SUMMARY
White adipose tissue (WAT) dysfunction plays a key role in the pathogenesis of type 2 diabetes (DM2). Unrestrained WAT lipolysis results in increased fatty acid release leading to insulin resistance and lipotoxicity, while impaired de novo lipogenesis in WAT decreases the synthesis of insulin sensitizing fatty acid species like palmitoleate. Here we show that insulin infused into the mediobasal hypothalamus (MBH) of Sprague Dawley rats increases WAT lipogenic protein expression, and inactivates hormone sensitive lipase (Hsl) and suppresses lipolysis. Conversely, mice that lack the neuronal insulin receptor exhibit unrestrained lipolysis and decreased de novo lipogenesis in WAT. Thus, brain and in particular hypothalamic insulin action play a pivotal role in WAT functionality.
Introduction
Adipose tissue functionality plays a critical role in normal glucose and lipid homeostasis. One of the major functions of WAT is the release of non–esterified fatty acids (NEFA) into the circulation during fasting and other energy demanding states such as exercise. Unrestrained lipolysis, which leads to increased circulating fatty acids in the absorptive state has been linked to muscle (Bergman and Ader, 2000; Boden, 2006) and liver (Boden et al., 1994) insulin resistance as well as hepatic steatosis (Ginsberg et al., 2006). Total fatty acid flux increases with adiposity, consistent with unrestrained lipolysis (Mittendorfer et al., 2009). Recently, it has been demonstrated that the insulin sensitizing fatty acid palmitoleate, termed a lipokine, is mainly produced in WAT via de novo lipogenesis (Cao et al., 2008). In obese humans there is evidence for reduced WAT de novo lipogenesis (Diraison et al., 2002; Roberts et al., 2009), which further supports the concept that WAT represents a key link between obesity and the insulin resistant state. However, the pathogenesis of adipose tissue dysfunction is still poorly understood.
Humans with insulin receptor mutations exhibit severe lipodystrophy and elevated circulating fatty acids which highlights the pivotal role that insulin signaling plays in maintenance of WAT functionality (Hegele, 2003). Insulin is considered the major anti–lipolytic hormone. Its anti–lipolytic effects are thought to be exclusively mediated through insulin receptors expressed on adipocytes (Degerman et al., 2003). Cyclic–AMP (cAMP) signaling represents the major pro–lipolytic pathway in WAT, which is chiefly regulated by the sympathetic nervous system (SNS). However, it is presently unknown whether insulin regulates WAT lipolysis by inhibiting sympathetic outflow through brain effects, and if so, in which anatomical brain structure. The proposition that insulin action in the brain could regulate WAT lipolysis is supported by the finding that deletion of the murine insulin receptors in both the brain and periphery results in severe lipodystrophy, yet deletion of insulin receptors in peripheral tissues (including WAT) alone but not the brain only leads to mild changes in adiposity (Koch et al., 2008). Therefore, we hypothesized that insulin acts in the brain to suppress lipolysis and induce lipogenesis maintaining adipose functionality.
Results
To test whether brain and specifically hypothalamic insulin signaling regulates WAT lipolysis and lipogenesis, we raised insulin levels locally in the brain by infusing insulin directly into the third ventricle (ICV) or the MBH of male Sprague Dawley (SD) rats via stereotactic cannulae. To first delineate insulin signaling in specific hypothalamic nuclei within the MBH we performed acute insulin signaling studies in ICV and MBH insulin infused rats and analyzed punch biopsies of the arcuate nucleus (ARC) and the venteromedial hypothalamus (VMH) by Western blot at doses that have been commonly used by others (Carvalheira et al., 2003; Rahmouni et al., 2004; Roman et al., 2005). Brain insulin infusion increased insulin receptor phosphorylation and activated downstream targets such as Akt, glycogen synthase kinase 3 (Gsk 3) and extracellular-signal regulated kinase (ERK) 1/2 predominantly in the ARC compared to the VMH (Figs. S1A and B). Next, we infused insulin ICV or intraparenchymally into the MBH for a 6 hr time period to study the role of brain insulin in the regulation of lipolysis in vivo. To reduce the likelihood of pharmacological effects of the insulin doses administered, we choose a dose of insulin that is more than 15,000–fold lower than those commonly used for ICV insulin infusions (Air et al., 2002; Brief and Davis, 1984; Rahmouni et al., 2004). Since the intracerebral infusion of hormones can alter pancreatic insulin secretion and circulating glucose levels, we subjected the animals to pancreatic clamps (protocol depicted in Fig. 1A) to maintain euglycemia during the brain infusion of insulin or artificial cerebrospinal fluid (vehicle) (Fig. S1C). To compare the effects of isolated brain insulin signaling with the effects of systemic insulin (which includes the direct effects of insulin on adipocytes) we kept plasma insulin either at baseline levels (1 mU · kg−1 · min−1) or induced hyperinsulinemia (3 mU · kg−1 · min−1) to mimic the fed state (Fig. 1F). As glucose metabolism and lipolysis are regulated by insulin, both were assessed simultaneously by employing tracer dilution techniques to determine glucose and glycerol fluxes utilizing [3H–3]–glucose and [2H–5]–glycerol, respectively.
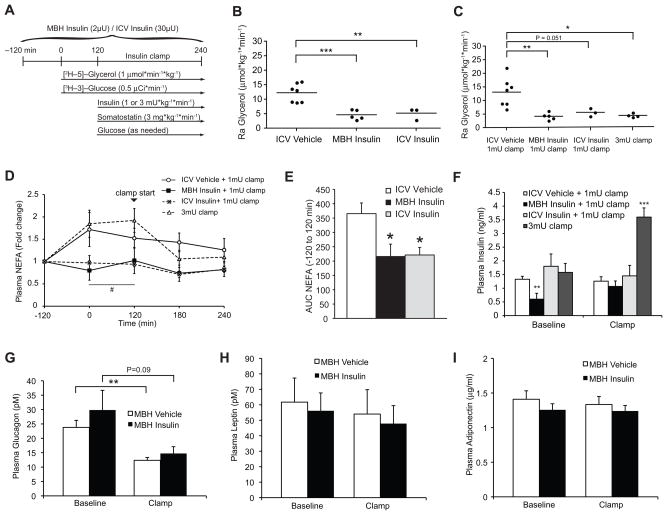
Figure 1. Brain insulin suppresses whole body lipolysis.
(A) Experimental protocol of the euglycemic clamp studies of SD rats. ICV or MBH insulin infusions were performed during basal insulin clamps (1 mU · kg−1 · min−1) and compared to rats subjected to hyperinsulinemic clamps (3 mU · kg−1 · min−1), while glycerol and glucose fluxes were determined through tracer dilution techniques. (B, C) Ra glycerol during basal (B) and clamped (C) conditions (n ≥ 3 per group). (D) Change of plasma NEFA levels compared to baseline during the 6 hr infusion protocol. Arrowhead marks the start of the clamp at time point 120 min (n ≥ 4 per group). (E) AUC of Fig. 1D comparing vehicle to ICV and MBH insulin infused rats prior to the start of the clamp study (time point -120 to 120 min, n ≥ 4 per group). (F) Plasma insulin levels during baseline (time point −120 to 120 min pre-clamp period) and the clamp (120 to 240 min, n ≥ 6 per group). (G, H, I) Plasma glucagon, leptin and adiponectin levels of MBH vehicle and insulin infused rats at baseline and the clamp (n ≥ 6). All error bars are s.e.m.; * P < 0.05, ** P < 0.01, *** P < 0.001 versus vehicle + 1 mU · kg−1 · min−1 clamp group if not otherwise indicated. # P < 0.05 versus vehicle + 3 mU· kg−1 · min−1 clamp group. (See also Fig. S1)
Brain insulin suppresses whole body lipolysis
Both ICV and MBH insulin administration markedly suppressed the rate of appearance (Ra) of glycerol under basal and clamped conditions indicating that brain insulin, and more specifically MBH insulin signaling, suppresses lipolysis (Figs. 1B and C). Systemic glucose and insulin levels were not different between ICV vehicle, ICV insulin and MBH insulin infused rats during a 1 mU · kg−1 · min−1 basal pancreatic clamp, while insulin levels were raised about three fold in the 3 mU · kg−1 · min−1 hyperinsulinemic clamp group (Figs. S1C and 1F). Thus, brain insulin infusion inhibited lipolysis independent of increases in peripheral insulin levels. Hyperinsulinemia induced by a 3 mU · kg−1 · min−1 clamp decreased the Ra glycerol by about 65% compared to a 1 mU · kg−1 · min−1 clamp in vehicle infused animals (Fig. 1C). Thus, at the doses administered, brain insulin infusion inhibited lipolysis to a similar extent as that achieved with peripheral hyperinsulinemia. In a separate series of studies we repeated MBH insulin infusions and compared this to MBH vehicle infused animals. Again, MBH insulin markedly suppressed Ra glycerol compared to MBH vehicle infused rats independent of circulating glucose and insulin levels (Figs. S2A, D and E).
These dynamic changes in the rates of whole body lipolysis were reflected by changes in static measurements of plasma parameters of fatty acid metabolism, albeit to a lesser degree. NEFA levels trended to increase during the basal clamp period in vehicle infused animals, which is due to the transitioning of the animal into the fasting state and the flushing of catheters with saline and heparin, a lipoprotein lipase activator, commonly used to prevent clotting of the vascular access. This rise in plasma NEFA levels was prevented by both MBH and ICV insulin administration (Fig. 1D), which was also reflected in a significantly lower AUC (Fig. 1E). Of note, the effects of centrally administered insulin closely mimicked the effects of systemic insulin infusion in humans, where heparin-induced increase in plasma NEFAs is completely blunted by insulin treatment (Chaudhuri et al., 2007). Plasma glycerol and triglyceride levels trended in the same direction, although this failed to reach statistical significance (Figs. S1D and E). As expected, due to the administration of somatostatin, glucagon levels decreased during the clamp, but neither glucagon nor the levels of the adipokines leptin and adiponectin were different between groups. (Figs. 1G–I).
Lipolytic flux tightly correlates with hepatic glucose production (GP)
Lipolytic flux can regulate hepatic GP potentially through two mechanisms (Mittelman and Bergman, 2000; Rebrin et al., 1996): 1) by providing the liver with the gluconeogenic precursor glycerol and 2) by enabling the synthesis of high energy NADH substrates required during gluconeogenesis through β–oxidation of NEFAs (Hers and Hue, 1983). Therefore, we simultaneously assessed glucose fluxes in the same studies to uncover a potential interdependence. MBH insulin infused rats required higher glucose infusion rates (GIR) compared to vehicle infused rats during the clamp (Figs. 2A and S2F) and suppressed hepatic GP to a similar extent as rats treated with systemic hyperinsulinemia (Figs. 2C, D and S2B), which is consistent with prior reports (Pocai et al., 2005). Notably, despite the ability of MBH insulin to suppress hepatic GP during the clamped period, circulating glucose levels (Fig. S1C and S2D) and hepatic GP (Fig. 2B) did not change during the basal period where MBH insulin was infused, but no IV somatostatin. Glucose disposal into peripheral tissues, as assessed through the use of [3H–3]–glucose tracer dilution technique, was not altered by central insulin infusion during basal clamps (Figs. 2E and S2C). As expected, systemic hyperinsulinemia increased glucose utilization in peripheral tissues like muscle and WAT, which is mainly a function of the direct effects of insulin on both tissues (Fig. 2E). Of note, the Ra glycerol tightly correlates with hepatic GP during the 1mU clamps (Fig. 2F), while neither of these parameters correlated with circulating insulin levels (Figs. S2H and I). Since we controlled glucose levels through euglycemic clamps we can rule out that hepatic glucose production drives WAT lipolysis in these studies. However, the tight correlation between lipolytic flux and hepatic GP during the MBH insulin infusion as well as the fact that MBH insulin did not change hepatic gluconeogenic gene expression of Pck1 and G6pc (Fig. S2G) suggests that one of the mechanisms through which hypothalamic insulin regulates hepatic GP is by controlling lipolytic flux from WAT.
Figure 2. MBH Insulin suppresses hepatic GP, which correlates with lipolytic flux.
(A) GIR required to maintain euglycemia. Right, AUC of line graph on the right (n ≥ 6 per group). (B) Baseline hepatic GP (n ≥ 6 per group). (C) Clamp hepatic GP (n ≥ 6 per group). (D) Percent suppression of GP during the clamp from baseline (n ≥ 6 per group). (E) Rate of glucose disposal during the clamp (n ≥ 6 per group). (F) Correlation between Ra glycerol and hepatic GP during the 1mU clamp (n = 11). All error bars are s.e.m.; * P < 0.05, ** P < 0.01, *** P < 0.001 versus vehicle + 1 mU · kg−1 · min−1 clamp group. (See also Fig. S2)
MBH insulin decreases systemic lipolysis by decreasing Hsl and Atgl activation in WAT by suppressing sympathetic outflow
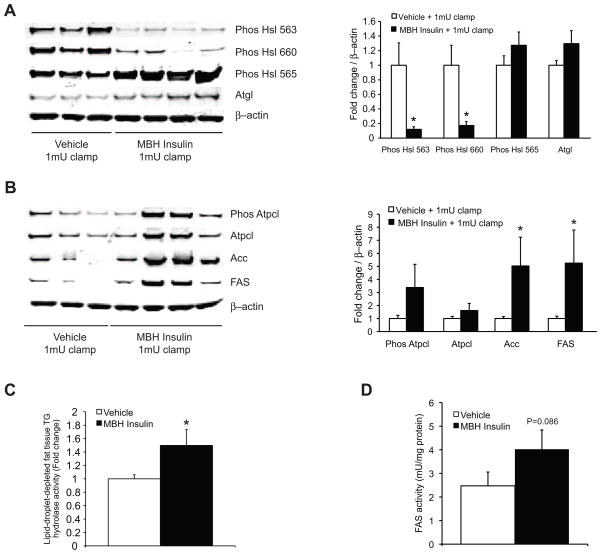
To delineate the molecular mechanisms through which hypothalamic insulin regulates lipolysis, we analyzed the expression and activation state of lipolytic proteins such as hormone sensitive lipase (Hsl, encoded by Lipe). Hsl hydrolyzes diacylglycerols to monoacylglycerols and serves as a marker for sympathetic nervous system outflow to WAT (Bartness et al., 2009; Buettner et al., 2008). Consistent with the glycerol flux data we found that central insulin suppressed the activation state of Hsl (Fig. 3A). Protein kinase A (PKA) activates Hsl through phosphorylation of Ser 563 and 660 (Anthonsen et al., 1998). Central insulin decreased phosphorylation at both these sites, while phosphorylation of Ser 565, which inactivates Hsl (Anthonsen et al., 1998), trended to be increased (Fig. 3A). Hsl is phosphorylated at Ser 565 by AMP–activated protein kinase (Ampk) (Anthonsen et al., 1998; Garton and Yeaman, 1990), which has been shown to inhibit adipocyte lipolysis (Anthony et al., 2009). Indeed, phospho–Ampk α (Thr 172) levels, an indicator of Ampk activation, was increased in WAT from MBH insulin infused animals (Fig. S3A). Next, we assessed triglyceride hydrolase activity in lipid–droplet–depleted adipose tissue homogenates harvested from the same MBH insulin and vehicle infused rats. The activation of Hsl by PKA leads to its translocation from the cytosol to the surface of the lipid droplet (Egan et al., 1992; Granneman et al., 2007). As total Hsl levels in total cell extracts were not different between MBH insulin and vehicle infused rats (data not shown), activation of Hsl should lead to its cytosolic depletion. Since MBH insulin infusion suppressed Hsl phosphorylation, we predicted higher cytosolic Hsl activity compared to vehicle infused controls after lipid droplet removal, which indeed was the case (Fig. 3C). Thus, MBH insulin suppresses Hsl activity in WAT by inhibiting its phosphorylation and thereby likely its translocation to lipid droplets.
Figure 3. MBH insulin suppresses Hsl activation and increases lipogenic protein expression.
(A, B) MBH insulin suppresses lipolysis and induces lipogenesis in WAT. Left, representative Western blot analyses of epidydimal fat pads from clamped animals. Right, quantification of the Western blot analyses (n ≥ 5 per group). (C) Lipid-droplet-depleted cytosolic triglyceride hydrolase activity (n ≥ 8 per group, one-tailed t-test was applied). (D) FAS activity measured in perirenal fat depots (n = 4 per group, one-tailed t-test was applied) Top, quantifications; Bottom, Western blot analyses (n ≥ 3 per group). All error bars are s.e.m.; * P < 0.05 vehicle group. (See also Fig. S3)
Adipose tissue triglyceride lipase (Atgl, encoded by Pnpla2) hydrolyzes triacylglycerols to diacylglycerols and, together with Hsl, accounts for over 90% of the WAT acyl–hydrolase activity (Zimmermann et al., 2004). MBH insulin infusion did not alter Atgl protein expression in WAT (Fig. 3A). However, Atgl activity is not solely determined by total protein levels, but also regulated through cAMP dependent and independent posttranslational modification. Atgl activity is increased upon β–adrenergic stimulation via a posttranslational mechanism (Haemmerle et al., 2006). This activation critically depends on the phosphorylation of the lipid droplet associated protein perilipin, which involves the dissociation of α/β hydrolase domain containing protein 5 (ABHD5; also known as comparative gene identification-58 (CGI-58)) from perilipin and its subsequent activation of Atgl (Granneman et al., 2007; Miyoshi et al., 2007; Zimmermann et al., 2009). Phospho–perilipin as assessed by a PKA substrate motif antibody trended (P = 0.053) to be suppressed in WAT after MBH insulin infusion (Fig. S3B). These results suggest that in addition to suppressing Hsl activity, MBH insulin reduces Atgl activation by decreasing perilipin phosphorylation and thereby favoring CGI-58 retention. Thus, brain insulin regulates Hsl activity and likely Atgl activation via PKA.
Insulin antagonizes cAMP signaling through activation of phosphodiesterase 3B (PDE3B) in an Akt dependent manner (Degerman et al., 2003). It has been shown that hypothalamic leptin signaling can increase insulin signal transduction as assessed by Akt phosphorylation in peripheral organs like the liver (German et al., 2009). Both leptin and insulin regulate autonomic outflow within the MBH (Buettner and Camacho, 2008), which could influence insulin signaling through crosstalk from cAMP signaling, for example. Although we controlled circulating insulin levels during the clamp studies, it is still conceivable that hypothalamic insulin could enhance insulin signaling in WAT. Thus, we assessed whether MBH insulin alters Akt activation in WAT as assessed by Akt phosphorylation at Ser473. We found that MBH insulin did not change Akt phosphorylation in WAT (Fig. S3C), indicating that the effects of brain insulin on WAT metabolism occur independent of changes in peripheral insulin signaling.
Brain insulin induces lipogenesis in WAT
Lipogenesis in WAT has recently been shown to regulate whole body insulin sensitivity by generating palmitoleate that improves insulin sensitivity (Cao et al., 2008). Furthermore, we have found that lipogenesis in WAT is upregulated in metabolically beneficial states even though adiposity is decreased. For example, lipogenesis is elevated in young versus aged rats (Fig. S3D), calorically restricted (CR) aged rats versus ad libitum (AL) fed aged rats (Fig. S3D) and exercised versus sedentary mice (data not shown). Since hyperinsulinemia induces lipogenesis in WAT (Assimacopoulos-Jeannet et al., 1995), we asked if brain insulin signaling is sufficient to increases lipogenesis in WAT in the absence of peripheral hyperinsulinemia and assessed lipogenic protein expression in the same tissue lysates that had been used for the Hsl Western blot analysis (Fig. 3A). MBH insulin increased the expression of the key lipogenic proteins fatty acid synthase (FAS, encoded by Fasn) and Acetyl–CoA carboxylase (Acc, encoded by Acaca) (Fig. 3B). Adipose tissue FAS enzyme activity of MBH insulin infused rats was increased by 60% just below statistical significance (P = 0.086) (Fig. 3D). Protein expression of ATP–citrate lyase (Atpcl) and its phosphorylation, a marker of the activated state, trended to be induced (Fig. 3B). These findings implicate that in vivo a) WAT lipogenesis and lipolysis are inversely regulated by hypothalamic insulin and that b) MBH insulin seems to oppose the acute actions of MBH leptin. We have shown in a prior study that MBH leptin induces lipolysis and inhibits lipogenesis by increasing sympathetic outflow to WAT (Buettner et al., 2008).
MBH insulin dampens SNS activity
Therefore, to delineate whether the effects of MBH insulin on WAT metabolism are mediated via the autonomic nervous system, we performed surgical denervation, as well as pharmacological sympathectomy, of rat epigonadal fat pads using 6–hydroxydopamine (6–OHDA). Surgical denervation and pharmacological sympathectomy suppressed basal WAT Hsl activation, a marker for sympathetic outflow to WAT (Bartness et al., 2009; Buettner et al., 2008). This effect was replicated with MBH insulin infusion (Fig. 4A). Although this experiment is not an ultimate proof of a linear pathway, these data suggest that hypothalamic insulin regulates Hsl activation via suppression of SNS outflow to WAT.
Figure 4. Acute inhibition of endogenous MBH insulin signaling is sufficient to unrestrain lipolysis in WAT, which depends on sympathetic innervation.
(A) MBH insulin suppresses Hsl activation to a similar degree as surgical denervation or selective pharmacological sympathectomy of the epidydimal fat pad, indicating that insulin suppresses lipolysis through a reduction of sympathetic nervous system outflow to WAT. (B) Validation of the insulin receptor antagonist in vivo. S961 was co–infused with insulin in equimolar amounts into the 3rd ventricle of male SD rats. S961 blocked insulin induced MBH insulin receptor phosphorylation (n = 3 per group) (C) Schematic study outline. Male SD rats were infused with glycerol tracer and received either vehicle S961 for 4 hrs into the MBH. (D) Ra glycerol of MBH vehicle and S961 infused rats as per protocol depicted in Fig. 4C (n ≥ 4 per group) (E) Ra glycerol of sham and pharmacologically sympathectomized rats that were either infused with MBH S961 or vehicle. A similar protocol as depicted in Fig. 5C was used (n ≥ 6 per group). (F) WAT NE levels in sham versus 6–OHDA denervated rats (n = 4 per group). All error bars are s.e.m.; * P < 0.05, ** P < 0.01 versus vehicle group. ### P < 0.001 versus ICV vehicle and ICV insulin + S961 group (See also Fig. S4).
The acute inhibition of brain insulin signaling is sufficient to unrestrain lipolysis
We next asked if the acute induction of brain insulin resistance is sufficient to alter lipolysis. S961 is a recently identified peptide inhibitor that blocks insulin binding to the insulin receptor, while not inhibiting insulin or insulin–like growth factor (IGF) 1 binding to the IGF receptor (Schaffer et al., 2008) or IGF 1 binding to the insulin receptor. First, we validated that S961 blocked insulin induced insulin receptor phosphorylation in the MBH after ICV infusion (Figs. 4B), which also obliterated downstream signaling (Fig. S4A). To assess if the inhibition of endogenous insulin in the MBH is sufficient to unrestrain lipolysis, we infused either the insulin receptor antagonist S961 or vehicle into male SD rats for 4 hrs, while simultaneously infusing [2H–5]–glycerol tracer systemically (Fig. 4C). During the infusion study systemic glucose and insulin levels were similar between groups (Figs. S4B and C). Indeed, inhibiting endogenous insulin signaling in the MBH doubled the Ra glycerol (Fig. 4D) suggesting that hypothalamic insulin resistance can contribute to the unrestrained lipolysis seen in the insulin resistant state. Since increased glucose production is a major component of widespread SNS activity (Gellhorn, 1954) and glucose levels throughout the entire infusion experiment were not different in S961 versus vehicle infused rats, it is unlikely that S961 is an unspecific activator of the SNS.
The denervation studies shown in Fig. 4A suggested that MBH insulin reduces WAT lipolysis via the autonomic nervous system, since MBH insulin suppressed Hsl phosphorylation comparable to chemical sympathectomy. Here we further tested this concept by asking if the unrestrained lipolysis induced by the acute inhibition of insulin signaling in the MBH depended on intact sympathetic innervations. To this end we induced systemic sympathectomy in male SD rats by injecting 6–OHDA. After a short recovery period, we subjected the animals to the same infusion studies depicted in Fig. 4C in order to test if MBH S961 would still be able to increase Ra glycerol. As expected systemic sympathectomy obliterated the pro–lipolytic effects of the insulin antagonist (Fig 4E). Importantly, WAT norepinephrine (NE) content was markedly reduced verifying the sympathectomy (Fig. 4F). Ra glycerol trended to be lower in the denervated animals compared to sham controls, suggesting that sympathectomy per se lowers pro-lipolytic input to WAT. Thus, these results provide further support for the concept that MBH insulin signaling controls WAT lipolysis by dampening sympathetic nervous system activity.
Genetic deletion of the neuronal insulin receptor increases lipolysis in mice and impairs the metabolic switch from fasting to refeeding
To test if neuronal insulin signaling mediates the role of brain insulin in regulating lipolysis, we studied glycerol fluxes in neuronal insulin receptor knock–out (NIRKO) and littermate lox-lox control mice (Fig. S5A) during hyperinsulinemic euglycemic clamp studies (Figs. S5B and C, protocol depicted in Fig. 5A). NIRKO mice have been reported to be susceptible to diet–induced obesity, although their body weight is not altered when they are young and fed a standard chow diet. Furthermore, they develop mild insulin resistance when they get older than 6 months (Bruning et al., 2000). We find that four-month-old NIRKO mice required equal GIR during a hyperinsulinemic clamp indicating no or only mild impairment of glucose homeostasis in young NIRKO mice (Fig. S5D). In the fasted state NIRKO mice exhibited a two–fold higher Ra glycerol compared to lox-lox littermate controls (Fig. 5B) although insulin levels were not different (Tab. S1), demonstrating that neuronal insulin signaling controls basal lipolytic rate. Induction of hyperinsulinemia during a 4 mU· kg−1 · min−1 euglycemic clamp raised insulin levels equally in both groups (Fig. S5B) and while glycerol fluxes were suppressed in both NIRKO and control mice (presumably through the direct actions of insulin on adipocytes), the Ra glycerol remained higher in the NIRKO mice (Fig. 5B), while glucose and glucagon levels were not different between groups (Figs. S5C and Tab. S1). These data indicate that lipolytic flux is exquisitely sensitive to brain insulin action, more so than hepatic glucose fluxes. Changes in levels of plasma NEFA and glycerol did not show significant differences (Fig. S5E). Since unrestrained lipolysis is expected to increase ketogenic substrate flux to the liver, we further assessed whether NIRKO mice display increased ketogenesis. Indeed, NIRKO mice challenged with a 16 hr fast had elevated β–hydroxybutyrate levels compared to control animals (Fig. 5C), supporting the concept that brain insulin signaling can regulate both lipolysis and ketogenesis. Plasma NEFA and glycerol levels were not different between groups following the 16 hr fast (Figs. S5F and G). The transition from fasting to re–feeding is a physiological challenge that requires adipose tissue to readily switch from NEFA release to NEFA retention. To assess the role of neuronal insulin receptor signaling in this metabolic adaptation, we fasted NIRKO and control littermates overnight and then re–fed them during the onset of their feeding cycle (Protocol depicted in Fig. 5D). This allowed us to assess the suppression of NEFA release after re–feeding. While fasting NEFAs were not different between NIRKO and control mice, the suppression of NEFAs following a 2 hr re-feeding period in NIRKO mice was significantly lower (Fig. 5F), despite higher peripheral insulin levels after feeding (Fig. 5E). Insulin levels of control mice were unchanged after feeding, which is consistent with prior reports that showed that lean insulin–sensitive mice do not exhibit a change in insulin levels after oral glucose administration (Andrikopoulos et al., 2008). Body weight and food intake during re–feeding were not different between groups (Figs. G and H). These data indicate that the neuronal insulin receptor regulates lipolysis in WAT during the fasting to feeding transition.
Figure 5. Genetic disruption of neuronal insulin signaling increases whole body lipolytic flux and impairs the switch from fasting to re–feeding.
(A) Schematic representation of the clamp studies in NIRKO mice. Following a 16 hr fast, NIRKO and littermate control mice were subjected to a 110 min 4 mU · kg−1 · min−1 hyperinsulinemic euglycemic clamp study. (B) Baseline and clamp glycerol fluxes as assessed by [2H–5]–glycerol tracer infusion are increased in the NIRKO mice (n ≥ 5 per group). (C) Plasma β–hydroxybutyrate levels are elevated in the NIRKO mice following a 16 hr fasting challenge (n = 9 per group). (D) Depiction of the fasting re–feeding protocol (E) Plasma NEFA levels before and after re–feeding. Insert depicts % suppression of plasma NEFA levels after food intake (n ≥ 7 per group) (F) Plasma insulin before and after re feeding (n ≥ 8 per group) (G) Food intake during re-feeding (n ≥ 9 per group) (H) Bodyweights after fasting (n ≥ 9 per group). All error bars are s.e.m.; * P < 0.05 versus control mice. (See also Fig. S5 and Tab. S1)
Neuronal insulin receptor signaling regulates de novo lipogenesis in WAT
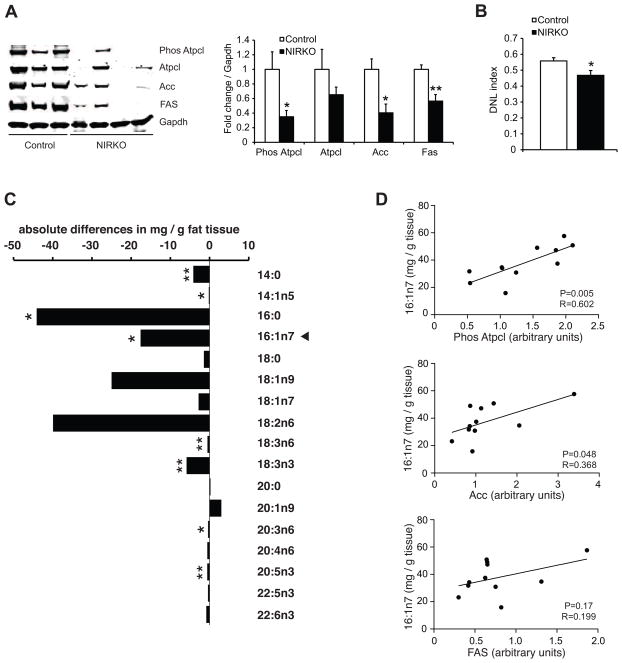
Consistent with our findings from the rat studies where MBH insulin increased lipogenic protein expression, loss of brain insulin signaling in the NIRKO mice decreased Acc and FAS protein expression as well as activated Phospho–Atpcl in WAT under clamped and overnight fasted conditions (Figs. 6A and S5H), suggesting that the lifelong absence of brain insulin signaling disrupts WAT lipogenesis. To test whether the decreased WAT lipogenic enzyme expression in the NIRKO mice translated into alterations in WAT fatty acid composition, we generated fatty acid lipid profiles from epidydimal fat pads of fasted control and NIRKO mice. We found a marked suppression of several fatty acid species (14:0, 14:1n5, 16:0, 16:1n7) associated with de novo lipogenesis in the epidydimal fat depots of the NIRKO mice (Fig. 6C), as well as a lower de novo lipogenesis index (Fig. 6B), which is used as a marker for lipogenesis (Chong et al., 2008; Hudgins et al., 1996). However, oleate (18:1n9), the most abundant fatty acid in WAT, was not significantly changed, suggesting that triglyceride content per se was not different between groups. Notably, the insulin sensitizing lipokine palmitoleate (16:1n7), which is produced during de novo lipogenesis in WAT (Cao et al., 2008), was decreased in the NIRKO mice (Fig. 6C). Furthermore, palmitoleate levels in WAT correlated closely with the expression of the lipogenic enzyme Acc and the activation state of Atpcl in the fasted state, further supporting the notion that brain insulin regulates palmitoleate synthesis through regulation of WAT lipogenesis (Fig. 6D). These findings assign a critical role to neuronal insulin signaling in maintaining WAT function. We speculate that hypothalamic insulin resistance could contribute to the decreased lipogenic protein expression in WAT observed in obesity. This in turn leads to decreased production of the insulin sensitizing fatty acid palmitoleate, further worsening systemic insulin resistance.
Figure 6. Loss of neuronal insulin receptor signaling impairs de novo lipogenesis in WAT.
(A) Left, representative Western blot analyses of lipogenic protein expression and the activation state of Atpcl in epidydimal fat pads obtained at the end of the clamp study. Right, quantification of Western blot data compared to littermate control mice (n ≥ 5 per group). (B) De novo lipogenesis (DNL) index calculated using the ratio of palmitic (16:0) to linoleic acid (18:2n6) (n ≥ 5 per group). (C) Differences of WAT fatty acid species of overnight fasted NIRKO and control mice. Arrowhead marks palmitoleate. (n = 6 per group) (D) Correlation between the expression of lipogenic proteins and palmitoleate levels (n = 11). All error bars are s.e.m.; * P < 0.05, ** P < 0.01 versus control mice.
Discussion
WAT has emerged as a critically important organ for whole body glucose and lipid homeostasis. WAT is an important metabolic sink, clearing and storing circulating lipids, thereby protecting other organs from ectopic lipid accumulation. In addition, WAT is an important source of adipokines like leptin and adiponectin, and inflammatory mediators like tumor necrosis factor alpha and interleukin 6 that circulate and can impair insulin signaling in distant organs including the hypothalamus (Zhang et al., 2008). During fasting, WAT is the main source of NEFAs that provide energy substrates to muscle and liver. Unrestrained lipolysis during the absorptive state induces lipotoxicity and a low–grade inflammation that is commonly associated with obesity and diabetes (Boden, 2006). Lastly, de novo lipogenesis in WAT produces insulin sensitizing fatty acid species like palmitoleate (Cao et al., 2008).
Our studies establish that neuronal and in particular hypothalamic insulin action is a critical regulator of WAT metabolism. We demonstrate that brain insulin action restrains lipolysis by reducing sympathetic outflow to WAT and controls de novo lipogenesis in adipose tissue. Impaired brain and hypothalamic insulin signaling increases lipolytic flux and decreases de novo lipogenesis hampering the production of the insulin sensitizing fatty acid palmitoleate. We come to this conclusion after delineating the role of brain insulin in regulating WAT metabolism in two independent models: 1) SD rats where we either increased or inhibited brain or MBH insulin signaling and 2) mice with a lifelong disruption of neuronal insulin signaling. The molecular mechanism through which MBH insulin regulates WAT metabolism within the adipocyte comprises of an increase in lipogenic protein expression and activity and a decrease in the activation state of lipolytic enzymes like Hsl (see proposed model in Fig. 7). Whether other anti-lipolytic regulators such as, adenosine, prostaglandin E2, neuropeptide Y or lactate participate in the regulation of WAT metabolism by brain insulin remains to be determined (Ahmed et al., 2010; Jaworski et al., 2009; Lafontan and Langin, 2009)
Figure 7. Proposed model of the role of brain insulin in regulating WAT metabolism.
Insulin inhibits WAT lipolysis through both direct and indirect effects: insulin binding to the insulin receptor expressed on adipocytes results in inactivation of PDE3B leading to the degradation of cAMP (Degerman et al., 1990; Smith et al., 1991). We propose that in addition to the direct effects of insulin on adipocytes, hypothalamic insulin signaling suppresses lipolysis and induces lipogenesis indirectly by dampening SNS outflow to WAT. The reduction in lipolysis contributes to a decrease in hepatic GP by limiting the flux of the gluconeogenic precursor glycerol and NEFAs, which provide energy substrates for gluconeogenesis.
These findings that ascribe a role of brain insulin signaling in the regulation of WAT functionality have implications not only for lipid metabolism, but are also likely to be relevant in glucose homeostasis, since lipolytic flux can contribute to hepatic gluconeogenesis (Mittelman and Bergman, 2000; Rebrin et al., 1996). Indeed, our data indicate that lipolytic flux is exquisitely sensitive to brain insulin action, more so than hepatic glucose fluxes. MBH insulin infusion in rats did not alter hepatic GP even after 4 hrs, while Ra glycerol flux was already altered (Fig. 2E). Only after somatostatin infusions were started an effect on hepatic GP became apparent.
There is growing evidence that in obesity and diabetes lipogenic capacity of adipose tissue is reduced in rodents as well as humans (Diraison et al., 2002; Moraes et al., 2003; Nadler et al., 2000). The stimulatory effects of brain insulin on WAT lipogenesis plus the finding that the absence of the neuronal insulin receptor impairs the lipogenic capacity of WAT suggest that the dysregulation of WAT adipose tissue lipogenesis in obesity is at least in part a function of brain insulin resistance.
Furthermore, our findings suggest that brain insulin action has important anabolic function in WAT maintenance, which is independent of, but complements cell–autonomous effects of insulin on adipocytes. In further support of this concept, a recent study showed that the presence of the brain insulin receptor is critically important to prevent lipodystrophy in mice (Koch et al., 2008). The same study showed that chronic ICV insulin infusion in mice leads to increased fat pad mass and hypertrophy of adipocytes, which the authors ascribed to increased lipogenesis. Equally, if not more important in the control of adiposity is likely the regulation of WAT lipolysis through brain insulin signaling. We draw this conclusion from the finding that denervation of WAT leads to no change in lipogenic protein expression, but completely abrogates Hsl activation leading to increased adipose depot mass (Buettner et al., 2008). The absence of WAT renders humans (Hegele, 2003; Petersen et al., 2002) and mice (Shimomura et al., 1999) severely insulin resistant, due to the ectopic accumulation of lipids in tissues such as muscle and liver. Ectopic lipids impair insulin signaling and induce a pro–inflammatory state. In an insulin sensitive organism, WAT is metabolically flexible and able to readily switch from a fatty acid storing to a fatty acid releasing mode according to metabolic needs.
Our studies raise several questions. One is which neuronal subtype within the CNS and the MBH mediates the effects of insulin on the regulation of WAT metabolism. Several neuron specific insulin receptor KO models have been generated (Belgardt et al., 2009), although to our knowledge an analysis of lipolytic flux or WAT lipogenesis has not been undertaken as of yet. Judging from the energy balance and glucose homeostasis phenotype of these diverse models it appears that the effects of brain insulin are not explained by a single neuronal population, but rather through an intricate and as of yet poorly understood communication within a complex neuronal network where in some instances insulin signaling in one neuron type can even antagonize the effects of insulin signaling in another neuronal population (Lin et al., 2009). Thus, even if a specific neuronal KO of the insulin receptor abolishes the ability of brain insulin to regulate WAT metabolism, this may mean that a certain balance within this neuronal network is disturbed, not that this particular neuron population is the chief target and mediator of hypothalamic insulin action. Another question is whether brain insulin signaling or adipocyte insulin signaling is predominant in the regulation of WAT metabolism by systemic insulin. Our studies clearly establish that in the absence of alterations of circulating insulin levels brain insulin action plays a critical role in the regulation of WAT metabolism.
Furthermore, our findings raise the possibility that hypothalamic insulin action may either 1) regulate both lipolysis and hepatic GP via a suppression of sympathetic outflow to the liver or 2) may indirectly regulate hepatic GP by decreasing the flux of glycerol and NEFA to the liver via suppression of lipolysis in WAT.
Finally, conditions in which brain insulin signaling is compromised, such as high fat feeding (Ono et al., 2008), chronic inflammatory conditions (Zhang et al., 2008) and obesity (Posey et al., 2009; Zhang et al., 2008), are likely to dysregulate the control of lipolysis and de novo lipogenesis in WAT due to impaired insulin action in the hypothalamus. Thus, hypothalamic insulin resistance unrestrains lipolysis and reduces the lipogenic capacity in WAT, which in turn induces lipotoxicity resulting in peripheral insulin resistance and thereby perpetuating a vicious cycle.
EXPERIMENTAL PROCEDURES
Animals
Rat experiments were performed in standard chow fed (Rodent Diet 5001, LabDiet, St. Louis; MO), male SD rats (Charles River Breeding Laboratories, Wilmington, MA) housed in a temperature and light controlled facility in separate cages. Prior to the clamp studies rats, were stereotactically fit with indwelling cannulae targeting the third ventricle (ICV) or the MBH (See supplementary experimental procedures). After a one–week recovery period carotid and jugular catheters were implanted for blood sampling and infusion, respectively. Rats were allowed to recover for an additional 4 days and required to return to within 10% of their pre–surgical body weight.
NIRKO mice were generated as described elsewhere (Bruning et al., 2000; Fisher et al., 2005), housed on a 12 hr light–dark cycle and fed a standard rodent diet (Mouse diet 9F, PMI Nutrition International, St. Louis, MO). All animal protocols were approved by the IACUC of Mount Sinai School of Medicine.
Rat pancreatic clamp studies
Rat clamp experiments were performed in ten–week old, conscious, non–restrained and ad libitum fed male SD rats. An ICV (5 μl/h) or MBH (0.18 μl/h per side) infusion with either vehicle (artificial cerebrospinal fluid (aCSF) (Harvard Apparatus, Holliston, MA) or insulin (ICV 30 μU; MBH 2 μU; Humulin R, Lilly) was started and maintained for 360 min. [3-3H]-glucose and [2H-5]-glycerol tracers were infused as depicted in Fig. 1A. The insulin clamp was initiated with a primed–continuous infusion of insulin (1 mU or 3 mU · kg−1 · min−1 infusion; Humulin R, Lilly) while also infusing somatostatin (3 μg · kg−1 · min−1). We collected plasma samples before and at the end of the clamp to determine Ra glycerol, during baseline and clamped period. See supplementary experimental procedures for a more detailed description.
Local denervation experiment
Epidydimal fat pads were denervated as previously described (Buettner et al., 2008). Rats were infused for 4 hrs with either MBH insulin (1.44 μU) or aCSF (vehicle). See supplementary experimental procedures for a more detailed description.
Insulin antagonist infusion experiment
Eight–week old male SD rats were equipped with MBH cannulae and catheters. Rats were infused for 4 hrs with either aCSF (vehicle) or 0.1 pmoles of the insulin receptor antagonist S961 (a gift from Novo Nordisk; Maaloev, Denmark) (Schaffer et al., 2008) into the MBH, while also employing a [2H-5]-glycerol tracer infusion.
Systemic pharmacological sympathectomy experiment
Eight week old male SD rats were simultaneously implanted with MBH cannulae and jugular catheters during ketamin–xylazine anesthesia. After a 3-day recovery period rats were given two IV bolus injections of 6-OHDA or vehicle (0.9% saline containing 0.1% ascorbic acid) 3 days apart (50 mg/kg BW freshly prepared in 0.9% saline containing 0.1% ascorbic acid). Vehicle injected animals were pair–fed to 6-OHDA injected rats to match their bodyweights. Three days following the last IV injection rats were either infused with MBH vehicle (aCSF) or S961 (0.1 pmoles) using the protocol depicted in Fig. 4C. After 2 hrs a [2H-5]-glycerol tracer infusion was started and maintained (30 μmol · kg−1 · min−1 for 4 min bolus followed by 3 μmol · kg−1 · min−1) for 2 hrs. Blood samples for Ra glycerol analysis were taken at time point (TP) 240 min by tail vein sampling. Only rats recovered within 10% of their pre–surgical BW were used.
Mouse clamp experiments
Mouse clamp studies were performed in 16 hr fasted 4 month–old male conscious, non– restrained NIRKO and littermate lox-lox control mice that were equipped with femoral artery and right jugular vein catheters. Surgical details are described in Supplementary experimental procedures. The protocol consisted of a 100 min tracer equilibration period followed by 110 min clamp period as depicted in Fig. 5A. A 10 μmol bolus of [2H–5]–glycerol (98 atom percent excess) was given at t = −100 min followed by a continuous infusion at 4 μmol · kg−1 · min−1 over 210 min. The insulin clamp was initiated at t = 0 min with a continuous infusion of human insulin (4 mU · kg−1 · min−1 infusion; Humulin R, Lilly) lasting 110 min. Euglycemia was maintained by measuring glucose every 10 min and infusing 50% dextrose as necessary. Plasma samples were collected before and at the end of the clamp to determine Ra glycerol during baseline and clamped period, respectively. To minimize blood loss, we re–infused spun–down erythrocytes, which were re–suspended in saline.
Mouse fasting–refeeding experiments
Ten-week old Nirko and littermate control mice were fasted for 24 hrs starting at 3 pm the day before the experiment, so the re–feeding period coincided with the onset of the feeding cycle (Protocol depicted in Fig. 5D). An initial blood sample was taken from the tail vein at TP 0 min. After 90 min mice were granted ad libitum access to regular chow food for 2 hrs. The final blood sample (TP 210 min) was sampled by tail.
Analytic procedures
Prior to tissue analyzes animals were anaesthetized, killed and their tissues snap frozen in liquid nitrogen. Blood was collected in EDTA tubes and glucose measured by Freestyle Freedom glucose analyzer (Abbot, Abott Park, IL). We analyzed glucose fluxes and Ra glycerol as described elsewhere (Kang et al., 2007; Liu et al., 1998; Obici et al., 2002; Pocai et al., 2005). Plasma metabolite assays, WAT NE measurements and tracer methodology details are described in Supplementary experimental procedures.
FAS activity
FAS activity in perirenal fat was measured spectrophotometrically using a modified protocol of Nepokroeff et al. (Nepokroeff et al., 1975). Detailed description can be found in Supplementary experimental procedures.
Triglyceride hydrolase activity
Assay was performed with epidydimal fat pads as previously described (Schweiger et al., 2006). Cytosolic and lipid–droplet fraction from rat adipose tissue were obtained using a modified protocol of Schweiger et al. (Schweiger et al., 2008). Detailed description can be found in Supplementary experimental procedures.
Fatty acid analysis
Fatty acid analysis was performed as previously described (Scheja et al., 2008). Detailed description can be found in Supplementary experimental procedures.
RNA extraction and quantitative real-time RT-PCR
Detailed description, forward and reverse customized primer pairs (Invitrogen, Carlsbad, CA) are described in Supplementary experimental procedures.
Western blot analyses
Western blots were performed as previously described (Buettner et al., 2008). Detailed description and antibodies used can be found in Supplementary experimental procedures.
Statistics
All data are represented as mean ± s.e.m. Comparisons among groups were made using one–way ANOVA followed by unpaired two tailed–Student’s t–tests if not otherwise indicated. Differences were considered statistically significant at P < 0.05. For Figs. 2D, 6D, S2I and J we used Pearson correlation and a two–tailed t–test performed in Graphpad Prism 5.0b for Mac (GraphPad Software, San Diego California USA)
Supplementary Material
Acknowledgments
We would like to thank Rui Chang, Martin Fasshauer, Sameer Halani, Harsha Madulla, Mark Real and Ashlie Sewdass for excellent technical assistance, Abott for providing Freestyle glucometers and strips, Drs Andrew Greenberg for the Perilipin antibody and Lauge Schaeffer from Novo Nordisk for the insulin antagonist. We would also like to thank Drs Ronald Kahn and Jens Bruening for making available the NIRKO mice, Case Western University MMPC, which is supported by U24 DK76169, for the mass spectrometry analyses, and the Yale Center of Clinical investigations for the NE measurements, which is supported by CTSA Grant UL1 RR024139 from the National Center for Research Resources. We further thank Drs Nir Barzilai and Radhika Muzumdar for WAT from calorically restricted, aged rats and Dr Gary Schwartz for his advice and helpful discussions. This work was supported by NIH Grants DK074873, DK083568 and DK082724 to C.B., DK073683 to S.F. and a European Foundation for the Study of Diabetes grant to T.S.. C.B. is the recipient of a Junior Faculty Award from the American Diabetes Association.
ABBREVIATIONS
- 6–OHDA
6-hydroxydopamine
- Acc
acetyl–CoA carboxylase
- aCSF
artificial cerebrospinal fluid
- Ampk
AMP–activated protein kinase
- ARC
arcuate nucleus of the hypothalamus
- Atgl
adipose tissue triglyceride lipase
- Atpcl
ATP–citrate lyase
- BW
bodyweight
- cAMP
cyclic-AMP
- DM2
diabetes mellitus type 2
- ERK
extracellular-signal regulated kinase
- FAS
fatty acid synthase
- GIR
glucose infusion rates
- GP
glucose production
- Gsk
glycogen synthase kinase
- Hsl
hormone sensitive lipase
- ICV
intracerebroventricular (3rd ventricle)
- IGF
insulin–like growth factor
- MBH
mediobasal hypothalamus
- NE
norepinephrine
- NEFA
non–esterified fatty acid
- NIRKO
neuronal insulin receptor knock out
- PDE3B
phosphodiesterase 3B
- PKA
protein kinase A
- Ra
rate of appearance
- SD
Sprague Dawley
- SNS
sympathetic nervous system
- VMH
venteromedial hypothalamus
- WAT
white adipose tissue
Footnotes
The authors declare that no competing financial interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, Hanson J, Offermanns S. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 2010;11:311–319. doi: 10.1016/j.cmet.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav. 2002;72:423–429. doi: 10.1016/s0091-3057(01)00780-8. [DOI] [PubMed] [Google Scholar]
- Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295:E1323–1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- Anthony NM, Gaidhu MP, Ceddia RB. Regulation of visceral and subcutaneous adipocyte lipolysis by acute AICAR-induced AMPK activation. Obesity (Silver Spring) 2009;17:1312–1317. doi: 10.1038/oby.2008.645. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F, Brichard S, Rencurel F, Cusin I, Jeanrenaud B. In vivo effects of hyperinsulinemia on lipogenic enzymes and glucose transporter expression in rat liver and adipose tissues. Metabolism. 1995;44:228–233. doi: 10.1016/0026-0495(95)90270-8. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol. 2009 doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt BF, Okamura T, Bruning JC. Hormone and glucose signalling in POMC and AgRP neurons. J Physiol. 2009;587:5305–5314. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep. 2006;6:177–181. doi: 10.1007/s11892-006-0031-x. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brief DJ, Davis JD. Reduction of food intake and body weight by chronic intraventricular insulin infusion. Brain Res Bull. 1984;12:571–575. doi: 10.1016/0361-9230(84)90174-6. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Buettner C, Camacho RC. Hypothalamic control of hepatic glucose production and its potential role in insulin resistance. Endocrinol Metab Clin North Am. 2008;37:825–840. doi: 10.1016/j.ecl.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Buettner C, Muse ED, Cheng A, Chen L, Scherer T, Pocai A, Su K, Cheng B, Li X, Harvey-White J, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalheira JB, Ribeiro EB, Araujo EP, Guimaraes RB, Telles MM, Torsoni M, Gontijo JA, Velloso LA, Saad MJ. Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats. Diabetologia. 2003;46:1629–1640. doi: 10.1007/s00125-003-1246-x. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Janicke D, Wilson M, Ghanim H, Wilding GE, Aljada A, Dandona P. Effect of modified glucose-insulin-potassium on free fatty acids, matrix metalloproteinase, and myoglobin in ST-elevation myocardial infarction. Am J Cardiol. 2007;100:1614–1618. doi: 10.1016/j.amjcard.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Chong MF, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am J Clin Nutr. 2008;87:817–823. doi: 10.1093/ajcn/87.4.817. [DOI] [PubMed] [Google Scholar]
- Degerman E, Landström TR, Holst LS, Göransson O, Härndahl L, Ahmad F, Choi Y-H, Masciarelli S, Liu H, Manganiello V. Role for Phosphodiesterase 3B in Regulation of Lipolysis and Insulin Secretion. In: LeRoith D, Olefsky JM, Taylor SI, editors. Diabetes Mellitus: A Fundamental and Clinical Text. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 374–381. [Google Scholar]
- Degerman E, Smith CJ, Tornqvist H, Vasta V, Belfrage P, Manganiello VC. Evidence that insulin and isoprenaline activate the cGMP-inhibited low-Km cAMP phosphodiesterase in rat fat cells by phosphorylation. Proc Natl Acad Sci U S A. 1990;87:533–537. doi: 10.1073/pnas.87.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2002;282:E46–51. doi: 10.1152/ajpendo.2002.282.1.E46. [DOI] [PubMed] [Google Scholar]
- Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos MC, Jr, Londos C. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci U S A. 1992;89:8537–8541. doi: 10.1073/pnas.89.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SJ, Bruning JC, Lannon S, Kahn CR. Insulin signaling in the central nervous system is critical for the normal sympathoadrenal response to hypoglycemia. Diabetes. 2005;54:1447–1451. doi: 10.2337/diabetes.54.5.1447. [DOI] [PubMed] [Google Scholar]
- Garton AJ, Yeaman SJ. Identification and role of the basal phosphorylation site on hormone-sensitive lipase. Eur J Biochem. 1990;191:245–250. doi: 10.1111/j.1432-1033.1990.tb19116.x. [DOI] [PubMed] [Google Scholar]
- Gellhorn E. Blood sugar and autonomic nervous system. Acta Neuroveg (Wien) 1954;9:74–94. doi: 10.1007/BF01232689. [DOI] [PubMed] [Google Scholar]
- German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology. 2009;150:4502–4511. doi: 10.1210/en.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HN, Zhang YL, Hernandez-Ono A. Metabolic syndrome: focus on dyslipidemia. Obesity (Silver Spring) 2006;14(Suppl 1):41S–49S. doi: 10.1038/oby.2006.281. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem. 2007;282:5726–5735. doi: 10.1074/jbc.M610580200. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- Hegele RA. Monogenic forms of insulin resistance: apertures that expose the common metabolic syndrome. Trends Endocrinol Metab. 2003;14:371–377. doi: 10.1016/s1043-2760(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Hers HG, Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem. 1983;52:617–653. doi: 10.1146/annurev.bi.52.070183.003153. [DOI] [PubMed] [Google Scholar]
- Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest. 1996;97:2081–2091. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski K, Ahmadian M, Duncan RE, Sarkadi-Nagy E, Varady KA, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Kim KH, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med. 2009;15:159–168. doi: 10.1038/nm.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE. Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem. 2007;282:28465–28473. doi: 10.1074/jbc.M705503200. [DOI] [PubMed] [Google Scholar]
- Koch L, Wunderlich FT, Seibler J, Konner AC, Hampel B, Irlenbusch S, Brabant G, Kahn CR, Schwenk F, Bruning JC. Central insulin action regulates peripheral glucose and fat metabolism in mice. J Clin Invest. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48:275–297. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Lin HV, Plum L, Ono H, Gutierrez-Juarez R, Shanabrough M, Borok E, Horvath TL, Rossetti L, Accili D. Divergent Regulation of Energy Expenditure and Hepatic Glucose Production by Insulin Receptor in AgRP and POMC Neurons. Diabetes. 2009 doi: 10.2337/db09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Karkanias GB, Morales JC, Hawkins M, Barzilai N, Wang J, Rossetti L. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J Biol Chem. 1998;273:31160–31167. doi: 10.1074/jbc.273.47.31160. [DOI] [PubMed] [Google Scholar]
- Mittelman SD, Bergman RN. Inhibition of lipolysis causes suppression of endogenous glucose production independent of changes in insulin. Am J Physiol Endocrinol Metab. 2000;279:E630–637. doi: 10.1152/ajpendo.2000.279.3.E630. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Magkos F, Fabbrini E, Mohammed BS, Klein S. Relationship between body fat mass and free Fatty Acid kinetics in men and women. Obesity (Silver Spring) 2009;17:1872–1877. doi: 10.1038/oby.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Perfield JW, 2nd, Souza SC, Shen WJ, Zhang HH, Stancheva ZS, Kraemer FB, Obin MS, Greenberg AS. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem. 2007;282:996–1002. doi: 10.1074/jbc.M605770200. [DOI] [PubMed] [Google Scholar]
- Moraes RC, Blondet A, Birkenkamp-Demtroeder K, Tirard J, Orntoft TF, Gertler A, Durand P, Naville D, Begeot M. Study of the alteration of gene expression in adipose tissue of diet-induced obese mice by microarray and reverse transcription-polymerase chain reaction analyses. Endocrinology. 2003;144:4773–4782. doi: 10.1210/en.2003-0456. [DOI] [PubMed] [Google Scholar]
- Nadler ST, Stoehr JP, Schueler KL, Tanimoto G, Yandell BS, Attie AD. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc Natl Acad Sci U S A. 2000;97:11371–11376. doi: 10.1073/pnas.97.21.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepokroeff CM, Lakshmanan MR, Porter JW. Fatty-acid synthase from rat liver. Methods Enzymol. 1975;35:37–44. doi: 10.1016/0076-6879(75)35136-7. [DOI] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- Ono H, Pocai A, Wang Y, Sakoda H, Asano T, Backer JM, Schwartz GJ, Rossetti L. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest. 2008;118:2959–2968. doi: 10.1172/JCI34277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin K, Steil GM, Mittelman SD, Bergman RN. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J Clin Invest. 1996;98:741–749. doi: 10.1172/JCI118846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, Hodson L, Dennis AL, Neville MJ, Humphreys SM, Harnden KE, Micklem KJ, Frayn KN. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52:882–890. doi: 10.1007/s00125-009-1300-4. [DOI] [PubMed] [Google Scholar]
- Roman EA, Cesquini M, Stoppa GR, Carvalheira JB, Torsoni MA, Velloso LA. Activation of AMPK in rat hypothalamus participates in cold-induced resistance to nutrient-dependent anorexigenic signals. J Physiol. 2005;568:993–1001. doi: 10.1113/jphysiol.2005.095687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer L, Brand CL, Hansen BF, Ribel U, Shaw AC, Slaaby R, Sturis J. A novel high-affinity peptide antagonist to the insulin receptor. Biochem Biophys Res Commun. 2008;376:380–383. doi: 10.1016/j.bbrc.2008.08.151. [DOI] [PubMed] [Google Scholar]
- Scheja L, Toedter K, Mohr R, Niederfellner G, Michael MD, Meissner A, Schoettler A, Pospisil H, Beisiegel U, Heeren J. Liver TAG transiently decreases while PL n-3 and n-6 fatty acids are persistently elevated in insulin resistant mice. Lipids. 2008;43:1039–1051. doi: 10.1007/s11745-008-3220-3. [DOI] [PubMed] [Google Scholar]
- Schweiger M, Schoiswohl G, Lass A, Radner FP, Haemmerle G, Malli R, Graier W, Cornaciu I, Oberer M, Salvayre R, et al. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J Biol Chem. 2008;283:17211–17220. doi: 10.1074/jbc.M710566200. [DOI] [PubMed] [Google Scholar]
- Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Vasta V, Degerman E, Belfrage P, Manganiello VC. Hormone-sensitive cyclic GMP-inhibited cyclic AMP phosphodiesterase in rat adipocytes. Regulation of insulin- and cAMP-dependent activation by phosphorylation. J Biol Chem. 1991;266:13385–13390. [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R, Lass A, Haemmerle G, Zechner R. Fate of fat: the role of adipose triglyceride lipase in lipolysis. Biochim Biophys Acta. 2009;1791:494–500. doi: 10.1016/j.bbalip.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.