Abstract
Here we document the case of a domestic ferret (Mustela putorius) that survived experimental inoculation with rabies virus of skunk origin. The ferret showed initial clinical signs of rabies (hindlimb paralysis) on day 81 after inoculation. The animal survived with paraplegia but otherwise was in an adequate nutritional state until the end of the observation period (PI day 181). At necropsy, no gross lesions were observed. Microscopic lesions were found in sections of cerebrum and spinal cord. In both tissues, the lesions were similar but were more severe with loss of neuronal parenchyma in the spinal cord. The lesions consisted of locally extensive areas with proliferation of astrocytes and moderate numbers of glial cells. Severely affected areas also contained clearly defined vacuoles in the neuropil. Multifocal areas of involvement showed mononuclear cuffing of blood vessels. In a few areas, the cuffing extended to the meninges. Rabies virus antigen was not detected by immunohistochemistry of tissue sections.
Abbreviations: DFA, direct fluorescent antibody; RFFIT, rapid fluorescent focus-inhibition test
Rabies is an acute, progressive, encephalomyelitis, with a case-to-fatality ratio ranking among the highest of any infectious disease. Once clinical signs appear, the disease usually is considered to be invariably fatal. However, throughout the 19th and 20th centuries, luminaries such as Pasteur and Koch both believed that animals could sicken and recover, as supported by numerous documented cases after natural or experimental infections.11Despite such exceptions, the belief is still widely held that the disease is always fatal. Therefore, claims for any purported recovery should be documented carefully and presented for scientific scrutiny. These cases become particularly important when considering potential therapeutic intervention, especially after the documentation of the first human case of survival after rabies in the absence of a history of vaccination.34 To this end, here we report the pathologic findings from a domestic ferret that recovered from rabies after experimental infection.
Case Report
The ferret in this study was part of an overall investigation into the pathogenesis of experimentally induced rabies in domestic ferrets for the formulation of recommendations concerning quarantine periods for domestic ferrets that bite people. A critical issue in virus transmission is shedding of virus in the saliva of a seemingly healthy animal.
The ferrets used in this study were donated by Marshall Farms (North Rose, NY) and were maintained according to the Guide for the Care and Use of Laboratory Animals16 and Animal Welfare Act.1 The protocol was approved by the Centers for Disease Control and Prevention's Institutional Animal Care and Use Committee. Ferrets were maintained in an AAALAC-accredited facility and given commercial ferret food (Teklad cat diet 7770, Harlan Laboratory Diets, Bartonville, IL) and water ad libitum. Ferrets were sedated with ketamine (25 mg/kg IM) and xylazine (2 mg/kg). Ferrets with severe clinical signs of rabies, including the inability to eat or drink for a 24-h period, were euthanized by intravenous or intracardiac administration (Beuthanasia D, Schering-Plough, Union, NJ).
The experimental protocol for this study has been described previously.26 Briefly, the ferret received a rabies virus (characterized as a North Central skunk rabies virus variant) inoculum prepared from the submaxillary salivary glands of a naturally infected skunk.23,27,29 On day 0, the ferret received rabies virus at a dose of 102.5 MICLD50 as a single 100-μL injection in the right gastrocnemius muscle. The ferret was monitored for 181 d after inoculation. Swab specimens of the oral cavity were collected on days 0 and 7, 3 times weekly between days 8 and 90, once or twice weekly between days 91 and 181, and daily after onset of clinical signs of rabies. Blood samples were obtained weekly by jugular venipuncture, and body weight was measured weekly. Rabies virus-neutralizing antibody levels were determined by use of the rapid fluorescent focus-inhibition test, as described.30A 4-fold or greater increase in titer of paired sera was deemed to indicate induction of rabies virus-neutralizing antibodies (seroconversion). At the end of the observation period (day 181), the ferret was euthanized. The carcass presented in an adequate nutritional state, based on mass and body fat examination. Apart from mild superficial skin abrasions on the hindlegs, no significant gross lesions were found.
Representative samples of major organs, including brain, and the spinal cord were immersion-fixed in 10% neutral buffered formalin. Tissues were embedded in paraffin wax, sectioned at 5 μm, and stained with hematoxylin and eosin. Immunohistochemistry using polyclonal and monoclonal (no. 802-2) antibodies was performed on CNS tissues for detection of rabies virus antigen, as described previously.12,13 Virus isolation was attempted from swab specimens and salivary glands by using the mouse inoculation technique.22 Primary rabies diagnosis was achieved by direct fluorescent antibody testing of the brainstem, as described.7
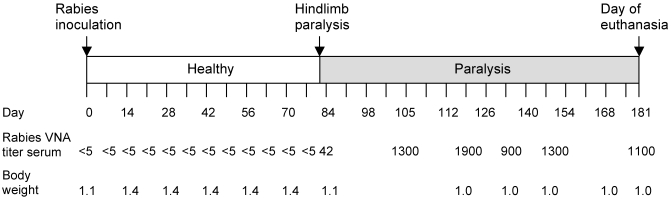
The basic response of ferrets to experimental rabies virus inoculation has been described previously.26 The ferret we report on here remained healthy for 80 d (Figure 1). On day 81 after inoculation, the ferret presented with hindlimb paresis and paralysis that progressed to paralysis of the hindquarters within 24 h. Over the next 2 wk, the ferret was moderately active, eating and drinking and producing fresh feces and urine. By day 96 after inoculation, the ferret was bright, alert, active, eating, and drinking and was observed playing with a plastic ring inside the cage. Over the course of clinical illness, the ferret lost approximately 29% of its body weight (from 1.4 to 1.0 kg) but maintained a stable body weight for the remainder of the observation period. Rabies virus-neutralizing antibodies were detected in the serum first on day 82 after inoculation (0.42 IU/mL). By day 116, the titer increased to 19.0 IU/mL, and the ferret remained seropositive throughout the remaining observation period. Rabies virus was not isolated from samples collected from the oral cavity at anytime during the observation period (a total of 52 oral swabs were collected: 30 prior to clinical signs and 22 during and after onset) or from the salivary glands after euthanasia.
Figure 1.
The ferret received rabies virus in the right gastrocnemius muscle (day 0) and remained healthy for 80 d. Initial clinical signs of rabies (hindlimb paralysis) were observed on day 81 after inoculation. The ferret survived rabies with paralytic sequelae for 100 d after onset, until the end of the observation period (day 181). Serum collected on day 77 after inoculation and prior was negative for rabies virus-neutralizing antibodies (VNA). On day 82 after inoculation, rabies virus-neutralizing antibodies were detected in serum (0.4 IU/mL), and the ferret remained seropositive for the duration of the observation period until euthanasia. Rabies virus-neutralizing antibodies were detected in cerebrospinal fluid collected on day 181 (24.0 IU/mL).
This ferret survived for 100 d after onset of clinical signs, with continued paraplegia. Euthanasia was performed on day 181 after inoculation. At this time, the ferret displayed normal behavior and was active, alert, and inquisitive. Hindlimb muscle atrophy was apparent at necropsy. Serum and cerebrospinal fluid collected at the time of euthanasia contained rabies virus-neutralizing antibodies (1:1100 and 1:2401, respectively), indicating viral infection of the CNS despite the prolonged survival. The brain was negative for rabies virus antigen by direct fluorescent antibody testing.
Microscopic examination of the heart, diaphragm, tongue, masseter muscle, lung (2 sections), liver, gall bladder, kidney, pancreas, skin (2 sections), spleen, mesenteric node, stomach, intestines (3 sections), thyroid, trachea, esophagus (2 sections), aorta, adrenal gland, urinary bladder, brain stem (2 sections), medulla, and cerebellum (4 sections) did not reveal any noteworthy histologic lesions. One section of the salivary gland showed multifocal distortion with dilatation of the ducts lumina, which contained inspissated, partially mineralized, eosinophilic material (secretion).
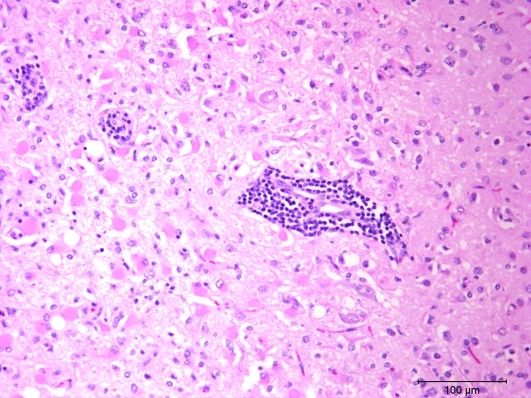
Two sections of the cerebrum revealed significant lesions, which appeared to be bilateral but were not symmetrical. The lesions consisted of locally extensive areas with proliferation of astrocytes and moderate numbers of glial cells (Figure 2). The affected areas contained multiple clear vacuoles in the neuropil. Multifocal areas with mononuclear cuffing of blood vessels were present in the periphery of the affected areas; at some sites, the cuffing extended into the meninges.
Figure 2.
This photomicrograph of cerebrum from the ferret shows a locally extensive area with large numbers of eosinophilic astrocytes (gemistocytic astrocytosis) and moderate numbers of small glial cells. Foci of blood vessels with mononuclear cell cuffing are visible also. Hematoxylin and eosin stain.
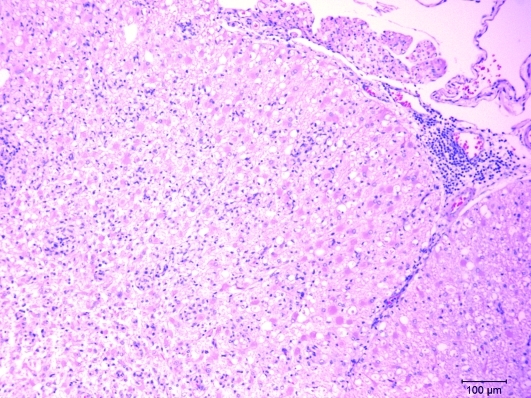
The spinal cord showed extensive multifocal to coalescing foci with loss of parenchyma that involved both the gray and white mater. Within these foci were many plump, eosinophilic astrocytes (gemistocytic astrocytes), moderate numbers of small glial cells, and some cuffing of blood vessels with mononuclear cells (Figure 3). As in the cerebrum, the cuffing extended into the meninges. Spinal cord lesions were progressively more severe in the thoracic and lumbosacral areas.
Figure 3.
This photomicrograph of lumbar spinal cord from the ferret shows locally extensive areas partially devoid of neuropil. The surrounding area is infiltrated with moderate numbers of glial cells. Also present are a few blood vessels with mononuclear cellular cuffing. At one site, the cellular infiltrate extends into the meninges. Hematoxylin and eosin stain.
Sections of the affected brain and spinal cord (n = 5) were examined by immunohistochemical staining for rabies virus antigens. None of the sections revealed rabies virus antigen by use of either rabies -specific polyclonal or monoclonal antibodies, in agreement with the findings from the direct fluorescent antibody testing performed on fresh CNS tissue.
Discussion
The pathogenesis of rabies is complex, from the classic productive, highly neurotropic concept to models of abortive infection and viral clearance.9 For more than a century, examples of recovery from clinical rabies have been documented in multiple species, including mammalian taxa as diverse as rodents to humans.8,11 As in the ferret that we present, most instances of documented recovery in rabies have been based on the relatively high concentrations of rabies virus antibodies documented rather than on documentation of viral antigens or amplicons or actual isolation of virus. Conditions conducive to such nonfatal outcomes after exposure to rabies virus may include the infectious dose, route of exposure, and particular virus variant, among other variables. For example, the intraperitoneal and mucosal routes appear to be associated with less severe outcomes than do typical intracerebral or intramuscular exposures.3,6 Similarly, less concentrated doses of virus may lead to greater opportunities for survival.4 Laboratory-attenuated rabies viruses have been adapted specifically for use as vaccines, and even minor base mutations in a single gene may mean the difference between a virulent virus and a more attenuated variant.10 Several authors have suggested that lyssaviruses may have evolved over time within wildlife, with more attenuated properties among their hosts.5,21,24 Particular host attributes, such as age, or environmental conditions, such as prior exposure, also may be operative.2 Although examples for occurrence are increasingly better documented in both natural and experimental rabies cases in a variety of species, mechanisms of abortive infection and recovery are poorly understood.
Current controversies abound over the ideal clinical management of human cases of rabies.14,15,17,18,28, 31-33 Given these discussions, the development of relevant animal models likely will enable greater insights into the potential therapy of this zoonosis. Laboratory rodents may provide ideal models for some aspects of rabies research.19,20,25 However, certain morphometric and logistical features limit the practical use of rodents to serve as surrogates for the prolonged and intensive care that accompanies human treatment. Similarly, although various anatomic and physiologic attributes of other mammals, such as nonhuman primates, may better mimic those of Homo sapiens in response and clinical manipulation, the more limited availability and comparative costs of these alternative animal models often precludes their consideration as suitable candidates. Considering their widespread purpose-bred availability and use as research subjects and the broad background of biomedical knowledge concerning the species, the domestic ferret is another candidate for rabies model development. The clinical case of spontaneous recovery after experimental infection of a ferret with street rabies virus that we report here supports this view.
Acknowledgments
We thank Gale Moser (New Bolton Center, Kennett Square, PA) and Martha Church (National Animal Disease Center, Ames, IA) for technical assistance.
References
- 1.Animal Welfare Act as Amended 2007. 7 USC §2131-2159.
- 2.Bell JF, Gonzalez MA, Diaz AM, Moore GJ. 1971. Nonfatal rabies in dogs: experimental studies and results of a survey. Am J Vet Res 32:2049–2058 [PubMed] [Google Scholar]
- 3.Bell JF, Moore GJ. 1971. Susceptibility of carnivora to rabies virus administered orally. Am J Epidemiol 93:176–182 [DOI] [PubMed] [Google Scholar]
- 4.Bell JF, Sancho MI, Diaz AM, Moore GJ. 1972. Nonfatal rabies in an enzootic area: results of a survey and evaluation of techniques. Am J Epidemiol 95:190–198 [DOI] [PubMed] [Google Scholar]
- 5.Constantine D. 1971. Bat rabies: current knowledge and future research, p 253–262 In: Nagano Y, Davenport FM. Rabies, 4th ed Baltimore (MD): Park Press [Google Scholar]
- 6.Correa-Giron EP, Allen R, Sulkin SE. 1970. The infectivity and pathogenesis of rabies virus administered orally. Am J Epidemiol 91:203–215 [DOI] [PubMed] [Google Scholar]
- 7.Dean DJ, Abelseth MK, Atanasiu P. 1996. The fluorescent antibody test, p 88–93 In: Meslin FX, Kaplan MM, Koprowski H. Laboratory techniques in rabies, 4th ed Geneva (Switzerland): World Health Organization [Google Scholar]
- 8.deDíaz AM, Fuenzalida E, Bell JF. 1975. Nonfatal rabies in dogs and cats. Ann Microbiol (Paris) 126:503–509 [PubMed] [Google Scholar]
- 9.Dietzschold B, Li J, Faber M, Schnell M. 2008. Concepts in the pathogenesis of rabies. Future Virol 3:481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietzschold B, Wunner WH, Wiktor TJ, Lopes AD, Lafon M, Smith CL, Koprowski H. 1983. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci USA 80:70–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fekadu M. 1991. Latency and aborted rabies, p 191–198 In: Baer GM. The natural history of rabies, 2nd ed Boston (MA): CRC Press [Google Scholar]
- 12.Hamir AN, Moser G, Fu ZF, Dietzschold B, Rupprecht CE. 1995. Immunohistochemical test for rabies: identification of a diagnostically superior monoclonal antibody. Vet Rec 136:295–296 [DOI] [PubMed] [Google Scholar]
- 13.Hamir AN, Moser G, Wampler T, Hattel A, Dietzschold B, Rupprecht CE. 1996. Use of a single antinucleocapsid monoclonal antibody to detect rabies antigen in formalin-fixed paraffin-embedded tissues. Vet Rec 138:114–115 [DOI] [PubMed] [Google Scholar]
- 14.Hemachudha T, Sunsaneewitayakul B, Desudchit T, Suankratay C, Sittipunt C, Wacharapluesadee S, Khawplod P, Wilde H, Jackson AC. 2006. Failure of therapeutic coma and ketamine for therapy of human rabies. J Neurovirol 12:407–409 [DOI] [PubMed] [Google Scholar]
- 15.Hu WT, Willoughby RE, Jr, Dhonau H, Mack KJ. 2007. Long-term follow-up after treatment of rabies by induction of coma. N Engl J Med 357:945–946 [DOI] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 17.Jackson AC. 2006. Bat rabies virus variants causing human rabies. Pediatr Infect Dis J 25:570. [DOI] [PubMed] [Google Scholar]
- 18.Jackson AC. 2009. Update on rabies diagnosis and treatment. Curr Infect Dis Rep 11:296–301 [DOI] [PubMed] [Google Scholar]
- 19.Jackson AC, Reimer DL, Ludwin SK. 1989. Spontaneous recovery from the encephalomyelitis in mice caused by street rabies virus. Neuropathol Appl Neurobiol 15:459–475 [DOI] [PubMed] [Google Scholar]
- 20.Jackson AC, Scott CA, Owen J, Weli SC, Rossiter JP. 2008. Human rabies therapy: lessons learned from experimental studies in mouse models. Dev Biol (Basel) 131:377–385 [PubMed] [Google Scholar]
- 21.Johnson HN. 1966. Sporadic cases of rabies in wildlife: relation to rabies in domestic animals and character of virus, p 25–30 National Rabies Symposium Proceedings. Atlanta (GA): Centers for Disease Control and Prevention [Google Scholar]
- 22.Koprowski H. 1996. The mouse inoculation test, p 80–86 In: Meslin FX, Kaplan MM, Koprowski H. Laboratory techniques in rabies, 4th ed Geneva (Switzerland): World Health Organization [Google Scholar]
- 23.Krebs JW, Strine TW, Smith JS, Noah DL, Rupprecht CE, Childs JE. 1996. Rabies surveillance in the United States during 1995. J Am Vet Med Assoc 209:2031–2044 [PubMed] [Google Scholar]
- 24.Lafon M, Lafon M. 2005. Bat rabies—the Achilles heel of a viral killer? Lancet 366:876–877 [DOI] [PubMed] [Google Scholar]
- 25.Lodmell DL, Bell JF, Moore GJ, Raymond GH. 1969. Comparative study of abortive and nonabortive rabies in mice. J Infect Dis 119:569–580 [DOI] [PubMed] [Google Scholar]
- 26.Niezgoda M, Briggs DJ, Shaddock J, Dreesen DW, Rupprecht CE. 1997. Pathogenesis of experimentally induced rabies in domestic ferrets. Am J Vet Res 58:1327–1331 [PubMed] [Google Scholar]
- 27.Orciari L. 1995. Genetic analysis of rabies virus isolates from skunks in the United States. [MS thesis]. Athens (GA): The University of Georgia Library [Google Scholar]
- 28.Rubin J, David D, Willoughby RE, Jr, Rupprecht CE, Garcia C, Guarda DC, Sohar Z, Stamler A. 2009. Applying the Milwaukee protocol to treat canine rabies in Equatorial Guinea. Scand J Infect Dis 41:372–375 [DOI] [PubMed] [Google Scholar]
- 29.Smith JS, Orciari L, Yager PA. 1995. Molecular epidemiology of rabies in the United States. Seminars Virol 6:387–400 [Google Scholar]
- 30.Smith JS, Yager PA, Baer GM. 1996. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus-neutralizing antibody, p 181–191 In: Meslin FX, Kaplan MM, Koprowski H. Laboratory techniques in rabies, 4th ed Geneva (Switzerland): World Health Organization [Google Scholar]
- 31.Wilde H, Hemachudha T, Jackson AC. 2008. Viewpoint: management of human rabies. Trans R Soc Trop Med Hyg 102:979–982 [DOI] [PubMed] [Google Scholar]
- 32.Willoughby RE., Jr 2009. “Early death” and the contraindication of vaccine during treatment of rabies. Vaccine 27:7173–7177 [DOI] [PubMed] [Google Scholar]
- 33.Willoughby RE, Opladen T, Maier T, Rhead W, Schmiedel S, Hoyer J, Drosten C, Rupprecht CE, Hyland K, Hoffmann GF. 2009. Tetrahydrobiopterin deficiency in human rabies. J Inherit Metab Dis 32:65–72 [DOI] [PubMed] [Google Scholar]
- 34.Willoughby RE, Jr, Tieves KS, Hoffman GM, Ghanayem NS, Amlie-Lefond CM, Schwabe MJ, Chusid MJ, Rupprecht CE. 2005. Survival after treatment of rabies with induction of coma. N Engl J Med 352:2508–2514 [DOI] [PubMed] [Google Scholar]