Abstract
It has been reported that premature infants in neonatal intensive care units are exposed to a high rate of bisphenol A (BPA), an endocrine disrupting chemical. Our previous studies demonstrated that corticothalamic projection was disrupted by prenatal exposure to BPA, which persisted even in adult mice. We therefore analyzed whether prenatal and lactational exposure to low doses of BPA affected the formation of the cortical barrel, the barreloid of the thalamus, and the barrelette of the brainstem in terms of the histology and the expression of genes involved in the barrel development. Pregnant mice were injected subcutaneously with 20 µg/kg of BPA daily from embryonic day 0 (E0) to postnatal 3 weeks (P3W), while the control mice received a vehicle alone. The barrel, barreloid and barrelette of the adult mice were examined by cytochrome C oxidase (COX) staining. There were no significant differences in the total and septal areas and the patterning of the posterior medial barrel subfield (PMBSF), barreloid and barrelette, between the BPA-exposure and control groups in the adult mice. The developmental study at postnatal day 1 (PD1), PD4 and PD8 revealed that the cortical barrel vaguely appeared at PD4 and completely formed at PD8 in both groups. The expression pattern of some genes was spatiotemporally altered depending on the sex and the treatment. These results suggest that the trigeminal projection and the thalamic relay to the cortical barrel were spared after prenatal and lactational exposure to low doses of BPA, although prenatal exposure to BPA was previously shown to disrupt the corticothalamic projection.
Keywords: endocrine disrupting chemicals, bisphenol A, barrel, barreloid, barrelette
I. Introduction
An endocrine-disrupting chemical [35], Bisphenol A (BPA; 2,2-bis(4-hydroxy-phenyl)propane) is produced in high volume and employed primarily to manufacture polycarbonate plastic and epoxy resins used in impact-resistant safety equipment, baby bottles, protective coatings inside metal food containers, and as composites and sealants in dentistry [31]. The widespread contamination of BPA in the environment, and the consequent BPA exposure among humans have led us to examine the potentially deleterious effects of this compound. It was recently reported that BPA levels did not decline rapidly with fasting time in urine samples obtained during the National Health and Nutrition Examination Survey (NHANES), which suggested substantial nonfood exposure, and accumulation in body tissues such as fat, or both [37]. The geometric mean urinary concentration of BPA (30.3 µg/L) among premature infants undergoing intensive therapeutic medical interventions was reported to be one order of magnitude higher than that among the general population [3]. Studies are urgently needed to determine the environmental source of BPA and the effects of exposure to BPA on premature infants.
We previously demonstrated that prenatal exposure to BPA affected fetal murine neocortical development by accelerating neuronal differentiation/migration during embryonic day 12 (E12) and E16, which were associated with up- and down-regulation of the genes critical for brain development, including the basic helix-loop-helix transcription factors [28]. Abnormal neocortical architecture was observed at postnatal 3 weeks (P3W) with prenatal exposure to BPA, and furthermore, the abnormal corticothalamic projection persisted in the BPA-exposure group at P3W, as well as at P12W [29]. Recently, we also demonstrated that epigenetic alterations in promoter-associated CpG-islands might underlie some of the effects on brain development after exposure to BPA [46].
The facial somatosensory map in the neocortex is derived from facial representations that are first established at the brainstem level: 1) The whiskers are innervated by the branch of the maxillary nerve which arises from the trigeminal ganglion, 2) In the brainstem, radial collaterals emerge from the central trigeminal axons and innervate the rostral principal nucleus (PrV) and the caudal spinal nucleus (SpV), where they form whisker-specific patterns (barrelettes). Trigeminothalamic axons from the PrV then project to the contralateral dorsomedial part of the ventral posteromedial nucleus (VPM) in the thalamus, where the whisker-related neural modules (barreloids) are formed. Finally, thalamocortical axons from the VPM convey the whisker patterning to the somatosensory cortex, where the barrel forms. An intact barrel representation in the neocortex is crucial for proper sensory function (e.g., [43]). The current view is that cortical maps develop through an interplay between mechanisms that are intrinsic to cortical progenitors and neurons, and extrinsic mechanisms imposed by thalamocortical input relaying information from the peripheries [8, 20, 33, 38]. The postnatal appearance of the cortical pattern coincides with a critical period of plasticity, during which wiring can be influenced by whisker-dependent neural activity. During this period, sensory loss or deprivation alters the development of the cortical pattern [2, 7]. A variety of developmental manipulations that alter sensory input from the vibrissae have been shown to affect the size, shape, and arrangement of the barrels in the PMBSF [9, 25].
In the present study we examined the size and the patterning of the cortical barrel, as well as the relay nuclei of the thalamus and brainstem after prenatal and lactational exposures to low-doses of BPA, since corticothalamic reciprocal projection was previously shown to be abnormal in prenatally BPA-exposure mice [29]. We also analyzed the spatiotemporal alteration in gene expression, with special reference to the genes involved in the serotonin system. Our results suggest that prenatal and lactational exposures to BPA do not affect the trigeminal projection or the thalamic relay to the cortical barrel, despite the fact that BPA induces transient alterations in the expression of genes involved in barrel development.
II. Materials and Methods
Animals and treatments
ICR/Jcl mice (Clea Japan, Tokyo, Japan) were housed under a 12:12-hr light-dark cycle in a conditioned environment of 23–24°C and 75% humidity with free access to food and water. All of the animal studies conducted for this study were approved by the Ethics Committee for Animal Experimentation of Kyoto Prefectural University of Medicine, and the animals were handled according to the institutional guidelines. Adult females were mated and the morning when a vaginal plug was observed was designated embryonic day 0 (E0). The dams were separated into two groups: BPA-exposure group and the control group. Each group had 10 pregnant females. The dams were subcutaneously injected daily with 20 µg/kg/day of BPA (Wako, Osaka, Japan) dissolved in sesame oil or the same amount of sesame oil, for the BPA-exposure group and the control group respectively, from E0 to postnatal 3 weeks (P3W). The offspring were weaned at P3W and housed according to the same sex and the litter groups until P11-15W.
Tissue preparation
Tissue preparation was performed essentially as previously described [18]. Adult mice were perfused transcardially with 0.1 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in 0.1 M PBS. The brain was removed and dissected hemispheres were flattened between two slide glasses overnight in 4% paraformaldehyde at 4°C. Body weight was measured prior to sacrifice. The hemispheres were tangentially sectioned at a thickness of 70 to 100 µm using a vibrating blade microtome (Leica VT1000 S, Leica, Germany). Serial sections were processed for cytochrome c oxidase (COX) histochemistry to visualize the PMBSF. Briefly, the sections were incubated free-floating at 37°C in the dark for 1–2 hr in a solution containing 60 mg 3,3'-diaminobenzidine in 27 ml 0.1 M sodium acetate, 3 ml 1% MnCl2, and 0.3 ml 0.1% H2O2, at pH 5.5. The reaction was stopped when individual barrels were clearly distinguishable from the background. After three rinses in water, sections were incubated in 1% CuSO4 for 5 min and mounted on APS coated glass slides (Matsunami Glass Ind., Ltd.) and cover slips applied with Permount. The ventral posteromedial (VPM) nucleus of the thalamus was coronally sectioned at the level of the bregma –1.58 to –1.82 mm, and the subnuclei principalis and subnuclei interpolaris (PrV) of the brainstem were coronally sectioned at the level of bregma –6.00 to –6.64 mm [10] and processed for COX histochemistry.
PMBSF reconstruction and the morphometry
Slides containing PMBSF barrels were viewed under a light microscope (Olympus, Japan) and photographed with a digital camera (Olympus DP20). PMBSF were reconstructed using Adobe Photoshop CS (Adobe Systems, Mountain View, CA, USA). Total PMBSF was measured using software, NIH ImageJ 1.33u. The PMBSF consisted of four straddler barrels (α, β, γ, δ), the first four barrels of rows A and B, and the first five barrels of rows C through E, resulting in a total of 27 barrels. Total PMBSF area and individual barrels were defined by drawing an outline around the 27 barrels, followed by measurement for total area of PMBSF and septum using ImageJ (Fig. 1). Photographed images of the barreloid of the thalamus and the barrelette of the brainstem were outlined and measured as described above. To avoid observer-based bias, morphometric analysis was performed without prior knowledge of the experimental group. All reconstructions and measurements were done by a single experimenter. Left and right PMBSF areas of all of the mice were measured and compared against one another.
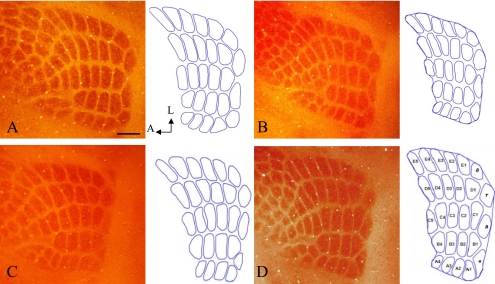
Fig. 1.
Cortical barrel field, including the PMBSF. Twenty-seven barrel areas of PMBSF were outlined for measurement using ImageJ. Control group (A, B), BPA-exposure group (C, D), males (A, C) and females (B, D). No significant differences were shown in the number and patterning of PMBSF between treatments and sex. COX staining. Bar=300 µm.
Morphological analyses of barrel development
Newborn mice at P1, P4 and at P8, after pre- and postnatal exposure to BPA or vehicle were perfused transcardially with 4% paraformaldehyde in 0.1 M PBS. The brain was removed, postfixed overnight at 4°C, and coronally sectioned at the level of the somatosensory cortex (S1), VPM of the thalamus and PrV of the brainstem. Sections were processed for COX histochemistry as described above.
RNA isolation and quantitative RT-PCR
The brains of newborn mice at P1, P4 and at P8 after pre- and postnatal exposure to low doses of BPA or vehicle were dissected into S1, VPM of the thalamus and PrV of the brainstem under a dissecting microscope and immediately frozen using liquid nitrogen until use. Total RNA was extracted from the dissected brains, followed by cDNA synthesis with Superscript II RNase H− reverse-transcriptase (Invitrogen, USA). The expression levels of the target transcripts and GAPDH were measured using the ABI PRISM 7000 Sequence Detection System (Applied Biosystems, USA) and Premix Ex Taq (Perfect Real Time). The expression level of the target transcripts in each sample was normalized with that of GAPDH. For multiple group comparisons, the significance of individual differences was evaluated using the Bonferroni test following one-way ANOVA (StatView, version 5.0, SAS Institute Inc., USA). The primer sequences are shown in Table 1.
Table 1.
Genes and their primers used for quantitative RT-PCR
| Genes | Forward primer | Reverse primer |
|---|---|---|
| Slc6a4 | agtcaaaacgtctggcaagg | gtttctgccagttgggtttc |
| Htr1b | ctatttcgttgccacccttc | tcggtgttcacaaagcagtc |
| Slc1a3 | acatgttccctcccaatctg | tttcgttggactggataggc |
| S100b | tgccctcattgatgtcttcc | tgctccttgatttcctccag |
| Maoa | atggaaggtgcagttgaagc | aagagctggaacatccttgg |
| Gap43 | aagataccaccatgctgtgc | ttatcttccggcttgacacc |
Statistical analysis
All values were expressed as means±STDEV. All data were analyzed by two-way ANOVA (treatment×sex) using Prism version 4.0 (GraphPad). When significant treatment×sex interaction was found, the Mann-Whitney U test was applied at a p<0.05 significance level.
III. Results
The size and the patterning of the PMBSF, barreloid and barrelette in adult mice (Table 2, Figs. 1, 2)
Table 2.
Average barrel field measurements (mm2) in adult mice in various treatment groups
| Exposure | Total PMBSF | Total barrels | Total septum | ||||
|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | ||
| Control | L | 1.66±0.17 | 1.62±0.19 | 1.30±0.14 | 1.26±0.17 | 0.37±0.04 | 0.36±0.19 |
| R | 1.53±0.04 | 1.67±0.09 | 1.17±0.04 | 1.31±0.07 | 0.36±0.03 | 0.37±0.03 | |
| BPA | L | 1.71±0.05 | 1.59±0.19 | 1.29±0.06 | 1.21±0.16 | 0.42±0.03 | 0.38±0.02 |
| R | 1.68±0.11 | 1.64±0.20 | 1.28±0.09 | 1.28±0.09 | 0.40±0.04 | 0.36±0.04 | |
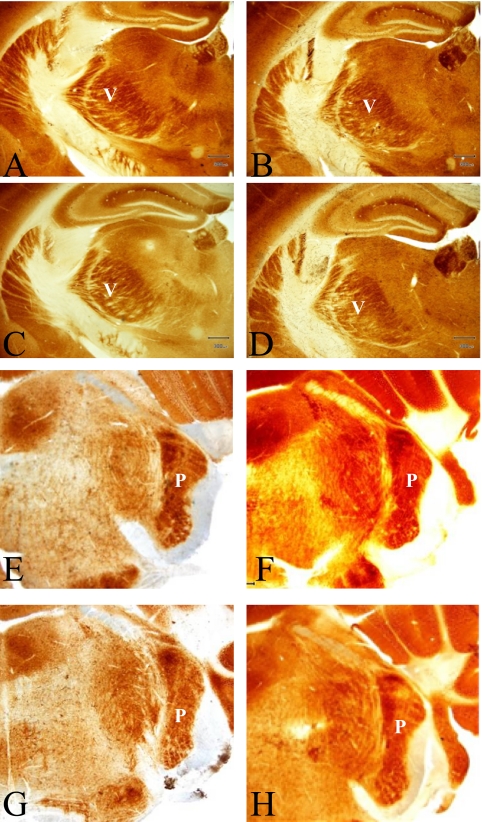
Fig. 2.
Barreloid (ventroposterior medial nucleus of the thalamus; VPM) and barrelette (subnuclei principalis and subnuclei interpolaris; PrV) in adult mice of control group (A, C, E, G), and the BPA-exposure group (B, D, F, H). males (A, B, E, F) and females (C, D, G, H), respectively. COX staining. Bar=300 µm. V, VPM; P, PrV.
The total area and the septum of the PMBSF showed no significant differences between the right and left PMBSF areas regardless of group or sex. There was no significant difference in the total area as well as the septum of the PMBSF between the BPA-exposure and the control groups. The areas of the barreloid of the thalamus were 0.635 ±0.0025 mm2 (n=3) in the control males, 0.691±0.0055 mm2 (n=3) in the control females, 0.620±0.0026 mm2 (n=3) in BPA-exposure males and 0.695±0.0085 mm2 (n=3) in BPA-exposure females. The area of the barrelette was 0.49±0.02 mm2 (n=7) in the control males, 0.51±0.04 mm2 (n=3) in the control females, 0.52±0.04 mm2 (n=5) in BPA-exposure males, and 0.49±0.03 mm2 (n=7) in BPA-exposure females. Two-way ANOVA revealed no significant differences in the morphometric data for the barreloid and the barrelette between the BPA-exposure and the control groups, or between male and female mice.
Development of the barrel, barreloid and barrelette (Fig. 3)
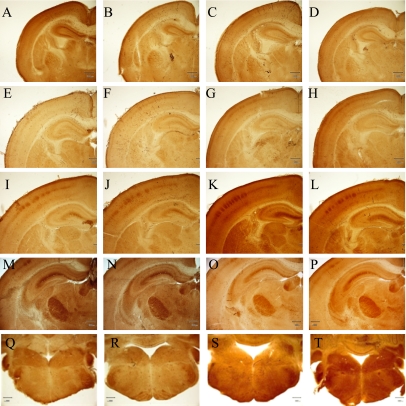
Fig. 3.
Cortical barrel, barreloid and barrelette at P1, P4 and P8. Cortical barrels at P1 (A–D), P4 (E–H) and at P8 (I–L): control males (A, E, I), control females (B, F, J), BPA-treated males (C, G, K) and BPA-treated females (D, H, L), respectively. Barreloid (M–P) and barrelette at P8 (Q–T): control males (M, Q), control females (N, R), BPA-treated males (O, S) and BPA-treated females (P, T), respectively. COX staining. Bar=300 µm.
The cortical barrel was not observed at P0, and only vaguely visualized at P4, but it was clearly identified at P8 in both groups and in both sexes. No significant differences were shown in the patterning and size of the cortical barrel between the BPA-exposure groups and the sex of the mice during development, from P1 to P8 (Fig. 3). The barreloid and the barrelette were not found by COX staining at P1 and P4, but were identified at P8. The areas of the barreloid were 0.361±0.0025 mm2 (n=3) in the control males, 0.356±0.0056 mm2 (n=3) in the control females, 0.347±0.0073 mm2 (n=4) in BPA-exposure males, and 0.358±0.013 mm2 (n=4) in BPA-exposure females. The development of the barrelette confirmed by COX staining showed no significant differences between the BPA-exposure and control groups or between the sex of the mice (data not shown).
Spatiotemporal gene expression in the barrel, the barreloid and the barrelette (Fig. 4 and Supplementary Fig. S1)
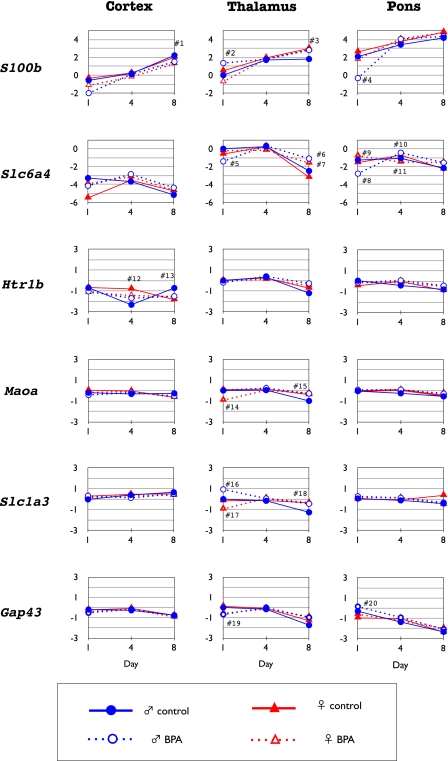
Fig. 4.
Temporal changes in the expression levels of S100b, Slc6a4, Htr1b, Maoa, Scla3, and Gap-43 in the cortex, thalamus and pons at PD1, PD4 and at PD8. Each level was compared to the expression level in the thalamus of the control mice at PD1. #1–#20: p<0.05 by Mann-Whitney U test. #1: BPA-exposure female<control female and male. #2: BPA-exposure male>BPA-exposure female. #3: BPA-exposure female and male, control female>control male. #4: BPA-exposure male<control female and male, BPA-exposure female. #5: BPA-exposure male<control female and male, BPA-exposure female. #6: BPA-exposure male>control male. #7: BPA-exposure female>control female. #8: BPA-exposure male<control male. #9: BPA-exposure female>control female. #10: BPA-exposure male>control male. #11: BPA-exposure female<control female. #12: BPA-exposure female<control female. #13: BPA-exposure male<control male. #14: BPA-exposure female<control female. #15: BPA-exposure male>control male. #16: BPA-exposure male>control male. #17: BPA-exposure female<control female. #18: BPA-exposure male>control male. #19: BPA-exposure male<control male. #20: BPA-exposure male>control male.
The expression of S100β was increased with early postnatal age in the cortex, thalamus and pons of all of the groups analyzed. It was up-regulated in the thalamus (Fig. 4, #2) and down-regulated in the pons (Fig. 4, #4) at PD1 in BPA-exposure males, whereas it was down-regulated in the thalamus at PD1 (Fig. 4, #2) in BPA-exposure females, which indicated that the exposure to BPA reversed the sex-difference found in the control mice. The Slc6a4 (5HT transporter) showed relatively low expression in the cortex and pons, compared to that of thalamus from PD1 to PD8 in all groups. Although there was a difference in the sex in terms of the expression in the cortex of the control mice at PD1, sexual differences disappeared in the BPA-exposure groups. Furthermore it was down-regulated in the thalamus (Fig. 4, #5) and pons (Fig. 4, #8) at PD1 in males exposed to BPA, and the expression became higher in the thalamus at PD8 in the BPA-exposure groups (Fig. 4, #6, 7), compared to the control groups. Slc6a4 expression in the pons was up-regulated in BPA-exposure male (Fig. 4, #10) and down-regulated in BPA-exposure female (Fig. 4, #11) at PD4. The expression of Htr1b in the cortex was down-regulated in BPA-exposure females as compared to control females at PD4 (Fig. 4, #12) and it was down-regulated in BPA-exposure males as compared to control males at PD8 (Fig. 4, #13). The expression of Maoa in the thalamus showed down-regulation at PD1 in BPA-exposure females (Fig. 4, #14) and up-regulation in BPA-exposure males at PD8 (Fig. 4, #15). The expression of Slc1a3 (GLAST; glutamate-aspartate transporter) was similar in all areas analyzed at any postnatal age except for up-regulation in BPA-exposure males at PD1 (Fig. 4, #16) and PD8 (Fig. 4, #18) and down-regulation in BPA-exposure females at PD1 (Fig. 4, #17) in the thalamus. Although the expression of Gap-43 was shown to be slightly higher in the cortex, thalamus and pons at an early postnatal age in all groups, it was down-regulated (Fig. 4, #19) in the thalamus and up-regulated in the pons (Fig. 4, #20) at PD1 in BPA-exposure males as compared to control males.
IV. Discussion
BPA is an endocrine-disrupting chemical, which shows adverse effects not only on the reproductive organs, but also on the central nervous system when rodents are exposed during development [26, 28–30, 34, 40, 46]. We have reported that prenatal exposure to low doses of BPA affected murine neocortical development and the corticothalamic projection associated with deranged expression patterns of some important genes [28, 29]. The pathway from the whisker to the barrel cortex originates in the mechanoreceptors at the base of the whisker hairs, whose sensory information is first relayed via afferent axons in the principal trigeminal nucleus of the brainstem, relayed mainly to the ventroposterior medial nucleus of the thalamus and finally projecting to the somatosensory barrel field. The thalamocortical afferent pattern is well-established in the barrel cortex at P6 in mice [12]. We therefore studied whether exposure to low doses of BPA affected development of the cortical barrel as well as the relayed nuclei of the brainstem and thalamus in this study.
The results of this study demonstrated that the size and patterning of the somatosensory barrel field were not affected by prenatal and lactational exposure to BPA. It is noteworthy that afferent axons projecting from the brainstem to somatosensory cortex developed normally, although the reciprocal corticothalamic pathway was disrupted after the exposure to BPA, as we showed previously [29]. In regard to the development of cortical barrel field, the VPM of the thalamus, and the PrV of the brainstem, the exposure to BPA did not affect the related morphological structures at P1, P4 and at P8. However, some changes were observed in the expression pattern of genes that have been reported to be indispensable for normal barrel formation [4–6, 23, 24, 27, 41, 45].
The patterning mechanisms of the thalamocortical (TC) and the corticothalamic circuits are spatiotemporally controlled by a combination of molecules, including the brain-derived neurotrophic factor, NeuroD2, and the fibroblast growth factor 8 [13–15, 19, 22, 32, 36, 39]. Abnormal cortical barrel formation or TC morphology has been reported in several mice lines. Cortex-specific knock-out (KO) mice that lacked NMDA receptor 1 only in cortical excitatory neurons (Cx-NR1KO mice) [18], and KO mice devoid of the type II regulatory subunit of PKA (PKARII KO mice) [42] showed defects in the barrel map. Knock-out mice for the metabotropic glutamate receptor 5 (mGluR5) [44], monoamine oxidase A (MAOA)-deficient mice [4, 41], and GAP-43 (growth-associated protein 43) KO mice [5, 6, 23] also manifested abnormal barrel formation. Barrelless (brl) mouse mutants, lacking the functional adenylyl cyclase I [1, 11, 16, 21, 43], exhibited defective barrel formation attributed to a disrupted clustering of TC axons within the barrel domains [43].
Xu et al. investigated the role of the transiently expressed serotonin (5-HT) transporter in the development of thalamic fibers projecting to the cortical barrels and the potential developmental changes in neuronal circuitry caused by a selective serotonin reuptake inhibitor (SSRI) [45]. In that study, the organization of the thalamic afferents to the barrels, shown by immunohistochemistry for 5-HT transporter or phospholipase C-h1 was altered in SSRI-treated pups. The authors suggested that the 5-HT transporter plays an important role in the refinement, but not the formation of barrel-like clusters of the thalamocortical fibers, and that the development of neural circuitry in rodent somatosensory cortex was affected by exposure to a SSRI during thalamocortical synaptic formation. It was also reported that the barrel formation was delayed in nutritionally-restricted rats due to a retarded reorientation of dendritic arbors of cortical neurons, which might occur as a secondary effect of decreased availability of 5-HT transporter and/or increased availability of 5-HT1B receptor early in postnatal life [24].
Muneoka et al. reported that much of the developmental role of serotonin is mediated by the release of the neurotrophic protein S-100β [27]. Expression of S-100β peaked in the ventral posterior nucleus of the thalamus at PD 7, and in layer IV of the parietal cortex from PD 7 to 15, showing a ‘barrel-like’ pattern. Their findings suggested that S-100β could be the mediator of the effects of serotonin on the barrel field formation. On the other hand, in a transgenic mouse line deficient for the gene encoding monoamine oxidase A (MAOA), the primary somatosensory cortex (S1) lacked the barrel-like clustering of layer IV neurons, whereas a normal pattern formation existed in the thalamus and the trigeminal nuclei [4, 41]. An excess of brain serotonin during the critical period of barrel formation induced by MAOA inhibition appeared to have a causal role in these cortical abnormalities, since early administration of an inhibitor of serotonin synthesis in the transgenic pups restored the formation of barrel in S1.
In our study, mice exposed to BPA showed complicated patterns of alterations in the expression of genes such as S100b, Slc6a4, Htr1b, and Maoa, all of which were involved in the serotonergic system. The alterations were most obvious at PD1. In BPA-exposure males, S100b was up-regulated in the thalamus and down-regulated in the pons, which was associated with the down-regulation of Slc6a4 in the thalamus and pons. In BPA-exposure females, we found down-regulation of Maoa and S100b in the thalamus. Therefore, it could be argued that BPA, albeit subtle, may interfere with the process of the development of the barrel and its related structures, especially in the early postnatal period. Our findings that serotonin and its metabolites increased in the thalamus in the BPA-exposure group, as measured by high-performance liquid chromatography [30] may support this notion.
Our results showed that the barrel architecture at adulthood and the development at an early postnatal age were not significantly different between the BPA-exposure group and the controls. These findings indicated that the prenatal exposure to low doses of BPA did not affect the trigeminal projection and the thalamic relay to the cortical barrel, although the developmental expression of genes especially associated with the serotonergic system was transiently altered in some areas by the exposure to BPA. The spatiotemporal expression of these genes is critical for the developmental process of the formation of the barrel, barrelloid, and barellette to proceed properly. We are currently studying how the cellular expression of mRNAs of those genes will be altered by exposure to BPA during postnatal development.
V. Conclusions
We studied the size and patterning of the cortical barrel as well as the relay nuclei of the thalamus and brainstem after prenatal and lactational exposure to low doses of BPA, since corticothalamic reciprocal projection was abnormally formed in mice exposed to BPA prenatally. Although the expression of genes responsible for the barrel development were varied by the exposure, sex, and age, no differences in the size and the patterning of the posterior medial barrel subfield, the barreloid and the barrelette were shown between the BPA-exposure and control groups. Our study indicated that the formation of the trigeminal projection and the thalamic relay to the cortical barrel is spared after BPA exposure, although prenatal exposure to low doses of BPA disrupted the corticothalamic projection.
VI. Disclosure of Conflicts of Interest
None of the authors has any conflict of interest to disclose.
VII. Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research (B) (15390334, 20310036) from the Japan Society for the Promotion of Science (JSPS).
Supplementary Material
Fig. S1
VIII. References
- 1.Abdel-Majid R. M., Leong W. L., Neumann P. E. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat. Genet. 1988;19:289–291. doi: 10.1038/980. [DOI] [PubMed] [Google Scholar]
- 2.Belford G. R., Killackey H. P. The sensitive period in the development of the trigeminal system of the neonatal rat. J. Comp. Neurol. 1980;193:335–350. doi: 10.1002/cne.901930203. [DOI] [PubMed] [Google Scholar]
- 3.Calafat A. M., Weuve J., Ye X., Jia L. T., Hu H., Ringer S., Huttner K., Hauser R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ. Health Perspect. 2009;117:639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cases O., Vitalis T., Seif I., De Maeyer E., Sotelo C., Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 5.Donovan S. L., Mamounas L. A., Anne M., Andrews A. M., Blue M. E., McCasland J. S. GAP-43 is critical for normal development of the serotonergic innervation in forebrain. J. Neurosci. 2002;22:3543–3552. doi: 10.1523/JNEUROSCI.22-09-03543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donovan S. L., McCasland J. S. GAP-43 is critical for normal targeting of thalamocortical and corticothalamic, but not trigeminothalamic axons in the whisker barrel system. Somatosens. Mot. Res. 2008;25:33–47. doi: 10.1080/08990220701830696. [DOI] [PubMed] [Google Scholar]
- 7.Durham D., Woolsey T. A. Effects of neonatal whisker lesions on mouse central trigeminal pathways. J. Comp. Neurol. 1984;223:424–447. doi: 10.1002/cne.902230308. [DOI] [PubMed] [Google Scholar]
- 8.Erzurumlu R. S., Kind P. C. Neural activity; sculptor of ‘barrels’ in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox K. The role of excitatory amino acid transmission in development and plasticity of SI barrel cortex. Prog. Brain Res. 1996;108:219–234. doi: 10.1016/s0079-6123(08)62542-x. [DOI] [PubMed] [Google Scholar]
- 10.Franklin K., Paxinos G. (ed) The Mouse Brain in Stereotaxic Coordinates, 3rd ed. Elsevier; UK: 2007. [Google Scholar]
- 11.Gheorghita F., Kraftsik R., Dubois R., Welker E. Structural basis for map formation in the thalamocortical pathway of the barrelless mouse. J. Neurosci. 2006;26:10057–10067. doi: 10.1523/JNEUROSCI.1263-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoerder-Suabedissen A., Paulsen O., Molnár Z. Thalamocortical maturation in mice is influenced by body weight. J. Comp. Neurol. 2008;511:415–420. doi: 10.1002/cne.21853. [DOI] [PubMed] [Google Scholar]
- 13.Inan M., Lu H. C., Albright M. J., She W. C., Crair M. C. Barrel map development relies on protein kinase A regulatory subunit II-mediated cAMP signaling. J. Neurosci. 2006;26:4338–4349. doi: 10.1523/JNEUROSCI.3745-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ince-Dunn G., Hall B. J., Ghosh A. Regulation of thalamocortical patterning and synaptic maturation by NeuroD2. Neuron. 2006;49:683–695. doi: 10.1016/j.neuron.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Itami C., Kimura F., Nakamura S. Brain-derived neurotrophic factor regulates the maturation of layer 4 fast-spiking cells after the second postnatal week in the developing barrel cortex. J. Neurosci. 2007;27:2241–2252. doi: 10.1523/JNEUROSCI.3345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwasato T., Inan M., Kanki H., Erzurumlu R. S., Itohara S., Crair M. C. Cortical adenylyl cyclase 1 is required for thalamocortical synapse maturation and aspects of layer IV barrel development. J. Neurosci. 2008;28:5931–5943. doi: 10.1523/JNEUROSCI.0815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jan T. A., Lu L., Li C-X., Williams R. W., Waters R. S. Genetic analysis of posterior medial barrel subfield (PMBSF) size in somatosensory cortex (SI) in recombinant inbred strains of mice. BMC Neurosci. 2008;9:3. doi: 10.1186/1471-2202-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee L. J., Iwasato T., Itohara S., Erzurumlu R. S. Exuberant thalamocortical axon arborization in cortex-specific NMDAR1 knockout mice. J. Comp. Neurol. 2005;485:280–292. doi: 10.1002/cne.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liguz-Lecznar M., Skangiel-Kramska J. Vesicular glutamate transporters VGLUT1 and VGLUT2 in the developing mouse barrel cortex. Int. J. Dev. Neurosci. 2007;25:107–114. doi: 10.1016/j.ijdevneu.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Bendito G., Molnar Z. Thalamocortical development: how are we going to get there? Nat. Rev. Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- 21.Lu H. C., She W. C., Plas D. T., Neumann P. E., Janz R., Crair M. C. Adenylyl cyclase I regulates AMPA receptor trafficking during mouse cortical ‘barrel’ map development. Nat. Neurosci. 2003;6:939–947. doi: 10.1038/nn1106. [DOI] [PubMed] [Google Scholar]
- 22.Lush M. E., Ma L., Parada L. F. TrkB signaling regulates the developmental maturation of the somatosensory cortex. Int. J. Dev. Neurosci. 2005;23:523–536. doi: 10.1016/j.ijdevneu.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Maier D. L., Mani S., Donovan S. L., Soppet D., Tessarollo L., McCasland J. S., Meiri K. F. Disrupted cortical map and absence of cortical barrels in growth-associated protein (GAP)-43 knockout mice. Proc. Natl. Acad. Sci. U S A. 1999;96:9397–9402. doi: 10.1073/pnas.96.16.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medina-Aguirre I., Gutiérrez-Ospina G., Hernández-Rodríguez J., Boyzo A., Manjarrez-Gutiérrez G. Development of 5-HT1B, SERT and thalamo-cortical afferents in early nutrionally restricted rats: An emerging explanation for delayed barrel formation. Int. J. Dev. Neurosci. 2008;26:225–231. doi: 10.1016/j.ijdevneu.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Micheva K. D., Beaulieu C. Development and plasticity of the inhibitory neocortical circuitry with an emphasis on the rodent barrel field: a review. Can. J. Physiol. Pharmacol. 1997;75:470–478. [PubMed] [Google Scholar]
- 26.Miyakoshi T., Miyajima K., Takekoshi S., Osamura R. Y. The influence of endocrine disrupting chemicals on the proliferation of ERalpha knockdown-human breast cancer cell line MCF-7: new attempts by RNAi technology. Acta Histochem. Cytochem. 2009;42:23–28. doi: 10.1267/ahc.08036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muneoka K. T., Borella A., Whitaker-Azmitia P. M. Transient expression of S-100b immunostaining in developing thalamus and somatosensory cortex of rat. Brain Res. Dev. Brain Res. 2003;142:101–104. doi: 10.1016/s0165-3806(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K., Itoh K., Yaoi T., Fujiwara Y., Sugimoto T., Fushiki S. Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of bisphenol A. J. Neurosci. Res. 2006;84:1197–1205. doi: 10.1002/jnr.21020. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura K., Itoh K., Sugimoto T., Fushiki S. Prenatal exposure to bisphenol A affects adult murine neocortical structure. Neurosci. Lett. 2007;420:100–105. doi: 10.1016/j.neulet.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura K., Itoh K., Yoshimoto K., Sugimoto T., Fushiki S. Prenatal and lactational exposure to low-doses of bisphenol A alters brain monoamine concentration in adult mice. Neurosci. Lett. 2010;484:66–70. doi: 10.1016/j.neulet.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 31.NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphnol A. NIH Publication No. 08-5994, 2008.
- 32.Ohsaki K., Nakamura S. Instructive role of a peripheral pattern for the central patterning of the trigeminal projection at the brainstem and thalamus reversed by an artificially altered whisker pattern. Neuroscience. 2006;141:1899–1908. doi: 10.1016/j.neuroscience.2006.04.082. [DOI] [PubMed] [Google Scholar]
- 33.O’Leary D. D., Chou S. J., Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Richter C. A., Bimbaum L. S., Farabollini F., Newbold R. R., Rubin B. S., Talsness C. E., Vandenbergh J. G., Walser-Kuntz D. R., vom Saal F. S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schafer T. E., Lapp C. A., Hanes C. M., Lewis J. B., Wataha J. C., Schuster G. S. Estrogenicity of bisphenol A and bisphenol A dimethacrylate in vitro. J. Biomed. Mater. Res. 1999;45:192–197. doi: 10.1002/(sici)1097-4636(19990605)45:3<192::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 36.Shimogori T., Grove E. A. Fibroblast growth factor 8 regulates neocortical guidance of area-specific thalamic innervation. J. Neurosci. 2005;25:6550–6560. doi: 10.1523/JNEUROSCI.0453-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stahlhut R. W., Welshons W. V., Swan S. H. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ. Health Perspect. 2009;117:784–789. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sur M., Rubenstein J. L. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- 39.Takasaki C., Okada R., Mitani A., Fukaya M., Yamasaki M., Fujihara Y., Shirakawa T., Tanaka K., Watanabe M. Glutamate transporters regulate lesion-induced plasticity in the developing somatosensory cortex. J. Neurosci. 2008;28:4995–5006. doi: 10.1523/JNEUROSCI.0861-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tando S., Itoh K., Yaoi T., Ikeda J., Fujiwara Y., Fushiki S. Effects of pre- and neonatal exposure to bisphenol A on murine brain development. Brain Dev. 2007;29:352–356. doi: 10.1016/j.braindev.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Vitalis T., Cases O., Callebert J., Launay J. M., Price D. J., Seif I., Gaspar P. Effects of monoamine oxidase A inhibition on barrel formation in the mouse somatosensory cortex: determination of a sensitive developmental period. J. Comp. Neurol. 1998;393:169–184. doi: 10.1002/(sici)1096-9861(19980406)393:2<169::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Watson R. F., Abdel-Majid R. M., Barnett M. W., Willis B. S., Katsnelson A., Gillingwater T. H., McKnight G. S., Kind P. C., Neumann P. E. Involvement of protein kinase A in patterning of the mouse somatosensory cortex. J. Neurosci. 2006;26:5393–5401. doi: 10.1523/JNEUROSCI.0750-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welker E., Armstrong-James M., Bronchti G., Ourednik W., Gheorghita-Baechler F., Dubois R., Guernsey D. L., Van der Loos H., Neumann P. E. Altered sensory processing in the somatosensory cortex of the mouse mutant barrelless. Science. 1996;271:1864–1867. doi: 10.1126/science.271.5257.1864. [DOI] [PubMed] [Google Scholar]
- 44.Wijetunge L. S., Till S. M., Gillingwater T. H., Ingham C. A., Kind P. C. mGluR5 regulates glutamate-dependent development of the mouse somatosensory cortex. J. Neurosci. 2008;28:13028–13037. doi: 10.1523/JNEUROSCI.2600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y., Sari Y., Zhou F. C. Selective serotonin reuptake inhibitor disrupts organization of thalamocortical somatosensory barrels during development. Brain Res. Dev. Brain Res. 2004;150:151–161. doi: 10.1016/j.devbrainres.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Yaoi T., Itoh K., Nakamura K., Ogi H., Fujiwara Y., Fushiki S. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem. Biophys. Res. Commun. 2008;376:563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1