Abstract
An adult, female, pig-tailed macaque (Macaca nemestrina) of Indonesian origin presented with profound weight loss, anemia (PCV, 29%; normal, 36% to 45%), hypoalbuminemia (1.0 g/dL; normal, 3.5 to 5.2 g/dL), elevated alkaline phosphatase (1990 U/L; normal, 26 to 98 U/L), and an elevated erythrocyte sedimentation rate (75 mm/h; normal, less than 20 mm/h). Abdominal ultrasonography demonstrated an enlarged liver with hyperechoic areas. Euthanasia was performed. Grossly, the liver had multifocal, effacing, white masses throughout and was enlarged with rounded edges. There were 2, small nodules in the right lung lobes. Histologically, the hepatic masses were densely fibrous-encapsulated granulomas with vast central necrosis. The lung nodules also were maturing granulomas, and one kidney and one atrium had small, early granulomas. Fite acid-fast stains of liver and lung revealed very few acid-fast bacilli. PCR analysis of paraffin-embedded liver identified Mycobacterium tuberculosis complex. Culture of the liver was negative twice. This macaque had 16 negative intradermal tuberculin skin tests over the course of 6 y. We hypothesize that the animal arrived with a latent hepatic or enteric infection that later recrudesced and disseminated. Primary hepatic mycobacteriosis is not a typical presentation of tuberculosis in macaques. Negative tuberculin skin tests can be seen with latent infections and extrapulmonary tuberculosis such as Pott disease. This case underscores the problems associated with current surveillance procedures and the risks associated with latent mycobacterial infections in macaques.
Abbreviation: TST, intradermal tuberculin skin test
Quarantine and surveillance procedures implemented by the Centers for Disease Control and Prevention in the 1970s have significantly reduced the incidence of Mycobacterium tuberculosis outbreaks in nonhuman primates.14 Early diagnosis and removal of infected animals has been the goal of quarantine and surveillance programs. However, outbreaks in the postquarantine period continue to occur and result in significant economic impact and loss of animals.8,14 Postquarantine outbreaks occur in part due to animals that have a latent infection that later reactivates.14 Latent infections are frequently difficult to detect and affected animals will frequently have a negative intradermal tuberculin skin test (TST).14,15 A reactivated Mycobacterium tuberculosis infection can be challenging to diagnose as well because the TST can be negative from anergy.3,14 In addition, clinical signs of tuberculosis are usually vague; weight loss, lethargy, and unthriftiness; or animals may die unexpectedly.3 Frequently, by the time tuberculosis is diagnosed the animal has already been shedding the bacteria and other animals have been exposed. This case report describes an unusual infection of Mycobacterium tuberculosis complex in a pig-tailed macaque.
Case Report
An approximately 12-y-old, female pig-tailed macaque of Indonesian origin completed a 90-d quarantine period and entered the colony at our facility (Washington National Primate Research Center, Seattle, WA) 6 y prior to presentation. The quarantine period included physical exams performed at entrance to and exit from quarantine. In addition, 5 TST each involving 0.1 mL mammalian tuberculin (15,000 tuberculin units per mL) were performed with 14 d between tests, as per facility policy at the time. The macaque was dewormed with ivermectin (400 μg/kg SC and repeated every 2 wk for 6 wk), fenbendazole (50 mg/kg PO once a day for 3 d), and pyrantel pamoate (10 mg/kg PO once a day for 3 d and repeated in 2 wk). After the macaque's entry into the colony, a member of the veterinary staff examined (including physical and TST) the animal every 6 mo. CBC and blood chemistries were performed at least yearly, and the macaque was consistently negative for simian T-lymphocyte virus 1 and simian retrovirus 2 during semiannual screening. She had a total of 16 negative TST while within the colony. This animal was being used as a social partner for an animal assigned to another project. She had previously been assigned to a rectal and vaginal topical microbicide project, from which she had been released at 4 mo prior to presentation; at release from that study, she was healthy, with a body condition score4 of 3 (scale of 1 to 5). Prior medical history included treatment for dental disease, allergic dermatitis, and conjunctivitis. The animal was housed in accordance with the regulations of the Animal Welfare Act2 and the recommendations of the Guide for the Care and Use of Laboratory Animals.9 The IACUC of the University of Washington approved all aspects of the studies to which this animal had been assigned.
Six years after entry into the colony, the macaque presented to the veterinary staff for weight loss of 1.3 kg over 6 wk. On cageside exam, she was bright, alert, and responsive with normal stool. On physical exam, body condition was poor (score of 1), with severe muscle wasting, a mildly enlarged spleen, and severe dental tartar deposition. Blood samples for CBC and blood chemistry analyses were obtained. The sample for CBC analysis clotted prior to analysis. Chemistry analysis revealed mild hypochloridemia (96 mEq/L; normal, 98 to 108 mEq/L), hyperproteinemia (8.6 g/dL; normal, 6.0 to 8.2 g/dL), severe hypoalbuminemia (1.0 g/dL; normal, 3.5 to 5.2 g/dL), hyperglobuminemia (7.6 g/dL; normal, 2.5 to 3.0 g/dL), low albumin:globulin ratio (0.13; normal, 0.94 to 1.14), elevated alkaline phosphatase (1990 U/L; normal, 26 to 98 U/L), and mildly elevated γ-glutamyl transferase (137 U/L; normal, 0 to 55 U/L). Additional CBC assessment and an erythrocyte sedimentation rate were obtained 3 d later. The CBC revealed anemia (PCV 29%; normal, 36% to 45%) and mild leukocytosis (11.3 × 103/µL; normal, 4.3 to 10.0 × 103/µL) characterized by mild neutrophilia (8.56 × 103/µL; normal, 1.8 to 7.00 × 103/µL). The erythrocyte sedimentation rate was elevated, at 75 mm/h (normal, less than 20 mm/h). A treatment regimen of nutritional support, prenatal vitamins, protein powder, and antibiotics were started at this time. Metronidazole (20 mg/kg PO twice daily) and enrofloxacin (5 mg/kg PO twice daily) were started due to suspicion of a bacterial infection in light of the neutrophilia and elevated liver enzymes.
Two weeks later, the macaque had no change in body weight or condition, and the splenomegaly persisted. Her CBC and blood chemistry values were essentially unchanged from the previous week. During treatment, this animal consistently was observed to be active with an excessive appetite and normal stools. A third exam was conducted 4 d later. She had lost another 0.1 kg, for a total body weight loss of 35%. Abdominal palpation revealed a mildly enlarged liver and a moderately enlarged spleen. On abdominal ultrasonography, the liver was enlarged with multifocal hyperechoic nodules, and the spleen was hyperechoic. Differential diagnosis included hepatic neoplasia, hepatic abscesses, and because of the host species, hepatic tuberculosis. The macaque never showed any respiratory signs, and her chest auscultated normally. Because of the profound weight loss, abdominal ultrasonographic findings, other clinical findings, and lack of response to treatment, clinical euthanasia and necropsy were performed. Prior to euthanasia, final CBC and chemistry assays were performed; findings were consistent with previous results.
Pathology.
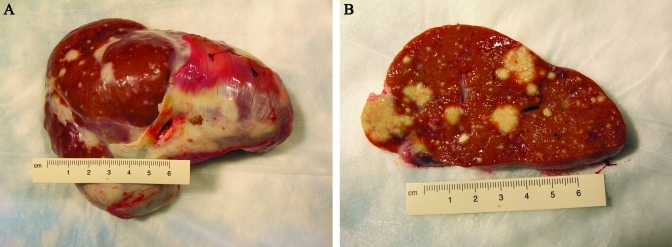
On gross exam, the body was in poor nutritional condition (scant to no adipose stores and poor muscling) and good postmortem condition. The liver had multifocal and coalescing, irregular, effacing, firm, white to tan nodules throughout and was overall moderately enlarged, with slightly round edges (Figure 1). Extensive, firm, white (fibrous) adhesions of the diaphragmatic aspect of the liver to the diaphragm were present. Multifocal, mild to moderate, firm, white (fibrous) adhesions were present between abdominal organs and visceral peritoneum to the parietal peritoneum. The spleen was moderately enlarged and homogenous. The right cranial and middle lung lobes each had a dorsal, moderately sized (diameter, 1 to 2 cm), firm, irregular, white tan focus. Multifocal, mild to moderate, fibrous adhesions of visceral pleural to parietal pleura were present.
Figure 1.
Gross photographs of liver. (A) Unsectioned liver observed from the diaphragmatic side with overlying, adhered diaphragm. (B) Cut section. Notice the multifocal, coalescing granulomas effacing large regions of parenchyma.
Tissue and organ samples were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 3 to 5 microns, and stained routinely with hematoxylin and eosin. In addition, sections of liver, lung and mesenteric lymph node were evaluated with Fite acid fast stain.
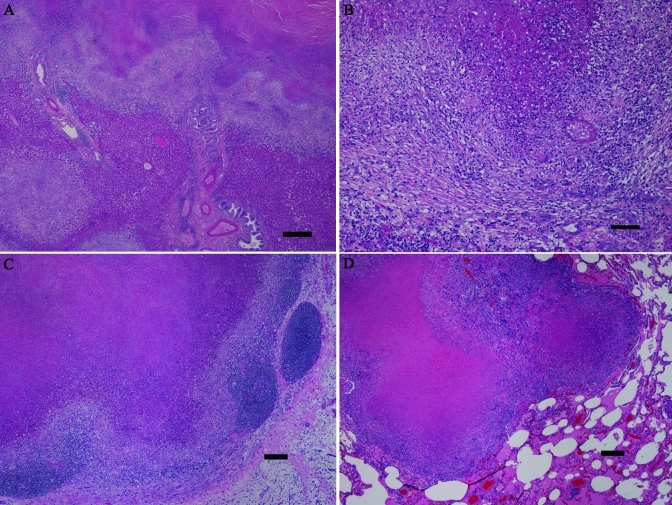
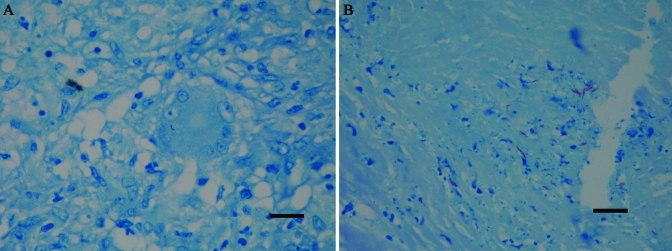
Histologic exam of the liver revealed multifocal to coalescing, moderate to effacing granulomas. The granulomas had central, often vast, necrosis, surrounded by few to moderate numbers of often degenerate neutrophils, which in turn were surrounded by large numbers of lymphocytes and macrophages with scattered giant cells. In addition, the granulomas had thin to broad walls of reactive to mature fibrous tissue (Figure 2 A, B). In many locations, the granulomas appeared to track and thus obliterate the biliary system. Scattered, small, early granulomas were present multifocally, and areas of moderate to extensive biliary hyperplasia and lobular collapse. The lesions extended through the capsule multifocally, with extensive adhesions to the diaphragm. The gall bladder was unremarkable. The axillary, inguinal, and mesenteric lymph nodes had moderately extensive lymphoid depletion, and some mesenteric nodes had scattered, small to moderate-sized granulomas (Figure 2 C). Sections of the grossly apparent lung nodules revealed maturing granulomas with thin walls of reactive fibrous tissue, and there were scattered, moderate to low numbers of microscopic, early granulomas with little to no associated fibrous tissue (Figure 2 D). The spleen had moderate lymphoid depletion, and gut-associated lymphoid tissue had moderate lymphoid depletion. One kidney and one atrium had small, microscopic, early granulomas. Fite acid-fast staining of liver and lung (Figure 3) revealed very few acid-fast positive bacilli, both intracellular and extracellular. Fite acid-fast staining of mesenteric lymph nodes was negative.
Figure 2.
Histologic findings. (A) This low-power photomicrograph of liver reveals extensive effacement of normal parenchyma by coalescing granulomas. (B) At higher magnification, the hepatic granulomas have central, massive necrosis, surrounded by intense granulomatous infiltrates including scattered giant cells, and with peripheral fibrosis. In the lower right portion of the image, biliary hyperplasia can be seen. (C) A mesenteric lymph node shows effacement of normal structure by a large granuloma. (D) One of the grossly identified nodules in the lung consists of coalescing granulomas. Note the lack of a thick, fibrous capsule in the lung granulomas, indicating more recent development. Hematoxylin and eosin stain; bar, 0.5 mm (A), 100 µm (B), 200 µm (C, D).
Figure 3.
Fite acid-fast staining. (A) In the liver, a giant cell contains a solitary Fite acid-fast organism. (B) This section of lung has a small cluster of Fite acid-fast organisms. Notably, organisms were present in very low numbers. Fite acid fast stain; bar, 20 µm.
Organism identification.
PCR was performed on paraffin-embedded tissues and identified Mycobacterium tuberculosis complex. PCR was performed as follows: total DNA was extracted from the tissue using the High Pure PCR Template Preparation Kit (Roche Diagnostics, Mannheim, Germany), according to manufacturer's instructions. To detect the pathogen, a 306 base pair section of the beta subunit of the RNA polymerase gene was amplified with primers MF (5′ CGA CCA CTT CGG CAA CCG 3′) and MR (5′ TCG ATC GGG CAC ATC CGG 3′).12 The reaction was 50 µL in volume, and contained 1× AmpliTaq buffer, 1.5 mM MgCl2, 200 µM deoxyribonucleotide triphosphates, 1.25 U AmpliTaq enzyme (all from Applied Biosystems, Foster City, CA), and 1 µM each primer. Amplification included an initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 60 s, annealing at 60 °C for 60 s, and extension at 72 °C for 60 s. Final extension occurred at 95 °C for 10 min, and then the products were held at 4 °C until analysis by gel electrophoresis. The length of the M. tuberculosis product was the same as that of the positive control (Mycobacterium chelonae DNA), when analyzed by gel electrophoresis. The PCR product was sequenced by using primers MF and MR as previously described.5 The sequence obtained was 100% identical to Mycobacterium tuberculosis H37Ra (GenBank accession no., CP000611.1) over 306 nucleotides. Two attempts were made to culture the organism. Briefly, cultures were set up as follows: the liver sample was homogenized in sterile water by using a disposable tissue grinder and inoculated into conventional solid and liquid media in an automated culture system for mycobacterial detection and recovery. The Middlebrook 7H11 agar plate (REMEL, Lenexa, KS) was incubated at 37 °C in 5% to 10% CO2 for 10 wk. The BACTEC Mycobacterial Growth Indicator Tube liquid medium was incubated in the BACTEC MGIT 960 Mycobacteria Culture System (BD Diagnostics, Sparks, MD) for 6 wk.10,11 Neither medium yielded growth of organisms.11
To confirm the diagnosis of tuberculosis, paraffin-embedded liver and lung were submitted to the Washington Animal Disease Diagnostic Lab (Pullman, WA). After DNA was extracted from samples, a portion of the 16S ribosomal RNA gene was amplified by PCR using universal Mycobacterium spp. primers. When sequenced and compared with GenBank sequences, the PCR product had 100% identity with Mycobacterium tuberculosis complex.
Quarantine procedures.
Animals exposed to this macaque were quarantined for 90 d and had 8 additional TST. All animals had negative skin tests and were released from quarantine. In addition, to our knowledge, no human exposed to this animal or her tissues has developed a positive TST. Finally, facility veterinary pathologists have performed necropsies with histopathology on multiple macaques that were exposed to this monkey, and none have shown pathology consistent with tuberculosis.
Discussion
Macaques are highly susceptible to tuberculosis.7,17 Current tuberculosis screening protocols of frequent TST have significantly reduced the frequency of tuberculosis outbreaks.14 However, although TST is still considered the ‘gold standard’ for diagnosing tuberculosis infection in vivo, it can lead to false-negative results for multiple reasons, including anergy and latent infections.14 False-negative tests can result in the release of infected animals to a colony, and such cases have the potential to shed organisms and infect other animals and human handlers. In the case we present here, no other animal or human exposed to this case had a positive TST. Therefore, the animal probably was infected prior to entering our facility. Her 16 negative TST were likely due to latent infection. The cause of the reactivation of the disease is unknown.
Some primate facilities have implemented PrimaGam testing, an in vitro assay for interferon γ response to tuberculin antigens,8,14,19 to screen for tuberculosis. However, PrimaGam's sensitivity is lower than that of the TST.8,14 Currently the PrimaGam test is not recommended as a sole screening tool.8,14 We did not add PrimaGam testing to the screening protocol for the macaques exposed to our index animal because of its low sensitivity when used for screening macaques for tuberculosis; the assay is more specific for confirming a diagnosis and differentiation of Mycobacterium species. Current tuberculosis screening protocols at our facility are based on TST and use PrimaGam as an adjunct in the differential diagnosis of suspicious TST results. Other diagnostic modalities for screening for and diagnosing tuberculosis include thoracic radiographs, gastric lavage with culture, and bronchial alveolar lavage and culture15 but these were not used in this case.
In the presented case, the specific identity of the organism could not be determined, although the organism was classified as Mycobacterium tuberculosis complex on the basis of PCR results. Mycobacterium tuberculosis complex includes Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium africanum, Mycobacterium microti, Mycobacterium pinnipedii, and Mycobacterium canetti.21 All of these species are highly pathogenic zoonoses. Although culture and identification of the organism is considered the ‘gold standard’ for diagnosis, we could not obtain growth of organisms from this macaque's liver, likely because of the low numbers of organisms present in the infected tissues. Initial PCR analysis yielded a sequence identical to H37Ra, a reference avirulent strain of Mycobacterium tuberculosis derived from the virulent strain H37.23 In addition, a commercial laboratory (Washington Animal Disease Diagnostic Lab) confirmed this result by PCR analysis of 16S ribosomal RNA on paraffin-embedded lung and liver.
Infection with tuberculosis in macaques usually occurs through inhalation of infected aerosols from an infected animal or human.14 Ingestion of organisms is an additional route of infection, although this mode generally is considered to be rare.3,14 The infection in our macaque was unusual because it was primary hepatic and appeared to track the biliary system. The granulomas in the atrium, kidney, lung, and mesenteric lymph nodes were less mature histologically and therefore likely developed much more recently than did the liver lesions. This pattern suggests that the route of entry was enteric, with subsequent progression to the liver and terminal dissemination hematologically to other organs, including the lungs. Reports of primary extrapulmonary tuberculosis in macaques are rare in the literature. In the United States, human cases of tuberculosis are on the decline.18 However, the proportion of extrapulmonary tuberculosis has increased and now comprises approximately 20% of all cases.18 Potentially any organ can be affected, including the spine (Pott disease), skin (lupus vulgaris), and lymph nodes (scrofula).6 Extrapulmonary tuberculosis presents a diagnostic challenge. Clinical signs are usually vague and vary depending on the organ infected. Hepatic tuberculosis frequently is misdiagnosed as a primary tumor or secondary metastasis.13,16 The ability of the TST to detect extrapulmonary tuberculosis has been controversial, and in one report,1 only 50% of extrapulmonary tuberculosis cases had positive TST results. Alternatively the macaque we present here may have had negative TST due to anergy. In humans, cell-mediated immunity testing has been used to evaluate a patient's potential for anergy.22 This procedure involves interdermal injections of antigens such as tetanus toxoid, diphtheria toxoid, and Streptococcus spp., which cause delayed hypersensitivity reactions in normal persons.22 A negative result would indicate a defect in the host's cell mediated immunity.22 However, this method of testing is controversial, and it is possible to react normally to other antigens while being anergic to tuberculosis.20 This macaque did not undergo testing for cell-mediated immunity.
This case underscores the problems with current tuberculosis screening protocols. TST is not always a reliable screening tool. Extrapulmonary tuberculosis can have vague clinical signs dependent on the organ(s) infected. In macaques, extrapulmonary tuberculosis is a great pretender of other diseases and should be included as a differential even if intradermal skin testing is negative.
Acknowledgments
We gratefully acknowledge the assistance of Mac Durning for histotechnology support, Trevor Pierce for computer technical assistance, and the Veterinary Technical Staff for their efforts with this case and subsequently during quarantine.
References
- 1.Alvarez S, McGabe WR. 1984. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine (Baltimore) 63:25–55 [PubMed] [Google Scholar]
- 2.Animal Welfare Act as Amended 2007. 7 USC §2131–2156.
- 3.Bernacky BJ, Gibson SV, Keeling ME, Abee CR. 2002. Nonhuman primates, p 676–777 In: Fox JG, Anderson LC, Loew FM. Laboratory animal medicine, 2nd ed San Diego (CA): Academic Press [Google Scholar]
- 4.Clingerman KJ, Summers L. 2005. Development of a body condition scoring system for nonhuman primates using Macaca mulatta as a model. Lab Anim (NY) 34:31–36 [DOI] [PubMed] [Google Scholar]
- 5.de Sanctis JT, Carpenter C, Sims M, SenGupta D, Prentice JL, Cookson BT, Boyanton BL. 2010. Culture-negative endocarditis and the use of molecular diagnostics. A case report. Infect Dis Clin Pract 18:120–123 [Google Scholar]
- 6.Elder NC. 1992. Extrapulmonary tuberculosis. A review. Arch Fam Med 1:91–98 [DOI] [PubMed] [Google Scholar]
- 7.Garcia MA, Bouley DM, Larson MK, Lifland B, Moorhead R, Simkins MD, Borie DC, Tolwani R, Otto G. 2004. Outbreak of Mycobacterium bovis in a conditioned colony of rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques. Comp Med 54:578–584 [PubMed] [Google Scholar]
- 8.Garcia MA, Yee J, Bouley DM, Moorhead R, Lerche NW. 2004. Diagnosis of tuberculosis in macaques, using whole-blood in vitro interferon γ (PRIMAGAM) testing. Comp Med 54:86–92 [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 10.Isenberg HD, American Society for Microbiology 2004. Clinical microbiology procedures handbook. Washington (DC): ASM Press [Google Scholar]
- 11.Kent PT, Kubica GP. 1985. Public health mycobacteriology: a guide for the level III laboratory. Atlanta (GA): US Department of Health and Human Services [Google Scholar]
- 12.Kim BJ, Lee SH, Lyu MA, Kim SJ, Bai GH, Chae GT, Kim EC, Cha CY, Kook YH. 1999. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J Clin Microbiol 37:1714–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köksal D, Köksal AS, Köklü S, Ciçek B, Altiparmak E, Sahin B. 2006. Primary tuberculous liver abscess: a case report and review of the literature. South Med J 99:393–395 [DOI] [PubMed] [Google Scholar]
- 14.Lerche NW, Yee JL, Capuano SV, Flynn JL. 2008. New approaches to tuberculosis surveillance in nonhuman primates. ILAR J 49:170–178 [DOI] [PubMed] [Google Scholar]
- 15.Lin PL, Yee JA, Klein E, Lerche NW. 2008. Immunological concepts in tuberculosis diagnostics for nonhuman primates: a review. J Med Primatol 37 Supp1:44–51 [DOI] [PubMed] [Google Scholar]
- 16.Parsak CK, Hanta I, Aslan A, Alabaz O. 2008. Isolated hepatic tuberculosis presenting as cystic-like and tumour-like mass lesions. Case Rep Gastroenterol 2:18–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsons SDC, de Villiers C, Grey van Pittius NC, Warren RM, van Helden PD. 2010. Detection of Mycobacterium kansasii infection in a rhesus macaque (Macaca mulatta) using a modified QuantiFERON-TB Gold assay. Vet Immunol Immunopathol 136:330–334 [DOI] [PubMed] [Google Scholar]
- 18.Rockwood RR. 2007. Extrapulmonary TB: what you need to know. Nurse Pract 32:44–49 [DOI] [PubMed] [Google Scholar]
- 19.Shipley ST, Coksaygan T, Johnson DK, McLeod CG, DeTolla LJ. 2008. Diagnosis and prevention of dissemination of tuberculosis in a recently imported rhesus macaque (Macaca mulatta). J Med Primatol 37:20–24 [DOI] [PubMed] [Google Scholar]
- 20.Slovis BS, Plitman JD, Haas DW. 2000. The case against anergy testing as a routine adjunct to tuberculin skin testing. J Am Med Assoc 283:2003–2007 [DOI] [PubMed] [Google Scholar]
- 21.Une Y, Mori T. 2006. Tuberculosis as a zoonosis from a veterinary perspective. Comp Immunol Microbiol Infect Dis 30:415–425 [DOI] [PubMed] [Google Scholar]
- 22.Vine MF, Stein L, Weigle K. 2000. Gender differences in response to the multitest CMI skin test in the general population. Ann Allergy Asthma Immunol 84:445–450 [DOI] [PubMed] [Google Scholar]
- 23.Zheng H, Lu L, Wang B, Pu S, Zhang Z, Zhu G, Shi W, Zhang L, Wang H, Wang S, Zhao G, Zhang Y. 2008. Genetic basis of virulence attenuation revealed by comparative genomic analysis of Mycobacterium tuberculosis strain H37Ra versus H37Rv. PLoS ONE 3:e2375. [DOI] [PMC free article] [PubMed] [Google Scholar]