Abstract
One of the major challenges of surgical neuropathology is the distinction of diffuse astrocytoma (World Health Organization [WHO] grade II) from astrocytosis. The most commonly used ancillary tool to solve this problem is p53 immunohistochemistry (IHC), but this is neither sensitive nor specific. Isocitrate dehydrogenase 1 (IDH1) mutations are common in lower grade gliomas, with most causing a specific amino acid change (R132H) that can be detected with a monoclonal antibody. IDH2 mutations are rare, but also occur in gliomas. In addition, gains of chromosome 7 are common in gliomas. In this study we assessed the status of p53, IDH1/2 and chromosome 7 to determine the most useful panel to distinguish astrocytoma from astrocytosis. We studied biopsy specimens from 21 WHO grade II diffuse astrocytomas and 20 reactive conditions. The single most sensitive test to identify astrocytoma is fluorescence in situ hybridization (FISH) for chromosome 7 gain (76.2%). The combination of p53 and mutant IDH1 IHC provides a higher sensitivity (71.4%) than either test alone (47.8%); this combination offers a practical initial approach for the surgical pathologist. The best overall sensitivity (95%) is achieved when FISH for chromosome 7 gain is added to the p53-mutant IDH1 IHC panel.
Keywords: Astrocytoma, Astrocytosis, Chromosome 7, FISH, IDH1, Immunohistochemistry, p53
INTRODUCTION
Diffuse astrocytoma (World Health Organization [WHO] grade II) is an infiltrating tumor that may be difficult to diagnose on standard hematoxylin and eosin stains, particularly on small biopsies. The major differential diagnosis is reactive astrocytosis in the setting of a non-neoplastic process. This differential diagnosis is a critically important one, i.e. if the pathologist cannot make the decision with confidence, proper treatment is delayed and there likely is a need for a second biopsy. The most commonly used markers to differentiate astrocytoma from astrocytosis are immunohistochemical stains for glial fibrillary acid protein (GFAP), proliferation markers (e.g. Ki-67) and p53.
GFAP is used to highlight astrocyte distribution, architecture and types of processes. Astrocytosis should feature evenly spaced astrocytes with multiple, thin long radiating glial processes. In contrast, diffuse astrocytoma has astrocytic cells that cluster and have shorter, thicker processes. However, these stains do not always permit accurate diagnosis, particularly because reactive astrocytosis occurs in astrocytomas, often at the infiltrating edges.
Proliferation markers such as the Ki-67 antibody have also been used to differentiate astrocytosis from astrocytoma. Reactive astrocytes proliferate, but usually have low proliferation indices of around 1%. However, proliferation indices can be high in some reactive conditions, for example, 13% in progressive multifocal leukoencephalopathy (PML) (1). Moreover, WHO grade II diffuse astrocytomas usually have low proliferation indices (ranging from 1.7 to 4.2%), similar to those of reactive conditions (2, 3). Nonetheless, although the proliferation index itself is not useful in making the distinction, because the Ki-67 antibody is a nuclear stain, it may help to highlight cytologically atypical, presumably neoplastic nuclei within a low cellularity biopsy. Such information, however, is not definitive for diagnosis.
TP53 mutations are common and early genetic alterations in astrocytomas, with mutations often affecting particular hotspots such as exons 248 and 273 (4). Nearly 60% of WHO grade II diffuse astrocytomas have TP53 mutations (5). Because most mutant forms of p53 have a longer half-life, strong and diffuse staining for p53 by immunohistochemistry (IHC) is used as a marker of astrocytoma. However, not all mutations lead to an increased half life (4); some wild-type proteins may accumulate (6) and some reactive conditions (notably PML) may show conspicuous p53 expression (7, 8). All of these factors limit the sensitivity and specificity of p53 IHC for diagnosing astrocytomas.
Other genetic changes that are common in low-grade astrocytomas include copy number gain of chromosome 7 (9, 10) and IDH gene mutations (11, 12); these changes have not been seen in astrocytosis (13–15). Chromosome 7 gain has been reported in up to two thirds of astrocytomas, including both diffuse and pilocytic forms (16, 17). Isocitrate dehydrogenase 1 (IDH1) gene mutations have been reported in up to 70% of grade II astrocytomas (18, 19). Mutations in the IDH2 gene also occur, but are less common (11, 12). More than 90% of IDH1 mutations involve substitution of arginine by histidine at codon 132 (R132H). This amino acid change can be detected by IHC with a monoclonal antibody (20). IDH mutations have not been found in reactive astrocytic conditions (15) and appear to be specific to gliomas. There are, however, rare exceptions that do not enter into the differential diagnosis of grade II astrocytoma vs. astrocytosis, such as acute myeloid leukemia, prostate cancer and paraganglioma (21–23). We have shown that mutant IDH1 (R132H)-specific IHC can be useful in the differential diagnosis of astrocytosis from astrocytoma, but this approach detected less than half of the studied astrocytomas (14).
To develop a practical panel to distinguish diffuse astrocytoma from reactive astrocytosis, we therefore evaluated a combination of immunohistochemical and molecular techniques to detect TP53 and IDH1/2 mutations as well as copy number gain of chromosome 7. The tests were analyzed individually and in combination to identify the most sensitive and practical combination for practicing neuropathologists.
MATERIALS AND METHODS
Cases
We analyzed 21 WHO grade II diffuse astrocytomas and 20 reactive conditions with astrocytosis (10 resections for epilepsy, 7 infarcts, 2 evacuated hematomas, and 1 traumatic brain injury), as previously described (14). All samples were formalin fixed-paraffin-embedded (FFPE) tissue from surgical pathology specimens collected at the Massachusetts General Hospital. The study was conducted following Institutional Review Board approval.
p53 and IDH1 (R132H) IHC
Immunohistochemical staining was performed on BenchMark XT automated tissue staining systems (Ventana Medical Systems, Inc., Tucson, AZ) using an anti-p53 mouse monoclonal antibody (IgG1/k) (Ventana) or an anti-human IDH1-R132H mouse monoclonal antibody culture supernatant (provided by Dr. Andreas von Deimling, University of Heidelberg, Heidelberg Germany), as previously described (14). Of note, the IDH1-R132H mouse monoclonal antibody used in this study is the same as the currently commercially available (Dianova, Hamburg, Germany). The results were scored as positive or negative staining independently by 2 authors (SCP and MJ).
FISH for Chromosome 7 Copy Number Gain
Four-µm-thick FFPE sections were used for 3-color FISH using the following probes: BAC clone (CTD-2113A18), specific for epidermal growth factor (EGFR), Spectrum Red (Abbott Molecular, Abbott Park, IL), for the short arm of chromosome 7; BAC clone (CTB-1013N12), specific for C-MET, Spectrum Green (Abbott Molecular), for the long arm of chromosome 7; and a control probe, Cep 7 (Abbott-Vysis 06J54-027), Spectrum Aqua (Abbott Molecular), for the centromere of chromosome 7. One hundred nuclei were scored by 1 author (SCP) for each case. The number of signals for each probe was recorded with 3 or more signals considered as copy number gain. Astrocytosis cases were counted first to determine the baseline percentage of non-neoplastic cells that could display copy number gain. We then evaluated the tumor cases and designated astrocytomas as positive when the percentage of cells with copy number gain was 2 or more standard deviations above the mean of the reactive cases.
TP53, IDH1 and IDH2 Mutation Analysis by SNaPshot Genotyping
A 1.5-mm-tissue core was obtained from paraffin blocks, deparaffinized at 95°C for 40 minutes and incubated overnight at 55°C with proteinase K solution. DNA extraction was then performed using the QIAamp DNA Blood mini kit (QIAGEN, Valencia, CA).
Mutational analysis of TP53 at hotspot codons R175, G245, R248, R273 and R306 was performed using a recently developed tumor genotyping protocol that applies SNaPshot multiplex technology (Life Technologies/Applied Biosystems, Carlsbad, CA) and that detects multiple mutations in tumor DNA extracted from FFPE tissue (24).
Four SNaPshot assays were used to perform targeted mutational analysis at codon R132 in IDH1 (nucleotide positions c.394 and c.395) and at codon R172 in IDH2 (c.514 and c.515). The following primers were used for PCR: IDH1 exon 4, 5’-ACGTTGGATGGGCTTGTGAGTGGATGGGTA-3’ (forward) and 5’-ACGTTGGATGGCAAAATCACATTATTGCCAAC-3’ (reverse) and IDH2 exon 4, 5’-ACGTTGGATGAACATCCCACGCCTAGTCC-3’ (forward), and 5’-ACGTTGGATGCAGTGGATCCCCTCTCCAC-3’ (reverse). The IDH1 and IDH2 amplification reactions were run independently using previously described conditions (24). Briefly, PCR was performed in a volume of 10 µl, containing 0.5 units of Platinum Taq polymerase (Invitrogen, Carlsbad, CA), 30 nmol MgCl2, 3 nmol dNTPs (Invitrogen), 0.6 pmol of amplification primers (IDT, Coralville, IA) and 20 ng of FFPE-derived tumor DNA. The IDH1 extension reactions were run individually and the IDH2 assays were run in a duplex SNaPshot reaction using the following primers: IDH1.394 extR 5’-GACTGACTGGACTGACTGACTGACTGACTGGACTGACTGACTGAGATCCCCATAAGCATGAC-3’, IDH1.395 extR 5’-TGATCCCCATAAGCATGA-3’, IDH2.514 extF 5’-GACTGACTGACTGACTGACTGACTGACTGGACTGACTGACTGACTGACTGGACTGACTGACCCATCACCATTGGC-3’ and IDH2.515 extR 5’-GACTGACTGACTGACTGACTGACTGACTGACTGACTGGACTGACTGACTGACTGACTGGACTGACTGAGCCATGGGCGTGC-3’. Single-base primer extension was performed in a volume of 10 µl, containing 3 µl of purified PCR product, 2.5 µl of SNaPshot Multiplex Ready Reaction mix, and extension primers (0.4 pmol for the IDH1.394 assay, 0.6 pmol for the IDH1.395 assay, and 0.4 pmol of each extension primer for the IDH2.514/IDH2.515 duplex reaction). Thermocycling conditions were as previously described (24).
RESULTS
p53 IHC
None of the astrocytosis cases had positive nuclear staining in astrocytes; 1 case had light nuclear staining of macrophages that were easily identifiable as such. Strong, diffuse nuclear staining was present in 10 of the 21 astrocytomas, providing a sensitivity of 47.6% (Fig. 1).
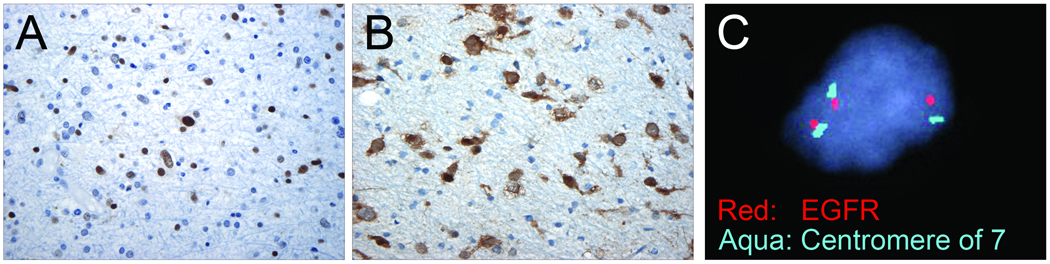
Figure 1.
World Health Organization grade II diffuse astrocytoma immunoreactive for p53 and mutant isocitrate dehydrogenase 1 (IDH1) and with copy number gain of chromosome 7. (A) p53 immunohistochemistry (IHC) shows strong, diffuse, nuclear positivity. (B) Mutant-specific IDH1 (R32H) IHC shows strong granular cytoplasmic and sometimes nuclear immunoreactivity in infiltrating tumor cells in the background of normal non-neoplastic brain parenchyma. (C) Fluorescence in situ hybridization analysis for chromosome 7 copy number detects copy number gain for two probes (Spectrum Red EGFR probe, Spectrum Aqua centromere 7). Similar results were obtained with the C-MET probe (data not shown). The majority of cases showed gain of all 3 markers, likely representing gain of the entire chromosome.
TP53 Mutation Analysis
The assay used screens for hotspot mutations at 5 codons, which include the 3 most common TP53 mutations found in astrocytomas. No mutations were present in the astrocytosis cohort, but 4 of the 21 astrocytomas had mutations: 2 R273C, 1 R248Q and 1 R175H. Therefore, the assay had a sensitivity of 19% for detecting tumor. The 4 cases with TP53 mutations were also positive on p53 IHC (Table 1).
Table 1.
Summary of Immunohistochemistry and Molecular Results for p53, IDH1/2 and Chromosome 7 in 21 WHO Grade II Diffuse Astrocytomas
| Immunohistochemistry | Molecular Tests | ||||||
|---|---|---|---|---|---|---|---|
| Case # | p53 | Mutant- specific (R132H) IDH1 |
IDH1 SNaPshot |
IDH2 SNaPshot |
TP53 SNaPshot |
FISH (+7) | |
| xT 6302 | -- | -- | WT | WT | WT | + | |
| xT 6303 | + | + | R132H | WT | R273C | + | |
| xT 6375 | + | -- | WT | WT | WT | + | |
| xT 5344 | + | + | R132H | WT | WT | + | |
| xT 6304 | -- | + | R132H | WT | WT | + | |
| xT 6029 | + | -- | R 132C | WT | WT | + | |
| xT 4852 | -- | -- | WT | WT | WT | + | |
| xT 4829 | + | + | R132H | WT | R175H | + | |
| xT 4917 | -- | -- | WT | WT | WT | -- | |
| xT 3991 | -- | -- | WT | R172K | WT | + | |
| xT 4237 | -- | + | R132H | WT | WT | -- | |
| xT 3798 | -- | + | R132H | WT | WT | -- | |
| xT 6305 | -- | + | R132H | WT | WT | -- | |
| xT 3362 | -- | -- | WT | WT | WT | + | |
| xT 3247 | -- | -- | R132G | WT | WT | + | |
| xT 3539 | + | -- | R132C | WT | R273C | + | |
| xT 6171 | + | + | R132H | WT | WT | -- | |
| xT 6376 | -- | + | R132H | WT | WT | + | |
| xT 6306 | + | + | R132H | WT | R248Q | + | |
| xT 4334 | + | -- | WT | WT | WT | + | |
| xT 6377 | + | -- | R132C | WT | WT | + | |
+7: copy number gain of chromosome 7
-- = negative; + = positive; FISH = fluorescence in situ hybridization; WT: wild-type IDH1/2 = isocitrate dehydrogenase 1/2.
Mutant-specific (R132H) IDH1 IHC
None of the astrocytosis cases were positive for mutant-specific IDH1 IHC, whereas 10 of 21 astrocytomas were immunoreactive (Fig. 1), yielding a sensitivity for detecting tumor of 47.6% (Table 1). Five of the 10 mutant IDH1-immunopositive astrocytomas were also immunoreactive for p53.
IDH1/2 Mutation Analysis
IDH1/2 mutations were identified in 15 tumors. The most common IDH1 mutation identified was R132H (10/21 astrocytomas). Three cases had IDH1 R132C and 1 had R132G mutation. Only 1 case was positive for an IDH2 mutation (R172K) (Table 1). The sensitivity for diagnosing astrocytoma with mutation analysis by IDH1 or IDH2 independently was, therefore, 66.7% and 4.7%, respectively; the sensitivity increased to 71.4% when used in combination (Table 2).
Table 2.
Sensitivity and Specificity Values and Positive and Negative Predictive Values of Single and Combined Tests for the Diagnosis of WHO Grade II Diffuse Astrocytomas
| p53 | Mutant-specific (R132H) IDH1 |
IDH1 SNaPshot |
FISH (+7) | Sensitivity (%) |
Specificity (%) |
PPV | NPV |
|---|---|---|---|---|---|---|---|
| X | 47.6 | 100 | 100 | 64.5 | |||
| X | 47.6 | 100 | 100 | 64.5 | |||
| X | 66.7 | 100 | 100 | 74 | |||
| X | 76.2 | 100 | 100 | 80 | |||
| X | X | 71.4 | 100 | 100 | 76.9 | ||
| X | X | 76.2 | 100 | 100 | 80 | ||
| X | X | 80.9 | 100 | 100 | 83.3 | ||
| X | X | 66.7 | 100 | 100 | 74 | ||
| X | X | 95 | 100 | 100 | 95 | ||
| X | X | 95 | 100 | 100 | 95 | ||
| X | X | X | 76.2 | 100 | 100 | 80 | |
| X | X | X | 95 | 100 | 100 | 95 | |
| X | X | X | 95 | 100 | 100 | 95 | |
| X | X | X | 95 | 100 | 100 | 95 | |
| X | X | X | X | 95 | 100 | 100 | 95 |
X = test applied; PPV = positive predictive value; NPV = negative predictive value; FISH = fluorescence in situ hybridization; IDH1 = isocitrate dehydrogenase.
Correlation of IDH1 Mutation Analysis and Mutant-specific IDH1 IHC
All IDH1 R132H mutant astrocytomas were strongly immunopositive for mutant-specific (R132H) IDH1 by IHC, with the exception of 1 block in case xT6305 (previously reported as negative in [14]). Repeat mutant-specific IDH1 IHC on this block revealed rare positive cells. Notably, this block was from previously frozen tissue, whereas no other blocks studied had been previously frozen. Thus, both the solid and infiltrating tumor blocks tested positive for the same mutation, but only the solid tumor stained strongly for mutant IDH1.
Copy Number Gain of Chromosome 7 by FISH Analysis
Reactive astrocytosis cases had a baseline mean of 2.5% of cells showing chromosome 7 centromere gain (range = 0–5% of cells). Most of these rare cells with copy number gain had 3 or 4 signals. Sixteen of 21 astrocytomas demonstrated chromosome 7 centromere gain, defined as 2 or more standard deviations (>6%) beyond the mean of the reactive cases (range = 7%–52%; mean = 19%); most of them exhibited gain at all 3 markers and therefore most likely representing gain of the entire chromosome (Fig. 1). The sensitivity of chromosome 7 FISH for diagnosing astrocytoma was therefore 76.2%.
Combination Panels
Of the 15 astrocytomas with IDH1/2 mutations, 7 did not have p53 alterations by IHC or TP53 mutations on the SNaPshot assay; of those 7 cases, 5 had IDH1 R132H mutation, 1 had IDH1 R132G mutation and 1 had the IDH2 R172K mutation. Of the 8 cases with p53 and IDH1 alterations, 5 had IDH1 R132H mutation and 3 had IDH1 R132C mutation.
Sensitivity was calculated for all possible combinations of tests to identify the best panel for identifying astrocytoma (Table 2). The assays with the lowest sensitivities when performed alone were TP53 hotspots and IDH2 mutation analyses (19% and 4.7%, respectively). When these 2 tests were combined with the others tests, they did not significantly increase sensitivity (data not shown).
The combination of p53 and mutant-specific IDH1 IHC has 100% specificity and a much higher sensitivity (71.4%) than that of either test by itself. FISH for chromosome 7 gain had the best overall sensitivity of a single test (76.2%), and had even better sensitivity when combined with IDH1 IHC or mutation analyses (95%) than when combined with p53 IHC alone (80.9%). When 3 or more tests are combined, the best sensitivity is achieved when chromosome 7 FISH is included in the panel (95%) (Table 2).
DISCUSSION
The distinction of infiltrating astrocytoma from astrocytosis is one of the most difficult differential diagnoses faced by surgical pathologists. Each of the commonly used approaches to address this differential diagnosis has problems with sensitivity, and some have problems with specificity. We, therefore, combined the currently most promising molecular and immunohistochemical assays to develop a diagnostic panel. We found that a combination of assays that included mutant IDH1 and p53 IHC with chromosome 7 FISH was 100% specific and 95% sensitive for diagnosing astrocytoma.
To detect copy number gain, we used a FISH assay that is commonly used in our molecular diagnostic laboratory: 3-color FISH for EGFR, C-MET and the centromere of chromosome 7. This assay proved the most sensitive single test to detect tumor in this study (sensitivity of 76.2%). Unfortunately, the technique has several practical limitations, particularly in the setting of a low cellularity infiltrating astrocytoma. Furthermore, this technique is time consuming and somewhat challenging because one must count individual signals from at least 100 nuclei to ensure adequate evaluation of diagnostic cells in low cellularity biopsies. In addition to low cellularity, brain autofluorescence can create difficulty counting the signals, particularly if the biopsy includes the cerebral cortex. Nonetheless, FISH analysis of interphase nuclei in FFPE tissue has become standard in many pathology laboratories (25), and for laboratories familiar with FISH these difficulties are readily surmountable. Therefore, FISH provides a powerful differential diagnostic tool in this setting, either by itself or in combination with IHC.
The second most useful individual assay involved the identification of IDH1 mutation. Because IDH1 and IDH2 mutations nearly always target a single codon in each gene (132 for IDH1 and 172 for IDH2), molecular approaches can be designed for efficient detection. In addition, because >90% of IDH1 mutations are substitutions of arginine for histidine (R132H), IHC can be used to identify the most common IDH1 mutations. We previously reported the utility of the mutant-specific (R132H) antibody in the differential diagnosis of astrocytoma vs. astrocytosis in the same series of cases used in this study (14). Here, we combined the previous immunohistochemical approach with mutation detection at the DNA level. We found the common IDH1 R132H mutation in 71.4% of the grade II astrocytomas with mutations (10/14), which is consistent with the literature (11, 12).
In the present series, all astrocytomas with the R132H IDH1 mutation were strongly positive by mutant-specific IDH1 IHC. Of note, in our initial series, case xT6305 had been reported as immunonegative on mutant IDH1 IHC (14). The tumor had the R132H IDH1 mutation on SNaPshot analysis and we therefore repeated IHC on 2 blocks of this tumor: 1) the original block, which contained low cellularity tumor infiltrating cerebral cortex and which had been previously frozen; and 2) a block of cellular, "solid" tumor. Repeat mutant IDH1 IHC showed strong staining in the "solid" component but only scattered positive cells in the infiltrating tumor. The explanation for this variance is not clear but could be related to prior freezing of the tissue (none of the other blocks in this study had been previously frozen), obfuscating immunoreactivity, or to greater sensitivity of mutation detection techniques over IHC in low cellularity biopsies with only rare infiltrating cells. A practical corollary of this observation could be that neuropathologists may need to select previously unfrozen tissue for mutant IDH1 IHC analysis; however, this question clearly requires further study. In general, therefore, mutant-specific (R132H) IDH1 IHC is a sensitive and practical tool to diagnose grade II astrocytoma in FFPE material. The mutant-specific (R132H) IDH1 antibody used in this study is now commercially available (Dianova) and we have standardized the commercial antibody to obtain strong immunoreactivity identical to that described in this study. On the other hand, if DNA mutation detection technology is available for IDH1/2 mutations, this may have better sensitivity than IHC for mutant specific IDH1 by itself.
TP53 mutations involve several regions of the gene, making screening for all mutations impractical and costly at present. In this study, we screened for the 3 most common mutations found in diffuse astrocytomas (exons 175, 248 and 273), which have been reported in approximately 26% of such tumors in a population-based study (5). In our study, only 4 of 21 cases (19%) had TP53 mutations by SNaPshot analysis, but 10 cases were strongly immunoreactive for p53 protein (including the 4 cases with TP53 mutations). Therefore, p53 IHC is a more sensitive and less expensive method for diagnosing astrocytoma, but by itself has a sensitivity of only 47%.
It has been suggested that IDH1 mutation is an early change in gliomagenesis that antedates TP53 mutation (19). In our study, 7 astrocytomas with IDH1/2 mutations did not show p53 alterations, supporting this hypothesis. On the other hand, 2 cases (xT 6375 and xT4334) had p53 accumulation on IHC but no identified IDH1/2 mutation. Of note, both of these cases had chromosome 7 gain. Thus, the combination of p53 and mutant-specific (R132H) IDH1 IHC is an easy and relative sensitive combination of tests (71.4%) to detect tumor and is more sensitive than the use of either mutant IDH1 or p53 IHC.
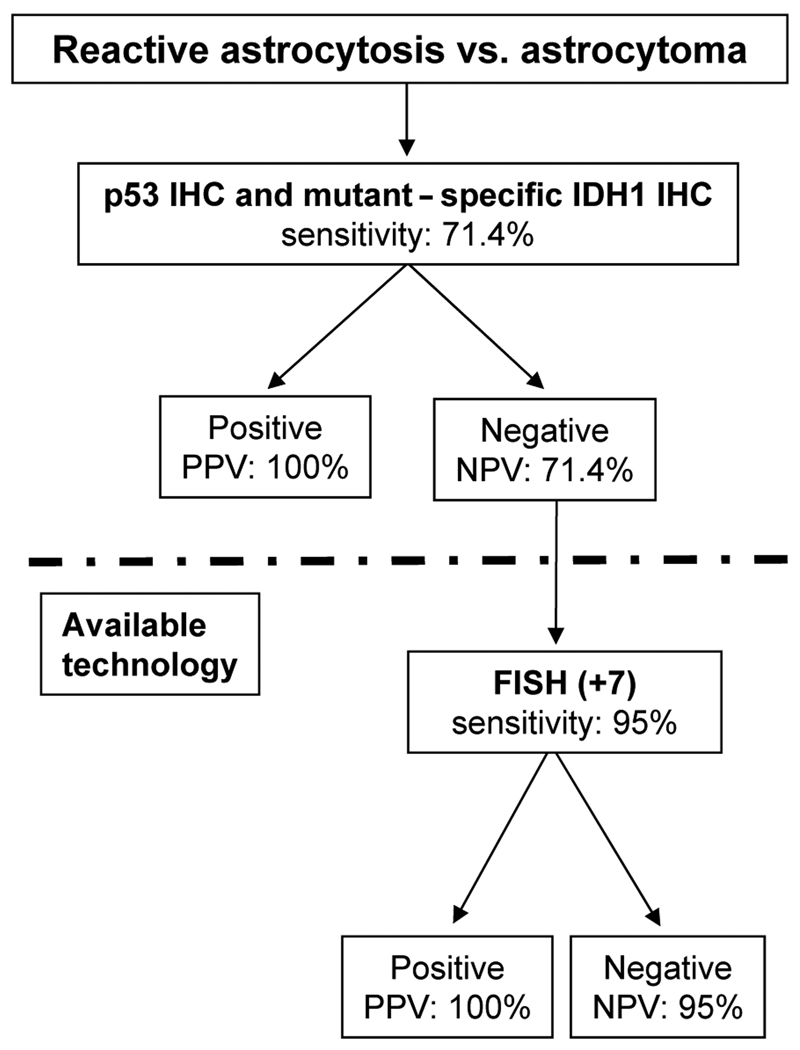
We have shown that a panel that includes IDH1, IDH2 and TP53 mutation analysis, FISH for chromosome 7 gain, as well as mutant-specific IDH1 and p53 IHC, is a powerful addition to the diagnostic armamentarium. In this study the assays were 100% specific. Moreover, only a single astrocytoma was negative on all assays. Our series was relatively small, but involved carefully selected, “classic” examples of grade II astrocytoma and reactive astrocytosis cases diagnosed at our hospital; the findings require follow-up with a larger series of cases and in other laboratories. Nonetheless, our results enable initial recommendations to be made. The single most sensitive test to diagnose astrocytoma is FISH for chromosome 7 gain; this assay adds the most sensitivity when used in combination with other assays. The best test combination of 2 assays is IDH1 mutation analysis and FISH for gain chromosome 7 (sensitivity 95% and specificity 100%), but both of these assays require technology that may not be available in all neuropathology laboratories. Taking into account ease, availability and practicality, the most attractive combination to detect diffuse astrocytoma is mutant-specific (R132H) IDH1 and p53 IHC (sensitivity 71.4%). Therefore, we propose that when faced with the differential diagnosis of reactive astrocytosis vs. diffuse astrocytoma, pathologists begin with mutant IDH1 and p53 IHC. If these 2 techniques are negative and if the chromosome 7 FISH assay is available and standardized, addition of FISH for chromosome 7 gain will still enable diagnosis of up to 95% of astrocytomas (Fig. 2).
Figure 2.
Suggested algorithm to approach the differential diagnosis of diffuse astrocytoma vs. astrocytosis. IHC = immunohistochemistry; +7 = copy number gain of chromosome 7; Sens = sensitivity; PPV = positive predictive value; NPV = negative predictive value.
The field of diagnostic glioma pathology is changing rapidly as a result of the new knowledge that is amassing from genomic inquiries. Each new genetic discovery requires careful clinical validation and subsequent evaluation of the sensitivity and specificity of the variety of available diagnostic assays for the alteration. As observed for both INI1/SMARC2B1 and IDH1 over the past few years, the most practical assay for a particular genetic change may be at the protein level, with a relatively easy immunohistochemical assay. Importantly though, for each new assay, evaluations must also be made in combinations with other already available approaches because the combinations may prove more powerful and practical than the individual assays.
ACKNOWLEDGMENTS
The authors thank Kristin Bergethon for technical support, Rebecca Betensky for statistical results review and Andreas von Deimling and the Deutsches Krebsforschungszentrum (DKFZ; German Cancer Research Center) for providing the R132H-mutant IDH1 antibody.
This work was supported by NIH CA57683 (DNL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Colodner KJ, Montana RA, Anthony DC, et al. Proliferative potential of human astrocytes. J Neuropathol Exp Neurol. 2005;64:163–169. doi: 10.1093/jnen/64.2.163. [DOI] [PubMed] [Google Scholar]
- 2.Burger PC, Shibata T, Kleihues P. The use of the monoclonal antibody Ki-67 in the identification of proliferating cells: Application to surgical neuropathology. Am J Surg Pathol. 1986;10:611–617. doi: 10.1097/00000478-198609000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Deckert M, Reifenberger G, Wechsler W. Determination of the proliferative potential of human brain tumors using the monoclonal antibody Ki-67. J Cancer Res Clin Oncol. 1989;115:179–188. doi: 10.1007/BF00397921. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN. The p53 gene and protein in human brain tumors. J Neuropathol Exp Neurol. 1994;53:11–21. doi: 10.1097/00005072-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Okamoto Y, Di Patre PL, Burkhard C, et al. Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol. 2004;108:49–56. doi: 10.1007/s00401-004-0861-z. [DOI] [PubMed] [Google Scholar]
- 6.Rubio MP, von Deimling A, Yandell DW, et al. Accumulation of wild type p53 protein in human astrocytomas. Cancer Res. 1993;53:3465–3467. [PubMed] [Google Scholar]
- 7.Ariza A, von Uexkull-Guldeband C, Mate JL, et al. Accumulation of wild-type p53 protein in progressive multifocal leukoencephalopathy: a flow of cytometry and DNA sequencing study. J Neuropathol Exp Neurol. 1996;55:144–149. doi: 10.1097/00005072-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Yaziji H, Massarani-Wafai R, Gujrati M, et al. Role of p53 immunohistochemistry in differentiating reactive gliosis from malignant astrocytic lesions. Am J Surg Pathol. 1996;20:1086–1090. doi: 10.1097/00000478-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Schrock E, Blume C, Meffert MC, et al. Recurrent gain of chromosome arm 7q in low-grade astrocytic tumors studied by comparative genomic hybridization. Genes Chromosomes Cancer. 1996;15:199–205. doi: 10.1002/(SICI)1098-2264(199604)15:4<199::AID-GCC1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Arslantas A, Artan S, Oner U, et al. Genomic alterations in low-grade, anaplastic astrocytomas and glioblastomas. Pathol Oncol Res. 2007;13:39–46. doi: 10.1007/BF02893439. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 12.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada Y, Ohno C, Ueki K, et al. Comparison of numerical change of epidermal growth factor receptor gene among pre- and postradiation glioma, and gliosis, and its clinical use. Brain Tumor Pathol. 2007;24:15–18. doi: 10.1007/s10014-007-0213-5. [DOI] [PubMed] [Google Scholar]
- 14.Camelo-Piragua S, Jansen M, et al. Mutant IDH1-specific immunohistochemistry distinguishes diffuse astrocytoma from astrocytosis. Acta Neuropathol. 2010;119:509–511. doi: 10.1007/s00401-009-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horbinski C, Kofler J, Kelly LM, et al. Diagnostic use of IDH1/2 mutation analysis in routine clinical testing of formalin-fixed, paraffin-embedded glioma tissues. J Neuropathol Exp Neurol. 2009;68:1319–1325. doi: 10.1097/NEN.0b013e3181c391be. [DOI] [PubMed] [Google Scholar]
- 16.Mott RT, Turner KC, Bigner DD, et al. Utility of EGFR and PTEN numerical aberrations in the evaluation of diffusely infiltrating astrocytomas. Laboratory investigation. J Neurosurg. 2008;108:330–335. doi: 10.3171/JNS/2008/108/2/0330. [DOI] [PubMed] [Google Scholar]
- 17.Wessels PH, Twijnstra A, Kessels AG, et al. Gain of chromosome 7, as detected by in situ hybridization, strongly correlates with shorter survival in astrocytoma grade 2. Genes Chromosomes Cancer. 2002;33:279–284. doi: 10.1002/gcc.10029. [DOI] [PubMed] [Google Scholar]
- 18.Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11:341–347. doi: 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capper D, Zentgraf H, Balss J, et al. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118:599–601. doi: 10.1007/s00401-009-0595-z. [DOI] [PubMed] [Google Scholar]
- 21.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaal J, Burnichon N, Korpershoek E, et al. Isocitrate dehydrogenase mutations are rare in pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2010;95:1274–1278. doi: 10.1210/jc.2009-2170. [DOI] [PubMed] [Google Scholar]
- 23.Kang MR, Kim MS, Oh JE, et al. Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int J Cancer. 2009;125:353–355. doi: 10.1002/ijc.24379. [DOI] [PubMed] [Google Scholar]
- 24.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol Med. 2010;2:146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuller CE, Perry A. Fluorescence in situ hybridization (FISH) in diagnostic and investigative neuropathology. Brain Pathol. 2002;12:67–86. doi: 10.1111/j.1750-3639.2002.tb00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]