominal approaches were measured using a digital timer and recorded. The time was noted from the moment the skin incision was made until tissue adhesive was applied.
Activity and temperature data.
Data on body temperatures and activity levels were collected by radiotelemetry in 30-s intervals every 5 min beginning 2 h after all surgeries were completed (time 0; day 0 at 1430) and extending through 1430 on day 14. On completion of the study, these data were imported into Excel 2007 (Microsoft). These data were organized into 11 consecutive time periods (study periods). This organization was necessary due to an unidentified failure in the radiotelemetry system resulting in 16 h of data loss in both surgical groups from 1700 on day 9 to 0900 on day 10. Period 1 included telemetry data for the first 2 d after surgery (study days 0 to 2); this organization helped minimize the effect of activation of the radiotelemetry devices on the day of surgery (instead of 24 h prior to surgery, as recommended by the manufacturer) on the initial data. Periods 2 through 6 included the telemetry data for day 3 through 1700 on day 8. Period 7 included data for days 8 (after 1700) to 10 (before 1430), corresponding to the period of data loss. Periods 8 through 11 contained the telemetry data from day 10 (after 1430) through day 14. Telemetry data were evaluated for each mouse in each group, and activity trends were analyzed to ensure that allocating telemetry data in this manner did not skew results. Telemetry data were analyzed by comparing mean activity and temperature over all 11 time periods and on a period-by-period basis. In addition, the mean time at a body temperature of less than 35.5 °C was compared between the surgical groups. This temperature 35.5 °C is consistent with published normal temperatures from other studies looking at individually caged mice but is lower than those reported for group-housed mice.15, 29
Body weight, food intake, gel intake, and vitality scoring.
Food and gel weights were recorded each morning as close to 0800 as possible. Food intake and gel intake levels were calculated as differences between the ‘beginning’ and ‘ending’ food and gel weights over a 24-h interval. Changes in food and gel intakes were defined as the differences in consumption on days 1 to 13 after surgery compared with the presurgical baseline values (on day −1). Body weight and vitality score were recorded together each morning as close to 0900 as possible. To standardize for the weight of the telemetry device in implanted mice, body weight measurements of all surgery mice were taken immediately after surgery on day 0, as a baseline measurement. Baseline measurements for control mice were taken immediately prior to injection with buprenorphine on day 0. The body weight measurements taken on subsequent days were subtracted from this baseline weight, resulting in a positive or negative change in weight. This change in weight was used for comparison. Vitality scores were taken by the same veterinarian for the entire study period, beginning 6 d prior to surgery. Vitality score criteria included appearance (0, normal; 1, dull or rough hair coat; and 3, absence of grooming or hunched posture) and provoked behavior (0, normal; 1, subdued but normal when stimulated, moves; 2, subdued even when stimulated, resists movement; and 4, unresponsive when stimulated, weak, unable to walk). The scores for the 2 categories were combined and interpreted as: 0, normal; 1 or 2, mild pain or distress response; 3, moderate pain or distress response, additional pain control necessary; and 4 or greater, severe pain, euthanize.
Statistics.
The data were analyzed inhouse (Department of Biostatistics, US Army Medical Research Institute of Infectious Diseases); the principal investigator generated graphs by using Excel 2007 (Microsoft). Differences were determined to be significant at a P value of less than 0.05. Trends were identified in cases where the data suggested that a difference exists but the P value was 0.05 to 0.10. Student t test analysis was used to compare mean surgery times between surgical groups. ANOVA was used to compare differences in mean activity and temperature levels between the surgery groups for 11 study periods and in daily body weight and gel and food intakes as compared with baseline levels. Correlation coefficients (R2) and regression equations were calculated for activity level. A Kaplan–Meier curve was developed to analyze the time each surgical group remained below a mean temperature of 35.5 °C. Because normality assumptions were not met in analysis of vitality score, nonparametric methods were used. Specifically, the nonparametric analog to ANOVA, the Kruskal–Wallis test, was used to assess overall group differences between the 3 groups at each time point between days 0 and 7. When the overall group difference was significant, the nonparametric analog to the independent-samples t test, the Wilcoxon–Mann–Whitney test, was used to assess pairwise differences between groups. Results for food intake, gel intake, and body weight were analyzed both directly between the 2 surgical groups and between the surgical groups and the buprenorphine-treated control group. When the control group was compared with the 2 surgical groups, adjustments were made by using posthoc Tukey tests to account for multiple comparisons. Standard errors were calculated for all variables examined.
Results
During the study, 2 mice in the group that underwent intraperitoneal implantation of radiotelemetry devices by the midline approach died during postsurgical recovery, one each on days 2 and 6. Their data were included in changes in food intake, gel intake, body weight, and vitality score and length of surgery. However, radiotelemetry data were analyzed only from mice surviving the study. For statistical calculations, adjustments were made in total numbers in groups after removal of the 2 mice that died. No other mice died during the study. A third mouse in the midline group did not reach 35.5 °C during the 14-d study period and was censured from temperature analysis. All remaining mice in both surgical groups received a single injection of ketamine–acepromazine–xylazine as described in Materials and Methods and recovered without obvious complications. Exclusion and censorship of the aforementioned data as well as the additional use of anesthetic in the single mouse skewed the results slightly toward the null hypothesis. The effects did not interfere with the overall study goals of evaluating approaches to intraperitoneal radiotelemetry device implantation. A variation in weighing times for food and gel occurred on days 12 and 13 of the study, affecting all groups equally but artificially altering amounts consumed for these days. In addition, study period 3 (day 4 after surgery) represents 48 to 72 h after buprenorphine administration.
Surgery time.
Surgery time was analyzed to determine whether one surgical approach was significantly advantageous in time to completion compared with the other. A statistically significant difference (P = 0.0010) existed in group surgery times, with surgery by the flank approach averaging approximately 1 min less time to perform than that by the midline approach.
Body temperature.
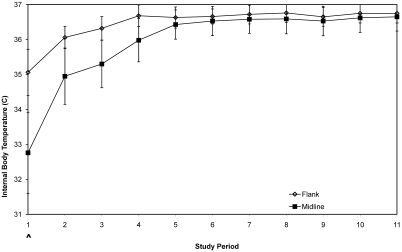
In general, an initial drop in temperature during and immediately after anesthesia is anticipated, followed by a rapid return to normal temperature as the animal recovers from anesthesia and surgery. A significant difference (P = 0.0070) in mean change in temperature between the flank and midline surgical groups occurred over the cumulative 11 time periods (Figure 1). However, significant differences were not noted at any single time period between groups. Data for time period 1 suggest (P = 0.0805) that a difference is present during the first 48 h after surgery, with the flank approach having a mean group positive difference in body temperature of nearly 2 °C compared with the midline group. In addition, the results for time below 35.5 °C suggest (P = 0.0881) that the midline group takes longer than the flank group, on average, to reach a normal temperature. The mean of the flank group exceeded 35.5 °C by study period 2, whereas the midline group did not exceed 35.5 °C until study period 5. In addition, variation in body temperature in individual mice (as reflected in the standard error) on a period-by-period basis was more pronounced in the midline group than the flank group, as far out as period 5. The lack of significance between the groups through period 4 appears to be related directly to the extreme intraindividual variation in the midline group. This variation continuously exceeds that seen in the flank group for all study periods, indicating less intraindividual variation in homeothermy in the flank group. Overall, the trends suggest that the flank incision has less of an effect on body temperature homeostasis after surgery than that of the midline approach, at least for periods 1 through 5.
Figure 1.
Postsurgery internal body temperatures. Each point represents the average group body temperature (mean ± SE) after surgery for each of 11 consecutive study periods measured by using RT. Open diamonds, flank surgery; solid squares, midline abdominal surgery; *, significant (P < 0.05) difference between groups; ^, suggested (P < 0.10) difference between groups.
Activity.
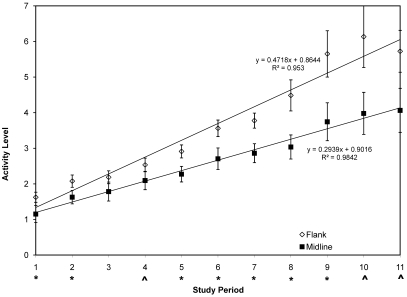
As an animal experiences increased pain or discomfort, activity decreases, with an animal staying in one place for abnormal lengths of time and with reluctance to move.25 A significant (P < 0.0001) overall difference in activity was noted over the cumulative 11 time periods, with greater activity as a whole noted in the group receiving radiotelemetry implantation by a flank approach (Figure 2). In addition, statistically significant differences between the 2 surgical groups occurred for time periods 1 (P = 0.0294), 2 (P = 0.0356), 5 (P = 0.0332), 6 (P = 0.0317), 7 (P = 0.0115), 8 (P = 0.0167), and 9 (P = 0.0350). The results were suggestive of a difference for study periods 4 (P = 0.0961), 10 (P = 0.0571), and 11 (P = 0.0631). These data indicate that the flank group was more active immediately after surgery than was the midline group. In addition, mean activity levels consistently increased over consecutive study periods in both groups in a near-linear fashion (see Figure 2 for regression equations and R2 values). However, after day 5, as supported by the P values noted earlier and the trend in R2, mean activity levels were consistently greater in the flank group than the midline group. In addition, the extremes in the standard error data themselves gradually increase in both groups beginning at period 6, with the greatest variation evident from day 8 forward. The variation in the midline group, however, is not similar to that of the flank group until day 11. This variation is related directly to the wide range of activity (or inactivity) levels between individuals within the groups over the entire study and supports a wider range of activity levels among members of the flank group than the midline group.
Figure 2.
Postsurgery activity levels. Each point represents the average group activity (mean ± SE) after surgery for each of 11 consecutive study periods measured by using RT. Open diamonds, flank surgery; solid squares, midline abdominal surgery; *, significant (P < 0.05) difference between groups; ^, suggested (P < 0.10) difference between groups (^). Coefficient of determination (R2) provides predictability between study period and change in activity showing correlation; straight-line regression equation serves as a measure of predictability between the independent variable ‘study period’ and the dependent variable ‘activity level,’ demonstrating strength of change in activity over time.
The significant (P < 0.05) differences in activity between the groups during periods 1 and 2 indicate less immediate impairment from surgery by the flank approach. The decline in average activity levels for period 11 is due to a shorter period of data analyzed at the end of the study, and both groups were affected similarly. Overall, activity appears to be less affected and more robust when intraperitoneal implantation of radiotelemetry devices is performed by the flank approach.
Body weight.
Mean presurgical body weights were similar among groups (flank group, 26 g; midline abdominal group, 25 g; and control mice, 26 g).
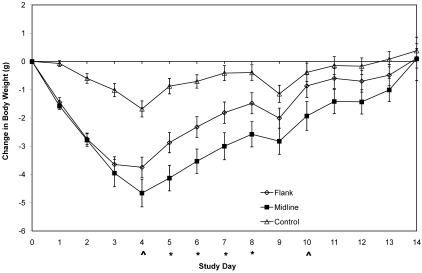
When standardized weights were compared directly between the surgical groups, statistically significant differences were present on days 5 (P = 0.0180), 6 (P = 0.0196), 7 (P = 0.0331), and 8 (P = 0.0424). These data reflect smaller changes in body weight over these time periods with the flank approach compared with those seen with the midline approach (Figure 3). In addition, data for days 4 (P = 0.0958) and 10 (P = 0.0804) suggest that a difference between groups exists.
Figure 3.
Change in body weight. Each point represents the average daily change in group body weights from weights taken immediately prior to the onset of surgery (mean ± SE); open diamonds, flank surgery; solid squares, midline abdominal surgery; open triangle, buprenorphine treated control group; statistically significant difference (P < 0.05) between groups (*); suggested difference (P < 0.10) between groups (^). A negative value indicates a decrease in body weight from baseline. Levels of significance apply only to the surgical groups.
Standardized body weights were significantly different between the control and flank surgical groups from day 1 through day 7 after surgery, as follows: days 1 through 3, P < 0.0001; days 4 and 5, P < 0.001; day 6, P < 0.01; and day 7, P = 0.0266. Data for day 8 (P = 0.0916) also suggest less of a change in body weight within the control mice than the flank surgical group. When comparing the control and midline groups, statistically significant differences emerged during days 1 through 10 as follows: days 1 through 7, P < 0.0001; 8, P < 0.001; 9, P < 0.01; and 10, P = 0.0338. Data for days 11 (P = 0.05), 12 (P = 0.0546), and 13 (P = 0.0924) are suggestive of a difference. The average cessation of weight loss for all groups was day 4, approximately 48 h after cessation of analgesics. The maximal average weight losses by day 4 were 1.68 g (6.5% of body weight) for the control group, 3.75 g (14%) for flank group, and 4.7 g (19%) for the midline group. The control group required 11 d to return to the presurgery body weight, whereas both surgical groups needed 14 d.
After discontinuing buprenorphine, the change in body weight significantly differed in the surgery groups to at least day 8 of the study. The difference in body weight between the surgery groups and the control group on day 1 demonstrates that the surgical procedure (including anesthesia) affected body weight. We cannot explain the decline in body weight on day 9 in all 3 groups; nearly all mice each lost 0.5 to 1.0 g on this day. This decline may be related to incorrect zeroing of the scale, changes in the way the mice were weighed on that day, change in time of day at which mice were weighed (before change of feed), or a recording or calculation error. The results do not alter the overall goal of the study and occur similarly across the groups.
Food intake.
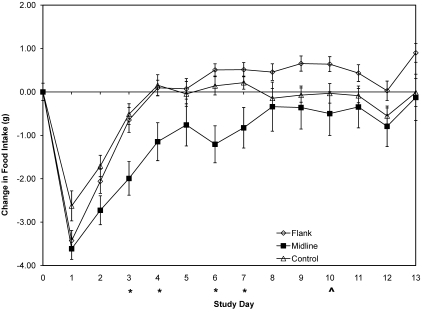
Significant differences in change in food consumption (Figure 4) between the 2 surgical groups were noted on days 3 (P = 0.0066), 4 (P = 0.0135), 6 (P = 0.0002), and 7 (P = 0.0069). Data for day 10 (P = 0.0987) are suggestive of a difference.
Figure 4.
Change in food intake. Each point represents the change in group food intake (mean ± SE) from a baseline level obtained the day prior to surgery. Open diamonds, flank surgery; solid squares, midline abdominal surgery; open triangles, buprenorphine-treated control group; *, significant (P < 0.05) difference between groups; ^, suggested (P < 0.10) difference between groups. A negative value indicates a decrease in body weight from baseline. Levels of significance apply only to the surgical groups. Note: day 13 measurements were 8 h late, thereby artificially elevating food intake for that period of time.
A statistically significant difference in change in food consumption among all groups occurred on days 1 (P = 0.0319), 2 (P = 0.0497), 3 (P = 0.0049), 4 (P = 0.0150), 6 (P = 0.0006), 7 (P = 0.0239), and 9 (P = 0.0314) after surgery (Figure 4). Data for day 10 (P = 0.0907) were suggestive of a difference between the 3 groups. A statistically significant difference in change in food consumption between the control and flank surgery group occurred only on day 9 (P = 0.0241); data for days 10 (P = 0.0971) and 13 (P = 0.0552) were suggestive of differences in food intake. A statistically significant difference between the control and midline surgical groups occurred on days 1 (P = 0.0291), 2 (P = 0.0424), 3 (P = 0.0077), 4 (P = 0.0245), and 6 (P = 0.0147). After day 7, food intake did not differ between the control and midline groups. Unlike the midline group but like the control group, the flank group returned to a presurgical baseline level of food intake by day 4, surpassing the control group in mean group change in food intake by day 6. The control group returned to baseline consumption by day 4 and remained at baseline through the remainder of the study. The midline surgery group did not return to a baseline level of food intake until at least day 8 and did not surpass the flank group in mean food consumption during the study. For the first 24 h after surgery, food consumption decreased significantly (P < 0.05) but in a similar manner from presurgical levels. This effect also was significant (P < 0.05) in the control group but to a smaller degree than for the surgical groups. The inflection point in food intake on day 1 in all groups indicates that buprenorphine affects food intake within the first 24 h of treatment. After this initial 24-h period, the anorexic side effects begin to reverse, with all groups appearing to normalize within 48 h after the last treatment dose. The similarity in change in food intake between the flank and control groups on day 2 suggests that any additive negative effects of anesthesia or procedure-associated discomfort on food intake began to dissipate within 48 h in these groups. Although change in food intake reversed in the midline group on day 1, these mice did not return to the food intake level of the control group until at least day 8.
Gel intake.
Daily fluid consumption was assessed by change in gel weight at time points prior to surgery through day 13. No significant group differences were noted between any of the comparative groups for any specific day.
Vitality score.
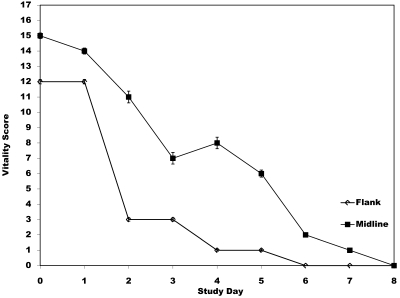
Visual evaluation of an animal is subjective but can be used to assess the pain and distress an animal is experiencing after a procedure. The average total vitality scores for each surgical group and the control group were compared (Figure 5). None of the control mice exceeded a score of 0 at any point during the study and therefore were not included in Figure 5. A significant difference (P < 0.0001; adjusted for multiple comparison) was noted between each surgery group and the control group for the first 2 d after surgery. No differences were noted between the surgical groups for any day or between either of the surgical groups and the control group after day 2. The statistically significant overall differences in vitality scores for days 0 and 1 between the control and surgical groups suggests that factors other than buprenorphine account for the clinical signs noted in the surgical groups. As evidenced in Figure 5, the total numerical scores were higher in the midline group than the flank group. However, this difference in score is reflective of the 2 mice in the midline group that died. Otherwise, overall differences in the number of mice reflecting a score greater than 0 on any given day were similar between the surgery groups.
Figure 5.
Change in vitality scores. Each point represents the total combined vitality score (total score ± SE) for all mice within a surgery group. Open diamonds, flank surgery; solid squares, midline abdominal surgery. Note: none of the control mice received a score greater than 0 and therefore are not represented graphically. The graph includes the vitality score for all mice in both surgical groups. All scores were 0 in all groups after day 7.
Discussion
The results of the current study show that intraperitoneal implantation of radiotelemetry devices in mice from a flank rather than a midline approach is beneficial in terms of food intake, changes in body weight, activity level, temperature homeostasis, and time for surgical implantation. Although all parameters examined in both surgical groups showed physiologic changes consistent with surgery, the negative effects were less substantial in the flank approach. The information provided is meant to build a framework for future studies by using a multidimensional analysis to support refinement of a procedure.
Continuously looking for ways to refine accepted surgical techniques should be a key aspect in minimizing pain and distress.25 The pain resulting from a surgical procedure may depend on the invasiveness of the procedure.12 One telemetric study that evaluated pain parameters after laparotomy in mice not given analgesics demonstrated significant changes in body temperature, body weight, food intake, electrocardiogram recordings, and heart rate as compared with those of mice provided analgesia.1 The current study changed the location of intraperitoneal radiotelemetry device implantation in an attempt to minimize invasiveness of the procedure and limit the direct effects of internal and external factors on the incision site. In a small animal, the incision size is proportionally large compared with body size, exposing more of the abdominal cavity than would the same procedure in larger animals. Female and young mice may have more negative effects related to radiotelemetry device implantation than do their larger counterparts. In this study, weight distributions after randomization were fairly consistent within and between the groups, indicating minimal to absent confounding of the results due to animal size.
Introducing the radiotelemetry device into the abdomen through a flank incision differs from the midline approach because the lateral abdominal muscles can be teased apart with hemostats in the flank approach, allowing for direct access to the peritoneum without incision of the subcutaneous tissue. In addition, the weight of the abdominal contents is not directly on the incision, thereby decreasing the risk of dehiscence and allowing for closure of the surgery site with a single cruciate ligature. Furthermore, tension on the flank muscles is minimal during stretching of the abdomen to reach food and gel in the overhead wire feeder, thereby minimizing discomfort to the animal. The opposite situation is true with a midline incision. Although the musculature paramedial to the midline can be teased apart and closed with a cruciate ligature, as in the flank approach, doing so is not recommended in light of the increased tension at the surgical site and the likely increased risk of dehiscence due to the decreased holding capacity of muscle itself. In addition, these modifications to the midline approach likely would result in increased mechanical stress at the surgical site due to stretching of the abdomen during acquisition of food and water, similar to observations in the current study. However, future studies might compare the flank and paramedial approaches to assess whether the paramedial approach is contraindicated as compared with access through the flank.
The composite measurements used in the current study to evaluate recovery in mice proved valuable in supporting refinement of the surgery method. Constructing means to rate pain, discomfort, or distress in an animal that cannot communicate is complex, objective criteria for doing so are not accepted universally, and relying on one parameter alone is dangerous to the welfare of the animal.12 Although the results of the current study are subtle in many areas, the advantages in refining the site of telemetry implantation are supported by the collective data as a whole. In this study, a difference in recovery or postoperative pain between the surgical groups is not evident based on vitality scores alone. The differences noted between the flank and midline approaches in total vitality scores, change in body weight, effects on temperature, and overall activity levels (in conjunction with slower rates of change over time present in the midline group) clearly support benefits of the flank surgical approach.
Simplifying a surgical procedure and reducing operating times promotes animal welfare.32 Decreasing the time associated with a procedure allows a quicker return to the recovery cage, less exposure of the open body cavity, better temperature homeostasis, and less postoperative pain.12 The longer the procedure, the greater the effects of external factors such as heat and loss of body fluids on vitality. The decreased time of surgery for the flank approach is related primarily to the ability to tease apart the muscles with hemostats rather than finding and incising the linea alba and to the application of a single suture to close the body wall before skin closure rather than multiple sutures along the midline to prevent evisceration related to dehiscence. The veterinary surgeon in the current study is well experienced in performing surgeries on mice by using both approaches. This experience was important in improving outcome of a procedure by ensuring that negative effects related to tissue handling and surgeon skills are minimized. Minimizing unnecessary trauma to tissues related to surgery limits the inflammatory response and helps control the amount of postoperative pain experienced.12 The minor decrease in surgery time likely accounts, at least in part, for some of the other results noted in the study. However, future studies might involve a set surgery time for both procedures to assess whether the surgical approach itself or the time involved in performing the procedure accounted for the differences noted in various parameters of this study.
Body temperatures in mice can remain suppressed for as long as 24 h after radiotelemetry device implantation by a midline approach.15 The mean temperatures in the current study were reduced in the midline group for as long as 5 d after surgery, suggesting that the effects on temperature may be more substantial in some cases. This outcome may be related to a variety of reasons, including surrounding ambient temperature, ease of access to food and water, and method of body temperature measurement, to name a few. The differences in the current study likely are due to direct effects of the surgical procedure but might also be explained by interindividual differences in the normal body temperature of mice, combined effects of buprenorphine and anesthesia, macro- and microenvironmental temperatures, and location on the rack. In addition, differences in activity, which is a means for mice to regulate body temperature, may play a key factor as well.
As noted earlier, one mouse in the study did not return to a normal temperature. The individual data for the mouse suggest that its normal body temperature was lower than 35.5 °C and represented individual variation. The mouse appeared otherwise healthy throughout the study. Individual temperatures of mice in both surgical groups showed similar variations in body temperatures. Administration of ketamine–acepromazine–xylazine anesthesia is known to decrease temperatures by several degrees, even without surgery,12 and this regimen can affect the wellbeing of mice over the long term as well.12 Furthermore, mice as a species are predisposed to hypothermia.30 In fact, rodents and other small mammals are particularly susceptible to irreversible hypothermia leading to death.25 Postsurgical internal body temperatures were less than 28.0 °C in the 2 mice that died in the midline group. Even though supplemental heat and warmed fluids were provided during and after surgery, the hypothermia in these 2 mice was irreversible. Overall, postsurgical internal body temperatures were suppressed below 35.5 °C consistently, with some temperatures falling and remaining below 30 °C, for several hours in both surgery groups. However, the randomized mice in this study all received the same anesthetic dosage (with one previously noted exception) and had similar durations of effects.
Environmental factors including ambient room temperatures as well as location in the ventilation rack affect the microenvironment of mice and thus their internal body temperatures. The cooler the ambient temperature, the higher the rate of metabolism needed to generate heat. The opposite effect is true with higher ambient temperatures. Warmer rooms may have helped the mice to recover better by decreasing metabolic demand. If the discomfort related to the surgery affects the ability of mice to thermoregulate, their postsurgical recovery may be delayed or decreased. Finally, cage location in an active ventilation system can lead to variations in humidity due to differences in airflow and hourly air exchange rates, thereby affecting heat loss and changing metabolic demands related to thermogenesis. However, the randomization of the mice in the current study likely limited the potentially confounding effects of these variables.
Of all parameters measured, the observed differences in activity levels were the most supportive of refining intraperitoneal radiotelemetry device implantation to a flank approach. The wellbeing of animals can be compromised for the first week after surgery, and an animal may require 5 to 7 d just to regain its circadian rhythm.2,4,5 Circadian rhythm is related directly to activity level. Activity increases body temperature, with different effects at different times of the day, and is very important in correcting a hypothermic state after anesthesia or surgery.31
Variations between individual animals in performance on a given task (such as food and fluid intake) during different phases of a study and on a day-to-day basis are expected.21The divergence in means and variation in standard errors in activity we noted in the current study may be related to differences in gender, weight, or age between groups. Additionally, the slight decreases in activity in both groups during study period 3 and the lack of significant or suggested differences between the groups likely are related to dissipation of buprenorphine. Regardless, animals in pain tend to stay in one location for abnormal lengths of time and can be reluctant to move unless absolutely necessary.25 This reluctance was evident in both surgical groups but most substantially in the midline group. In addition, isolation (individually housing) is known to decrease animal wellbeing and may affect sleep patterns also impacting activity.12 However, the groups in the current study were randomized, thus making the potentially confounding effects of isolation similar between the groups. Abdominal discomfort, similar to that associated with a flank incision, has been reported to cause quadrupeds to take additional steps when turning toward the painful side in an effort not to bend laterally.12 This compensation may have played a role in the increase in activity we noted for the flank group. However, activity in the mice that underwent the flank approach increased consistently over the study periods and did not plateau as the study progressed. This pattern indicates that similar accommodation was not the cause of the increased activity in the flank group. If so, the activity levels would have reflected a correction in activity level as the flank incision healed (that is, day 8 or later). In addition, the greater activity and randomization of the groups support a stronger recovery in activity by the flank group.
Metabolic rate influences recovery, and depending on the surgical procedure, smaller species may recover more rapidly.12 Body weight is used frequently in literature as a measure of postsurgical recovery. Short-term changes in body weight have been noted in other studies after intraperitoneal radiotelemetry device implantation surgery by a midline approach in mice, indicating impairment.4,2 Mice can take from 10 to 14 d in the absence of analgesia to recover from midline intraperitoneal radiotelemetry device implantation.3,2, 29 Mice with a substantial loss of weight after midline intraperitoneal implantation of radiotelemetry devices need as long as 14 d to recover to presurgical weights.21 The same report suggests that the change in body weight may never recover to the same level as that of nonsurgical groups and that the laparotomy procedure itself was responsible for a decrease of approximately 30% body weight in mice in the study.21 Other studies measuring body weight after sham and actual intraperitoneal radiotelemetry device placement in mice with midline incisions have demonstrated loss of body weight to day 4 (as in the current study), with a return to normal weight between days 4 and 14.30 In a study in rats comparing subcutaneous incision with midline laparotomy, the largest reduction in weight was seen after laparotomy and visceral agitation.22
As the current study demonstrates, the effects of the surgical procedure and analgesic on body weight are long-lasting, requiring considerable time for recovery. The fact that the control group does not return to a baseline weight for several days suggests a residual long-term effect on body weight related to the use of buprenorphine. However, the 6.5% change in body weight in the control group is not as substantial as the 14% loss in the flank group and 19% in the midline group, suggesting additional effects directly related to the surgical approach.
The changes in activity, temperature variation, and food intake in the midline group explain why weight loss in that group exceeds that of the flank group and why it persists to at least day 9 and likely beyond. Weight is a lagging indicator and requires time for recovery. Although mice receiving a radiotelemetry device by a flank approach did not return to the baseline weight until at least day 10, they recovered in food intake, activity, and temperature sooner than the midline group and may be fully recovered in terms of other parameters by 7 to 8 d after surgery.
Pain is known to decrease how much work an animal will do to gain access to food.12 Feeding behavior can be used in postoperative pain assessment and provides valuable information regarding the wellbeing of an animal.12 In the current study, the cause for the change in food intake in the surgery groups for the first 24 h cannot be differentiated definitively between the effects of analgesia, anesthesia, and the surgical approach. However, a substantial decrease is also evident, to nearly the same extent, in the control group, suggesting that buprenorphine alone plays a large role in the initial negative changes in food intake noted. The results demonstrate that after reversal in the effects of buprenorphine, the flank group normalized and exceeded presurgical food intake levels for most of the first 7 d after surgery. This situation, as well as the positive difference in activity level, suggests the mice were actively recovering from surgery and meeting metabolic needs. The fact that the midline group does not follow the same pattern in recovery of food intake to a baseline level supports impairment in this group directly related to aspects of the surgical procedure (anesthesia, time in surgery, hypothermia, and decreased activity level). The differences noted in change in food intake in the current study likely are affected by the location of the food (and gel) in the overhead wire feeder, which required the mice to work harder to achieve nutrition and hydration. The inability to access food due to abdominal pain is known to affect food intake.12 Tension on the midline surgery site due to actively stretching for food and gel likely increased the pain and discomfort in the mice in this group. In facilities where the current practice is to place food in an overhead wire feeder after surgery, placing food in an easily accessible location, such as a container on the cage floor, for at least the first 24 h after surgery may improve recovery by decreasing the physical demands required to access nourishment. Future studies might examine the effects of the location of food and gel (or water) on recovery between the 2 surgical approaches.
The use of gel provides an alternative to the use of liquid water in rodent studies. However, if gel use is considered, factors including evaporation of the gel, location of the gel in the cage, frequency of gel change, effects on food intake, and gel friability should be considered. In the current study, technical errors in weighing the gel, destruction of the gel square by the mice, dehydration of the gel, and hoarding of pieces of gel all altered results regarding gel intake in the study. These factors should be controlled if gel is used to monitor fluid intake in future studies.
Visual pain scoring systems are highly subjective and should not be relied on as a sole source of determining pain or other effects on wellbeing. Generally accepted objective criteria are not available for assessing the degree of pain. Many numeric scoring systems are available that attempt to look at behavioral, physical, and physiologic observations in a single dimension.12 The current study limited the subjectivity by using the same person to evaluate and assign a pain category to each mouse.
In general, untreated or undertreated pain has a variety of negative effects, including increased morbidity and mortality, delayed wound healing, changes in natural behavior patterns such as sleep, extended postoperative recovery, immunosuppression, anorexia, decreased natural activities such as nest building, and many other physical, physiologic, and behavioral parameters.12 Although the experience of pain cannot be assumed to be equal across species or within strains, the opposite effect cannot be assumed either, making examination of the stimuli in the respective species imperative to animal welfare.12 Studies have shown that some differences in physiologic parameters are strain-associated.15 Even subjects within a species may react differently to noxious stimuli in any single parameter, and it should not be assumed that the absence of clinical signs consistent with the stimuli suggests that pain is not present either.12 The current study specifically assesses parameters in CF1 mice. This selectivity demands caution in assuming results of any study can be correlated to another strain or species. However, negative effects noted in the current study were less severe on the welfare of the mice by using a flank approach. More substantial effects on welfare were noted with the midline approach. At the same time, positive benefits were noted in recovery after the flank approach that may be beneficial to conspecific welfare after the procedure. Therefore, we believe that implanting intraperitoneal radiotelemetry devices through a flank incision is beneficial to all mice until proven otherwise.
The current study also suggests that pain and discomfort directly correlate to recovery. Pain affects many of the parameters used in this study. However, the evaluation of pain itself is extremely subjective and complex. For instance, evidence suggests that pain tolerance can vary based on individual characteristics including gender and age.12 Still, tolerance implies that a level of pain or distress is present—the difference is that one individual better copes with the pain than another. To this extent, variation between members of a group may be present in the data of a study skewing their statistical significance but maintaining their clinical relevance. This consideration is important in a study because even randomization does not guarantee normal distribution between groups. In addition, pairwise comparison itself cannot account for differences behavioral factors have on the study.
Accounting for confounding variables is desirable and necessary to confidently accept or reject a hypothesis. However, controlling for all variables in a study often is not practical or even justified. The benefits of the data obtained must be weighed against the welfare of the animal. Many potential confounders likely affected the results of the current study and were not or could not be controlled, including the effects of ketamine–acepromazine–xylazine anesthesia alone on recovery; the effects of the combined anesthesia and analgesia on recovery; comparison in recovery to other strains of mice or species of animals; the effects of body weight differences, age differences, gender differences, and estrus cycle on recovery; activity and internal temperatures through use of recovered, preimplanted mice with intraperitoneal radiotelemetry devices; effect of group housing, including mice with a single approach per cage as well as a heterozygous mixture of approaches and controls per cage; frequency of buprenorphine dosing; type of analgesic used (incorporating control groups given common nonsteroidal anti-inflammatory drugs as a comparison to buprenorphine on surgical recovery in both approaches); location within a ventilated rack for effects related to variations in airflow, ambient temperatures, and humidity; effects in a nonventilated caging system; and use of multiple sham surgical groups. In particular, mice preimplanted with intraperitoneal radiotelemetry devices were unavailable for use as a control group and would have provided valuable data regarding comparative activity and temperature data.
Because of the paucity of published data on the effects of buprenorphine in normal healthy mice and the known effects of buprenorphine on activity, body weight, food intake, and fluid consumption, we elected to control for its use in the current study. The control group served primarily to complement the direct comparison between the 2 surgical groups. The data for the control group were retained in the study because, in the opinions of the authors and the consulting biostatistician, the control group complements the study from period 2 (48 h after surgery) forward, providing valuable comparative data, especially those involving body weight and food intake. In addition, these data provide valuable information on the effects of buprenorphine in normal, healthy mice that can be beneficial to other studies.
Sham surgeries are informative and can be used to eliminate effects related to the incision itself. In the current study, incorporating 2 sham control groups per surgical approach may have proven beneficial in determining the effects of both skin incision alone and full-thickness incision, without placement of an intraperitoneal radiotelemetry device, on postsurgical recovery. However, sham surgeries were not performed in this study because of space constraints, limits in the numbers of mice available for the study, and the availability of comparative information from sham procedures in other studies.
Other studies using sham approaches have provided valuable information. One study that used sham surgeries involving midline intraperitoneal radiotelemetry device placement showed that the radiotelemetry device itself is responsible for some of the negative effects related to implantation.21 In another study of midline abdominal operations in rats using implanted and sham approaches, the midline intraperitoneal radiotelemetry surgical procedure caused significant reduction in food and water consumption, body weight, and locomotor activity.22 In contrast, the sham group, after receiving only a midline skin incision, showed significantly less depression of food and water consumption and body weight than did groups that underwent laparotomy.22 Another study looking at the effects of mock ova implantation by a flank incision in ICR mice (in particular, a control group not provided analgesic), albeit by a smaller flank incision than used in the current study (0.5 cm versus 1.0 cm, respectively), suggested that the pain induced by the sham procedure was not debilitating.13 These results support other published information that suggest that the location of the surgical incision may be as important as the size of the device itself.21,26 In the current study, the size of the radiotelemetry device was constant between the 2 surgical groups and the final location of the radiotelemetry device within the abdomen was similar, even though different approaches were used. Based on this information and despite the lack of inclusion of sham surgeries, the results of the current study support the flank approach as creating less debilitation than does the midline approach. Regardless, the incorporation of sham surgeries to eliminate the effects of the radiotelemetry device and to control for those of the skin incision itself should be considered for future studies.
Postoperative analgesia is an essential component in the recovery of mice after invasive surgery.32 One study using radiotelemetry evaluated pain parameters after laparotomy in mice not provided analgesics. The study demonstrated significant negative changes in body temperature, body weight, food intake, electrocardiogram recordings, and heart rate, as compared with those of mice provided analgesia.1 Currently, buprenorphine is a drug of choice for pain management in rodents.12 As with most opioids, buprenorphine has a wide range of side effects that substantially affect recovery, with results differing between studies. Negative effects of buprenorphine itself on food intake, water intake, and body weight have been reported for mice and rats.3,13,27 However, in other studies looking at rats treated with buprenorphine, food and water consumption was greater in treated groups than in those provided saline only.12 These results may be related to many factors, including the previously mentioned confounders and species-specific variation in responses to buprenorphine. In addition, buprenorphine has been shown to increase activity in some rodents,3 and in studies between treated and untreated mice, mice treated with buprenorphine prior to a laparotomy were more active than were untreated mice, which failed to move.12 However, an increase in activity level in conjunction with a decrease in food and water intake might create a metabolic crisis in an animal. In this way, buprenorphine likely contributed to the substantial decrease in body weight after day 2 that we noted. Our study demonstrates that changes related directly to buprenorphine occur in normal healthy mice and are substantial. In particular, the effects on food intake and gel intake were immediate, with direct and residual effects on baseline body weight lasting as long as 8 d after the last dose (10 d after the initial dose). Furthermore, studies on the effects of nonsteroidal anti-inflammatory drugs (such as ketoprofen and carprofen) in rats after abdominal surgery did not show significant differences in analgesia compared with buprenorphine.12 Buprenorphine greatly affects the health and wellbeing of mice. Its noted effect in normal healthy mice in the current study supports limiting the use of buprenorphine in investigative studies in favor of analgesics with similar pain controlling properties and fewer side effects, such as various nonsteroidal anti-inflammatory drugs described elsewhere.3,14
The current study provides a variety of topics for future investigation. First, many of the factors previously mentioned could be considered, including incorporation of sham surgeries; use of preimplanted mice; effects of environmental factors such as ambient temperatures, ventilation rates, humidity levels, and location of food and water; effects of physical factors such as gender, age, weight, and estrus cycle; combined effects of anesthesia and buprenorphine; effects on recovery related to type of analgesic used; and comparison with conspecifics. Examining the effects of the location of surgical implantation across a myriad of different species and assessing the effect of size of the different devices should also be considered.
Our direct comparison involving intraperitoneal radiotelemetry device implantation by either a flank or midline approach demonstrated subtle but positive benefits to mice in which devices were implanted through a flank incision. We noted no contraindications for use of the flank approach compared with a midline approach for this application. Future studies incorporating intraperitoneal radiotelemetry devices in mice should consider implantation by the flank approach instead of a midline approach to limit effects on mouse wellbeing and improve the quality of scientific data. In addition, the current study demonstrated negative effects in changes in body weight, food intake, and gel intake in normal, healthy mice treated only with buprenorphine. These findings support other studies with similar findings and further suggest that improvements in animal wellbeing might be gained by limiting the use of buprenorphine in favor of another equally effective analgesic.
Acknowledgments
We gratefully acknowledge the contributions of the following animal veterinary, technical, and caretaking staff at USAMRIID for assistance in animal care, data collection, manuscript review, and study advice: LTC(P) James Sheets, LTC Rebecca Holt, MAJ Christine Ege, MAJ Scott Willens, SFC Stephanie McClain, SGT Daisy Novenario, Mr Joshua Moore, Mr William Agee, Mr Carlton Rice, Mr Jim Franklin, Mr Joe Williams, Mr Chuck Stover, and Ms Deb Harrison. We further acknowledge the assistance of Ms Diana Fisher (MA, Biostatistician, USAMRIID) for assisting with data analysis, Dr Michael Parker for arrangement of the study in conjunction with an approved protocol, and the members of the USAMRIID IACUC. The research described herein was the result of a Cooperative Research and Development Agreement (51008544) between DynPort Vaccine Company (CSC Co, Frederick, Maryland) and USAMRIID. The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the US Army.
References
- 1.Arras M, Rettich A, Cinelli P, Kasermann HA, Burki K. 2007. Assessment of postlaparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. Biomed Central Vet Res 3:16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumans V, Bouwknecht JA, Boere H, Kramer K, van Lith HA, van de Weerd HA, Van Herck H. 2001. Intraabdominal transmitter implantation in mice: effects on behavior and body weight. Anim Welf 10:291–302 [Google Scholar]
- 3.Blaha MD, Leon LR. 2008. Effects of indomethacin and buprenorphine analgesia on the postoperative recovery of mice. J Am Assoc Lab Anim Sci 47:8–19 [PMC free article] [PubMed] [Google Scholar]
- 4.Braga VA, Prabhakar NR. 2009. Refinement of RT for measuring blood pressure in conscious rats. J Am Assoc Lab Anim Sci 48:268–271 [PMC free article] [PubMed] [Google Scholar]
- 5.Butz GM, Davvison RL. 2001. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics 5:89–97 [DOI] [PubMed] [Google Scholar]
- 6.Charles River Laboratories International [Internet]. Research animal models: CF1 mouse. [Cited 18 May 2009]. Available at: http://www.criver.com/en-US/ProdServ/ByType/ResModOver/ResMod/Pages/CF_1_Mice.aspx
- 7.Clement JG, Mills P, Brockway B. 1989. Use of telemetry to record body temperature and activity in mice. J Pharmacol Methods 21:129–140 [DOI] [PubMed] [Google Scholar]
- 8.Coe RJ, Grint NJ, Tivers MS, Moore AH, Holt PE. 2006. Comparison of flank and midline approaches to the ovariohysterectomy of cats. Vet Rec 159:309–313 [DOI] [PubMed] [Google Scholar]
- 9.Data Sciences International (DSI) 2008. Guide to the DSI system. St Paul (MN): Data Sciences International [Google Scholar]
- 10.Data Sciences International (DSI) [Internet]. 2009. PhysioTel TA transmitters specifications. [Cited 4 February 2009]. Available at: http://www.datasci.com/pdf/products/PhysioTel_Transmitter_Specifications.pdf
- 11.Data Sciences International (DSI) 2009. Temperature device surgical manual. St Paul (MN): Data Sciences International [Google Scholar]
- 12.Fish R, Danneman PJ, Brown M, Karas A. 2008. Anesthesia and analgesia in laboratory animals, 2nd ed, chapters 1, 4, 8, and 10 New York (NY): Academic Press [Google Scholar]
- 13.Goecke JC, Awad H, Lawson JC, Bolvin GP. 2005. Evaluating postoperative analgesics in mice using telemetry. Comp Med 55:37–44 [PubMed] [Google Scholar]
- 14.Hayes KE, Raucci JA, Gades NM, Toth LA. 2000. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp Top Lab Anim Sci 39:18–23 [PubMed] [Google Scholar]
- 15.Johnston NA, Bosgraaf C, Cox L, Reichensperger J, Verhulst S, Patten C, Toth LA. 2007. Strategies for refinement of abdominal device implantation in mice: strain, carboxymethylcellulose, thermal support, and atipamezole. J Am Assoc Lab Anim Sci 46:46–53 [PubMed] [Google Scholar]
- 16.Kaïdi S, Brutel F, Van Deun F, Kramer K, Remie R, Dewé W, Remusat P, Delaunois A, Depelchin O. 2007. Comparison of 2 methods (left carotid artery and abdominal aorta) for surgical implantation of radiotelemetry devices in CD1 mice. Lab Anim 41:388–402 [DOI] [PubMed] [Google Scholar]
- 17.Keckler MS, Gallardo-Romero NF, Langham GL, Damon IK, Karem KL, Carroll DS. 2010. Physiologic reference ranges for captive black-tailed prarie dogs (Cynomys ludovicianus). J Am Assoc Lab Anim Sci 49:274–281 [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer K, Kinter L, Brockway BP, Voss HP, Remie R, VanZutphen BL. 2001. The use of radiotelemetry in small laboratory animals: recent advances. Contemp Top Lab Anim Sci 40:8–16 [PubMed] [Google Scholar]
- 19.Kramer K, Kinter LB. 2003. Evaluation and applications of radiotelemetry in small laboratory animals. Physiol Genomics 13:197–205 [DOI] [PubMed] [Google Scholar]
- 20.Krzaczynski J. 1974. The flank approach to feline ovariohysterectomy (an alternate technique). Vet Med Small Anim Clin 69:572–574 [PubMed] [Google Scholar]
- 21.Leon LR, Walker LD, DuBose DA, Stephenson LA. 2004. Biotelemetry transmitter implantation in rodents: impact on growth and circadian rhythms. Am J Physiol Regul Integr Comp Physiol 286:R967–R974 [DOI] [PubMed] [Google Scholar]
- 22.Liles JH, Flecknell PA. 1993. The effects of surgical stimulus on the rat and the influence of analgesic treatment. Br Vet J 149:515–525 [DOI] [PubMed] [Google Scholar]
- 23.Martin SS, Bakken RR, Lind CM, Reed DS, Price JL, Koeller CA, Parker MD, Hart MK, Fine DL. 2009. Telemetric analysis to detect febrile responses in mice following vaccination with a live-attenuated virus vaccine. Vaccine 27:6814–6823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGrath H, Hardie RJ, Davis E. 2004. Lateral flank approach for ovariohysterectomy in small animals. Compendium 26:922–930 [Google Scholar]
- 25.National Research Council 2003. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 26.Prabhakar NR, Marek W, Loeschcke HH. 1985. Altered breathing pattern elicited by stimulation of abdominal visceral afferents. J Appl Physiol 58:1755–1760 [DOI] [PubMed] [Google Scholar]
- 27.St A, Stewart L, Martin WJ. 2003. Evaluation of postoperative analgesia in a rat model of incisional pain. Contemp Top Lab Anim Sci 42:28–34 [PubMed] [Google Scholar]
- 28.Van Loo PLP, Kuin N, Sommer R, Avasaroglu H, Pham T, Baumans V. 2007. Impact of ‘living apart together’ on postoperative recovery of mice compared with social and individual housing. Lab Anim 41:441–455 [DOI] [PubMed] [Google Scholar]
- 29.Vlach KD, Boles JW, Stiles BG. 2000. Telemetric evaluation of body temperature and physical activity as predictors of mortality in a murine model of staphylococcal enterotoxic shock. Comp Med 50:160–166 [PubMed] [Google Scholar]
- 30.Weiergraber M, Henry M, Hescheler J, Smyth N, Schneider T. 2005. Electrocorticographic and deep intracerebral EEG recording in mice using a RT system. Brain Res Brain ResProtoc 14:154–164 [DOI] [PubMed] [Google Scholar]
- 31.Weinert D, Waterhouse J. 1998. Diurnally changing effects of locomotor activity on body temperature in laboratory mice. Physiol Behav 63:837–843 [DOI] [PubMed] [Google Scholar]
- 32.Working Party Report 2003. Seventh report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement, part A. Refinements in telemetry procedures. Lab Anim 37: 261–299 [DOI] [PubMed] [Google Scholar]
- 33.Working Party Report 2004. Seventh report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement, part B. Husbandry refinements for rats, mice, dogs, and nonhuman primates used in RT procedures. Lab Anim 38: 1–10 [DOI] [PubMed] [Google Scholar]