Abstract
The transmission of herpes simplex virus (HSV)-1 by corneal transplantation has rarely been reported. It is believed that these cases have resulted either from reactivated virus traveling from the trigeminal ganglion to the cornea or from latent HSV-1 in the donor cornea itself. Studies of long-term viral presence in corneal tissue have sought to determine whether there is evidence of true non-neuronal latency, although there are problems in its definition. Recent studies provide new insights into neuronal latency, while similar HSV-1 gene regulation in the cornea may implicate corneal latency in pathophysiology and as a potential risk for transplant recipients. This issue has led to concerns over eye banking, which currently screens for other infectious agents but not HSV-1. Here we review the literature regarding corneal latency and the transmission of HSV-1.
Keywords: corneal latency, donor-host transmission, eye banking, HSV, transplant
Herpes simplex virus (HSV) is believed to be the leading cause of infectious blindness in the developed world, with 400,000 people infected ocularly in the USA [1]. The two serotypes of HSV are HSV-1 and HSV-2, which have different tropisms. Both are able to cause ocular disease, which is facilitated by the ability of the virus to spread in tissue as well as neuronally (Figure 1). However, the vast majority of ocular HSV has been attributed to HSV-1, which is known for infections above the waist, although it is also a cause of genital disease. Most humans are probably infected with HSV-1 orally, with subsequent viral spread to the trigeminal ganglia. Viral replication may occur in the trigeminal ganglia until CD8+ T-cell activity and possibly an inefficiency of the DNA repair mechanism drive the virus into latency [2,3]. During latency the virus remains in a dormant state, characterized by restricted gene expression with the exception of latency-associated transcripts (LATs). Reactivation may be triggered by several factors including stress, UV radiation and fever.
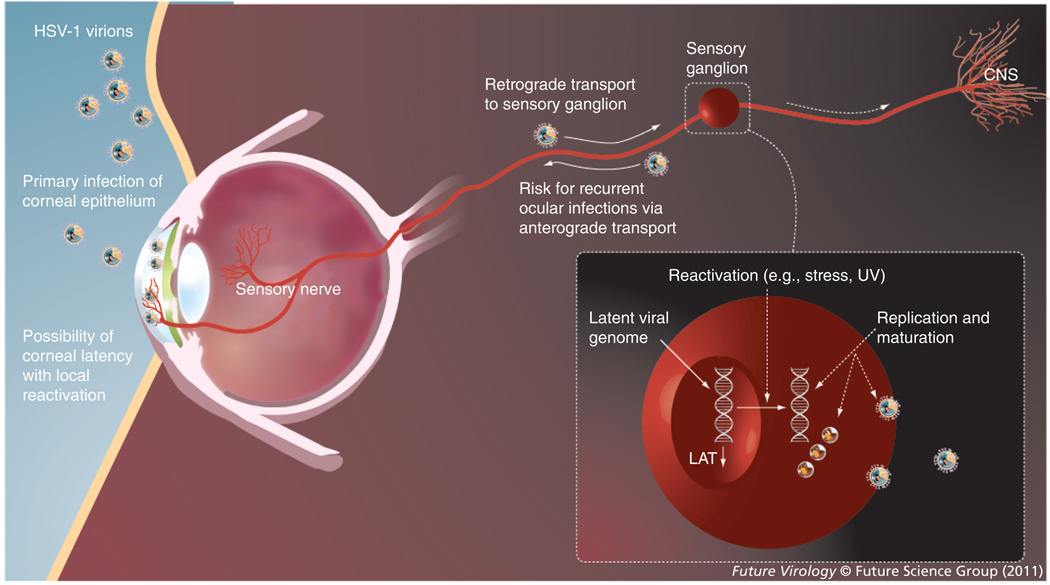
Figure 1. Herpes simplex virus ocular infection, neuronal spread, latency and reactivation.
The HSV virion is shown. The spikes on the envelope represent glycoproteins required for entry and spread. The icosahedral nucleocapsid contains viral DNA. HSV may initially infect the eye or (more commonly) oral mucosa, leading to retrograde spread of the virus along sensory nerves to a sensory ganglion, where it develops a lifelong latent infection. Latency-associated transcripts are expressed in high amounts during latency. Reactivation from latency can lead to ocular infection via anterograde viral spread. The virus may also develop latency in the cornea with local reactivations or occasional migrate to the CNS.
HSV: Herpes simplex virus; LAT: Latency-associated transcripts.
The entry of HSV into ocular tissue may occur with exogenous exposure to the virus or reactivated virus that has traveled to the site of infection. Initial ocular exposure to HSV most often results in infection of the conjunctiva [4]. The mechanism of entry into ocular cells relies on glycoprotein receptors on the cell surface, including nectin-1, herpesvirus entry mediator (HVEM), 3-O sulfated heparan sulfate (3-OS HS) and paired immunoglobulin-like receptor α (PILR-α) [5,6]. Access to these entry receptors may determine if the virus enters from the apical or basolateral surface of cells [7]. Local replication and spread allows the virus to access sensory nerve branches followed by neuronal spread to the trigeminal ganglion or elsewhere.
The issue of corneal latency of HSV has been discussed in the literature and several studies have sought to investigate its molecular and/or clinical basis [8]. This has potential significance in the pathophysiology of ocular herpes, as localized reactivation may contribute to virus and immune-mediated morbidity. Infection of the corneal stroma, in particular, most often occurs in the setting of recurrent disease. Although rare, the virus can also be transmitted by corneal transplantation [9], raising the possibility of non-neuronal, cornea-specific latency. The current article focuses on important aspects of HSV-1 latency in the context of eye infections.
Eye bank screening
Despite many advances in eye banking to allow corneal transplantation to become the most performed allograft procedure today, virtually none of the donor corneas are prescreened for HSV-1. In the 1940s Richard Townley Paton founded the first eye bank in New York (NY, USA) [10]. Donor corneas in the USA were originally kept in a humidified chamber at 4°C until the development of Optisol™, which contained chondroitin sulfate, dextran, vitamins and adenosine triphosphate precursors [11–13]. Corneas in the USA and many parts of the world today are stored in Optisol-GS at 4°C, which has a combination of gentamycin and streptomycin. In much of Europe, organ culture at or near 34°C is commonly used as a method of storage [11,14].
The screening and storage of donor corneas in eye banks evolved as reports of infection and other issues followed transplant procedures [12]. The Eye Bank Association of America (EBAA) now requires that all member eye banks assess corneas by slit-lamp biomicroscopy and perform serologic testing for HIV types 1 and 2, hepatitis B surface antigen (HBsAg), hepatitis C virus (HCV) and syphilis. Donors are also screened based on medical history and cause of death. The EBAA reviews all reported adverse events associated with corneal transplantation and regularly assesses the need for changes in eye banking standards. The European Eye Bank Association and other organizations have also continued to update guidelines, although there is much greater variability in the developing world [15]. Donor corneas are not currently screened for HSV.
Pros & cons of storing donor corneas at 4°C versus 34°C
Among the advantages of the organ culture system are longer preservation (up to 4 weeks versus up to 7–10 days in hypothermic storage), more time for transport, increased possibility of detecting infectious pathogens, as well as HLA typing in some instances [11]. However, disadvantages stem from the need for repeated evaluation of the endothelium, expensive equipment, trained personnel, requirement for changing culture media and variations in media components. Both methods of cornea storage appear to have similar outcomes in terms of graft survival. It is unknown which storage method has a lower possibility of HSV transmission.
HSV recurrence & ocular disease
Much of the ocular morbidity associated with HSV occurs in recurrences over time rather than the initial ocular infection. Recurrences also account for the majority of annual incidence rates in major epidemiologic studies [1]. The majority of adults have seropositivity to HSV-1, and studies using the more sensitive PCR assay suggest that nearly all adults are latently infected in sensory neurons [16]. Interestingly, the virus is intermittently shed via tears among asymptomatic individuals, raising the possibility that HSV-1 may reside in the cornea [17]. Until now the main treatments for ocular herpes inhibit viral replication, and long-term antiviral prophylaxis only partially reduces the recurrence rate [18]. The development and modulation of latency is, therefore, an important topic for clinicians and researchers. Much about this process remains unknown, although it appears to involve both host immune and virus-related factors.
Neuronal latency
The development of neuronal latency occurs in part due to deficiencies in viral replication, although the exact mechanism remains unknown [2]. Some studies have recently demonstrated an active role of the immune response; for example, while CD8+ T cells are responsible for destroying infected cells, they also appear to be involved in latency induction through granzyme B degradation of ICP4, an immediate-early protein required for HSV-1 replication [3]. Evidence also suggests that CD8+ T cells that infiltrate HSV-infected trigeminal ganglia are strain-specific based on interactions with viral proteins, which may explain their frequent co-localization in latently infected neurons [19,20]. Upregulation of several genes involved in the host adaptive immune response occurs during viral reactivation, reflecting either direct or indirect involvement [21].
The circularized genome associated with latent HSV produces LATs, which represent the only portion of the viral genome transcribed abundantly during neuronal latency [4,22,23]. LATs are enhanced by the LAT promoter binding factor, and their inhibition of antiapoptotic activity may protect HSV from CD8+ T-cell cytolytic destruction [24–26]. LATs are also involved in regulation of the ICP0 gene, which may modulate other genes, including ICP4 and CD83, and is believed to be an important factor in viral reactivation [21,27–29]. Differential expression of viral proteins, such as VP16, may also play a role in viral exit from latency [30]. Reactivated virus in the neuron depends on the viral proteins US9, gE, and gI for anterograde transport along sensory nerve branches, whereby it may gain access to various anatomic structures in the eye [31,32]. A higher level of latency as indicated by LAT expression in the trigeminal ganglion may correlate to increased corneal scarring over time [33].
Corneal latency
During the last few decades there has been increasing interest in the possibility of corneal latency of HSV-1. Many studies describe long-term viral presence in the cornea or the propensity for transmission by transplant in experimental models [34–40]. Some of the difficulty regarding this issue has been in defining non-neuronal latency. There is nonetheless evidence in support of corneal latency or persistence of HSV-1. However, while immune system involvement and viral gene expression in neuronal latency is increasingly known, a mechanism for conversion of HSV-1 to a latent state in the cornea remains to be effectively demonstrated. Although some have sought to establish requirements for proving non-neuronal latency, investigations have often focused on varying aspects of viral presence in the cornea [4,41–43].
A major category of investigations has looked into the potential role of LAT in facilitating corneal latency. One study found that regulation of the LAT promoter was similar in neuronal cells and corneal stromal keratocytes in a manner distinct from other non-neuronal cells [44]. Specifically, similar regions increased LAT promoter activity in neuronal cells and keratocytes, which either did not affect or downregulated activity in other cell types. This is consistent with the more frequent recovery of HSV-1 antigens from the stroma than other parts of the cornea [34]. A study of excised corneas from patients with recurrent stromal keratitis but without active disease found that while most were positive for HSV DNA by PCR, LATs were not detected by in situ hybridization (ISH), which has substantially lower sensitivity [45]. By contrast, ISH has shown high levels of LAT expression in latently infected neurons. In another study LATs were detected in a majority of corneal specimens by PCR but not ISH, which may also result from differences in sensitivity [46].
It has been noted that the virus undergoes spontaneous reactivation in rabbits but not mice [4]. In a study of rabbits infected with HSV-1, LAT expression was detected in two out of 22 corneas weeks after exposure [47]. However, in another study LATs were detected in all trigeminal ganglia but no corneas in latently infected rabbits [48]. Differences in LAT detection in the cornea may therefore result from: HSV strain variations; host differences; antiviral treatment and the method of detection. LAT-positive strains of HSV have increased transmissibility from infected to naive rabbits undergoing corneal transplantation, although the implications of these results for corneal latency are unclear [49,50]. It remains unknown at this time whether LATs play a similar role in neuronal versus corneal latency.
A second category of investigations into corneal latency has described the detection of HSV related to corneal transplantation. HSV DNA was detected in ten out of 24 rabbit corneas months after transplantation without ocular disease, tear shedding or seroconversion [51]. A study of donor culture medium detected HSV DNA in three out of 80 samples but did not lead to ocular disease in recipients [52]. In another study, murine corneas known to be infected with HSV were subjected to organ culture conditions in which some corneas remained clear; this was described as a potential transplant risk [53]. The presence of the virus in donor culture media has been suggested as a possible cause of endothelial failure [54]. HSV DNA in excised recipient corneas was correlated in one study to endothelial failure despite a lack of ocular infection history, although the virus has been detected with and without clinical infection in multiple studies [55–60].
The long-term presence of HSV DNA in the cornea has been demonstrated. HSV DNA load in excised corneas from patients with recurrent keratitis correlates with several parameters, including time since previous transplant, severity of disease and neovascularization [34,61,62]. Finally, a mutant strain of HSV lacking US9 protein caused stromal keratitis in mice, although it was recently suggested that gE and gI may be more important for anterograde transport [31,32].
Corneal latency of HSV-1 versus long-term viral persistence
Many studies appear to collectively provide some evidence for corneal latency of the virus, although varying methodologies and definitions suggest a need to revisit the requirements for establishing non-neuronal latency. It is possible that the virus maintains a low replicative state that differs from latency. Distinct features of the human immune response to HSV also require further investigation and may limit the external validity of animal studies.
Donor-host transmission of HSV
There have been several published reports of primary graft failure due to HSV that may represent donor-host transmission through corneal transplantation (Table 1). Primary graft failure attributed to HSV was first reported in 1994, in which virus was detected in both the failed graft and the fellow cornea of the donor, the latter of which had undergone significant endothelial loss in organ culture [63]. A series published in 1997 reported that HSV was present in two out of three cases of primary graft failure; it was suggested that latent virus in the donor cornea may have reactivated [64]. Another series found that HSV was present in a third of primary graft failures, of which three had developed necrotizing herpetic disease [65]. Biswas et al. reported the cases of two patients without a history of ocular HSV that experienced graft failure after transplant [66]. HSV was subsequently found in donor corneas by culture, immunohistochemistry, electron microscopy and PCR.
Table 1.
Published cases of primary graft failure due to herpes simplex virus.
| Detection method | Corneas (n) | Year | Ref. |
|---|---|---|---|
| Culture, PCR | 1 | 1994 | [63] |
| PCR, Southern blotting | 2 | 1997 | [64] |
| IHC, PCR | 7 | 2000 | [65] |
| Culture, IHC, PCR, EM | 2 | 2000 | [66] |
| Culture, IHC, PCR | 4 | 2001 | [67,68] |
| PCR-based DNA fingerprint assay | 1 | 2001 | [9] |
| Culture | 3 | 2004 | [69] |
EM: Electron microscopy; IHC: Immunohistochemistry.
De Kesel et al. reported a group of four patients who had primary graft failure due to HSV within 17 days at one institution [67,68]. In a series of three patients without a history of ocular HSV, two experienced primary graft failure related to keratouveitis and one developed a herpetic dendrite [69]. In 2001 a case was published in the Lancet in which a PCR-based DNA fingerprint assay was used to show that HSV from the donor was identical to that found in the recipient [9]. This patient subsequently developed blindness from stromal keratitis. Finally, another specific PCR-based assay was used to demonstrate donor–host transmission, while electron microscopy showed mature, replicating virus in remnant donor tissue [70]. It should be noted that in most reported cases there was no definitive evidence that the donor rather than the recipient was the source of virus. The suspicion of donor–host transmission is often based on a lack of previous ocular herpes in the recipient [71,72].
Conclusion
The issue of corneal latency of HSV-1 has been a topic of much research. The current understanding of ocular morbidity in HSV suggests that recurrent episodes lead to damage through viral, as well as immune-mediated effects. As most humans are latently infected, it is of great interest whether this applies to the cornea in particular. One of the challenges of this issue is in defining non-neuronal latency. Although a number of studies have investigated this topic, they have used varied methodologies and reveal aspects of viral presence in the cornea that leave many questions unanswered. Furthermore, recent studies have not adequately confirmed earlier work that initially suggested the possibility of corneal latency. It does appear that HSV-1 may either develop latency in the cornea or persist in a low-replicative state in a time-dependent manner, both of which may have similar implications.
This is important not only for understanding the pathophysiology of ocular HSV but also due to the potential for transmission of the virus by corneal transplant. The latter has been described in several case reports. In the absence of genotypic analyses, it cannot be verified whether most of these cases represent actual donor–host transmission versus reactivated virus in the host. Nonetheless, there is evidence suggesting that a transmission risk related to HSV-1 may be a true clinical problem. It should be noted that corneal latency is not essential to donor–host transmission as reactivated virus can spread neuronally to the cornea at the time of surgery.
Standards for screening donor corneas for infectious agents are based not only on the possibility of transmission, but also the level of risk to the recipient and the benefit of detection. Therefore, donors with a known history of rabies, HIV, Creutzfeldt-Jakob disease and other lethal infections are excluded. Serologic testing is also performed for HIV-1 and -2, HBsAg, HCV and syphilis, as each of these is associated with substantial risk. HSV has been detected in association with primary graft failure, and rarely has been verified to originate from the donor using a highly specific assay. The significance of HSV-1 presence in donor corneas remains unknown. It is possible that long-term viral presence in the cornea is a sign of non-neuronal latency, and that the virus could reactivate after transplantation.
Future perspective
Improved technologies in the detection and quantification of HSV-1 may facilitate screening of donor corneas in the future as well as provide an improved understanding of corneal latency. This will require further investigation of viral gene regulation as well as the mechanism of long-term HSV-1 presence in the cornea. It appears that whether or not the issue of corneal latency is resolved, the role of the virus as a cause of graft failure and its transmission by transplant will be increasingly demonstrated.
Recent history suggests that the screening and storage of donor corneas will continue to evolve with improvements driven by necessity and/or innovation. As the majority of humans are infected latently by HSV-1, screening for the virus may potentially eliminate a large proportion of tissue. An improved understanding of the clinical significance of detection by various assays would therefore be required to establish a set of guidelines for deeming tissue unusable. The role of antiviral agents in tissue storage medium also requires investigation. Whether the risk of transmitting (usually) nonlethal infectious agents such as HSV-1 will influence eye banking standards remains to be seen. The value of exclusion versus diminishing the donor supply will be important to consider.
Executive summary
Introduction
-
▪
Herpes simplex virus-1 is an important cause of monocular infectious blindness. Most people are initially infected orally, after which the virus develops latency in sensory ganglia.
-
▪
Ocular infection is most directly linked to viral latency in the ipsilateral trigeminal ganglion, which provides sensory innervation to the cornea.
-
▪
The cornea itself has been discussed as an additional site of HSV-1 latency. There is also some concern over the implications of corneal latency for eye banking and transplant procedures.
Eye bank screening
-
▪
The history of eye banking shows that since the 1940s, substantial improvements have been made to screening and storage methods in developed nations. These include improved antibiotic usage standards and screening for several infectious agents, including HIV, hepatitis B, hepatitis C and syphilis.
-
▪
The Eye Bank Association of America regularly reviews reported adverse events.
-
▪
Donor corneas are not currently screened for HSV, which has been rarely transmitted.
HSV recurrence & ocular disease
-
▪
Much of the morbidity and the majority of annual incidence rates in ocular HSV are related to recurrent episodes.
-
▪
Latency and reactivation occur as a result of interplay between viral and host factors.
Neuronal latency
-
▪
The development of neuronal latency may occur through active immune system involvement and deficiencies in viral replication. CD8+ T cells act through cytotoxic and noncytotoxic mechanisms, the latter of which was recently described; the cells release granzyme B, which degrades ICP4, one of the intermediate-early proteins required for replication.
-
▪
Latency-associated transcripts are the only portion of the viral genome abundantly transcribed during neuronal latency and appear to have an active role in latency modulation.
Corneal latency
-
▪
The long-term presence of HSV DNA in corneas has been effectively demonstrated and virus has been detected in the absence of disease. However, it is unclear whether this is a result of latency and if non-neuronal latency has unique characteristics.
-
▪
The detection of viral genes in the cornea shows variability based on method of detection and other factors.
-
▪
There is substantial evidence for corneal latency or persistence of the virus that may have implications for pathogenesis and transplantation.
Donor–host transmission of HSV-1
-
▪
There are some reports of possible transmission by corneal transplant, with rare cases in which a highly specific PCR-based assay demonstrated that the virus was donor-derived.
Conclusion
-
▪
The transmissibility of HSV-1 by corneal transplant may be enhanced by, but is not dependent on, corneal latency. Improved detection methods may enhance our understanding of HSV presence in the cornea and also its role in primary graft failure.
Acknowledgments
This work was supported by NIH grants AI057860 (Deepak Shukla), AI081869 (Deepak Shukla) and a Core Grant EY01792. Deepak Shukla is a recipient of the Lew Wasserman Merit award from Research to Prevent Blindness, Inc. (RPB) and a research award from the Glaucoma Foundation. Asim V Farooq was supported by an RPB Medical Student Eye Research Fellowship.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Liesegang TJ, Melton LJ, Daly PJ, et al. Epidemiology of ocular herpes simplex: incidence in Rochester, Minn, 1950 through 1982. Arch. Ophthalmol. 1989;107:1155–1159. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 2.Ghiasi H, Cai S, Perng GC, et al. Both CD4+ and CD8+ T cells are involved in protection against HSV-1 induced corneal scarring. Br. J. Ophthalmol. 2000;84:408–412. doi: 10.1136/bjo.84.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knickelbein JE, Khanna KM, Yee MB, et al. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322(5899):268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaye S, Choudhary A. Herpes simplex keratitis. Prog. Retin. Eye Res. 2006;25:355–380. doi: 10.1016/j.preteyeres.2006.05.001.. ▪ Excellent review of herpes simplex keratitis pathophysiology.
- 5.Akhtar J, Tiwari V, Oh M-J, et al. HVEM and nectin-1 are the major mediators of herpes simplex virus 1 (HSV-1) entry into human conjunctival epithelium. Invest. Ophthalmol. Vis. Sci. 2008;49(9):4026–4035. doi: 10.1167/iovs.08-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiwari V, Shukla SY, Yue BY, et al. Herpes simplex virus type 2 entry into cultured human corneal fibroblasts is mediated by herpesvirus entry mediator. J. Gen. Virol. 2007;88:2106–2110. doi: 10.1099/vir.0.82830-0. [DOI] [PubMed] [Google Scholar]
- 7.Galen B, Cheshenko N, Tuyama A, Ramratnam B, Herold BC. Access to nectin favors herpes simplex virus infection at the apical surface of polarized human epithelial cells. J. Virol. 2006;80(24):12209–12218. doi: 10.1128/JVI.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill JM, Clement C. Herpes simplex virus type 1 DNA in human corneas: what are the virological and clinical implications? J. Infect. Dis. 2009;200:1–4. doi: 10.1086/599330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Remeijer L, Maertzdorf J, Doornenbal P, et al. Herpes simplex virus 1 transmission through corneal transplantation. Lancet. 2001;357:442. doi: 10.1016/S0140-6736(00)04011-3.. ▪▪ Describes a case in which a patient developed blinding herpes simplex virus (HSV) keratitis after corneal transplantation in which the virus was found to be donor-derived using a highly specific assay.
- 10.Moffatt SL, Cartwright VA, Stumpf TH. Centennial review of corneal transplantation. Clin. Exp. Ophthalmol. 2005;33:642–657. doi: 10.1111/j.1442-9071.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 11.Rijneveld WJ, Beekhuis WH, van Rij G, et al. Clinical comparison of grafts stored in McCarey–Kaufman media at 4°C and in organ culture at 31°C. Arch. Ophthalmol. 1992;110:203–205. doi: 10.1001/archopht.1992.01080140059027. [DOI] [PubMed] [Google Scholar]
- 12.Chu W. The past twenty-five years in eye banking. Cornea. 2000;19(5):754–765. doi: 10.1097/00003226-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Lindstrom RL, Kaufman HE, Skelnik DL, et al. Optisol corneal storage medium. Am. J. Ophthalmol. 1992;114:345–356. doi: 10.1016/s0002-9394(14)71803-3. [DOI] [PubMed] [Google Scholar]
- 14.Ehlers N, Hjortdal J, Moller-Pederson T. Corneal storage and complications related to corneal grafting. Curr. Opin. Ophthalmol. 1994;5:75–80. doi: 10.1097/00055735-199408000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Garg P, Krishna PV, Stratis AK, et al. The value of corneal transplantation in reducing blindness. Eye. 2005;19:1106–1114. doi: 10.1038/sj.eye.6701968. [DOI] [PubMed] [Google Scholar]
- 16. Hill JM, Ball MJ, Neumann DM, et al. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J. Virol. 2008;82:8230–8234. doi: 10.1128/JVI.00686-08.. ▪ Shows that nearly all adults have been exposed to HSV-1 and that the virus remains latent in trigeminal ganglia. Previous studies using serology have suggested that a majority of adults have been exposed and that this is age dependent.
- 17. Kaufman HE, Azcuy AM, Varnell ED, et al. HSV-1 DNA in tears and saliva of normal adults. Invest. Ophthalmol. Vis. Sci. 2005;46:241–247. doi: 10.1167/iovs.04-0614.. ▪ Demonstrated a high rate of asymptomatic shedding in adults without a history of ocular HSV.
- 18.The Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N. Engl. J. Med. 1998;339:300–306. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 19.Khanna KM, Bonneau RH, Kinchington PR, et al. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18(5):593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheridan BS, Cherpes TL, Urban J, et al. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J. Virol. 2009;83(5):2237–2245. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clement C, Bhattacharjee PS, Kaufman HE, et al. Heat-induced reactivation of HSV-1 in latent mice: upregulation in the TG of CD83 and other immune response genes and their LAT-ICP0 focus. Invest. Ophthalmol. Vis. Sci. 2009;50(6):2855–2861. doi: 10.1167/iovs.08-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steiner I, Spivack JG, O’Boyle DR, et al. Latent herpes simplex virus type 1 transcription in human trigeminal ganglia. J. Virol. 1988;62:3493–3496. doi: 10.1128/jvi.62.9.3493-3496.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens JG, Wagner EK, Devi-Rao GB, et al. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 24.Zwaagstra JC, Ghiasi H, Nesburn AB, et al. Identification of a major regulatory sequence in the latency associated transcript (LAT) promoter of herpes simplex virus type 1 (HSV-1) Virology. 1991;182:287–297. doi: 10.1016/0042-6822(91)90672-x. [DOI] [PubMed] [Google Scholar]
- 25.Perng G, Jones C, Ciacci-Zanella J, et al. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science. 2000;287(5457):1500–1503. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 26.Branco FJ, Fraser NW. Herpes simplex virus type 1 latency-associated transcript expression protects trigeminal ganglion neurons from apoptosis. J. Virol. 2005;79(14):9019–9025. doi: 10.1128/JVI.79.14.9019-9025.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leib DA, Coen DM, Bogard CL, et al. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton EA, Hong CS, Glorioso JC. The stable 2.0-kilobase intron of the herpes simplex virus type 1 latency-associated transcript does not function as an antisense repressor of ICP0 in nonneuronal cells. J. Virol. 2003;77:3516–3530. doi: 10.1128/JVI.77.6.3516-3530.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu M, Rakowski B, Gershburg E, et al. ICP0 antagonizes ICP4-dependent silencing of the ICP0 gene. PLoS ONE. 2010;5(1):E8837. doi: 10.1371/journal.pone.0008837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson RL, Preston CM, Sawtell NM. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog. 2009;3:e1000352. doi: 10.1371/journal.ppat.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGraw HM, Awasthi S, Wojcechowskyj JA, Friedman HM. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not US9. J. Virol. 2009;83(17):8315–8326. doi: 10.1128/JVI.00633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polcicova K, Biswas PS, Banerjee K, et al. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc. Natl Acad. Sci. USA. 2005;102(32):11462–11467. doi: 10.1073/pnas.0503230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mott KR, Bresee CJ, Allen SJ, et al. Level of herpes simplev virus rype 1 latency correlates with severity of corneal scarring and exhaustion of CD8+ T cells in trigeminal ganglia of latently infected mice. J. Virol. 2009;83(5):2246–2254. doi: 10.1128/JVI.02234-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaye S, Baker K, Bonshek R, et al. Human herpesviruses in the cornea. Br. J. Ophthalmol. 2000;84(6):563–571. doi: 10.1136/bjo.84.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abghari SZ, Stulting R. Recovery of herpes simplex virus from ocular tissues of latently infected inbred mice. Invest. Ophthalmol. Vis. Sci. 1988;29:239–243. [PubMed] [Google Scholar]
- 36.Abghari SZ, Stulting R, Petrash J. Detection of herpes simplex virus type 1 latency-associated transcripts in corneal cells of inbred mice by in situ hybridization. Cornea. 1998;11:433–438. doi: 10.1097/00003226-199209000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Cook SD, Brown SM. Herpes simplex virus type 1 persistence and latency in cultured rabbit corneal epithelial cells, keratocytes, and endothelial cells. Br. J. Ophthalmol. 1986;70:642–650. doi: 10.1136/bjo.70.9.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook SD, Batra SK, Brown SM. Recovery of herpes simplex virus from the corneas of experimentally infected rabbits. J. Gen. Virol. 1987;68(7):2013–2017. doi: 10.1099/0022-1317-68-7-2013. [DOI] [PubMed] [Google Scholar]
- 39.Coupes, Klapper P, Cleator G, et al. Herpesvirus simplex in chronic human stromal keratitis. Curr. Eye Res. 1986;5:735–738. doi: 10.3109/02713688609000013. [DOI] [PubMed] [Google Scholar]
- 40.Easty DL, Shimeld C, Claoue CM, et al. Herpes simplex virus isolation in chronic stromal keratitis: human and laboratory studies. Curr. Eye Res. 1987;6:69–74. doi: 10.3109/02713688709020071. [DOI] [PubMed] [Google Scholar]
- 41.Kaye SB, Lynas C, Patterson A, et al. Evidence for herpes simplex viral latency in the human cornea. Br. J. Ophthalmol. 1991;75:195–200. doi: 10.1136/bjo.75.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicholls SM, Shimeld C, Easty DL, et al. Recurrent herpes simplex after corneal transplantation in rats. Invest. Ophthalmol. Vis. Sci. 1996;37:425–435. [PubMed] [Google Scholar]
- 43.Gordon YJ, Romanowski E, Araullo-Cruz T, et al. HSV-1 corneal latency. Invest. Ophthalmol. Vis. Sci. 1991;32:663–665. [PubMed] [Google Scholar]
- 44.Perng G, Zwaagstra JC, Ghiasi H, et al. Similarities in regulation of the HSV-1 LAT promoter in corneal and neuronal cells. Invest. Ophthalmol. Vis. Sci. 1994;35:2981–2989. [PubMed] [Google Scholar]
- 45.Laycock KA, Lee SF, Stulting RD, et al. Herpes simplex virus type 1 transcription is not detectable in quiescent human stromal keratitis by in situ hybridization. Invest. Ophthalmol. Vis. Sci. 1993;34(2):285–292. [PubMed] [Google Scholar]
- 46.Rong BL, Pavan-Langston D, Weng QP, et al. Detection of herpes virus thymidine kinase and latency-associated transcript gene sequences in human herpetic corneas by polymerase chain reaction amplification. Invest. Ophthalmol. Vis. Sci. 1991;32:1808–1815. [PubMed] [Google Scholar]
- 47.Cook SD, Hill JM, Lynas C, et al. Latency-associated transcripts in corneas and ganglia of HSV-1 infected rabbits. Br. J. Ophthalmol. 1991;75(11):644–648. doi: 10.1136/bjo.75.11.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Brien WJ, Tsao LS, Taylor JL. Tissue-specific accumulation of latency-associated transcripts in herpes virus-infected rabbits. Invest. Ophthalmol. Vis. Sci. 1998;39(10):1847–1853. [PubMed] [Google Scholar]
- 49.Zheng X. Reactivation and donor-host transmission of herpes simplex virus after corneal transplantation. Cornea. 2002;21:S90–S93. doi: 10.1097/01.ico.0000263126.76392.cf. [DOI] [PubMed] [Google Scholar]
- 50.Zheng X, Marquart ME, Loutsch JM, et al. HSV-1 migration in latently infected and naive rabbits after penetrating keratoplasty. Invest. Ophthalmol. Vis. Sci. 1999;40(11):2490–2497. [PubMed] [Google Scholar]
- 51.Openshaw H, McNeill JI, Lin XH, et al. Herpes simplex virus DNA in normal corneas: persistence without viral shedding from ganglia. J. Med. Virol. 1995;46(1):75–80. doi: 10.1002/jmv.1890460116. [DOI] [PubMed] [Google Scholar]
- 52.Morris DJ, Cleator GM, Klapper PE, et al. Detection of herpes simplex virus DNA in donor cornea culture medium by polymerase chain reaction. Br. J. Ophthalmol. 1996;80:654–657. doi: 10.1136/bjo.80.7.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Robert P, Ducher V, Pleyer U, et al. The survival of herpes simplex virus in preserved murine corneas. Ophthalmic Res. 2008;40:329–332. doi: 10.1159/000151245. [DOI] [PubMed] [Google Scholar]
- 54.Builles N, Kodjikian L, Burillon C, et al. Major endothelial loss from corneas in organ culture: importance of second endothelial count. Cornea. 2006;25(7):815–820. doi: 10.1097/01.ico.0000230253.62730.85. [DOI] [PubMed] [Google Scholar]
- 55.Aydemir O, Türkcüoglu P, Bulut Y, Kalkan A. The relationship of graft survival and herpes simplex virus latency in recipient corneal buttons. Clin. Ophthalmol. 2007;1(2):127–131. [PMC free article] [PubMed] [Google Scholar]
- 56.Robert PY, Adenis JP, Denis F, et al. Herpes simplex virus DNA in corneal transplants: prospective study of 38 recipients. J. Med. Virol. 2003;71:69–74. doi: 10.1002/jmv.10454. [DOI] [PubMed] [Google Scholar]
- 57.Sabbaga EM, Pavan-Langston D, Bean KM, et al. Detection of HSV nucleic acid sequences in the cornea during acute and latent ocular disease. Exp. Eye Res. 1988;47:545–553. doi: 10.1016/0014-4835(88)90093-0. [DOI] [PubMed] [Google Scholar]
- 58.Shimeld C, Tullo AB, Easty DL, et al. Isolation of herpes simplex virus from the cornea in chronic stromal keratitis. Br. J. Ophthalmol. 1982;66:643–647. doi: 10.1136/bjo.66.10.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tullo AB, Easty DL, Shimeld C, et al. Isolation of herpes simplex virus from corneal discs of patients with chronic stromal keratitis. Trans. Ophthalmol. Soc. UK. 1985;104:159–165. [PubMed] [Google Scholar]
- 60.van Gelderen BE, Van der Lelij A, Treffers WF, et al. Detection of herpes simplex virus type 1, 2 and varicella zoster virus DNA in recipient corneal buttons. Br. J. Ophthalmol. 2000;84:1238–1243. doi: 10.1136/bjo.84.11.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Remeijer L, Duan R, van Dun JM, et al. Prevalence and clinical consequences of herpes simplex virus type 1 DNA in human cornea tissues. J. Infect. Dis. 2009;200:11–19. doi: 10.1086/599329. [DOI] [PubMed] [Google Scholar]
- 62.Shimomura Y, Deai T, Fukuda M, et al. Corneal buttons obtained from patients with HSK harbor high copy numbers of the HSV genome. Cornea. 2007;26(2):190–193. doi: 10.1097/ICO.0b013e31802eaee6. [DOI] [PubMed] [Google Scholar]
- 63.Cleator GM, Klapper PE, Dennett C, et al. Corneal donor infection by herpes simplex virus: herpes simplex virus DNA in donor corneas. Cornea. 1994;13(4):294–304. doi: 10.1097/00003226-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Cockerham GC, Krafft AE, McLean IW. Herpes simplex virus in primary graft failure. Arch. Ophthalmol. 1997;115(5):586–589. doi: 10.1001/archopht.1997.01100150588001. [DOI] [PubMed] [Google Scholar]
- 65.Cockerham GC, Bijwaard K, Sheng ZM, et al. Primary graft failure: a clinicopathologic and molecular analysis. Ophthalmology. 2000;107(11):2083–2090. doi: 10.1016/s0161-6420(00)00361-4. [DOI] [PubMed] [Google Scholar]
- 66.Biswas S, Suresh P, Bonshek RE, et al. Graft failure in human donor corneas due to transmission of herpes simplex virus. Br. J. Ophthalmol. 2000;84:701–705. doi: 10.1136/bjo.84.7.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Kesel RJ, Koppen C, Ieven M, et al. Primary graft failure caused by herpes simplex virus type 1. Cornea. 2001;20(2):187–190. doi: 10.1097/00003226-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 68.Cockerham G. Primary graft failure caused by herpes simplex virus type 1. Cornea. 2001;20(7):774–775. doi: 10.1097/00003226-200110000-00024. [DOI] [PubMed] [Google Scholar]
- 69.Borderie WM, Meritet JF, Chaumeil C, et al. Culture-proven herpetic keratitis after penetrating keratoplasty in patients with no previous history of herpes disease. Cornea. 2004;23(2):118–124. doi: 10.1097/00003226-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Thuret G, Acquart S, Gain P, et al. Ultrastructural demonstration of replicative herpes simplex virus type 1 transmission through corneal graft. Transplantation. 2004;77:325–326. doi: 10.1097/01.TP.0000102458.30516.13. [DOI] [PubMed] [Google Scholar]
- 71.Remeijer L, Doornenbal P, Geerards AJM, et al. Newly acquired herpes simplex virus keratitis after penetrating keratoplasty. Ophthalmology. 1997;104:648–652. doi: 10.1016/s0161-6420(97)30257-7. [DOI] [PubMed] [Google Scholar]
- 72.Rezende RA, Uchoa UBC, Raber IM, et al. New onset of herpes simplex virus epithelial keratitis after penetrating keratoplasty. Am. J. Ophthalmol. 2004;137:415–419. doi: 10.1016/j.ajo.2003.09.057. [DOI] [PubMed] [Google Scholar]