Abstract
Adrenal corticosteroids (cortisol in humans or corticosterone in rodents) exert numerous effects on the central nervous system that regulates the stress response, mood, learning and memory, and various neuroendocrine functions. Corticosterone (CORT) actions in the brain are mediated via two receptor systems: the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR). It has been shown that GR and MR are highly colocalized in the hippocampus. These receptors are mainly distributed in the cytoplasm without hormones and translocated into the nucleus after treatment with hormones to act as transcriptional factors. Thus the subcellular dynamics of both receptors are one of the most important issues. Given the differential action of MR and GR in the central nervous system, it is of great consequence to clarify how these receptors are trafficked between cytoplasm and nucleus and their interactions are regulated by hormones and/or other molecules to exert their transcriptional activity. In this review, we focus on the nucleocytoplasmic and subnuclear trafficking of GR and MR in neural cells and non-neural cells analyzed by using molecular imaging techniques with green fluorescent protein (GFP) including fluorescence recovery after photobleaching (FRAP) and fluorescence resonance energy transfer (FRET), and discuss various factors affecting the dynamics of these receptors. Furthermore, we discuss the future directions of in vivo molecular imaging of corticosteroid receptors at the whole brain level.
Keywords: glucocorticoid receptor, mineralocorticoid receptor, hippocampus, GFP, real-time imaging
I. Introduction
Glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) show a high degree of colocalization in the hippocampus [1]. Since MR has about 10-fold higher affinity for corticosterone (CORT) than does GR, hippocampal MR responds strongly to CORT [32]. Thus, in the hippocampus, one compound, CORT, serves to regulate two signaling pathways via MR and GR [31]. The progressive activation of MR at a low CORT concentration and additional activation of GR when CORT levels increase can cause extreme alterations of neuronal integrity for responding to stress conditions [11] and of neuronal excitability [16] associated with changes in neuroendocrine regulation and behavior.
These corticosteroid receptors are localized predominantly in the cytoplasm in the absence of ligand associated with various chaperone proteins such as heat shock protein 90 (hsp90). After binding with hormone, the hormone-receptor complex becomes activated leading to dynamic conformational changes of protein complex, and translocates into the nucleus. For inducing transactivation, the hormone-receptor complex binds to glucocorticoid responsive elements (GRE) in the promoter regions in a homodimer or a heterodimer form. Thus, the elucidation of mechanisms for subcellular and subnuclear trafficking of these receptors is a remarkably important issue. Furthermore, recent studies prompted the hypothesis that corticosteroids possess membrane-associated receptors through which nongenomic signaling may evoke rapid effects on physiology and behavior [4, 21]. In this review, we would like to just focus on the intracellular type of corticosteroid receptors.
II. Nucleocytoplasmic Trafficking of GR and MR
Differential responses to the common natural ligand, corticosterone
Adrenal corticosteroids regulate their own secretion via a negative feedback system at the level of the hypothalamus and pituitary that is mediated by GR. In addition to these regulatory systems, recent studies have indicated that the tonic inhibitory action of CORT on hypothalamus-pituitary-adrenal (HPA) activity is exerted via MR in the suprahypothalamic structures including the hippocampus. The limbic structure controls HPA activity via the inhibitory GABAergic system [13]. At the lower level of CORT during the circadian trough, MR is predominantly occupied and operated in a pro-active fashion in the maintenance of homeostasis, while at the higher level of CORT observed at the circadian peak or after stress, GR is mainly activated. Thus, the cytoplasm-to-nuclear translocation of these two receptors in response to CORT in single cells is intriguing.
We investigated the trafficking manners of GR and MR in response to the common natural ligand, CORT, in single living cells cotransfected with GR and MR using dual-color labeling with two different GFP spectral variants, CFP (cyan fluorescent protein) and YFP (yellow fluorescent protein). The double labeling strategies with CFP and YFP have allowed time-lapse imaging of two different receptors in single living cells simultaneously [26]. In the absence of CORT, CFP-GR was predominantly localized in the cytoplasmic regions, whereas YFP-MR was distributed in both the cytoplasm and nucleus. The trafficking manners of these fusion proteins in the cotransfected cells were basically the same as those in the singly transfected cells.
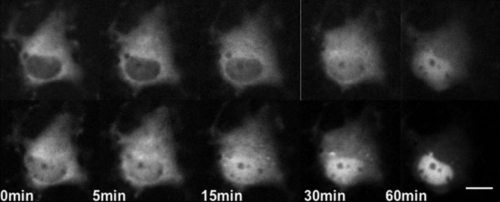
In COS-1 cells, YFP-MR was accumulated in the nucleus faster than CFP-GR in the presence of 10−9 M CORT (Fig. 1), a concentration between the Kd values of MR and GR. In contrast, no significant difference was observed in the accumulation rate in the presence of 10−6 M CORT, a concentration much higher than the Kd values of both receptors. Since COS-1 cells have no endogenous MR or GR, the difference in trafficking kinetics detected in the presence of 10−9 M CORT is considered to directly reflect the difference in affinities for CORT between MR and GR; more specifically, MR has about 10-fold higher affinity for CORT than that of GR. The findings suggest that both MR and GR are saturated in cells treated with 10−6 M CORT, causing the lack of difference in trafficking kinetics. These results are in agreement with the present understanding that MR plays major roles at physiological concentrations of CORT, while GR is mainly effective at high concentrations of CORT [6].
Fig. 1.
Dual-color imaging of GR and MR with GFP color variants in a single COS-1 cell. COS-1 cells co-transfected with CFP-GR and YFP-MR were cultured in the absence of serum and steroids for 24 hr before observation. Upper images were representative time-lapse images of CFP-GR, and bottoms were those of YFP-MR. Note that YFP-MR was accumulated in the nuclear region faster than CFP-GR after treatment with 10−9 M CORT. Bar=10 µm.
Contrary to COS-1 cells, hippocampal neurons did not show any obvious difference in the nuclear accumulation rates of MR and GR in the presence of either 10−9 M or 10−6 M CORT. Since hippocampal neurons express endogenous MR and GR, these endogenous receptors may affect the trafficking of YFP-MR and CFP-GR. Another possible explanation is that hippocampal neurons may have a unique nuclear transporting system for accumulating MR and GR in the nucleus together, which is different from that in COS-1 cells, expressing no endogenous receptors. Recent study showed that vesicles containing NMDA receptor 2B are transported along microtubules by KIF (kinesin superfamily) 17, a neuron-specific molecular motor [33]. Although our previous data indicated that microtubules are not essentially involved in the nuclear import of GFP-MR or GFP-GR, the results of Setou and coworkers suggest that some receptors expressed in neuronal cells are transported by a neuron-specific molecular motor [33]. These results led us to speculate that MR and GR could be translocated into the nucleus at mostly the same speed using specific motor molecules in cultured hippocampal neurons.
Role of carrier proteins
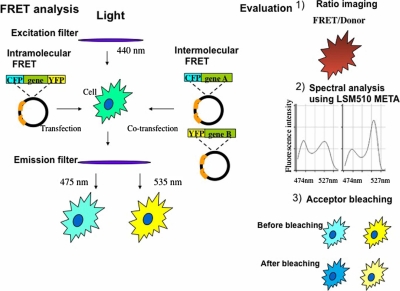
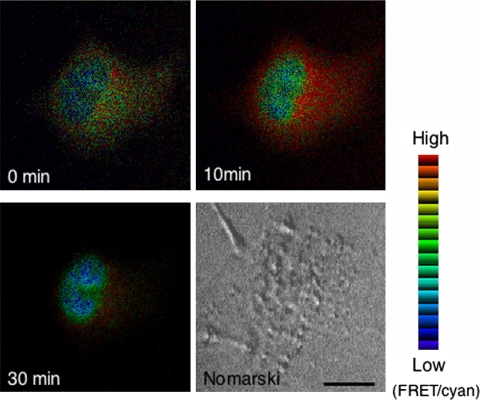
It has been conjectured that the cytoplasmic/nuclear distribution of steroid hormone receptors is primarily regulated by the conditional interaction of nuclear localization signals (NLS) with the import/export apparatus in the nuclear pores [39]. In the absence of hormones, steroid hormone receptors are associated with a complex set of chaperones in a large complex, and the interaction of the cognate ligand with these receptors induces a conformational change resulting in dissociation of the complex and loss of many associated factors. This reconstruction is thought in some cases to expose previously masked NLS, and the receptors are then recognized by the transport machinery, such as importin family members [12]. Because macromolecules greater than about 40 kDa, including corticosteroid receptors, are transported through gated channels of the nuclear pore complex (NPC) by active mechanisms, whereas molecules less than 20–40 kDa can passively diffuse through NPC [5]. In the classical nuclear import pathway, importin α recognizes and binds to the NLS on the cargo protein, and also binds to importin β, which then docks the NPC and mediates translocation from the cytoplasm to the nucleus [44]. We showed that corticosteroid receptors were translocated from the cytoplasm to the nucleus in association with importin α after ligand binding in single living COS-1 cells coexpressing fusion proteins with GFP color variants, which means importin α was also translocated from the cytoplasm to the nucleus in mostly the same time course as that of corticosteroid receptors [37]. In contrast, the distribution of importin β was predominantly around perinuclear sites and little changed after ligand binding. Furthermore, analysis using fluorescence resonance energy transfer (FRET) (Fig. 2) proved that GR directly interacted with importin α in the whole area of the cytoplasm upon ligand treatment and detached importin α shortly after nuclear import (Fig. 3). However, direct interaction between GR and importin β was not detected. The study of a mutant in NLS of corticosteroid receptors supports these data [37].
Fig. 2.
Procedure and evaluation of fluorescence resonance energy transfer (FRET) experiment using fusion proteins with cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP). In our experiment, FRET is evaluated with three ways: 1) Ratio imaging (FRET image was divided by Donor image). Ratio images were pseudocolored where the red range indicated a high ratio and the blue range indicated a low ratio. 2) For detecting an emission spectral change in FRET imaging, Emission Fingerprinting method using confocal laser-scanning microscope LSM 510 META (Zeiss) was employed. First, spectral signatures of the fluorescence within the specimen were captured by means of lambda stack acquisition with excitation at 458 nm and detection at 10 nm-intervals from 458 through 596 nm using an HFT 458/543 dichroic mirror. Several regions of interest (ROIs) with a diameter of 2 µm were then randomly selected for obtaining emission spectral patterns, and the mean ratio of fluorescence intensity of 527 nm and 474 nm was calculated from selected ROIs at each time point after ligand addition. 3) For acceptor photobleaching, we used the confocal laser-scanning microscope. Energy transfer was detected as an increase in donor fluorescence (CFP) after photobleaching of the acceptor molecules (YFP). The acceptor was photobleached by using a 514 nm laser for 1 min at maximum power (25 mW).
Fig. 3.
Ratio images of the cell co-expressing CFP-GR and YFP-importin α detected by FRET. COS-1 cells were co-expressed with CFP-GR and YFP-importin α and cultured in the absence of serum and steroids for at least 15 hr before observation. Fluorescent images of CFP-GR and YFP-importin α were captured using a filter set of CFP (440AF21 excitation, 480AF30 emission, and 455DRLP dichroic mirror) and YFP (500AF25 excitation, 545AF35 emission, and 525DRLB dichroic mirror), respectively. FRET image was detected using a filter set with 440AF21 excitation and 535AF26 emission, and 455DRPL dichroic mirror at 0, 10, and 30 min after treatment with 10−6 M CORT. Filter sets were purchased from Omega Optical Inc. The ratio of the FRET image was divided by donor image to obtain the ratio images using MetaMorph software (Universal Imaging Corp.). The ratio images were pseudo colored. The red range showed high ratio and blue range showed low ratio. High ratio was observed in the cytoplasm, indicating an interaction of CFP-GR and YFP-importin, whereas low ratio was observed in the nucleus, indicating a dissociation of these two molecules.
III. Subnuclear Trafficking of GR and MR
Nuclear profile after translocated in the nucleus
After GR and MR enter the nucleus affected by various kinds of factors, what is happening in the nucleus? In live cell studies using GFP-GR and GFP-MR, the GFP fluorescence appears to be accumulated in certain specific nuclear regions, and is distributed in heterogeneous dot-like distributions in the nucleus [15, 25]. Fejes-Toth [9] reported that agonist-activated GFP-MR accumulates in discrete clusters in the nucleus, and that this phenomenon occurs only with transcriptionally active MR. In contrast, van Steensel and colleagues [41] demonstrated the spatial distribution of MR and GR in clusters in specific nuclear domains using an immunofluorescence technique with confocal microscopy. They indicated that there is no correlation between the clusters of receptor and the distribution of newly synthesized pre-mRNA, suggesting that the clusters of receptor are not directly involved in active transcription.
Recent studies showed that various nuclear proteins such as transcription factors, splicing factors and chromatin remodeling factors, continuously and rapidly associate and dissociate with nuclear compartments such as regulatory sites in living cells [22]. These studies investigated the nuclear dynamics of GFP-labeled proteins in living cells using FRAP (fluorescence recovery after photobleaching) and FLIP (fluorescence loss in photobleaching) to ask the question how fast these nuclear proteins move within the nucleus. McNally and colleagues elegantly visualized the direct interaction of GR with hormone response elements in living cells by applying a large tandem array of a mouse mammary tumor virus/Harvey viral ras reporter [22]. Using FRAP techniques, they proposed a dynamic “hit-and-run” model in which the receptor undergoes continuous exchange between chromatin regulatory elements and the nucleocytoplasmic compartment during a constant existence of ligand. In the case of cultured hippocampal and cortical neurons, FRAP study showed mostly the same rapid movement of GR and MR as detected in non-neural cells (Nishi unpublished data). The techniques of FRAP and FLIP showed the possibilities that the nuclear proteins are freely diffusing or constrained by structure, perhaps actively recruited from one place to the next.
Previous studies demonstrated that proteasome inhibition increased accumulation of GR and reduced its mobility within the nucleus [8, 35] as has also been observed for other steroid receptors such as the estrogen receptor, androgen receptor, and progesterone receptor [20, 34, 36]. We first reported that not only GR, but also MR, showed a reduced mobility in the nucleus as a result of proteasome inhibition. Nuclear accumulation of GR was significantly increased by proteasome inhibition in the presence of 10−9 M CORT, while no significant difference in accumulation was detected at 10−6 M CORT. In contrast, the nuclear MR level did not show a significant difference by proteasome inhibition either in the presence of 10−6 M or 10−9 M CORT. Wang et al. indicated that GR levels are not affected by MG132 in the cultured hippocampal neurons [43], but they used whole cell lysates, not nuclear fractions. The difference in the subcellular fractions used can cause discrepancies in the receptor levels. Together with the results of differential effects of CORT concentration and proteasome inhibition on nuclear mobility, these findings of retention patterns of GR and MR suggest that GR might be cleared from the nucleus by proteasome-mediated mechanisms leaving activated MR in the nucleus at the lower concentration of 10−9 M CORT, which is similar to physiological conditions. In contrast, at the higher concentration of 10−6 M CORT, which mimics stressful conditions, both GR and MR are activated and accumulated in the nucleus, and GR exhibits predominant actions. The results also support the idea that the probability of heterodimerization of GR and MR increased at the higher concentration of 10−6 M CORT than 10−9 M CORT [27].
Studies where the proteasome is inhibited with drugs such as MG132 demonstrate that proteasome activity is required for activation of transcription by some steroid receptors, but not all steroid receptors [7, 8, 20, 42]. This has led to a widely accepted model in which proteolytic activity of the proteasome might be critical to promote the exchange of transcriptional factors on chromatin which probably facilitates various steps of transcription initiation, and consequently regulates receptor-mediated gene expression [3, 24, 30]. Proteasome inhibition synergistically increases GR-mediated transactivation, which is unlike other steroid receptors [8, 43]. We also found that corticosterone-induced GR- and MR-mediated transactivation increased in the cultured hippocampal neurons after inhibition of the proteasome. The present findings suggest that while altered nuclear mobility of steroid receptors is a common property of proteasome inhibition, GR and MR are unique in their enhanced transactivation activity that occurs when proteasome function is compromised.
Interaction of corticosteroid receptors in the nucleus
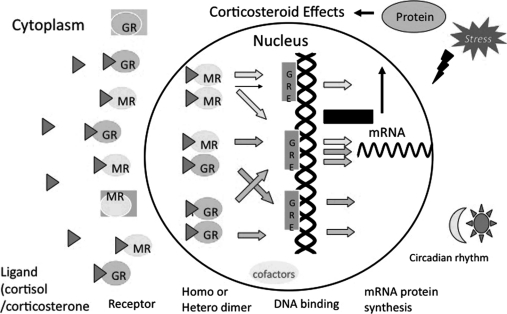
Heterodimerization between transcription factors is not uncommon and seems to increase with the level of functional diversity [29]. Likewise, the formation of heterodimers between members of the nuclear receptor superfamily is a common property. Interactions have been reported between the retinoic X receptor and the retinoic acid receptor, and between the vitamin D receptor and the thyroid receptor [17]. The same could be true for the case of GR and MR. Previous molecular biological studies have indicated that in cells expressing only one of the receptors, transcriptional regulation from hormone response element (HRE), many of which are imperfect inverted hexanucleotide repeats, is mediated by receptor homodimers [10]. However, physiological studies in various systems suggest that GR and MR also functionally interact with each other [16]. Biogenetic evidence demonstrated that GR and perhaps MR form homodimers through a dimer interface within their zinc finger regions (ZFRs), and these receptors share complete sequence identity in this ZFR dimer interface, suggesting that this region might mediate heterodimerization as well [19]. To visualize such an interaction in spatio-temporal specific manner in living cells, we performed a FRET analysis coupled with a new technique, spectral imaging fluorescence microscopy [18, 23], to compensate for varying levels of protein expression (Fig. 2). This technique allowed us to detect spectral changes in fluorescence in living cells and to address several argued points of intermolecular FRET [23]. We calculated mean ratio of fluorescence intensity at acceptor and donor emission maximum wave lengths, 527 nm and 474 nm, respectively. By using these methods, we observed that CFP-GR and YFP-MR directly interact with each other in the nucleus, but not in the cytoplasm, after treatment with CORT, both in COS-1 cells and cultured hippocampal neurons. These results suggest that heterodimer formation depends on the content of GR and MR in the nucleus. Then we investigated whether GR and MR heterodimer formation is affected by concentrations of CORT, because there is a possibility that GR and MR exert various functions reflecting the differences in affinity for the common ligand, CORT. Particularly, in structures such as the hippocampus where both GR and MR are co-expressed in the same cells [41], heterodimerization of these receptors may have a decisive influence on the regulation of corticosteroid-responsive genes in the brain. We employed two different concentrations, 10−6 M and 10−9 M, and found that content of heterodimer of CFP-GR and YFP-MR detected at 10−6 M was higher than that at 10−9 M. These results suggest that MR, with 10-fold higher affinity than GR, may form predominantly homodimers at a lower concentration, whereas at a higher concentration mimicking stressful conditions, occupancy of GR increases the probability of heterodimerization. Our findings could be consistent with the previous demonstrations that MR is dominantly activated at lower concentrations of CORT to explore tonic influences, while the additional occupancy of GR with higher levels of CORT mediates the feedback actions to restore disturbances of homeostasis [6]. The physiological significance of the formation of GR-MR heterodimers has been proposed from the co-localization of these receptors in a variety of tissues and cells [2]. Some studies have shown that GR-MR heterodimerization may play a crucial role in corticosteroid action in the brain, especially in structures such as the hippocampus where both receptors are co-expressed in single cells [19]. Hence, having two types of receptors may allow a more flexible response to widely varying corticosteroid concentrations that may be present under physiological and pathological conditions [28, 38]. The availability of a variety of corticosteroid receptor dimers gives the potential to provide a more finely orchestrated regulation of corticosteroid-responsive genes than the previous model of corticosteroid action based on homodimerization (Fig. 4). But the real functional role of heterodimerization in vivo remains controversial. Furthermore, the recruitment of transcriptional cofactors is affected by dimerization manner, homodimer or heterodimer, which leads to differential regulation of transcription activities of GR and MR. These findings indicate that heterodimerization of GR and MR may provide a more fine tuned regulation of gene expression in response to various cellular environments such as fluctuating CORT concentrations.
Fig. 4.
A schematic model for dimer formation of corticosteroid receptor. A variety of corticosteroid receptor dimers including homodimers and heterodimers may give the potential to provide a more finely tuned regulation of corticosteroid-regulated gene for responding to fluctuations in plasma cortisol/corticosterone level affected by stress responses, circadian rhythm, and so on.
In vivo imaging
Two-photon excitation laser scanning microscopy (2PLSM), together with expression of fluorescent proteins such as GFP, allows high-resolution imaging of intact tissue including neurons in vivo [14]. Recent studies employed a chronic cranial window to obtain optical access to the mouse cerebral cortex for long-term in vivo imaging, which enables imaging of ongoing structural plasticity and the neuronal circuits remodeling [14]. Thus, by using these techniques, we can analyze the dynamics of corticosteroid receptors in response to fluctuating hormonal environments induced by stress response and circadian rhythm in living animals. In order to address this issue, we recently employed GFP-GR knockin (KI) mice [40]. As a preliminary data, we can observe the nuclear localized GR in the layer I~II of frontal cortex of GFP-GR KI mice by using 2PLSM after CORT treatment (unpublished data). Moreover, in vivo imaging of double KI mice expressing CFP-GR and YFP-MR is intriguing, which will clarify the real regulation of balance of GR and MR dynamics corresponding to fluctuating CORT concentrations.
IV. Conclusion
These studies of receptor trafficking in living cells reveal a dynamic alteration in the subcellular localization of receptors in response to various extracellular and intracellular environments. Although there are still problems in tagging proteins with GFP and overexpressing the receptors, this approach makes it possible to observe various events in living cells which have never been detected in fixed cells. Finally, it is particularly important to elucidate whether the functional significance of the observations in living neurons hold true at the whole brain level. This could come true in the near future with the recent progress in multidisciplinary studies of life science areas including various combinations of genetically engineered animals with sophisticated optical instruments.
V. Acknowledgments
The author would like to thank present and past members of the Dr. Kawata’s laboratory at Kyoto Prefectural University of Medicine for their contributions to this work.
This work was supported by Grant-in-Aid for Scientific Research from MEXT.
Footnotes
This paper was presented at the Japan/US Joint Symposium on “New Insight in Bio-imaging”, 51st Annual Meeting of Japan Society of Histochemistry and Cytochemistry (Tokyo, 2010)
VI. References
- 1.Arriza J. L., Simerly R. B., Swanson L. W., Evans R. M. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron. 1988;1:887–900. doi: 10.1016/0896-6273(88)90136-5. [DOI] [PubMed] [Google Scholar]
- 2.Bohn M. C., O’Banion M. K., Young D. A., Giuliano R., Hussain S., Dean D. O., Cunningham L. A. In vitro studies of glucocorticoid effects on neurons and astrocytes. Annl. N.Y. Acad. Sci. 1994;746:243–259. doi: 10.1111/j.1749-6632.1994.tb39241.x. [DOI] [PubMed] [Google Scholar]
- 3.Collins G. A., Tansey W. P. The proteasome: A utility tool for transcription? Curr. Opin. Genet. Dev. 2006;16:197–202. doi: 10.1016/j.gde.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Dallman M. F. Fast glucocorticoid actions on brain: back to the future. Front. Neuroendocrinol. 2005;26:103–108. doi: 10.1016/j.yfrne.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Davis L. I. The nuclear pore complex. Annu. Rev. Biochem. 1995;64:865–896. doi: 10.1146/annurev.bi.64.070195.004245. [DOI] [PubMed] [Google Scholar]
- 6.de Kloet E. R., Joels M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 7.Dennis A. P., O’Malley B. W. Rush hour at the promoter: how the ubiquitin-proteasome pathway polices the traffic flow of nuclear receptor-dependent transcription. J. Steroid Biochem. Mol. Biol. 2005;93:139–151. doi: 10.1016/j.jsbmb.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Deroo B. J., Rentsch C., Sampath S., Young J., DeFranco D. B., Archer T. K. Proteasomal inhibition enhances glucocorticoid receptor transactivation and alters its subnuclear trafficking. Mol. Cell. Biol. 2002;22:4113–4123. doi: 10.1128/MCB.22.12.4113-4123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fejes-Toth G., Pearce D., Naray-Fejes-Toth A. Subcellualr localization of mineralocorticoid receptors in living cells: effects of receptor agonists and antagonists. Proc. Natl. Acad. Sci. U S A. 1998;95:2973–2978. doi: 10.1073/pnas.95.6.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forman B. M., Umesono K., Chen J., Evans R. M. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 11.Gould E., Tanapat P., McEwen B. S. Activation of the type 2 adrenal steroid receptor can rescue granule cells from death during development. Brain Res. Dev. Brain Res. 1997;101:265–268. doi: 10.1016/s0165-3806(97)00023-0. [DOI] [PubMed] [Google Scholar]
- 12.Hager G. L., Lim C. S., Elbi C., Baumann C. T. Trafficking of nuclear receptors in living cells. J. Steroid Biochem. Mol. Biol. 2000;74:249–254. doi: 10.1016/s0960-0760(00)00100-x. [DOI] [PubMed] [Google Scholar]
- 13.Herman J. P., Cullinan W. E. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 14.Holtman A., Bonhoeffer T., Chow D. K., Chucjowree J., Dr Paola V., Hofer S. B., Hubener M., Keck T., Knott G., Lee W-C. A., Mostany R., Mrsic-Flogel T. D., Nedivi E., Portera-Cailliau C., Svoboda K., Trachtenberg J. T., Wilbrecht L. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protocol. 2009;4:1128–1144. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Htun H., Barsony J., Renyi I., Gould D., Hager G. L. Visualization of glucocorticoid receptor translocation and intranuclear organization in living cells with a green fluorescent protein chimera. Proc. Natl. Acad. Sci. U S A. 1996;93:4845–4850. doi: 10.1073/pnas.93.10.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joels M., de Kloet E. R. Mineralocorticoid and glucocorticoid receptors in the brain. Implication for ion permeability and transmitter systems. Prog. Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 17.Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signaling. Nature. 1992;355:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lansford R., Bearman G., Fraser S. E. Resolution of multiple green fluorescent protein color variants and dyes using two-photon microscopy and imaging spectroscopy. J. Biomed. Opt. 2001;6:311–318. doi: 10.1117/1.1383780. [DOI] [PubMed] [Google Scholar]
- 19.Liu W., Wang J., Sauter N. K., Pearce D. Steroid receptor heterodimerization demonstrated in vitro and in vivo. Proc. Natl. Acad. Sci. U S A. 1995;92:12480–12484. doi: 10.1073/pnas.92.26.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonard D. M., Nawaz Z., Smith C. L., O’Malley B. W. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol. Cell. 2000;5:939–948. doi: 10.1016/s1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- 21.McEween B. S. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res. Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNally J. G., Muller W. G., Walker D., Wolford R., Hager G. L. The glucocorticoid receptor: Rapid exchange with regulatory sites in the living cells. Science. 2000;287:1262–1265. doi: 10.1126/science.287.5456.1262. [DOI] [PubMed] [Google Scholar]
- 23.Miyawaki A. Innovations in the imaging of brain functions using fluorescent proteins. Neuron. 2005;48:189–199. doi: 10.1016/j.neuron.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Nawaz Z., O’Malley B. W. Urban renewal in the nucleus: Is protein turnover by proteasome absolutely required for nuclear receptor-regulated transcription? Mol. Endocrinol. 2004;18:493–499. doi: 10.1210/me.2003-0388. [DOI] [PubMed] [Google Scholar]
- 25.Nishi M., Takenaka N., Morita N., Ito T., Ozawa H., Kawata M. Real-time imaging of glucocorticoid receptor dynamics in living neurons and glial cells in comparison with non-neural cells. Eur. J. Neurosci. 1999;11:1927–1936. doi: 10.1046/j.1460-9568.1999.00616.x. [DOI] [PubMed] [Google Scholar]
- 26.Nishi M., Ogawa H., Ito T., Matsuda K., Kawata M. Dynamic changes in subcellular localization of mineralocorticoid receptor in living cells: in comparison with glucocorticoid receptor using dual-color labeling with green fluorescent protein spectral variants. Mol. Endocrinol. 2001;15:1077–1092. doi: 10.1210/mend.15.7.0659. [DOI] [PubMed] [Google Scholar]
- 27.Nishi M., Tanaka M., Matsuda K., Sunaguchi M., Kawata M. Visualization of Glucocorticoid receptor and mineralocorticoid receptor interactions in living cells with GFP-based fluorescent resonance energy transfer (FRET) J. Neurosci. 2004;24:4918–4927. doi: 10.1523/JNEUROSCI.5495-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishi M., Kawata M. Brain corticosteroid receptor dynamics and trafficking: implications from live cell imaging. Neuroscientist. 2006;12:119–133. doi: 10.1177/1073858405279691. [DOI] [PubMed] [Google Scholar]
- 29.Power R. F., Conneely O. M., O’Malley B. W. New insights into activation of the steroid hormone receptor superfamily. Trends Pharmacol. Sci. 1992;13:318–323. doi: 10.1016/0165-6147(92)90099-r. [DOI] [PubMed] [Google Scholar]
- 30.Reid G., Hübner M. R., Métivier R., Brand H., Denger S., Manu D., Beaudouin J., Ellenberg J., Gannon F. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol. Cell. 2003;11:695–707. doi: 10.1016/s1097-2765(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 31.Reul J. M., de Kloet E. R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 32.Rupprecht R., Reul J. M., van Steensel B., Spengler D., Soder M., Berning B., Holsboer F., Damm K. Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands. Eur. J. Pharmacol. 1993;247:145–154. doi: 10.1016/0922-4106(93)90072-h. [DOI] [PubMed] [Google Scholar]
- 33.Setou M., Nakagawa T., Seog D-H., Hirokawa N. Kinesin superfamily motor protein KIF 17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- 34.Sheflin L., Keegan B., Zhang W., Spaulding S. W. Inhibiting proteasomes in human HepG2 and LNCaP cells increases endogenous androgen receptor levels. Biochem. Biophys. Res. Commun. 2000;276:144–150. doi: 10.1006/bbrc.2000.3424. [DOI] [PubMed] [Google Scholar]
- 35.Stavreva D. A., Müller W. G., Hager G. L., Smith C. L., McNally J. G. Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes. Mol. Cell. Biol. 2004;24:2682–2697. doi: 10.1128/MCB.24.7.2682-2697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stenoien D. L., Patel K., Mancini M. G., Dutertre M., Smith C. L., O’Malley B. W., Mancini M. A. FRAP reveals that mobility of oestrogen receptor-alpha is ligand- and proteasome-dependent. Nat. Cell Biol. 2001;3:15–23. doi: 10.1038/35050515. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka M., Nishi M., Morimoto M., Kawata M. Nuclear import of glucocorticoid receptor in association with importin α and importin β: Analysis with real-time fluorescence imaging and fluorescence resonance energy transfer in living cells. Endocrinology. 2003;144:4070–4079. doi: 10.1210/en.2003-0282. [DOI] [PubMed] [Google Scholar]
- 38.Trapp T., Holsber F. Heterodimerization between mineralocorticoid and glucocorticoid receptors increases the functional diversity of corticosteroid action. Trends Pharmacol. Sci. 1996;17:145–149. doi: 10.1016/0165-6147(96)81590-2. [DOI] [PubMed] [Google Scholar]
- 39.Tyagi R. K., Amazit L., Lescop P., Milgrom E., Guiochon-Mantel A. Mechanisms of progesterone receptor export from nuclei: role of nuclear localization signal, nuclear export signal, and ran guanosine triphosphate. Mol. Endocrinol. 1998;12:1684–1695. doi: 10.1210/mend.12.11.0197. [DOI] [PubMed] [Google Scholar]
- 40.Usuku T., Nishi M., Morimoto M., Sugimoto T., Brewer J., Muglia L., Kawata M. Visualization of glucocorticoid receptor in the brain of green fluorescent protein-glucocorticoid receptor knockin mice. Neuroscience. 2005;135:1119–1128. doi: 10.1016/j.neuroscience.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 41.van Steensel B., van Binnendijk E. P., Hornsby C., van der Voort T. M., Krozowski Z. S., de Kloet E. R. Partial colocalization of glucocorticoid and mineralocorticoid receptors in discrete compartments in nuclei of rat hippocampal neurons. J. Cell Sci. 1996;109:787–792. doi: 10.1242/jcs.109.4.787. [DOI] [PubMed] [Google Scholar]
- 42.Wallace A. D., Cidlowski J. A. Proteasome-mediated glucocorticoid receptor degradation restricts transcriptional signaling by glucocorticoids. J. Biol. Chem. 2001;276:42714–42721. doi: 10.1074/jbc.M106033200. [DOI] [PubMed] [Google Scholar]
- 43.Wang X., Pongrac J. L., DeFranco D. B. Glucocorticoid receptors in hippocampal neurons that do not engage proteasomes escape from hormone-dependent down-regulation but maintain transactivation activity. Mol. Endocrinol. 2000;16:1987–1998. doi: 10.1210/me.2001-0287. [DOI] [PubMed] [Google Scholar]
- 44.Weis K. Nucleocytoplasmic transport: cargo trafficking across the border. Curr. Opin. Cell Biol. 2002;14:328–335. doi: 10.1016/s0955-0674(02)00337-x. [DOI] [PubMed] [Google Scholar]