Abstract
Degenerative joint disease (DJD), also known as osteoarthritis, has been well documented in aging populations of captive and free-ranging macaques; however, successful treatments for DJD in nonhuman primates have not been published. Published data on chimpanzees show little to no DJD present in the wild, and there are no published reports of DJD in captive chimpanzees. We report here the first documented case of DJD of both the right and left femorotibial joints in a captive male chimpanzee. Progression from minimal to moderate to severe osteoarthritis occurred in this animal over the course of 1 y. Treatment with chondroprotective supplements (that is, glucosamine chondroitin, polysulfated glycosaminoglycan) and intraarticular corticosteroid injections (that is, methylprednisolone, ketorolac), together with pain management (that is, celecoxib, tramadol, carprofen), resulted in increased activity levels and decreased clinical signs of disease. DJD has a considerable negative effect on quality of life among the human geriatric population and therefore is likely to be one of the most significant diseases that will affect the increasingly aged captive chimpanzee population. As this case study demonstrates, appropriate treatment can improve and extend quality of life dramatically in these animals. However, in cases of severe osteoarthritis cases, medication alone may be insufficient to increase stability, and surgical options should be explored.
Abbreviation: DJD, degenerative joint disease
Degenerative joint disease (DJD) or osteoarthritis is the most common joint disorder seen in humans, affecting some 60% to 70% of adults 60 y of age and older.1,27Among nonhuman primates, DJD has been documented in both captive3-6,9,18,26 and free-ranging21,29 macaque populations, with the disease affecting as much as 55% to 79% of the aged (older than 15 y) population.3-5,9,21 In addition, osteoarthritis has been reported among captive New World monkeys33,35 and prosimians.32,35 However, existing data for great apes show little to no DJD present in the wild.14,22,24,30,31 In addition, there are no published reports of DJD in captive chimpanzee populations; however, the few published reports of captive gorillas suggest that DJD may be more prevalent than previously reported.25,39
Osteoarthritis is a complex and progressive disease resulting in damage to and loss of articular cartilage, attrition of subarticular bone, diminished joint space, formation of osteophytes, and often synovial distension and inflammation.1 Among men and women, DJD occurs most frequently in the hands (50% to 70% of cases),15,19,36 followed by knee joints (25% to 40%),15,36 hip joint (10% to 20%),15,19,36 and upper back and shoulders (5% to 10%).15,19,36 Among free-ranging21,29 and captive3-6 macaques, DJD occurs in the hands (55% of cases),19 lumbar spine (69% to 78%),5,6,9 and knee and hip joints (32% to 67%).4,29 In addition to the similarity in prevalence rates, osteoarthritis increases with age and obesity in both humans1,27 and macaques4,23 and is radiologically and histologically similar.
The increasing numbers of geriatric (older than 30 y) chimpanzees in captivity likely will generate new challenges for veterinary care and captive management. Given that DJD is one of the leading causes of disability in aged Americans (older than 60 y) and has considerable influence on quality of life,1,19 the increasingly aged chimpanzee population likely will also be affected by the disease. We report here the diagnosis and treatment of severe osteoarthritis (DJD) in a chimpanzee (Pan troglodytes).
Case Study
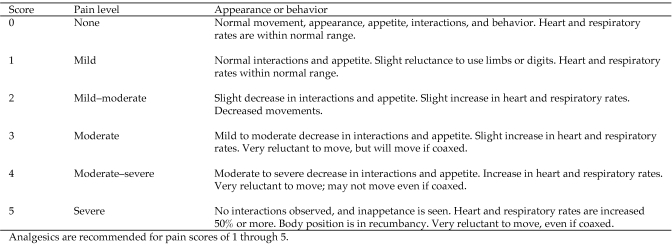
Each animal at our facility (Alamogordo Primate Facility, Holloman Air Force Base, NM) annually receives a complete physical examination under sedation with tiletamine hydrochloride–zolazepam (3.5 mg/kg; Telazol, Fort Dodge Animal Health, Fort Dodge, IA), CBC, clinical chemistry, electrocardiography, abdominal ultrasonography, tuberculosis testing, dental prophylaxis, and blood pressure assessment. Chimpanzees are housed socially and maintained in accordance with the Guide for the Care and Use of Animals.13 All procedures were approved by an institutional animal care and use committee. The facility and its program are fully AAALAC-accredited. No research occurs at the facility. Pain and locomotion of all animals are assessed by veterinary and animal care staff on a regular basis by using a pain score scale (Figure 1).
Figure 1.
Pain assessment scores.
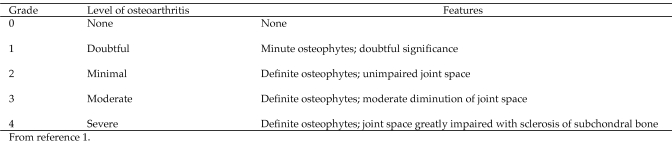
In June 2006, animal care staff at our facility noted that a 25-y-old ,95-kg, captive-born, socially housed male chimpanzee (negative for hepatitis B virus, hepatitis C virus, and HIV1) was slow-moving and slightly lame on his left leg during morning observation sessions. However during the afternoon, the animal would appear to have no lameness, pain, or discomfort and was active. During physical examination, the left femorotibial joint had decreased range of motion and a considerable amount of crepitus. The knee area had minimal swelling, with no joint effusion noted. Radiographs revealed marginal osteophyte formation at the joint capsule attachment and a slight narrowing of the joint space, resulting in a diagnosis of minimal to moderate osteoarthritis (that is, grade 2 to 3 according to the Kellgren–Lawrence Grading System, Figure 2).1 The right femorotibial joint appeared normal. The chimpanzee was placed on a course of celecoxib (200 mg daily for 30 d; Celebrex, Pfizer, New York, NY). The lameness resolved, and the animal did not exhibit any further clinical signs until June 2007.
Figure 2.
Kellgren–Lawrence system of grading osteoarthritis.
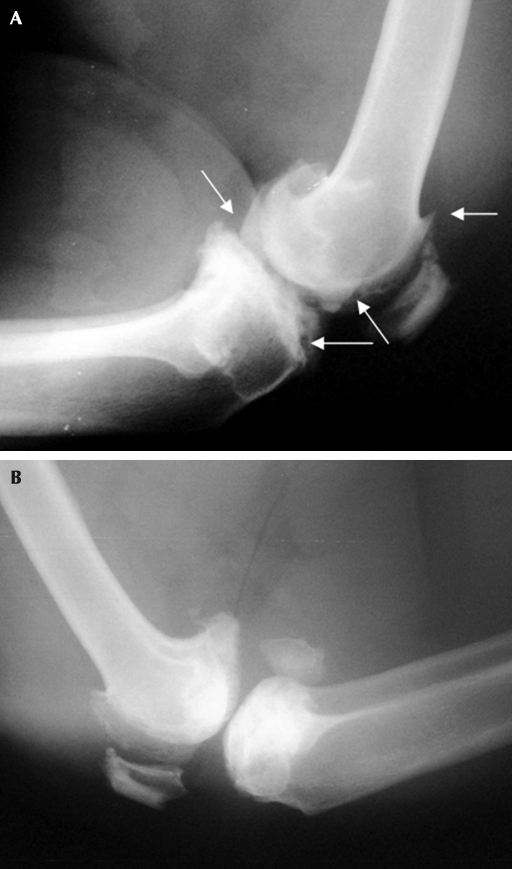
Prior to the animal's annual physical examination in June 2007, animal care staff noted minimal lameness in the chimpanzee's right leg and assigned a pain score of 2 (Figure 1). During examination, mild swelling was noted on the medial aspect of the right femorotibial joint. An orthopedic evaluation was performed, with both cranial drawer and tibial compression tests being negative. Radiographs of the left femorotibial joint (Figure 3 A) showed an increase in marginal osteophyte formation at the joint capsule attachment, periarticular remodeling, and a continued narrowing of the joint space, resulting in a diagnosis of moderate osteoarthritis (grade 3 on the Kellgren–Lawrence Grading System, Figure 2).1 Radiographs showed no osteal changes to the right femorotibial joint (Figure 3 B), resulting in a diagnosis of minimal osteoarthritis (grade 1 on the Kellgren–Lawrence Grading System).1 The animal was given ketoprofen (180 mg IM; Ketofen, Fort Dodge Animal Health) and was prescribed carprofen (75 mg twice daily; Rimadyl, Pfizer). The chimpanzee was placed on cage rest for 5 d and was returned to his den after increased use of the right leg was noted.
Figure 3.
Radiographs of the (A) left and (B) right femorotibial joints in June 2007. Arrows indicate formation of osteophytes, narrowing of joint space, and presence of periarticular remodeling of the left femorotibial joint.
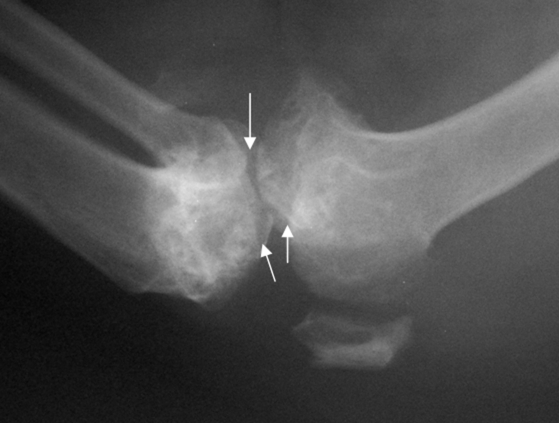
An increase in mobility was observed over the next 45 d; however, mild lameness continued and alternated between the left and right legs (pain score 1, Figure 1). During a follow–up examination in August 2007, radiographs showed osteophytes and periarticular bone proliferation on the right femur and tibia (Figure 4). In addition, the right femorotibial joint showed 40% decreased range of motion and mild crepitus; however, little joint laxity was demonstrated with the anterior drawer technique. These findings resulted in a diagnosis of minimal to moderate osteoarthritis (Kellgren–Lawrence grade 2 to 3, Figure 2).1 Radiographs and range-of-motion tests showed no change in the left femorotibial joint. A regimen of glucosamine chondroitin (1500 mg daily; Solgar, Leonia, NJ) and tramadol (50 mg twice daily, Amneal Pharmaceuticals, Glasgow, KY) was initiated; carprofen (75 mg twice daily) was continued. Polysulfated glycosaminoglycan (350 mg IM; Adequan Novartis, Larchwood, IA) was administered every 14 d for 2 mo.
Figure 4.
Radiographs of the right femorotibial joint in August 2007. Arrows indicate formation of osteophytes, narrowing of joint space, and presence of periarticular remodeling.
In October 2007, a follow-up physical examination was performed to reassess the chimpanzee's health status and progress of DJD. Radiographs showed no new osteophytes or periarticular bone proliferation of either the right or left femur or tibia. Range of motion in both joints was unchanged, lameness was noted in both legs, and the pain score had increased to level 2 (Figure 1). In addition, the anterior drawer technique revealed considerable laxity of the right femorotibial joint. A tear in the anterior cruciate ligament was suspected to have occurred secondary to osteoarthritis.
In October 2007, after consultation of veterinary staff with a board-certified orthopedic surgeon, the chimpanzee began intraarticular injections of ketorolac tromethamine (20 mg; Toradol, Roche, Nutley, NJ) and methylprednisolone sodium succinate (40 mg; SoluMedrol, Pfizer). Injections were administered by using the medial approach to the femorotibial joint. Alternate femorotibial joints were injected every 2 wk for 8 wk, resulting in 2 injections per joint. The regimen of glucosamine chondroitin, tramadol, and carprofen was continued.
The intraarticular injections were continued for 3 mo, with serial radiographs and evaluations every 1 to 2 mo. The chimpanzee has shown a modest decrease in clinical signs, and his comfort level has improved, as have his activity level and interaction with denmates. His pain score has decreased from 2 to level 0 to 1, and the animal has been observed to use both limbs, although he still tends to favor the right leg. Surgical options have been explored in an effort to further stabilize the joint and slow the progression of the disease.
Discussion
Degenerative joint disease is a progressive deterioration of the articular cartilage with various degrees of periarticular remodeling.1 Synovitis usually occurs first and results in progressive degradation of the joint cartilage, sometimes leading to degeneration of the anterior cruciate ligament and subsequent instability of the joint.7,17 DJD is common among aged humans1 and aging populations of captive and free-ranging macaques.5,6,29 However, the disease has rarely been reported for great ape species and is believed to be absent among wild chimpanzees.14,22,24 This difference is likely due to variations in life expectancies and body weights between wild and captive populations, given that both increased age and obesity are significant risk factors for DJD in humans.1 Captive chimpanzees are more likely to reach old age than are their wild counterparts, in that by the age of 15 y, captive chimpanzees have a life expectancy of 45 y,10 compared with a life expectancy of 30 y for wild chimpanzees.12 In addition, captive chimpanzees (females, 52 to 55 kg; males, 58 to 63 kg)38 tend to weigh significantly more than their wild counterparts (females, 31 to 41 kg; males, 39 to 47 kg).28 Therefore, as they age, captive chimpanzees may show increased clinical signs of DJD, as compared with their wild counterparts, due to the increased weight of captive chimpanzees and the effect of hard floor surfaces on joint stress.20 Increased attention and screening for DJD among captive chimpanzees, as well as the development of alternative treatment plans, will likely be required.
Successful treatment of DJD requires not only pain management but also should attempt to slow down or halt progression of the disease.16 Initial treatments for DJD in humans generally address pain management through the use of either nonsteroidal antiinflamatory drugs or selective cyclooxygenase 2 inhibitors (that is, celecoxib) as well as chondroprotective supplements, such as glucosamine and chondroitin sulfates.2 The use of glucosamine and chondroitin sulfates remains controversial, and results of their effectiveness in pain management and reversing joint degeneration have been mixed.2,16,37 Treatments for moderate to severe DJD often include opioids (that is, tramadol, txycodone) and intraarticular injections of either corticosteroids (that is, triamcinolone, methylprednisolone) or hyaluronic acid.2,11 Corticosteroid and hyaluronic acid injections both can result in pronounced pain relief, but hyaluronic acids tend to have a slower onset of efficacy and a longer effect.9 Disadvantages of hyaluronic acid include the need for a series of weekly injections (typically 5 or more) and its cost prohibitiveness.11,17
DJD of the human knee typically progresses slowly, taking several years to progress from mild to severe.1 In contrast, the disease progressed much more quickly in this chimpanzee case study, taking only 1 y to progress from mild to severe DJD in both knees. Previous studies of ground force during locomotion and articular joint surfaces have demonstrated that chimpanzee locomotion results in much greater joint stress than does human bipedalism.8,20,34 This high level of joint stress, coupled with habitual locomotion on hard surfaces, likely makes captive chimpanzees more prone to DJD than their wild counterparts. Successful treatment of DJD in captive chimpanzees can be problematic because of the high frequency of dosing (as for hyaluronic acid) and the high activity level of group-housed chimpanzees. However, in light of the appropriate levels of pain management and inclusion of intraarticular injection therapy, the current case study demonstrates that we can extend the quality of life for captive chimpanzees as they progress into old age.
Acknowledgments
This study was supported by NIH contract no. NO2-RR-1-2079. We are grateful for Dr Frank Bryant (Southwest Orthopedic) for assistance with this case. We are grateful to Drs Maggie McTighe, Roger Black, Paul Langner, and John Ely for editorial assistance.
References
- 1.Arden N, Nevitt MC. 2006. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol 20:3–25 [DOI] [PubMed] [Google Scholar]
- 2.Bjordal JM, Klovning A, Ljunggren AE, Slǿrdal L. 2007. Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: a meta-analysis of randomized placebo-controlled trials. Eur J Pain 11:125–138 [DOI] [PubMed] [Google Scholar]
- 3.Black A, Tilmont EM, Handy AM, Scott WW, Shapses SA, Ingram DK, Roth GS, Lane MA. 2001. A nonhuman primate model of age-related bone loss: a longitudinal study in male and premenopausal female rhesus monkeys. Bone 28:295–302 [DOI] [PubMed] [Google Scholar]
- 4.Carlson CS, Loeser RF, Purser CB, Gardin JF, Jerome CP. 1996. Osteoarthritis in cynomolgus macaques III: effects of age, gender, and subchondral bone thickness on the severity of disease. J Bone Miner Res 11:1209–1217 [DOI] [PubMed] [Google Scholar]
- 5.Colman RJ, Kemnitz JW, Lane MA, Abbott DH, Binkey N. 1999. Skeletal effects of aging and menopausal status in female rhesus macaques. J Clin Endocrinol Metab 84:4144–4148 [DOI] [PubMed] [Google Scholar]
- 6.Colman RJ, Lane MA, Binkley N, Wegner FH, Kemnitz JW. 1999. Skeletal effects of aging in male rhesus monkeys. Bone 24:17–23 [DOI] [PubMed] [Google Scholar]
- 7.Cushner FD, LaRosa DF, Vigorita VJ, Scuderi GR, Scott WN, Insall JN. 2003. A quantitative histologic comparison: ACL degeneration in the osteoarthritic knee. J Arthroplasty 18:687–692 [DOI] [PubMed] [Google Scholar]
- 8.Demes B, Larson SG, Stern JT, Jr, Jungers WL, Biknevicius AR, Schmidt D. 1994. The kinematics of primate quadrupedalism: ‘hindlimb drive’ reconsidered. J Hum Evol 26:353–374 [Google Scholar]
- 9.DeRousseau CJ. 1985. Aging in the musculoskeletal system of rhesus monkeys: II. Degenerative joint disease. Am J Phys Anthropol 67:177–184 [DOI] [PubMed] [Google Scholar]
- 10.Dyke B, Gage TB, Alford PL, Swenson B, Williams-Blangero S. 1995. Model life table for captive chimpanzees. Am J Primatol 37:25–37 [DOI] [PubMed] [Google Scholar]
- 11.Gossec L, Dougados M. 2006. Do intra-articular therapies work and who will benefit most? Best Pract Res Clin Rheumatol 20:131–144 [DOI] [PubMed] [Google Scholar]
- 12.Hill K, Boesch C, Goodall J, Pusey A, Williams J, Wrangham R. 2001. Mortality rates among wild chimpanzees. J Hum Evol 40:437–450 [DOI] [PubMed] [Google Scholar]
- 13.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 14.Jurmain R. 2000. Degenerative joint disease in African great apes: an evolutionary perspective. J Hum Evol 39:185–203 [DOI] [PubMed] [Google Scholar]
- 15.Kellgren JH, Lawrence JS. 1958. Osteoarthrosis and disk degeneration in an urban population. Ann Rheum Dis 17:388–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly GS. 1998. The role of glucosamine sulfate and chondroitin sulfate in the treatment of degenerative joint disease. Altern Med Rev 3:27–39 [PubMed] [Google Scholar]
- 17.Kirwan J. 2001. Is there a place for intraarticular hyaluronate in osteoarthritis of the knee? Knee 8:93–101 [DOI] [PubMed] [Google Scholar]
- 18.Kramer PA, Newell-Morris LL, Simkin PA. 2002. Spinal degenerative disk disease (DDD) in female macaque monkeys: epidemiology and comparison with women. J Orthop Res 20:399–408 [DOI] [PubMed] [Google Scholar]
- 19.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F. 1998. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 41:778–799 [DOI] [PubMed] [Google Scholar]
- 20.Lewton KL. 2005. Epigenetic differences in articular surface area in captive and wild chimpanzees (Pan troglodytes). Am J Phys Anthropol 126 Suppl 40:138 [Google Scholar]
- 21.Lim KKT, Kessler MJ, Pritzker KPH, Turnquist JE, Dieppe PA. 1996. Osteoarthritis of the hand in nonhuman primates: a clinical, radiographic, and skeletal survey of Cayo Santiago rhesus macaques. J Med Primatol 25:301–308 [DOI] [PubMed] [Google Scholar]
- 22.Lovell NC. 1990. Patterns of injury and illness in great apes: a skeletal analysis. Washington (DC): Smithsonian Institution Press [Google Scholar]
- 23.Miller LM, Novatt JT, Hamerman D, Carlson CS. 2004. Alterations in mineral composition observed in osteoarthritic joints of cynomolgus monkeys. Bone 35:498–506 [DOI] [PubMed] [Google Scholar]
- 24.Morbeck ME, Galloway A, Sumner DR. 2002. Getting old at Gombe: skeletal aging in wild-ranging chimpanzees, p 48–62 In: Erwin JM, Hoff PR. Aging in nonhuman primates. Basel (Switzerland): Karger [Google Scholar]
- 25.Nichols KA, Zihlman AL. 2001. Skeletal and dental evidence of aging in captive and wild African apes: a preliminary report, p 340–343 In: The Apes: Challenges for 21st Century Conference Proceedings. Brookfield (IL): Brookfield Zoo [Google Scholar]
- 26.Nuckley DJ, Kramer PA, Del Rosario A, Fabro N, Baran S, Ching RP. 2008. Intervertebral disc degeneration in a naturally occurring primate model: radiographic and biomechanical evidence. J Orthop Res 26:1283–1288 [DOI] [PubMed] [Google Scholar]
- 27.Petersson IF, Jacobsson LTH. 2002. Osteoarthritis of the peripheral joints. Best Pract Res Clin Rheumatol 16:741–760 [DOI] [PubMed] [Google Scholar]
- 28.Pusey AE, Oehlert GW, Williams JM, Goodall J. 2005. Influence of ecological and social factors on body mass of wild chimpanzees. Int J Primatol 26:3–31 [Google Scholar]
- 29.Rothschild BM, Hong N, Jurnquist JE. 1999. Skeletal survey of Cayo Santiago rhesus macaques: osteoarthritis and articular plate excrescences. Semin Arthritis Rheum 29:100–111 [DOI] [PubMed] [Google Scholar]
- 30.Rothschild BM, Rühli FJ. 2005. Comparison of arthritis characteristics in lowland Gorilla gorilla and mountain Gorilla beringei. Am J Primatol 66:205–218 [DOI] [PubMed] [Google Scholar]
- 31.Rothschild BM, Rühli FJ. 2005. Etiology of reactive arthritis in Pan paniscus, P. troglodytes troglodytes, and P. troglodytes schweinfurthii. Am J Primatol 66:219–231 [DOI] [PubMed] [Google Scholar]
- 32.Rothschild BM, Woods RJ. 1992. Erosive arthritis and spondyloarthpathy in Old-World primates. Am J Phys Anthropol 88:389–400 [DOI] [PubMed] [Google Scholar]
- 33.Rothschild BM, Woods RJ. 1993. Arthritis in New-World monkeys: osteoarthritis, calcium pyrophosphate deposition disease, and spondyloarthopathy. Int J Primatol 14:61–78 [Google Scholar]
- 34.Sockol MD, Raichlen DA, Pontzer H. 2007. Chimpanzee locomotor energetics and the origin of human bipedalism. Proc Natl Acad Sci USA 104:12265–12269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stringfield CE, Wynne JE. 1999. Nurtraceutical chondroprotectives and their use in osteoarthritis in zoo animals, p 63–68 In: Baer CK. Proceedings of the American Association of Zoo Veterinarians. Los Angeles (CA): Los Angeles Zoo [Google Scholar]
- 36.Van Saase JLCM, Van Romunde LKJ, Cats A, VandenBroucke JP, Valkenburg HA. 1989. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis 48:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verbruggen G. 2006. Chrondroprotective drugs in degenerative joint diseases. Rheumatology (Oxford) 45:129–138 [DOI] [PubMed] [Google Scholar]
- 38.Videan EN, Fritz J, Murphy J. 2007. Development of guidelines for assessing obesity in captive chimpanzees (Pan troglodytes). Zoo Biol 26:93–104 [DOI] [PubMed] [Google Scholar]
- 39.White W. 1992. St. Louis zoo loses its patriarch. Gorilla Gazette 6:3 [Google Scholar]