Abstract
Nonhuman primates are used frequently in cardiovascular research. Cardiac time intervals derived by phonocardiography have long been used to assess left ventricular function. Electronic stethoscopes are simple low-cost systems that display heart sound signals. We assessed the use of an electronic stethoscope to measure cardiac time intervals in 48 healthy bonnet macaques (age, 8 ± 5 y) based on recorded heart sounds. Technically adequate recordings were obtained from all animals and required 1.5 ± 1.3 min. The following cardiac time intervals were determined by simultaneously recording acoustic and single-lead electrocardiographic data: electromechanical activation time (QS1), electromechanical systole (QS2), the time interval between the first and second heart sounds (S1S2), and the time interval between the second and first sounds (S2S1). QS2 was correlated with heart rate, mean arterial pressure, diastolic blood pressure, and left ventricular ejection time determined by using echocardiography. S1S2 correlated with heart rate, mean arterial pressure, diastolic blood pressure, left ventricular ejection time, and age. S2S1 correlated with heart rate, mean arterial pressure, diastolic blood pressure, systolic blood pressure, and left ventricular ejection time. QS1 did not correlate with any anthropometric or echocardiographic parameter. The relation S1S2/S2S1 correlated with systolic blood pressure. On multivariate analyses, heart rate was the only independent predictor of QS2, S1S2, and S2S1. In conclusion, determination of cardiac time intervals is feasible and reproducible by using an electrical stethoscope in nonhuman primates. Heart rate is a major determinant of QS2, S1S2, and S2S1 but not QS1; regression equations for reference values for cardiac time intervals in bonnet macaques are provided.
Abbreviations: CTI, cardiac time intervals; QS1, electromechanical activation time; QS2, electromechanical systole; S1S2, interval between first and second heart sounds; S2S1, interval between second and first heart sounds
Nonhuman primates are used widely in biomedical research. Macaques and baboons are the most widely studied species.6,12,20,31,38,39,42,45 Macaques are anatomically similar to humans and exhibit similar cardiovascular physiology and metabolism,2,4,15-17,22,24,28 which make them useful as a model of left ventricular dysfunction and heart failure.2,23,30,32,37,44 Echocardiography has emerged as the most commonly used technique to assess left ventricular function, because it provides a variety of indices pertaining to left ventricular systolic and diastolic function. However, the availability of high-quality ultrasound equipment, transducers, technical expertise in image acquisition, and interpretation are factors that can limit the use of echocardiography in nonhuman primate research.
Left ventricular function has long been assessed by using cardiac time intervals (CTI) derived by phonocardiography and carotid pulse tracings. In humans, CTI have been found highly correlated with echocardiographic, angiographic, and hemodynamic measures of left ventricular function11,13. Low cost, ease of use, high accuracy, and excellent reproducibility have led to the frequent use of CTI in human cardiovascular research. However, electrical bioimpedence and phonocardiography with external carotid wave recordings have in large part been replaced by echocardiography as techniques to measure CTI.35
In recent years, electronic advances in the stethoscope have enhanced quantitative assessment of heart sounds.5,9,33,34,43 Electronic stethoscopes are capable of recording heart sounds that can be digitally processed for display, storage, and analysis on computer. Therefore, electronic stethoscopes provide a portable low-cost alternative means to obtain CTI. Several investigators have incorporated analytical techniques to derive information pertaining to left ventricular function and pulmonary artery pressure from humans by using electronic stethoscopes.1,5,9,43
The results of several studies have led to the use of the electronic stethoscope as an alternative to the phonocardiogram, an older technology for determining CTI.1,7,27,40 Data pertaining to the uses of CTI and electronic stethoscopes in nonhuman primates is lacking. We are unaware of a single study that has either evaluated left ventricular function by measuring CTI or has used the electronic stethoscope for cardiac assessment in monkeys. The ability to obtain CTI data and reference values may be very useful for the identification of cardiovascular abnormalities in primates. The objective of the current study was to ascertain the feasibility of determining CTI from recorded heart sounds in apparently healthy bonnet macaques (Macaca radiata). Bonnet macaques have a close physical resemblance to rhesus monkeys but are relatively smaller and lighter.26
Materials and Methods
The characteristics of the SUNY Downstate Medical Center primate colony have been described previously.16 In summary, there are 125 laboratory-born and -raised bonnet macaques (Macaca radiata) living in social groups of 6 to 10 and maintained on commercial monkey chow. The SUNY Downstate Medical Center Division of Laboratory Animal Research approved this prospective study. All procedures were performed in careful conformance with the Guide for the Care and Use of Laboratory Animals.14 We studied 48 bonnet macaques, of which 46 were female, without recent or ongoing participation in physiologic or pharmacologic studies.
Laboratory methods.
Animals.
Anesthesia was administered by using ketamine (15 mg/kg IM) as clinically indicated to achieve sedation throughout the procedure. Immediately after sedation, each monkey was weighed, crown–rump length was measured, and blood pressures were recorded by sphygmomanometry of the right lower extremity. Monkey body mass index was calculated by dividing weight in kilograms by the square of the crown–rump length in meters.15 Monkey body surface area was calculated according to a previously published formula.22
Electronic stethoscope.
Recordings of heart sounds were made by using an electronic stethoscope (model ds32a, Thinklabs Medical, Denver, CO). The electronic stethoscope consists of a conventional stethoscope design equipped with an electromagnetic diaphragm, loud-speaker drivers at the eartips, and a lightweight analog recorder at the junction of the single tubing to the double tubing. The system records sounds directly to a laptop computer; the data can be uploaded and displayed as waveforms (Dell Computer, Round Rock, TX) with the signal processed through the computer's sound card.
Analysis of ECG and stethoscope recordings.
Amplified (auto gain) and band-limited 3-lead electrocardiographic signals were preprocessed. Each audio signal was digitized to 10 bits at 1000 samples per second on individual channels of an analog-to-digital module (DI-148U, Dataq Instruments, Akron, OH) attached to a laptop PC. The digital output was recorded and displayed in strip-chart format by using WinDaq software (Dataq Instruments). The recorded data were converted by using Dataq conversion software, into a comma-separated-value array and written to a file in plain text. Electrocardiographic data were low-pass–filtered with a sliding-averaging filter, with an α coefficient of 0.1, to attenuate motion artifacts and 60-Hz interference. Next, the absolute value of the first derivative was obtained and the average subtracted, and large inflections were used to determine the temporal spacing between electrocardiographic packets. The spacing distribution was used to set a temporal threshold. Electrocardiographic event timing that fell outside the threshold was used to eliminate that event. The initial filtered data set was examined backward from the flagged time in an attempt to find local minima that would correspond to the Q wave. These points in time were taken as the zero reference time for S1 and S2 timing. The electrocardiographic plot, with the timing flags, was examined and edited by the user to eliminate spurious time points. The array containing the S1 and S2 signals was high-pass–filtered to attenuate low-frequency background noise while preserving the fidelity of the heart sounds. This resulted in a mean signal:background ratio of 7.25:1. The array was divided logically into sections that corresponded temporally with individual Q–Q intervals. Each section was examined for positive and negative inflection. Each inflection was weighed by the absolute derivatives of the signal before and after each inflection. This process determined the center-of-mass for the each heart-sound packet. These signal sections subsequently were divided temporally, so that the first one-third of the signal section was processed to locate S1 and the remaining two-thirds S2. Values of 0.5 times the center-of-mass values for each heart sound were used, respectively, as a threshold to locate S1 and S2 time-points. The final data set of Q-, S1-, and S2-detected times were processed to determine the elapsed time between Q and S1, Q and S2, S1and S2, and S2 and S1. These intervals represent the following: electromechanical activation time (QS1), electromechanical systole (QS2), mechanical systole, defined as the interval between the first and second sounds (S1S2), and mechanical diastole, defined as the interval between the second and first sounds (S2S1).
Echocardiographic studies.
Two experienced echocardiographers (JL and LS) performed transthoracic echocardiographic studies. Each study was inspected carefully to assure adequate endocardial definition. Left ventricular septal wall thickness, posterior wall thickness, and end-diastolic diameter were measured from M-mode images according to American Society of Echocardiography standards.19 When the scanning axis was not perpendicular to the axis of the heart, 2D images were used. The transmitral pulsed Doppler velocity recordings from 3 consecutive cardiac cycles were used to derive the peak E wave and A wave velocities of diastolic transmitral flow. Left ventricular ejection time was measured from the apical 5-chamber view with the pulse wave 1 cm below the aortic outflow tract. Left ventricular mass and ejection fraction were calculated by the American Society of Echocardiography corrected cube formula and indexed by crown–rump length.19
Statistics.
Descriptive data for all variables included in Table 1 were expressed as mean ± 1 SD. Univariate associations between variables in Table 2 were analyzed by using Spearman correlation coefficients. Multiple linear-regression analysis was performed to determine independent predictors of cardiac time intervals and to provide regression equations for the relationship between cardiac time interval and heart rate. Reproducibility of cardiac time intervals was assessed by type C intraclass correlation coefficients using a consistency definition with 2-way random-effects model in which subject effects and measures effects both were random and reported as 95% confidence interval. All statistical analyses were achieved by using Statistical Package for Social Sciences version 17.0 software (SPSS, Chicago, IL). A P value less than 0.05 was considered to be statistically significant.
Table 1.
Anthropometrics and echocardiographic data from 48 bonnet macaques
| Minimum | Maximum | Mean ± 1 SD | |
| Age (y) | 3 | 20 | 8 ± 5 |
| Weight (kg) | 3.5 | 13.4 | 5.8 ± 1.9 |
| Systolic blood pressure (mm Hg) | 101 | 173 | 133 ± 15 |
| Diastolic blood pressure (mm Hg) | 46 | 153 | 82 ± 19 |
| Heart rate (bpm) | 152 | 240 | 198 ± 21 |
| Pulse pressure (mm Hg) | 19 | 86 | 52 ± 15 |
| Mean arterial pressure (mm Hg) | 69 | 160 | 99 ± 17 |
| Crown–rump length (m) | 0.43 | 0.46 | 0.44 ± 0.01 |
| Body surface area (m2) | 0.20 | 0.27 | 0.24 ± 0.02 |
| Body mass index (kg/m2) | 22.96 | 38.67 | 30.8 ± 5.1 |
| Ejection fraction (%) | 53 | 93 | 80 ± 10 |
| Left ventricular mass (g) | 1.94 | 15.64 | 7.1 ± 3.5 |
| Left ventricular ejection time (s) | 70 | 176 | 132 ± 24 |
| Left ventricular mass index (g/m) | 14.31 | 28.53 | 18.9 ± 6 |
| E/A (cm/s) | 0.68 | 2.8 | 1.02 ± 0.41 |
| QS1 (ms) | 1.86 | 28.03 | 13.3 ± 5.8 |
| QS2 (ms) | 119.84 | 223.63 | 171 ± 21 |
| S1S2 (ms) | 112.13 | 202.88 | 157.33 ± 18.9 |
| S2S1 (ms) | 100.78 | 219.68 | 148.23 ± 22.5 |
| QS1/S1S2 | 0.01 | 0.21 | 0.08 ± 0.04 |
| S1S2/S2S1 | 0.75 | 1.47 | 1.08 ± 0.16 |
A, late Doppler velocity component of mitral inflow ; E, early Doppler velocity component of mitral inflow; QS1, electromechanical activation time; QS2, electromechanical systole; S1S2, interval between the first and second heart sounds; S2S1, interval between second and first heart sounds.
Table 2.
Correlations between cardiac time intervals and echocardiographic and anthropometric data
| QS1(ms) | QS2(ms) | S1S2(ms) | S2S1(ms) | S1S2/S2S1 | ||
| Heart rate (bpm) | r | –0.198 | –0.795 | –0.820 | –0.800 | 0.106 |
| P | 0.177 | <0.001 | <0.001 | .<001 | 0.499 | |
| Systolic blood pressure (mm Hg) | r | 0.110 | –0.243 | –0.270 | –0.431 | 0.294 |
| P | 0.478 | 0.112 | 0.076 | 0.004 | 0.045 | |
| Diastolic blood pressure (mm Hg) | r | –0.050 | –0.337 | –0.344 | –0.442 | 0.173 |
| P | 0.748 | 0.025 | 0.022 | 0.003 | 0.262 | |
| Mean arterial pressure (mm Hg) | r | –0.028 | –0.364 | –0.374 | –0.487 | 0.209 |
| P | 0.851 | 0.015 | 0.012 | 0.001 | 0.174 | |
| Age (y) | r | –0.019 | 0.262 | 0.335 | 0.192 | 0.040 |
| P | 0.898 | 0.076 | 0.021 | 0.196 | 0.787 | |
| Left ventricular ejection time (ms) | r | 0.064 | 0.534 | 0.621 | 0.324 | 0.150 |
| P | 0.763 | 0.006 | 0.001 | 0.034 | 0.475 |
QS1, electromechanical activation time; QS2, electromechanical systole; S1S2, interval between the first and second heart sounds; S2S1, interval between second and first heart sounds.
Results
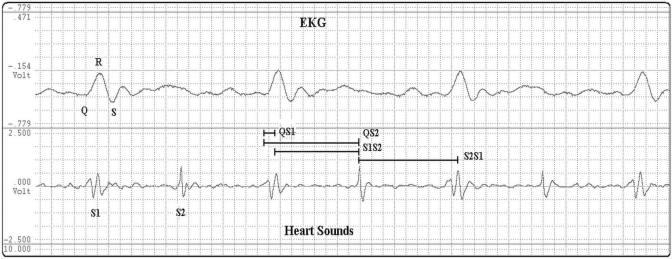
Clinical and echocardiographic parameters including mean ± 1 SD and ranges (minimum to maximum) are reported for all 48 bonnet macaques in Table 1. Technically adequate heart-sound recordings were obtained in all of the monkeys tested. Figure 1 shows an example of a heart-sound recording from a 9.5-kg female macaque. Table 2 summarizes the relations between cardiac time intervals and the anthropometric and echocardiographic data. QS2 was inversely correlated with heart rate (r = −0.795, P < 0.001), mean arterial pressure (r = −0.364, P = 0.015), and diastolic blood pressure (r = −0.337, P = 0.025) and directly correlated with left ventricular ejection time (r = 0.534, P = 0.006) as determined by using echocardiography. S1S2 inversely correlated with heart rate (r = −0.820), mean arterial pressure (r = −0.374, P = 0.012), and diastolic blood pressure (r = −0.344, P = 0.022) and directly correlated with left ventricular ejection time (r = 0.621, P = 0.001) and age (r = 0.335, P = 0.021). S2S1 inversely correlated with heart rate (r = −0.800, p ≤ 0.001), mean arterial pressure (r = −0.487, P = 0.001), diastolic blood pressure (r = −0.442, P = 0.003), and systolic blood pressure (r = −0.431, P = 0.004) and directly correlated with left ventricular ejection time (r = 0.324, P = 0.034). The S1S2/S2S1 relation correlated with systolic blood pressure (r = 0.294, P = 0.045). On multivariate analyses adjusted for age, sex, weight, and blood pressure, heart rate was the only independent predictor of QS2 (R2 = 0.62), S1S2 (R2 = 0.67), S2S1 (R2 = 0.73; P = 0.001). QS1 did not correlate with any demographic or echocardiographic parameters on univariate or multivariate analysis.
Figure 1.
Simultaneous recording of electrocardiogram (EKG) and heart sounds used in assessing cardiac time intervals. Q, start of QRS complex; QS1, electromechanical activation time; QS2, electromechanical systole; S1, first heart sound; S2, second heart sound; S1S2, interval between the first and second heart sounds; S2S1, interval between second and first heart sounds.
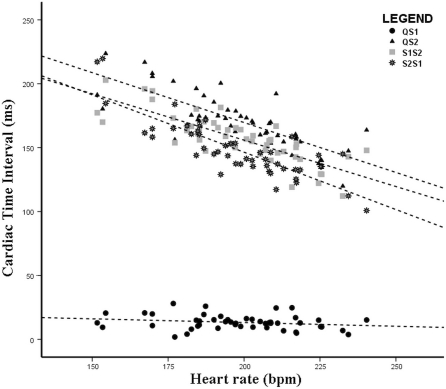
Figure 2 depicts the relation between heart rate and individual cardiac time intervals as expressed by the following regression equations: QS2 = 325.826 − (0.78 × heart rate); S1S2 = 301.055 − (0.726 × heart rate); and S2S1 = 325.890 − (0.897 × heart rate). The figure depicts a negative association between heart rate and the time intervals QS2, S1S2 and S2S1 but no association with QS1. Intraclass correlation coefficients for reproducibility of cardiac time intervals was 98.7% (95% confidence interval, 95.0 to 99.7) for QS2, 98.7% (94.9 to 99.7) for S1S2, 99.5% (98.1 to 99.9) for S2S1, and 87.9% (58.9 to 96.9) for QS1.
Figure 2.
Association between various cardiac time intervals and heart rate among the 48 bonnet macaques. All 4 intervals were obtained from all monkeys. QS1, electromechanical activation time; QS2, electromechanical systole; S1, first heart sound; S2, second heart sound; S1S2, interval between the first and second heart sounds; S2S1, interval between second and first heart sounds.
Discussion
We determined the feasibility of measuring cardiac time intervals in nonhuman primates by the simultaneous recording of heart sounds by electronic stethoscope and single-lead electrocardiography. We used this method to measure QS1, QS2, S1S2, and S2S1 in a group of adult bonnet macaques. The monkeys that were studied represented a wide range of ages and weights in animals of both sexes, with a mean heart rate of 199 bpm. Cardiac time intervals were obtained in 100% of macaques studied. Cardiac time intervals derived by a variety of techniques have been used frequently in human cardiovascular research but not in nonhuman primates. Although prior studies have shown various cardiac time intervals to correlate with hemodynamic and echocardiographic measures of cardiovascular function, few human studies have used an electronic stethoscope to derive cardiac time intervals;1,7,27,40 we are unaware of a prior study that has evaluated either cardiac time intervals or the electronic stethoscope in primates.
We assessed the intervals of QS1, QS2, S1S2, and S2S1. QS1, defined as the time from Q-wave onset to the peak first heart sound, is also known as the electromechanical activation time.8 Higher QS1 values are associated with reduced left ventricular ejection fraction and reduced left ventricular rate of pressure rise.27 Although some studies have normalized QS1 to heart rate, this has not been a consistent approach.8 The present study found QS1 to be unrelated to heart rate. This finding refutes the need for QS1 to be corrected for heart rate. In addition, because QS1 has previously been normalized to S1S2 to more closely approximate the left ventricular ejection fraction,36,46 we reported these values as well. Mean values for QS1/S1S2 were lower in nonhuman primates than previously reported for humans (0.09 versus 0.29).29
The QS2 interval represents electromechanical systole. Although unaffected by lowering of the ejection fraction, QS2 is shortened by excessive adrenergic tone that results in settings such as acute coronary syndromes.21 This interval has been found to be dependent on heart rate in humans.13 QS2 can be used to calculate the preejection period as QS2 – left ventricular ejection time; among the various cardiac time intervals, left ventricular ejection time and the preejection period have been used the most frequently in prior studies. The left ventricular ejection time is the time needed for the left ventricle to empty into the arterial system.41 The preejection period is the time interval between ventricular depolarization and the onset of ventricular ejection. Therefore, in the setting of left ventricular dysfunction the preejection period shortens, left ventricular ejection time lengthens, and the total QS2 remains the same.41 In contrast, adrenergic stimulation normally shortens both preejection period and left ventricular ejection time and decreases the ratio of preejection period to left ventricular ejection time in contrast.21
Left ventricular ejection time can be approximated by the S1S2 interval. The S1S2 interval is the time of mechanical systole, whereas S2S1 is the duration of mechanical diastole. Both time intervals were related strongly to heart rate, as expected. The observed slope of the regression line for S2S1 was similar to that for S1S2. This finding is again similar to that reported for humans.3 Recent studies suggest the systolic to diastolic ratio to be a useful index of left ventricular systolic function.10 We determined this ratio by using the S1S2/S2S1 ratio and found values similar to those reported previously in humans.3 Moreover, S1S2/S2S1 was significantly correlated with systolic BP, thereby suggesting the load dependence of this index.
Collectively, these findings serve to characterize cardiac time intervals in nonhuman primates and extend the findings of prior studies showing systolic and diastolic time intervals to vary with heart rates of 60 to 110 bpm.25 The mean heart rate of the macaques in the present study (199 bpm) was more than 100 bpm higher than that in humans in whom systolic time intervals were first proposed (199 bpm compared with 81 bpm).
Accordingly, we report regression equations for cardiac time interval reference values for use in nonhuman primates. Although their rapid heart rates might be expected to complicate assessment, cardiac time intervals were measurable in all monkeys. According to the manufacturer (Thinklabs Medical), the electronic stethoscope records data points within a frequency range of approximately 20 to 1000 Hz (bell mode), and most heart-sound energy typically is within the range of 20 to 200 Hz. Therefore, this device can easily record arterial heart sounds occurring at a rate of 250 bpm. These findings are likely applicable to rhesus monkeys which are the most commonly studied macaques. Bonnet macaques have a close physical resemblance to rhesus although are relatively smaller and lighter.26 This technique also may be useful in cynomolgous macaques, a model of atherosclerosis and coronary artery disease.
The current study suggests a great potential for electronic stethoscopes in determining cardiac time intervals to quantify left ventricular function in nonhuman primate models. Overall, high-quality heart-sound recordings were obtained which is likely due to the small distance from the heart to the surface of the chest wall in bonnet macaques and other nonhuman primates in general. Other advantages of electronic stethoscopes are their portability, low cost, and utility in serial evaluations and in colonies, for which echocardiography is less readily applied.
The current assessment is a cross-sectional study of apparently healthy bonnet macaques. All measurements were obtained during sedation with ketamine. Although anesthetic agents are known to influence left ventricular performance, ketamine has been shown to have minimal effects on cardiac contractility and heart rate.18 The mean heart rate of our macaques was similar to those observed in other monkey experiments.20 Left ventricular ejection fraction was based on measurements from the parasternal view, which was obtained easily in all of our animals. Cardiac time intervals can be influenced by hemodynamics and conduction disturbances, which were not evaluated formally.11 Although the prevalence of conduction disturbances was probably very low, this factor merits further study. Measurements were made from the onset of the Q wave to the heart sound. Although the peak of the R wave would be easier to measure than the Q wave of the electrocardiogram, we opted to follow measures that were used in prior studies and that demonstrated excellent reproducibility.
In conclusion, the current study demonstrates the feasibility and supports the applicability of recording heart sounds with an electronic stethoscope concurrent with electrocardiographic monitoring to determine cardiac time intervals in bonnet macaques. Our data yielded preliminary regression equations for CTI reference values, which may be useful for identification of cardiovascular abnormalities in similar primates. Validation by comparison of calculated indices to those obtained invasively merits further study.
References
- 1.Abelson D, Kamens EA. 1977. Bedside measurement of systolic and diastolic time intervals using the stethometer. Cardiovasc Res 11:270–274 [DOI] [PubMed] [Google Scholar]
- 2.Abrahams C, Janicki JS, Weber KT. 1987. Myocardial hypertrophy in Macaca fascicularis. Structural remodeling of the collagen matrix. Lab Invest 56:676–683 [PubMed] [Google Scholar]
- 3.Bombardini T, Gemignani V, Bianchini E, Venner L, Petersen C, Pasanisi E, Pratali L, Alonso-Rodriguez D, Mascia Pianelli M, Francesco Faita F, Giannoni M, Arpesella G, Picano E. 2008. Diastolic time–frequency relation in the stress echo lab: filling timing and flow at different heart rates. Cardiovasc Ultrasound 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buss DD, Hyde DM, Poulos PW., Jr 1982. Coronary artery distribution in bonnet monkeys (Macaca radiata). Anat Rec 203:411–417 [DOI] [PubMed] [Google Scholar]
- 5.Collins SP, Lindsell CJ, Kontos MC, Zuber M, Kipfer P, Attenhofer Jost C, Kosmicki D, Michaels AD. 2009. Bedside prediction of increased filling pressure using acoustic electrocardiography. Am J Emerg Med 27:397–408 [DOI] [PubMed] [Google Scholar]
- 6.Crawford MH, Walsh RA, Cragg D, Freeman GL, Miller J. 1987. Echocardiographic left ventricular mass and function in the hypertensive baboon. Hypertension 10:339–345 [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira Neto NR, Pinheiro MA, Carriço AS, Santos de Oliveira MM, Camara MF, Lagreca RJ. 2008. Abnormalities of the systolic time intervals obtained by electronic stethoscope in heart failure. Internet J Cardiol 5(2). [Google Scholar]
- 8.Efstratiadis S, Michaels AD. 2008. Computerized acoustic cardiographic electromechanical activation time correlates with invasive and echocardiographic parameters of left ventricular contractility. J Card Fail 14:577–582 [DOI] [PubMed] [Google Scholar]
- 9.Erne P. 2008. Beyond auscultation–acoustic cardiography in the diagnosis and assessment of cardiac disease. Swiss Med Wkly 138:439–452 [DOI] [PubMed] [Google Scholar]
- 10.Friedberg MK, Silverman NH. 2006. Cardiac ventricular diastolic and systolic duration in children with heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol 97:101–105 [DOI] [PubMed] [Google Scholar]
- 11.Gillebert TC, Van de Veire N, De Buyzere ML, De Sutter J. 2004. Time intervals and global cardiac function. Use and limitations. Eur Heart J 25:2185–2186 [DOI] [PubMed] [Google Scholar]
- 12.Haider B, Yeh CK, Thomas G, Oldewurtel HA, Lyons MM, Regan TJ. 1978. Altered myocardial function and collagen in diabetic rhesus monkeys on atherogenic diet. Trans Assoc Am Physicians 91:197–203 [PubMed] [Google Scholar]
- 13.Hassan S, Turner P. 1983. Systolic time intervals: a review of the method in the noninvasive investigation of cardiac function in health, disease and clinical pharmacology. Postgrad Med J 59: 423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 15.Jen KL, Hansen BC, Metzger BL. 1985. Adiposity, anthropometric measures, and plasma insulin levels of rhesus monkeys. Int J Obes 9:213–214 [PubMed] [Google Scholar]
- 16.Kaufman D, Smith EL, Gohil BC, Banerji M, Coplan JD, Kral JG, Rosenblum LA. 2005. Early appearance of the metabolic syndrome in socially reared bonnet macaques. J Clin Endocrinol Metab 90: 404–408 [DOI] [PubMed] [Google Scholar]
- 17.Kawashima T, Sato K, Akita K, Sasaki H. 2005. Comparative anatomical study of the autonomic cardiac nervous system in macaque monkeys. J Morphol 266: 112–124 [DOI] [PubMed] [Google Scholar]
- 18.Koenig SC, Ludwig DA, Reister C, Fanton JW, Ewert D, Convertino VA. 2001. Left ventricular, systemic arterial, and baroreflex responses to ketamine and TEE in chronically instrumented monkeys. Comp Med 51:513–517 [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. 2005. European Society of Echocardiography recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463 [DOI] [PubMed] [Google Scholar]
- 20.Lazar JM, Qureshi G, Qureshi MR, Smith E, Scharf B, Rosenblum LA, Signaevsky M, Kral JG, Salciccioli L. 2008. Left ventricular systolic and diastolic function in healthy adult bonnet macaques (Macaca radiata). New echocardiographic indices in Old World monkeys. Cardiology 113: 116–121 [DOI] [PubMed] [Google Scholar]
- 21.Lewis RP, Boudoulas H, Welch TG, Forester WF. 1976. Usefulness of systolic time intervals in coronary artery disease. Am J Cardiol 37: 787–796 [DOI] [PubMed] [Google Scholar]
- 22.Liu CT, Higbee GA. 1976. Determination of body surface area in the rhesus monkey. J Appl Physiol 40:101–104 [DOI] [PubMed] [Google Scholar]
- 23.Liu XB, Li J, Xiao Y, Pang LY, Jiang X, Wang L. 2009. Effects of neregulin on rhesus monkey heart failure induced by rapid pacing. Sichuan Da Xue Xue Bao Yi Xue Ban 40:93–96 [PubMed] [Google Scholar]
- 24.Nussmeier NA, Benthuysen JL, Steffey EP, Anderson JH, Carstens EE, Eisele JH, Jr, Stanley TH. 1991. Cardiovascular, respiratory, and analgesic effects of fentanyl in unanesthetized rhesus monkeys. Anesth Analg 72: 221–226 [DOI] [PubMed] [Google Scholar]
- 25.Oilinki OI, Takkunen JT, Linnaluoto MM. 1978. The influence of heart rate, age, blood pressure, obesity, and work on systolic and diastolic time intervals. Ann Clin Res 10:14–18 [PubMed] [Google Scholar]
- 26.Rao AJ, Ramesh V, Ramachandra SG, Krishnamurthy HN, Ravindranath N, Moudgal NR. 1998. Growth and reproductive parameters of bonnet monkey (Macaca radiata). Primates 39:97–107 [Google Scholar]
- 27.Roos M, Toggweiler S, Zuber M, Jamshidi P, Erne P. 2006. Acoustic cardiographic parameters and their relationship to invasive hemodynamic measurements in patients with left ventricular systolic dysfunction. Congest Heart Fail 12: 19–24 [DOI] [PubMed] [Google Scholar]
- 28.Sandhyamani S. 1992. Vasculopathic and cardiomyopathic changes induced by low-protein high-carbohydrate tapioca-based diet in bonnet monkeys. Vasculopathic and cardiomyopathic changes in induced malnutrition. Am J Cardiovasc Pathol 4:41–50 [PubMed] [Google Scholar]
- 29.Shapiro M, Moyers B, Marcus GM, Gerber IL, McKeown BH, Vessey JC, Jordan MV, Huddleston M, Foster E, Chatterjee K, Michaels AD. 2007. Diagnostic characteristics of combining phonocardiographic third heart sound and systolic time intervals for the prediction of left ventricular dysfunction. J Card Fail 13: 18–24 [DOI] [PubMed] [Google Scholar]
- 30.Stalder M, Tye T, Lam TT, Chan MC, Berry GJ, Borie DC, Morris RE. 2005. Improved assessment of graft function by echocardiography in cynomolgus monkey recipients of hDAF-transgenic pig cardiac xenografts. J Heart Lung Transplant 24: 215–221 [DOI] [PubMed] [Google Scholar]
- 31.Swindle MM, Blum JR, Weiss JL. 1986. Morphometric studies of the heart in normal rhesus monkeys (Macaca mulatta). J Med Primatol 15:433–440 [PubMed] [Google Scholar]
- 32.Tang HL, Wang LL, Cheng G, Wang L, Li S. 2008. Evaluation of the cardiovascular function of older adult rhesus monkeys by ultrasonography. J Med Primatol 37:101–108 [DOI] [PubMed] [Google Scholar]
- 33.Tavel ME. 2006. Cardiac auscultation: a glorious past—and it does have a future! Circulation 113: 1255–1259 [DOI] [PubMed] [Google Scholar]
- 34.Tavel ME, Brown DD, Shander D. 1994. Enhanced auscultation with a new graphic display system. Arch Intern Med 154: 893–898 [PubMed] [Google Scholar]
- 35.Tei C. 1995. New noninvasive index for combined systolic and diastolic ventricular function. J Cardiol 26:135–136 [PubMed] [Google Scholar]
- 36.Toggweiler S, Zuber M, Kobza R, Roos M, Jamshidi P, Meier R, Erne P. 2007. Improved response to cardiac resynchronization therapy through optimization of atrioventricular and interventricular delays using acoustic cardiography: a pilot study. J Card Fail 13: 637–642 [DOI] [PubMed] [Google Scholar]
- 37.Tsusaki H, Yonamine H, Tamai A, Shimomoto M, Kuwano K, Iwao H, Nagata R, Kito G. 2007. Left ventricular volume and function in cynomolgus monkeys using real-time 3D echocardiography. J Med Primatol 36:39–46 [DOI] [PubMed] [Google Scholar]
- 38.Vaitkevicius PV, Lane M, Spurgeon H, Ingram DK, Roth GS, Egan JJ, Vasan S, Wagla DR, Ulrich P, Brines M, Wuerth JP, Cerami A, Lakatta EG. 2001. A crosslink breaker has sustained effects on arterial and ventricular properties in older rhesus monkeys. Proc Natl Acad Sci USA 98:1171–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallrabe D, Wolter F, Heine H, Martin G, Urmantscheeva T, Storrer W. 1989. The application of the Doppler echocardiography for the determination of the prompt hemodynamic reactors in the rhesus monkey. Z Klin Med 44:483–492 [Google Scholar]
- 40.Wanderman KL, Hayek Z, Ovsyshcher I, Loutaty G, Cantor A, Gussarsky Y, Gueron M. 1981. Systolic time intervals in adolescents. Normal standards for clinical use and comparison with children and adults. Circulation 63:204–209 [DOI] [PubMed] [Google Scholar]
- 41.Weissler AM. 1983. Interpreting systolic time intervals in man. J Am Coll Cardiol 2: 1019–1020 [DOI] [PubMed] [Google Scholar]
- 42.Williams JK, Anthony MS, Clarkson TB. 1991. Coronary heart disease in rhesus monkeys with diet-induced coronary artery atherosclerosis. Arch Pathol Lab Med 115:784–790 [PubMed] [Google Scholar]
- 43.Xu J, Durand LG, Pibarot P. 2002. A new, simple, and accurate method for noninvasive estimation of pulmonary arterial pressure. Heart 88: 76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zabalgoitia M, Ventura J, Anderson L, Carey KD, Williams JT, Vandeberg JL. 2003. Morphologic and functional characterization of Chagasic heart disease in nonhuman primates. Am J Trop Med Hyg 68:248–252 [PubMed] [Google Scholar]
- 45.Zhang Y, Tang H, Huang H. 2005. Echocardiography of rhesus monkeys (Macaca mulatta). Chinese J Zoology (Peking) 40:95–98 [Google Scholar]
- 46.Zuber M, Toggweiler S, Roos M, Kobza R, Jamshidi P, Erne P. 2008. Comparison of different approaches for optimization of atrioventricular and interventricular delay in biventricular pacing. Europace 10: 367–373 [DOI] [PubMed] [Google Scholar]