Abstract
Replication-deficient adenoviral (Ad) vectors are an attractive platform for a vaccine against lung infections caused by Pseudomonas aeruginosa. Ad vectors based on non-human serotypes have been developed to circumvent the problem of pre-existing anti-Ad immunity in humans. The present study analyzes the anti-P.aeruginosa systemic and lung mucosal immunity elicited by a non-human primate-based AdC7 vector expressing the outer membrane protein F (AdC7OprF) of P. aeruginosa. Intramuscular immunization of mice with AdC7OprF induced similar levels of serum and mucosal anti-OprF IgG and increased levels of anti-OprF IgA in lung epithelial lining fluid (ELF) compared to immunization with a human serotype Ad5OprF vector (p>0.05). OprF-specific INF-γ in splenic T cells stimulated with OprF-pulsed syngeneic splenic dendritic cells (DC) was similar following immunization with AdC7OprF compared to Ad5OprF (p>0.05). In contrast, OprF-specific INF-γ responses in lung T cells stimulated with either spleen or lung DC were increased following immunization with AdC7OprF compared to Ad5OprF (p<0.05). Interestingly, direct administration of AdC7OprF to the respiratory tract resulted in an increase of OprF-specific IgG in serum, OprF-specific IgG and IgA in lung ELF, and OprF-specific INF-γ in lung T-cells compared to immunization with Ad5OprF, and survival following challenge with a lethal dose of P. aeruginosa. These data demonstrate that systemic or lung mucosal immunization with an AdC7-based vaccine vector induces superior pulmonary humoral and cellular anti-transgene immunity compared to immunization with an Ad5-based vector and favors AdC7-based vectors as vaccines to induce lung mucosal immunity.
Introduction
Pulmonary infections with P. aeruginosa are a frequent problem for patients with cystic fibrosis, immunodeficiency or bronchiectasis [1;2]. There is no current vaccine available against P. aeruginosa. The P. aeruginosa OprF protein, a major outer membrane protein that is surface exposed and antigenically conserved in various strains of P. aeruginosa, is a promising antigen for a vaccine [3]. Antibodies against OprF are associated with protection in animal models [4-7] and are present following immunization in humans [8-14]. Expression of OprF by a human serotype Ad5 vector induces anti-OprF humoral and cellular immunity and provides protection pulmonary infections with P. aeruginosa in mice [15].
Ad vectors are attractive platforms as vaccines due to their immunogenicity and their function as adjuvants [16-19]. The most commonly used human Ad serotypes 2 and 5 have the disadvantage of being less effective in the presence of the prevalent anti-human Ad 2 and 5 immunity among many populations [20-24]. Wild-type Ad5 is a ubiquitous pathogen and neutralizing titers are found in up to 50% of the adult US population impeding the efficacy of Ad5 based vaccines [20-24]. Less prevalent human or non-human Ad serotypes, including non-human primate-derived Ad vectors, have become attractive alternatives as Ad-based vaccines [21;25-27;27-36]. Non-human primate Ad serotypes do not circulate in the human population and should therefore not be affected by pre-existing immunity when used as vaccine carries in humans [29;37]. However this concept may not be completely viable for all populations, as neutralizing antibodies against chimpanzee Ad serotypes have been detected in a small percentage of people living in Sub-Saharan Africa [38;39]. Vaccine vectors based on the non-human serotype C3, C68, C6 and C7 can generate potent transgene-specific immune responses [28-30;33;40]. These immune responses can also be boosted by the sequential use of heterologous vectors [33;35;41-43].
The present study analyzes the systemic and airway mucosal immunogenic properties of an AdC7-based vector expressing OprF (AdC7OprF) to induce anti-P. aeruginosa protection compared to an Ad5-based vector. Interestingly, while weaker in inducing systemic and mucosal humoral anti-OprF IgG responses initially, immunization with AdC7OprF induced sustained higher levels of mucosal anti-OprF IgA and lung T-cell immunity and also resulted in protection against a lethal pulmonary challenge with P. aeruginosa. These data suggest that C7-based Ad vectors have a favorable profile to induce protective and mucosal immunity against a pulmonary pathogen such as P. aeruginosa.
Methods
Adenovirus Vectors
The recombinant Ad vectors used in this study are replication-defective E1-, E3- Ad vectors based on the human Ad5 or the chimpanzee AdC7 genome [35;44]. For construction of the AdC7OprF vector, the AdC7 plasmid pPan-GFP (kindly provided by JM Wilson, University of Pennsylvania) was digested with I-Ceul and PI-Scel. For both serotypes, an OprF expression cassette [15] was inserted into the E1 region, containing the human cytomegalovirus intermediate-early enhancer/promoter, the OprF cDNA, and a simian virus 40 poly(A) stop signal. Ad5Null, an Ad5 vector with no transgene [45] or AdC7Null (kindly provided by JM Wilson), an AdC7 vector with the green fluorescent protein cDNA under control of a prokaryotic promoter that does not lead to transgene expression in mammalian cells, were used as controls. The vectors were used on the basis of equal number of particle units (pu) and were propagated and purified as described previously [46;47].
Purification of Recombinant OprF Protein
A recombinant bacterial expression vector (pSUMO-OprF) with an N-terminal His tag was constructed and the recombinant OprF protein was purified as described previously[15]. Briefly, the PCR-amplified OprF gene was cloned into the expression vector pET SUMO (Invitrogen, Carlsbad, CA). The recombinant plasmid pSUMO-His-OprF was transformed into Escherichia coli BL21 (DE3), and the recombinant protein was purified by Ni-chelating affinity chromatography under native conditions [15].
Mice
Female C57BL/6 mice, obtained from Taconic Farms (Tarrytown, NY), were housed under specific pathogen-free conditions and used at 6 to 8 wk of age. The mice were immunized with the Ad vectors diluted in 50 μl PBS intramuscularly to the right thigh or intratracheally through a 24G angiocath inserted into the trachea.
Anti-OprF Humoral Responses
Mice were immunized with AdC7OprF, Ad5OprF, Ad5Null or AdC7Null at a doses from 109 - 1011 pu/animal via the intramuscular or intratracheal route. Serum and lung epithelial lining fluid (ELF), were collected after 4, 8 and 12 wk. Lung ELF was collected by three intratracheal instillations and aspirations of 1 ml PBS, pH 7.4, which was then centrifuged at 6000 rpm at 4° C for 10 min, and the supernatant was stored at −80° C. Anti-OprF total IgG and isotype antibody levels were assessed by ELISA using flat bottomed 96-well EIA/RIA plates (Corning, New York, NY) coated with recombinant OprF (0.5 μg/well in 0.05 M carbonate buffer, pH 7.4). The plates were blocked with 5% dry milk in PBS for 1 h at 23° C and serial serum dilutions were added to each well and incubated for 1 h at 23°C. Following three washes with PBS containing 0.05% Tween (PBS-Tween) a peroxidase-conjugated sheep anti-mouse IgG (Sigma), diluted 1:10,000 in PBS containing 1% dry milk, was added and incubated for 1 h at 23° C. Anti-OprF IgA and IgG isotypes (IgG1, IgG2a, IgG2b and IgG3) were determined using an isotyping kit (Bio-Rad Laboratories, Hercules, CA). Absorbance at 415 nm was measured with a microplate reader (Bio-Rad Laboratories) and the antibody titers were calculated with a log(OD)-log(dilution) interpolation model and a cutoff value equal to 2-fold the absorbance of the background.
OprF-specific Cellular Responses
OprF-specific local and systemic cellular immune responses were assessed in T cells isolated from spleens 7 days following intramuscular and from lungs 8 wk following intratracheal administration of AdC7OprF, Ad5OprF, Ad5Null or AdC7Null (1010 pu per animal) using an interferon-γ (IFN-γ) enzyme-linked immunospot (ELISPOT) assay. CD3+T cells were purified from the spleen and lung using CD3 Microbeads (Miltenyi Biotec, Auburn, CA). Dendritic cells (DC) were purified from spleen and lung of naive syngeneic animals by positive selection using anti-CD11c microbeads (Miltenyi Biotec) and two consecutive purifications over MACS LS columns (Miltenyi Biotec) to serve as antigen-presenting cells. Purity for CD3 T cells was >95% and for DC was >90%, as determined by flow cytometry. DC (5 ×106/ml) were incubated for 2 hr with purified recombinant OprF protein (100 μg/ml) in RPMI medium supplemented with 2% fetal calf serum (FCS; HyClone, Logan, UT), 10 mM HEPES, pH 7.5 (Bio-Source International, Camarillo, CA) and 105 μM β-mercaptoethanol (Sigma-Aldrich). T cells (2 ×105) were incubated for 48 hr with splenic DC at a ratio of 4:1 with or without recombinant OprF protein on anti-IFN-γ-coated plates (R&D Systems), followed by incubation with the biotinylated anti-IFN-γ antibodies (R&D) for 14 hr at 4□C and with streptavidin-alkaline phosphatase conjugate (R&D) and the 3-amino-9-ethylcarbazole substrate (R&D). Spots were counted by computer-assisted ELISPOT image analysis (Zellnet Consulting, New York, NY).
Respiratory Tract Challenge with P. aeruginosa
To assess protective anti-P. aeruginosa immunity, mice that had received AdC7OprF, Ad5OprF, Ad5Null or AdC7Null (all at 1010 pu/mouse), were challenged after 8 wk with 50 μl of P. aeruginosa encapsulated in agar beads (5 × 106 CFU) via the intratracheal route. This model usually results in death of the mice 2-6 days following administration of the beads depending on the growth of the bacteria, which have to be prepared fresh, and the encapsulation efficiency into the agar beads [48;49].All mice were monitored daily for 14 days after the infection. Animals that appeared moribund were sacrificed, and this was recorded as the date of death.
Statistical Analysis
The data are presented as mean ± standard error of the mean. Statistical analyses were performed using ANOVA. Survival evaluation was carried out using Kaplan-Meier analysis. Statistical significance was determined at p<0.05.
Results
Systemic Humoral and Protective Immunity Following Intramuscular Immunization
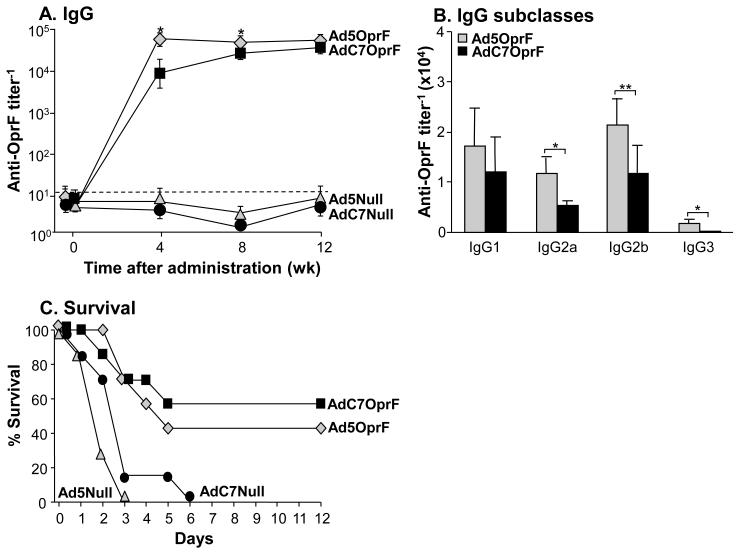
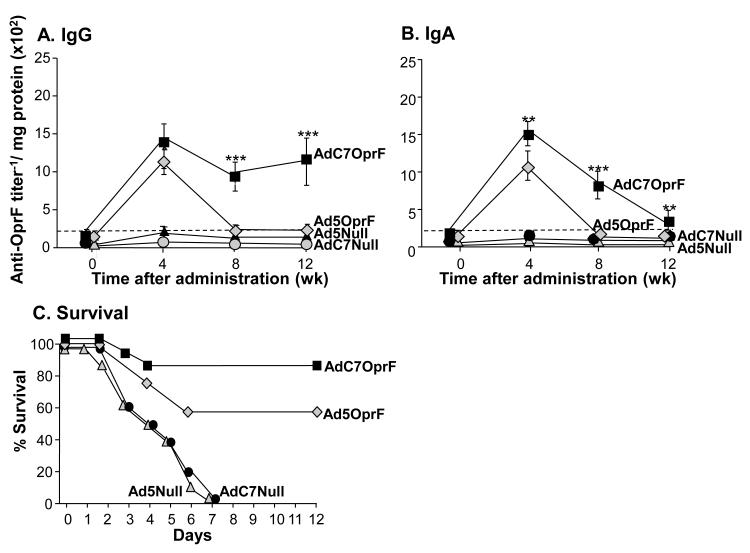
Serum anti-OprF IgG was detected 4, 8 and 12 wk following intramuscular administration of AdC7OprF and Ad5OprF (Supplemental Figure 1). The anti-OprF titers increased with increasing doses. At a dose of 1010 pu the titers following immunization with AdC7OprF were initially lower at 4 and 8 wk compared to Ad5OprF (p<0.05, both time points), but were similar at 12 wk (p>0.1). The dose of 1010 pu was used for all subsequent experiments. No anti-OprF antibodies were detected in the serum of mice that had received Ad5Null or AdC7Null at all time points. Analysis of the AdC7OprF-induced IgG isotypes at 8 wk showed that the anti-OprF IgG antibodies were predominantly of the IgG1 and IgG2b isotype, followed by IgG2a and IgG3 (Figure 1B), with lower IgG2a, IgG2b and IgG3 isotype titers compared to Ad5-immunized animals (p<0.01 for IgG2b, p<0.05 for IgG2a and IgG3).
Figure 1.
Systemic humoral and protective anti-OprF immunity following intramuscular immunization with AdC7OprF and Ad5OprF. C57BL/6 mice were immunized with AdC7OprF, Ad5OprF, Ad5Null or AdC7Null at a dose of 1010 pu/mouse. A. Serum anti-OprF IgG antibodies 0, 4, 8 and 12 wk following administration analyzed by ELISA using recombinant OprF as antigen. B. Serum anti-OprF IgG1, IgG2a, IgG2b and IgG3 antibodies 8 wk following administration. Data are shown as mean ± SEM, n=4/group, of one of three representative experiments. The dashed line represents the limit of detection. C. Protection against pulmonary challenge with P. aeruginosa. Eight wk following immunization mice (n=7/group) were challenged with a lethal intratracheal dose of agar-encapsulated P. aeruginosa (106 cfu), and survival was monitored for 12 days. * and ** denote significance of p<0.05 and ** p<0.01, respectively, between AdC7OprF and Ad5OprF.
To evaluate the protective effect of the immunizations with AdC7OprF and Ad5OprF against pulmonary infections with P. aeruginosa, mice were challenged with a lethal dose of agar-encapsulated P. aeruginosa 8 wk following vector administration. Dose escalation experiments showed dose-dependant protection, with 100% protection of the mice following immunization with Ad5OprF or AdC7OprF at a dose of 1011 pu (Supplemental Figure 1B). At an immunization dose of 1010 pu/mouse all mice that had received Ad5Null or AdC7Null died within the first 6 days after challenge (Figure 1C). In contrast, mice that had been administered with AdC7OprF or Ad5OprF showed prolonged survival (p< 0.01 AdC7OprF and Ad5OprF vs Ad5Null and AdC7Null, all comparisons; p= 0.058 Ad5OprF vs AdC7OprF). These data suggest, that systemic immunization with AdC7OprF induces protective immunity against P. aeruginosa, even in the presence of lower total systemic anti-OprF IgG titers compared to Ad5OprF.
AdC7OprF Induces Lung Mucosal Humoral Immunity
To evaluate if the protective anti-P. aeruginosa immunity was related to mucosal anti-OprF humoral immune responses, anti-OprF IgG and IgA levels were analyzed in lung ELF 4, 8 and 12 wk following administration of Ad5OprF, AdC7OprF, Ad5Null or AdC7Null. Mucosal anti-OprF IgG titers were higher 4 wk following administration of Ad5OprF compared to AdC7OprF (Figure 2A; p<0.05), but were similar at 8 and 12 wk. In contrast, mucosal anti-OprF IgA was higher following administration of AdC7OprF compared to Ad5OprF at all time points (p<0.05; Figure 2B). Similar results and statistical significance was observed in the repeat experiments (not shown). This suggests that transgene-specific lung mucosal humoral immunity is increased following immunization with the AdC7-based vector compared to Ad5.
Figure 2.
Mucosal humoral anti-OprF response following intramuscular immunization. C57BL/6 mice were immunized with AdC7OprF, AdOprF, Ad5Null or AdC7Null at a dose of 1010 pu/mouse. A. Anti-OprF IgG antibodies in the lung epithelial lining fluid 0, 4, 8 and 12 wk following vector administration. B. Anti-OprF IgGA antibodies in lung epithelial lining fluid 0, 4, 8 and 12 wk following administration. Data are shown as mean ± SEM, n=4 /group, of one of three representative experiments. The dashed line represents the limit of detection. * denotes significance of p<0.05, respectively, between AdC7OprF and Ad5OprF.
AdC7OprF Induces Systemic and Lung Cellular Immunity after Intramuscular Administration
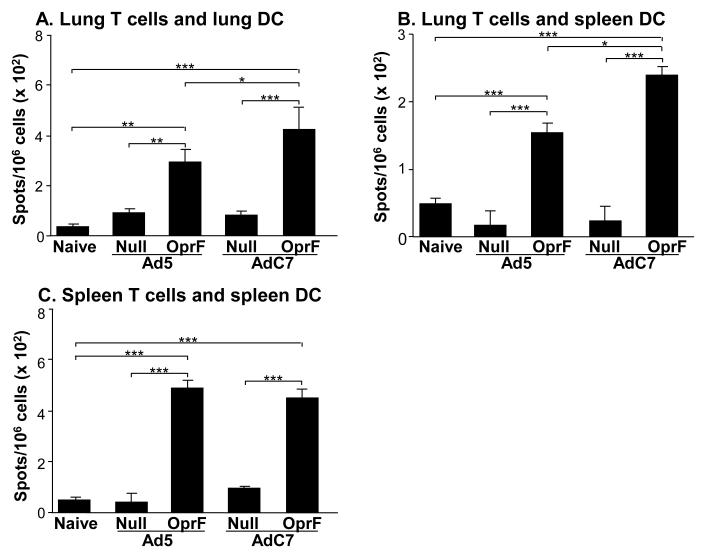
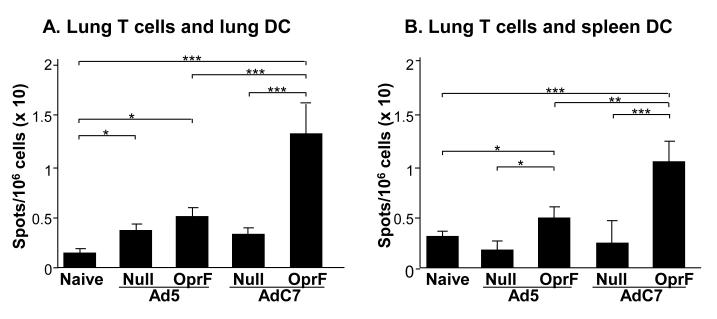
To evaluate the systemic and lung cellular responses following immunization with AdC7OprF and Ad5OprF vectors, OprF-specific IFN-γ responses in CD3 T cells from spleen and lung of immunized mice were analyzed. Seven days following administration of Ad5OprF, AdC7OprF, Ad5Null or AdC7Null, lung CD3+ T cells from immunized animals were stimulated with syngeneic pulmonary or splenic DC from naive mice, that were pulsed with recombinant OprF. Both Ad5OprF and AdC7OprF, induced an OprF-specific IFN-γ response in pulmonary CD3+ T cells, which was higher in cells from AdC7OprF-immunized mice stimulated with pulmonary (p<0.05; Figure 3A) or splenic DC (p<0.05; Figure 3B). The systemic anti-OprF cellular response, determined in splenic CD3+ T cells stimulated with OprF-pulsed splenic DC, showed no difference in the OprF-specific IFN-γ response between cells from Ad5OprF and AdC7OprF immunized mice (p>0.05, Figure 3C). IFN-γ levels in CD3+ T cells derived from mice that had received Ad5Null or AdC7Null were low and not different to those in cells from naive control mice (Figure 3A,B,C). This suggests that intramuscular administration of AdC7OprF can induce a higher cellular anti-OprF response in the lung compared to Ad5OprF.
Figure 3.
Systemic and mucosal cellular IFN-γ response to OprF following intramuscular immunization. AdC7OprF, AdOprF, Ad5Null or AdC7Null were administered intramuscularly at a dose of 1010 pu/mouse. Seven days following administration, CD3+ T cells were isolated from spleens and lungs and incubated in vitro with splenic or lung DC alone or pulsed with recombinant OprF and OprF-specific IFN-γ responses were determined in an ELISPOT assay. A. INF-γ in lung CD3+ T cells stimulated with pulsed syngenic pulmonary DC. B. INF-γ in lung CD3+ T cells stimulated with pulsed syngenic splenic DC. C. INF-γ in splenic CD3 T cells stimulated with pulsed syngenic splenic DC. The data represent the mean of pooled cells from five mice per group (n =5). Values are presented after subtraction of background IFN-γ secretion by corresponding CD3+ T cells induced by DC alone. *, ** and *** denote significance of p<0.05, p<0.01 and p<0.001, respectively
Systemic Humoral Immune Responses Following Lung Mucosal Immunization
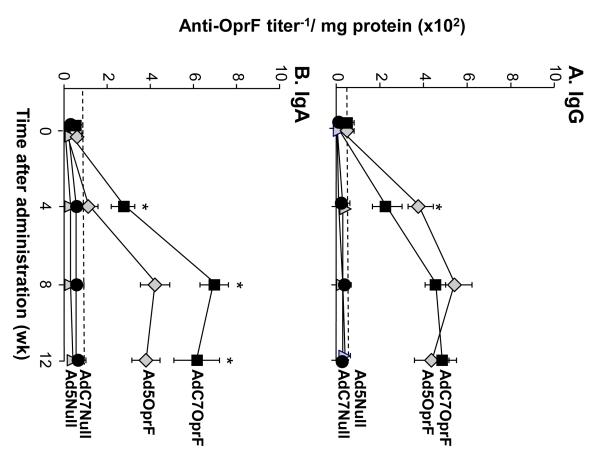
Based on the above results that intramuscular administration of AdC7OprF resulted in better lung mucosal immunity against P. aeruginosa compared to administration of Ad5OprF, anti-OprF humoral and anti-P. aeruginosa protective immunity following direct administration of the vectors to the respiratory tract by intratracheal administration was evaluated. Anti-OprF serum IgG levels were similar 4 wk following administration of AdC7OprF and Ad5OprF (p>0.05; Figure 4A). Interestingly, anti-OprF IgG level was higher in the AdC7OprF group after 8 and 12 wk (p<0.01 and p<0.05, respectively). Anti-OprF titers increased until 8 wk and were sustained up to 12 wk, whereas in the Ad5OprF group titers decreased at 8 wk and reached background levels at 12 wk following administration. No anti-OprF IgG was detected in mice that had received AdC7Null or AdNull at all time points. Similar results and statistical significance was observed in the repeat experiments (not shown). The serum anti-OprF IgG isotype response in the AdC7OprF group at 8 wk was predominantly IgG2b followed by IgG2a, IgG3 and IgG1 (Figure 4B). All IgG isotypes were higher with AdC7OprF compared to Ad5OprF (p<0.001 for IgG2a and IgG2b; p<0.01 for IgG1 and IgG3).
Figure 4.
Systemic anti-OprF humoral response following mucosal immunization. C57BL/6 mice were immunized with AdC7OprF, AdOprF, Ad5Null or AdC7Null at a dose of 1010 pu/mouse. A. Serum anti-OprF IgG antibodies 0, 4, 8 and 12 wk after administration analyzed by ELISA using recombinant OprF as antigen. B. Serum anti-OprF IgG1, IgG2a, IgG2b and IgG3 antibodies 8 wk following administration. Data are shown as mean ± SEM, n=4/group, of one of three representative experiments. The dashed line represents the limit of detection. *, ** and *** denote significance of p<0.05, p<0.01 and p<0.001, respectively, between AdC7OprF and Ad5OprF.
Mucosal Humoral and Protective Immune Responses Following Lung Mucosal Immunization
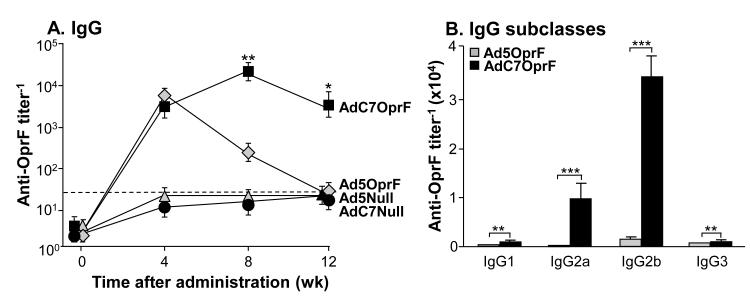
Lung mucosal anti-OprF antibodies were determined in the lung ELF 4, 8 and 12 wk following intratracheal administration of AdC7OprF, Ad5OprF, Ad5Null and AdC7Null. Lung mucosal anti-OprF IgG titers were similar 4 wk following intratracheal administration of AdC7OprF and Ad5OprF (p>0.1, Figure 5A). Mice immunized with AdC7OprF showed a sustained anti-OprF IgG response up to 12 wk, whereas no anti-OprF IgG titer was detected 8 and 12 wk following administration of Ad5OprF (p<0.001, both time points; Figure 5A). Anti-OprF IgA titers were higher following administration of AdC7OprF compared to Ad5OprF at all time points (p<0.01 at 4 and 12 wk, p<0.001 at 8 wk; Figure 5B), suggesting that, similar to intramuscular administration, intratracheal administration of the AdC7-based vector induces higher mucosal humoral immunity compared to an Ad5-based vector.
Figure 5.
Mucosal humoral and protective anti-OprF immunity following mucosal immunization. C57BL/6 mice were immunized with AdC7OprF, AdOprF, Ad5Null or AdC7Null at a dose of 1010 pu/mouse. A. Anti-OprF IgG antibodies in lung epithelial lining fluid 0, 4, 8 and 12 wk following vector administration. B. Anti-OprF IgA antibodies in the lung epithelial lining fluid 0, 4, 8 and 12 wk following administration. Data are shown as mean ± SEM, n=4/group, of one of three representative experiments. The dashed line represents the limit of detection. C. Protection against pulmonary challenge with P. aeruginosa following intratracheal immunization. AdC7OprF, AdOprF, Ad5Null or AdC7Null were administered intratracheally at a dose of 1010 pu/mouse. Mice (n=10/group) were challenged 8 wk later with a lethal intratracheal dose of agar-encapsulated P. aeruginosa (106 cfu), and survival was monitored for 12 days. Shown is a representative of 1 out of 2 experiments. ** and *** denote significance of p<0.01 and p<0.001, respectively, between AdC7OprF and Ad5OprF.
To evaluate the protective effect of lung mucosal immunization against a pulmonary challenge with P. aeruginosa, AdC7OprF, Ad5OprF, Ad5Null and AdC7Null were administered intratracheally and the mice were challenged with a lethal dose of agar-encapsulated PAO1 after 8 wk. All mice that had received Ad5Null or AdC7Null died within 7 days (Figure 5C). In contrast, mice that had received AdC7OprF or Ad5OprF showed similar prolonged survival (p<0.01 AdC7OprF and Ad5OprF vs Ad5Null and AdC7Null, all comparisons; p= 0.023 Ad5OprF vs AdC7OprF), although they differed in systemic and mucosal anti-OprF immunity.
AdC7OprF Induces Lung Cellular Immunity Following Lung Mucosal Administration
To address the systemic and lung cellular responses following immunization with AdC7OprF and Ad5OprF vectors, OprF-specific IFN-γ responses in CD3 T cells from lung of immunized mice were analyzed. 8 wk following administration of Ad5OprF, AdC7OprF, Ad5Null or AdC7Null, lung CD3+ T cells from immunized animals were stimulated with syngeneic pulmonary or splenic DC from naive mice, that were pulsed with recombinant OprF. Both Ad5OprF and AdC7OprF, induced an OprF-specific IFN-γ response in pulmonary CD3+ T cells, but the response was higher in cells from AdC7OprF-immunized mice stimulated with pulmonary (p<0.001; Figure 6A) or splenic DC (p<0.001; Figure 6B). The overall systemic anti-OprF cellular response, determined in splenic CD3+ T cells stimulated with OprF-pulsed splenic DC, was under the detectable level (data not shown). IFN-γ levels in CD3+ T cells derived from mice that had received Ad5Null or AdC7Null were low and not different to those in cells from naive control mice (Figure 6 A and B). This shows that intratracheal administration of AdC7OprF can induce persistent cellular anti-OprF response in the lung. Overall, these data suggest that, as seen for systemic immunization, administration of AdC7OprF to the respiratory tract results in humoral, cellular and protective anti-P. aeruginosa immunity and this protective effect may be based on different immune parameters compared to Ad5OprF.
Figure 6.
Mucosal cellular IFN-γ response to OprF following mucosal immunization. AdC7OprF, AdOprF, Ad5Null or AdC7Null were administered intratracheally at a dose of 1010 pu/mouse. Eight wk following administration, CD3+ T cells were isolated from lungs and incubated in vitro with splenic or lung DC alone or pulsed with recombinant OprF and OprF-specific IFN-γ responses were determined in an ELISPOT assay. A. INF-γ in lung CD3+ T cells stimulated with pulsed syngenic pulmonary DC. B. INF-γ in lung CD3+ T cells stimulated with pulsed syngenic splenic DC. The data represent the mean of pooled cells from five mice per group (n =5). Values are presented after subtraction of background IFN-γ secretion by corresponding CD3+ T cells induced by DC alone. *, ** and *** denote significance of p<0.05, p<0.01 and p<0.001, respectively.
Discussion
A vaccine against pulmonary infections with P. aeruginosa is needed for patients at risk, in particular individuals with CF before their lungs become colonized with this pathogen. The present study demonstrates that an AdC7-based vector expressing OprF induces longterm anti-P. aeruginosa systemic, mucosal and protective immunity following systemic and airway mucosal immunization. Furthermore, mucosal immunization with AdC7OprF led to sustained high levels of mucosal anti-OprF IgG, IgA and lung T-cell immunity. In comparison, Ad5OprF induces anti-P. aeruginosa protective immunity with lower anti-OprF IgA levels after systemic administration or short term mucosal anti-OprF IgG and IgA response after mucosal administration, suggesting that C7-based Ad vectors may use a different immune profile to induce protective and mucosal immunity against P. aeruginosa.
Vaccine-induced Protective Immunity Against P. aeruginosa
Several P. aeruginosa vaccine candidates have been assessed in experimental animals and humans, including sub-cellular fractions, capsule components, purified proteins and recombinant proteins [3;50]. The utility of most of these vaccines has been demonstrated in rodent models of P. aeruginosa infection, including the agar-encapsulated bacteria challenge model used in the present study. Humoral immunity seems to be paramount in evoking protection against P. aeruginosa in this model [51]. Since P. aeruginosa is an extracellular pathogen, humoral immunity induced by a vaccine should prevent infection. OprF is one of the promising P. aeruginosa antigen candidates for a vaccine [3] as humoral systemic immunity against OprF is associated with protection in animal models [4-7] and serum antibodies are present following immunization in humans [8-14]. An Ad5 vector expressing the OprF cDNA as transgene and containing an OprF-epitope in the Ad capsid has been shown to provide protective immunity in mice [15]. Both, Ad5OprF and AdC7OprF induced systemic humoral anti-OprF immunity, with Ad5OprF more superior after 4 and 8 wk following intramuscular administration and AdC7OprF more superior after 8 and 12 wk following intratracheal administration at equal doses of 1010 particles. This dose was chosen for based on the similar levels at 12 wk following intramuscular immunization and the antibody response for both viruses at this dose being increased compared to the next lower dose in the dose escalation studies. Although, T cell-mediated anti-P. aeruginosa immunity has received less attention in the vaccine development, human T cell proliferation in response to P. aeruginosa [51;52]and protective CD4 and CD8 T cell-dependent immunity was achieved in several models [52-54]. Comparing CF patients with and without chronic lung infection suggested that a Th2 type response correlated with infection, implying that a Th1 type response may be more protective [55;56] and that the Th1 cytokine IFN-γ seems to play a important role in the clearance of P. aeruginosa infection in the lung [55;56]. OprF-specific IFN-γ responses were detected in spleen and lung T cells 7 days and 8 wk following immunization with both OprF-expressing vectors, with a higher mucosal anti-OprF specific IFN-γ response induced by AdC7OprF.
AdC7-based Vectors as Vaccine Platforms
AdC7 and other primate-derived Ad vectors are closely related to human adenoviruses and share many of their structural and biological characteristics including the capacity to act as a vaccine carrier [29;42]. Humoral responses against transgenes following intramuscular or subcutaneous administration have been lower compared Ad5-based vectors after administration in rodents [28;40;42]. In contrast, following intranasal administration both, simian Ad vector and Ad5 vectors, induce comparable levels of serum and mucosal humoral immunity up to 2 wk [28], as observed in the present study after intratracheal administration up to 4 wk. However, after12 wk both vectors induced equal levels of systemic and mucosal OprF-specific IgG following intramuscular administration. In contrast, a sustained systemic and mucosal OprF-specific IgG level was only present following lung mucosal administration of AdC7OprF and not with Ad5OprF indicating a longterm predominance of mucosal immune induction by a primate-derived Ad vector. Different parameters could play a role including infectivity and stimulation of different target cells or differences in the adjuvant effect of the Ad vectors itself [57;58]. Particularly in the respiratory tract, infectivity and stimulation of various cell types as well as interaction with innate immune molecules such as Toll-like receptors by primate-derived Ad vector has so far not been evaluated and could be at last partially responsible for the prolonged mucosal immune response.
Mucosal Immunity Induced by AdC7
Mucosal surfaces are sites of entry for numerous pathogens and mucosal immune responses, including secretory IgA and cytotoxic T cells play crucial roles in host protection against these pathogens [59-64]. In general, induction of mucosal immunity, especially for the respiratory tract, requires efficient antigen delivery and adjuvant systems, that can target the mucosal immune-inductive sites and appropriately stimulate the innate immune system to generate effective adaptive immunity [64]. Several vaccine strategies, including Ad-based vaccines have been studied as mucosal vaccines [65;66]. Ad-based vaccines, administered by the intramuscular route, have been shown to also induce mucosal immune responses to some extent [67-71]. As adenovirus can invade and replicate in mucosal surfaces of the respiratory and gastrointestinal tract, mucosal immunization may even elicit a more potent immune response. Intranasal administration of Ad-based vaccines generated mucosal immune responses and protective immunity [67;68;72-75]. Transgene-specific humoral and T-cell responses at mucosal sites were detected following intramuscular administration, however at much lower levels compared to the systemic responses [67-71]. In accordance with these studies, systemic administration of AdC7Oprf and Ad5OprF resulted in similar but time dependant systemic humoral and cellular immune responses, but interestingly, mucosal anti-OprF IgA levels were higher following immunization with AdC7OprF. Immunization with AdC7OprF also resulted in increased mucosal T cell-mediated anti-OprF immune response compared to Ad5OprF. This effect was even more pronounced following administration of AdC7OprF to the respiratory tract, with longterm sustained systemic and mucosal anti-OprF IgG and mucosal anti-OprF IgA compared to Ad5OprF. Previous studies have shown that chimpanzee-based Ad induce similar levels of transgene-specific humoral mucosal immunity compared to Ad5 [28], but these studies were only extended to 2 wk post administration.
Simian Ad vectors stimulate transgene-specific Th1 T cell responses in mice that are similar or slightly superior to those achieved with Ad5 [30;41] based on their increased induction of type I interferons in DC [58]. An even more striking effect was seen when we analyzed the OprF-specific T cell mediated response in the lung. AdC7OprF induced more OprF-specific INF-γ producing lung T cells after intramuscular administration compared to Ad5OprF and similar results were obtained after administration to the respiratory tract (not shown). Although the mucosal anti-OprF immunity, particular the anti-OprF IgA responses, induced by AdC7OprF was higher compared to Ad5OprF, the protective immunity induced by AdC7OprF against a lethal challenge with P. aeruginosa was comparable. The protective values of IgA has been demonstrated for a variety of pathogens [76], but has not been studied in detail for Ad-based anti-P. aeruginosa vaccines. Further studies are needed to investigate if the prevalent mucosal immune induction by AdC7 vectors may lead to a higher protective outcome in other mucosal pathogen models.
Overall, the favorable immunological mucosal response in mice following immunization with the AdC7 vaccine vector favors the further development of non-human primate based Ad vectors as vaccines to induce protective pulmonary mucosal immunity.
Supplementary Material
Acknowledgments
We thank N. Mohamed and T. Virgin-Bryan for help in preparing this manuscript. These studies were supported by U01 AI069032, P01 HL51746 and U01 HL66952.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15(2):194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stryjewski ME, S DJi. In: Severe Infections Caused by Pseudomonas aeruginosa. Hauser AR, R J, editors. Kluwer Academic; Boston: 2003. pp. 1–15. [Google Scholar]
- [3].Stanislavsky ES, Lam JS. Pseudomonas aeruginosa antigens as potential vaccines. FEMS Microbiol Rev. 1997;21:243–77. doi: 10.1111/j.1574-6976.1997.tb00353.x. [DOI] [PubMed] [Google Scholar]
- [4].Matthews-Greer JM, Gilleland HE., Jr. Outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine against heterologous immunotype strains in a burned mouse model. J Infect Dis. 1987;155:1282–91. doi: 10.1093/infdis/155.6.1282. [DOI] [PubMed] [Google Scholar]
- [5].Moon MM, Hazlett LD, Hancock RE, Berk RS, Barrett R. Monoclonal antibodies provide protection against ocular Pseudomonas aeruginosa infection. Invest Ophthalmol Vis Sci. 1988;29(8):1277–84. [PubMed] [Google Scholar]
- [6].Price BM, Galloway DR, Baker NR, Gilleland LB, Staczek J, Gilleland HE., Jr. Protection against Pseudomonas aeruginosa chronic lung infection in mice by genetic immunization against outer membrane protein F (OprF) of P. aeruginosa. Infect Immun. 2001;69(5):3510–5. doi: 10.1128/IAI.69.5.3510-3515.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gilleland HE, Gilleland LB, Staczek J, et al. Chimeric animal and plant viruses expressing epitopes of outer membrane protein F as a combined vaccine against Pseudomonas aeruginosa lung infection. FEMS Immunol Med Microbiol. 2000;27:291–7. doi: 10.1111/j.1574-695X.2000.tb01442.x. [DOI] [PubMed] [Google Scholar]
- [8].Bumann D, Behre C, Behre K, et al. Systemic, nasal and oral live vaccines against Pseudomonas aeruginosa: a clinical trial of immunogenicity in lower airways of human volunteers. Vaccine. 2010;28(3):707–13. doi: 10.1016/j.vaccine.2009.10.080. [DOI] [PubMed] [Google Scholar]
- [9].Gocke K, Baumann U, Hagemann H, et al. Mucosal vaccination with a recombinant OprF-I vaccine of Pseudomonas aeruginosa in healthy volunteers: comparison of a systemic vs. a mucosal booster schedule. FEMS Immunol Med Microbiol. 2003;37(2-3):167–71. doi: 10.1016/S0928-8244(03)00094-4. [DOI] [PubMed] [Google Scholar]
- [10].Hancock RE, Mouat EC, Speert DP. Quantitation and identification of antibodies to outer-membrane proteins of Pseudomonas aeruginosa in sera of patients with cystic fibrosis. J Infect Dis. 1984;149(2):220–6. doi: 10.1093/infdis/149.2.220. [DOI] [PubMed] [Google Scholar]
- [11].Jang IJ, Kim IS, Park WJ, et al. Human immune response to a Pseudomonas aeruginosa outer membrane protein vaccine. Vaccine. 1999;17:158–68. doi: 10.1016/s0264-410x(98)00159-5. [DOI] [PubMed] [Google Scholar]
- [12].Kim DK, Kim JJ, Kim JH, et al. Comparison of two immunization schedules for a Pseudomonas aeruginosa outer membrane proteins vaccine in burn patients. Vaccine. 2000 Dec 8;19(9-10):1274–83. doi: 10.1016/s0264-410x(00)00235-8. 2000;19:1274-83. [DOI] [PubMed] [Google Scholar]
- [13].Larbig M, Mansouri E, Freihorst J, et al. Safety and immunogenicity of an intranasal Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers. Vaccine. 2001;19:2291–7. doi: 10.1016/s0264-410x(00)00550-8. [DOI] [PubMed] [Google Scholar]
- [14].Mansouri E, Gabelsberger J, Knapp B, et al. Safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers. Infect Immun. 1999;67:1461–70. doi: 10.1128/iai.67.3.1461-1470.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Worgall S, Krause A, Qiu J, Joh J, Hackett NR, Crystal RG. Protective immunity to pseudomonas aeruginosa induced with a capsid-modified adenovirus expressing P. aeruginosa OprF. J Virol. 2007;81(24):13801–8. doi: 10.1128/JVI.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hackett NR, Kaminsky SM, Sondhi D, Crystal RG. Antivector and antitransgene host responses in gene therapy. Curr Opin Mol Ther. 2000;2(4):376–82. [PubMed] [Google Scholar]
- [17].Jooss K, Yang Y, Fisher KJ, Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72(5):4212–23. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jooss K, Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 2003;10(11):955–63. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- [19].Molinier-Frenkel V, Lengagne R, Gaden F, et al. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J Virol. 2002;76(1):127–35. doi: 10.1128/JVI.76.1.127-135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].D’Ambrosio E, Del Grosso N, Chicca A, Midulla M. Neutralizing antibodies against 33 human adenoviruses in normal children in Rome. J Hyg (Lond. 1982;89(1):155–61. doi: 10.1017/s0022172400070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hofmann C, Loser P, Cichon G, Arnold W, Both GW, Strauss M. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J Virol. 1999;3(8):6930–6. doi: 10.1128/jvi.73.8.6930-6936.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Molnar-Kimber KL, Sterman DH, Chang M, et al. Impact of preexisting and induced humoral and cellular immune responses in an adenovirus-based gene therapy phase I clinical trial for localized mesothelioma. Hum Gene Ther. 1998;9(14):2121–33. doi: 10.1089/hum.1998.9.14-2121. [DOI] [PubMed] [Google Scholar]
- [23].Piedra PA, Poveda GA, Ramsey B, McCoy K, Hiatt PW. Incidence and prevalence of neutralizing antibodies to the common adenoviruses in children with cystic fibrosis: implication for gene therapy with adenovirus vectors. Pediatrics. 1998;101(6):1013–9. doi: 10.1542/peds.101.6.1013. [DOI] [PubMed] [Google Scholar]
- [24].Vogels R, Zuijdgeest D, van Rijnsoever R, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77(15):8263–71. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bangari DS, Mittal SK. Development of nonhuman adenoviruses as vaccine vectors. Vaccine. 2006;24(7):849–62. doi: 10.1016/j.vaccine.2005.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Seregin SS, Amalfitano A. Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert Opin Biol Ther. 2009;9(12):1521–31. doi: 10.1517/14712590903307388. [DOI] [PubMed] [Google Scholar]
- [27].Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17(8):1333–9. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xiang Z, Gao G, Reyes-Sandoval A, et al. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol. 2002;76(6):2667–75. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Farina SF, Gao GP, Xiang ZQ, et al. Replication-defective vector based on a chimpanzee adenovirus. J Virol. 2001;75(23):11603–13. doi: 10.1128/JVI.75.23.11603-11613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fitzgerald JC, Gao GP, Reyes-Sandoval A, et al. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J Immunol. 2003;170(3):1416–22. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- [31].Mittal SK, Prevec L, Graham FL, Babiuk LA. Development of a bovine adenovirus type 3-based expression vector. J Gen Virol. 1995;76(Pt 1):93–102. doi: 10.1099/0022-1317-76-1-93. [DOI] [PubMed] [Google Scholar]
- [32].Moffatt S, Hays J, Hogen EH, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology. 2000;272(1):159–67. doi: 10.1006/viro.2000.0350. [DOI] [PubMed] [Google Scholar]
- [33].Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson JM, Ertl HC. Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J Immunol. 2003;171:6774–9. doi: 10.4049/jimmunol.171.12.6774. [DOI] [PubMed] [Google Scholar]
- [34].Reddy PS, Idamakanti N, Hyun BH, Tikoo SK, Babiuk LA. Development of porcine adenovirus-3 as an expression vector. J Gen Virol. 1999;80(Pt 3):563–70. doi: 10.1099/0022-1317-80-3-563. [DOI] [PubMed] [Google Scholar]
- [35].Reyes-Sandoval A, Fitzgerald JC, Grant R, et al. Human immunodeficiency virus type 1-specific immune responses in primates upon sequential immunization with adenoviral vaccine carriers of human and simian serotypes. J Virol. 2004;78(14):7392–9. doi: 10.1128/JVI.78.14.7392-7399.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Roy S, Gao G, Lu Y, et al. Characterization of a family of chimpanzee adenoviruses and development of molecular clones for gene transfer vectors. Hum Gene Ther. 2004;15(5):519–30. doi: 10.1089/10430340460745838. [DOI] [PubMed] [Google Scholar]
- [37].Basnight M, Rogers NG, Gibbs CJ, Gajdusek DC. Characterization of four new adenovirus serotypes isolated from chimpanzee tissue explants. Am J Epidemiol. 1971;94(2):166–71. doi: 10.1093/oxfordjournals.aje.a121308. [DOI] [PubMed] [Google Scholar]
- [38].Xiang Z, Li Y, Cun A, et al. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg Infect Dis. 2006;12(10):1596–9. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dudareva M, Andrews L, Gilbert SC, et al. Prevalence of serum neutralizing antibodies against chimpanzee adenovirus 63 and human adenovirus 5 in Kenyan children, in the context of vaccine vector efficacy. Vaccine. 2009;27(27):3501–4. doi: 10.1016/j.vaccine.2009.03.080. [DOI] [PubMed] [Google Scholar]
- [40].Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10(4):616–29. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pinto AR, Fitzgerald JC, Gao GP, Wilson JM, Ertl HC. Induction of CD8+ T cells to an HIV-1 antigen upon oral immunization of mice with a simian E1-deleted adenoviral vector. Vaccine. 2004;22(5-6):697–703. doi: 10.1016/j.vaccine.2003.08.029. [DOI] [PubMed] [Google Scholar]
- [42].Ertl HC. Immunological insights from genetic vaccines. Virus Res. 2005;111(1):89–92. doi: 10.1016/j.virusres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- [43].Santra S, Sun Y, Korioth-Schmitz B, et al. Heterologous prime/boost immunizations of rhesus monkeys using chimpanzee adenovirus vectors. Vaccine. 2009;27(42):5837–45. doi: 10.1016/j.vaccine.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hashimoto M, Boyer JL, Hackett NR, Wilson JM, Crystal RG. Induction of Protective Immunity to Anthrax Lethal Toxin with a Nonhuman Primate Adenovirus-Based Vaccine in the Presence of Preexisting Anti-Human Adenovirus Immunity. Infection and Immunity. 2005;73(10):6885–91. doi: 10.1128/IAI.73.10.6885-6891.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hersh J, Crystal RG, Bewig B. Modulation of gene expression after replication-deficient, recombinant adenovirus-mediated gene transfer by the product of a second adenovirus vector. Gene Ther. 1995;2(2):124–31. [PubMed] [Google Scholar]
- [46].Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70(11):7498–509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rosenfeld MA, Yoshimura K, Trapnell BC, et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68(1):143–55. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- [48].Kikuchi T, Worgall S, Singh R, Moore MA, Crystal RG. Dendritic cells genetically modified to express CD40 ligand and pulsed with antigen can initiate antigen-specific humoral immunity independent of CD4+ T cells. Nat Med. 2000;6(10):1154–9. doi: 10.1038/80498. [DOI] [PubMed] [Google Scholar]
- [49].Worgall S, Busch A, Rivara M, et al. Modification to the capsid of the adenovirus vector that enhances dendritic cell infection and transgene-specific cellular immune responses. J Virol. 2004;78:2572–80. doi: 10.1128/JVI.78.5.2572-2580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pier G. Application of vaccine technology to prevention of Pseudomonas aeruginosa infections. Expert Rev Vaccines. 2005;4(5):645–56. doi: 10.1586/14760584.4.5.645. [DOI] [PubMed] [Google Scholar]
- [51].Worgall S, Kikuchi T, Singh R, Martushova K, Lande L, Crystal RG. Protection against pulmonary infection with Pseudomonas aeruginosa following immunization with P. aeruginosa-pulsed dendritic cells. Infect Immun. 2001;69(7):4521–7. doi: 10.1128/IAI.69.7.4521-4527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Porwoll JM, Gebel HM, Rodey GE, Markham RB. In vitro response of human T cells to Pseudomonas aeruginosa. Infect Immun. 1983;40(2):670–4. doi: 10.1128/iai.40.2.670-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dunkley ML, Clancy RL, Cripps AW. A role for CD4+ T cells from orally immunized rats in enhanced clearance of Pseudomonas aeruginosa from the lung. Immunology. 1994;83(3):362–9. [PMC free article] [PubMed] [Google Scholar]
- [54].Stevenson MM, Kondratieva TK, Apt AS, Tam MF, Skamene E. In vitro and in vivo T cell responses in mice during bronchopulmonary infection with mucoid Pseudomonas aeruginosa. Clin Exp Immunol. 1995;99(1):98–105. doi: 10.1111/j.1365-2249.1995.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hartl D, Griese M, Kappler M, et al. Pulmonary T(H)2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. J Allergy Clin Immunol. 2006;117(1):204–11. doi: 10.1016/j.jaci.2005.09.023. [DOI] [PubMed] [Google Scholar]
- [56].Moser C, Jensen PO, Kobayashi O, et al. Improved outcome of chronic Pseudomonas aeruginosa lung infection is associated with induction of a Th1-dominated cytokine response. Clin Exp Immunol. 2002;127(2):206–13. doi: 10.1046/j.1365-2249.2002.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cohen CJ, Xiang ZQ, Gao GP, Ertl HCJ, Wilson JM, Bergelson JM. Chimpanzee adenovirus CV-68 adapted as a gene delivery vector interacts with the coxsackievirus and adenovirus receptor. J Gen Virol. 2002;83(1):151–5. doi: 10.1099/0022-1317-83-1-151. [DOI] [PubMed] [Google Scholar]
- [58].Varnavski AN, Schlienger K, Bergelson JM, Gao GP, Wilson JM. Efficient transduction of human monocyte-derived dendritic cells by chimpanzee-derived adenoviral vector. Hum Gene Ther. 2003 Apr 10;14(6):533–44. doi: 10.1089/104303403764539323. 2003;14:533-44. [DOI] [PubMed] [Google Scholar]
- [59].Kyd JM, Foxwell AR, Cripps AW. Mucosal immunity in the lung and upper airway. Vaccine. 2001;19(17-19):2527–33. doi: 10.1016/s0264-410x(00)00484-9. [DOI] [PubMed] [Google Scholar]
- [60].Lavelle EC. Generation of improved mucosal vaccines by induction of innate immunity. Cell Mol Life Sci. 2005;62(23):2750–70. doi: 10.1007/s00018-005-5290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Twigg HL., III Humoral Immune Defense (Antibodies): Recent Advances. Proc Am Thorac Soc. 2005;2(5):417–21. doi: 10.1513/pats.200508-089JS. [DOI] [PubMed] [Google Scholar]
- [62].Pilette C, Ouadrhiri Y, Godding V, Vaerman JP, Sibille Y. Lung mucosal immunity: immunoglobulin-A revisited. Eur Respir J. 2001;18(3):571–88. doi: 10.1183/09031936.01.00228801. [DOI] [PubMed] [Google Scholar]
- [63].Mestecky J, Russell MW, Elson CO. Perspectives on mucosal vaccines: is mucosal tolerance a barrier? J Immunol. 2007;179(9):5633–8. doi: 10.4049/jimmunol.179.9.5633. [DOI] [PubMed] [Google Scholar]
- [64].Holmgren J, Harandi AM, Czerkinsky C. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Expert Rev Vaccines. 2003;2:205–17. doi: 10.1586/14760584.2.2.205. [DOI] [PubMed] [Google Scholar]
- [65].Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(4 Suppl):S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- [66].Vajdy M, Srivastava I, Polo J, Donnelly J, O’Hagan D, Singh M. Mucosal adjuvants and delivery systems for protein-, DNA- and RNA-based vaccines. Immunol Cell Biol. 2004;82(6):617–27. doi: 10.1111/j.1440-1711.2004.01288.x. [DOI] [PubMed] [Google Scholar]
- [67].Park KS, Lee J, Ahn SS, et al. Mucosal immunity induced by adenovirus-based H5N1 HPAI vaccine confers protection against a lethal H5N2 avian influenza virus challenge. Virology. 2009;395(2):182–9. doi: 10.1016/j.virol.2009.09.018. [DOI] [PubMed] [Google Scholar]
- [68].Lemiale F, Kong Wp, Akyurek LM, et al. Enhanced Mucosal Immunoglobulin A Response of Intranasal Adenoviral Vector Human Immunodeficiency Virus Vaccine and Localization in the Central Nervous System. J Virol. 2003;77(18):10078–87. doi: 10.1128/JVI.77.18.10078-10087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Baig J, Levy DB, McKay PF, et al. Elicitation of Simian Immunodeficiency Virus-Specific Cytotoxic T Lymphocytes in Mucosal Compartments of Rhesus Monkeys by Systemic Vaccination. J Virol. 2002;76(22):11484–90. doi: 10.1128/JVI.76.22.11484-11490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gao W, Soloff AC, Lu X, et al. Protection of Mice and Poultry from Lethal H5N1 Avian Influenza Virus through Adenovirus-Based Immunization. J Virol. 2006;80(4):1959–64. doi: 10.1128/JVI.80.4.1959-1964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hoelscher MA, Garg S, Bangari DS, et al. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet. 2006;367(9509):475–81. doi: 10.1016/S0140-6736(06)68076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hoelscher MA, Jayashankar L, Garg S, et al. New Pre-pandemic Influenza Vaccines: An Egg- and Adjuvant-independent Human Adenoviral Vector Strategy Induces Long-lasting Protective Immune Responses in Mice. Clin Pharmacol Ther. 2007;82(6):665–71. doi: 10.1038/sj.clpt.6100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Croyle MA, Patel A, Tran KN, et al. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice. PLoS One. 2008;3(10):e3548. doi: 10.1371/journal.pone.0003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kim S, Jang JE, Yu JR, Chang J. Single mucosal immunization of recombinant adenovirus-based vaccine expressing F1 protein fragment induces protective mucosal immunity against respiratory syncytial virus infection. Vaccine. 2010;28(22):3801–8. doi: 10.1016/j.vaccine.2010.03.032. [DOI] [PubMed] [Google Scholar]
- [75].Guo L, Wang J, Zhou H, et al. Intranasal administration of a recombinant adenovirus expressing the norovirus capsid protein stimulates specific humoral, mucosal, and cellular immune responses in mice. Vaccine. 2008;26(4):460–8. doi: 10.1016/j.vaccine.2007.11.039. [DOI] [PubMed] [Google Scholar]
- [76].Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1(1):11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.