Abstract
Objective
To investigate the associations between obesity-predisposing genetic variants, cardiovascular biomarkers, and cardiovascular disease (CVD) risk in women with preexisting type 2 diabetes.
Methods and Results
We genotyped polymorphisms at nine established obesity loci in 1,395 women with diabetes from the Nurses’ Health Study; 449 of these women developed CVD and 946 did not. A genetic risk score (GRS) was derived by summing risk alleles for each individual. Four polymorphisms, rs9939609 (FTO), rs11084753 (KCTD15), rs10838738 (MTCH2), and rs10938397 (GNPDA2), showed nominally significant associations with CVD. The GRS combining all obesity loci was linearly related to CVD risk (P for trend = 0.013). The OR was 1.08 per risk allele (95% CI: 1.02–1.15; P = 0.01) after adjustment for BMI and other conventional risk factors. Women with the highest quartile of GRS had 53% (6% – 122%) increased CVD risk, compared with those in the lowest quartile (P = 0.024). In addition, higher GRS was associated with lower adiponectin levels (P = 0.02). Further adjustment for BMI and other covariates did not change the association (P = 0.006). Higher GRS was also correlated with lower levels of HDL (P= 0.01).
Conclusions
Obesity-predisposing variants may jointly affect CVD risk among women with diabetes.
Keywords: Cardiovascular disease, type 2 diabetes, obesity gene, polymorphism
Obesity is a major risk factor for the development of type 2 diabetes and cardiovascular disease (CVD)1. The underlying mechanisms involve insulin resistance, endothelial damage, inflammation, and dyslipidemia2 resulting largely from over-activation of adipose depots. Because of a strong link between obesity and the risk of diabetes and CVD, obesity-predisposing genetic variants may adversely affect CVD risk in people with diabetes. Identification of genetic risk markers might help elucidate the mechanisms underlying the relationship between diabetes and CVD.
Recently, genome-wide association studies (GWAS) have identified multiple risk variants that are associated with risk of obesity. These variants localize to the FTO, MC4R, TMEM18, SH2B1, KCTD15, MTCH2, NEGR1, and GNPDA2 genes3–6. To our knowledge, no study has examined associations between these variants and CVD risk among people with diabetes. We aimed to address this issue in women from the Nurses’ Health Study. We also examined the associations between these polymorphisms and levels of adipocytokines, lipids, and inflammatory and endothelial biomarkers.
Methods
Study population
The Nurses’ Health Study began in 1976 with the recruitment of 121,700 female registered nurses (aged 30–55 years). In 1989 and 1990, a total of 32,826 women provided blood samples. Medical history, lifestyle information and disease diagnoses were updated every 2 years using a validated questionnaire7. Women with type 2 diabetes were identified by self-report methods that were confirmed with a validated supplementary questionnaire. For cases diagnosed before the 1998 cycle, we used the National Diabetes Data Group criteria8 to define diabetes. The validity of this method has been established9. We used the 1997 American Diabetes Association [ADA] diagnostic criteria for diabetes diagnoses from 1998 onwards10. The Human Research Committee at the Brigham and Women’s Hospital in Boston approved this study. All participants gave written informed consent.
CVD ascertainment
CVD cases were defined as those in whom fatal CHD, nonfatal myocardial infarction (MI), coronary artery bypass grafting or percutaneous transluminal coronary angioplasty, fatal stroke, or nonfatal stroke had occurred between baseline and follow-up through 2004. Fatal CHD was confirmed if there was fatal MI confirmed by hospital records or on autopsy with the next of kin’s permission. Nonfatal MI was confirmed according to the criteria of the World Health Organization by reviewing medical records for documentation of symptoms plus either typical electrocardiographic changes or elevated cardiac enzymes levels. The methods used to ascertain CVD events were described in detail elsewhere11. Only CVD events that occurred after the onset of diabetes are included in the present report. In total, 1,395 Caucasian women with European ancestry were included. Among them, 449 CVD (including 347 CHD and 102 fatal and nonfatal stroke) events occurred. 946 women remained event free during follow-up.
Assessment of covariates and plasma levels of biochemical markers
Blood samples were collected in1989 and 1990 and were analyzed by previously reported methods12. To minimize bias and interassay variation, study samples were selected from randomly ordered case-control pairs. The methods for measuring lipids and other biomarkers are described in detail elsewhere11, 13–15. The coefficients of variation were 3.8% for plasma C- reactive protein (CRP), 3.4% for adiponectin, 3.4–8.3% for leptin, 3.3–4.8% for intracellular cell adhesion molecule-1 (ICAM-1), 2.6–4.8% for tumor necrosis factor-α receptor 2 (TNF-R2), 5.7–8.8% for E-selectin and 3.0% for HbA1c.
BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2). We used the baseline measurement (1976) to examine the genetic associations with BMI and CVD outcome and validated the associations using BMI in 1990. BMI measured at 1990, when blood samples were collected, were used in the analysis of the associations with biomarkers. Physical activity was expressed as metabolic equivalents (MET) per hour, which were calculated with data from a self-report questionnaire focused on types and durations of .activities over the previous year. The validity of the self-reported body weight and physical activity data for this cohort has been described previously16.
DNA extraction, single nucleotide polymorphisms (SNPs) selection and genotyping methods
DNA was extracted from the buffy coat fraction of centrifuged blood using the QIAmp Blood kit (Qiagen, Chatsworth, CA). We selected nine SNPs that showed a significant association with obesity in recently published GWASs3–6. These polymorphisms are FTO (rs9939609), MC4R (rs17782313 and rs17700633), TMEM18 (rs6548238), SH2B1 (rs7498665), KCTD15 (rs11084753), MTCH2 (rs10838738), NEGR1 (rs2815752), and GNPDA2 (rs10938397). Genotyping was performed using the OpenArray™ SNP Genotyping System (BioTrove, Woburn, MA). Replicate quality control samples (10%) were included and genotyped with > 99% concordance. All SNPs were in Hardy-Weinberg equilibrium (HWE; P > 0.05), with one exception, which was the SH2B1 rs7498665 variant in the group of women free of CVD (P = 0.002).
Genetic Risk Score (GRS) calculation
To evaluate the cumulative effect of the nine obesity variants on CVD risk, we constructed a GRS, using previously reported methods17. We assumed that each SNP in the panel acts independently and contributes equally to the risk of obesity in an additive manner. For each individual we summed the number of risk alleles for each of the SNPs producing a score out of 18 (the total number of risk alleles). Because the two SNPs rs17782313 and rs17700633 near to MC4R gene showed low correlation (r2 = 0.16), both were included in calculating the GRS. Scores for individuals with missing genotypes were standardized to those for individuals with complete data, assuming that the missing genotypes were not associated with disease status. In sensitivity analyses, results were similar when subjects with missing genotypes were excluded.
Statistical analyses
HWE was assessed by a chi-square test. We used unconditional logistic regression to estimate ORs for CVD and CHD risk, adjusting for age (in years), BMI (kg/m2), physical activity (< 1.5, 1.5–5.9, 6.0–11.9, 12–20.9, or ≥ 21.0 MET h/week), smoking (never, past, or current [1–14.9, 15–24.9, ≥25 pack-years]), alcohol intake (nondrinker or drinker [0.1–4.9, 5.0–9.9, 10–14.9 or ≥ 15g/day]), duration of diabetes, menopausal status (pre- or postmenopausal [never, past, or current hormone use]), and HbA1c levels (in quintiles). To normalize the distributions, plasma CRP, TNF-R2, E-selectin, adiponectin, ICAM-1 and leptin were logarithmically transformed. Generalized linear models were used to compare geometric mean values of quantitative traits across quartiles of the GRS after adjustment for covariates. In addition, we used restricted cubic spline regressions model18 to examine the associations between GRS (as continuous variables) and risk of CVD among women with diabetes. The SAS statistical package was used for the analyses (SAS, version 9.0 for UNIX). All P values are two sided.
Results
Baseline characteristics of diabetic CVD cases and controls
Table 1 presents the baseline characteristics of persons who did or did not develop CVD during follow-up. Women who developed CVD were older, more likely to be postmenopausal, had higher HbA1c levels, and had diabetes for a longer duration than those who remained CVD-free. There were no significant differences in BMI, alcohol intake, physical activity or smoking between CVD cases and those did not have CVD events.
Table 1.
Baseline characteristics of study participants
| Variables | CVD subjects | CHD subjects | Control subjects | P (CVD)* | P (CHD)* |
|---|---|---|---|---|---|
| n | 449 | 347 | 946 | -- | -- |
| Age (years) | 60 ± 6 | 60 ± 6 | 57 ± 7 | <0.01 | <0.01 |
| BMI (kg/m2) | 30.1 ± 6.0 | 30.1 ± 6.0 | 30.1 ± 7.0 | 0.92 | 0.87 |
| Alcohol intake (g/day) | 2.3 ± 6.2 | 2.1 ± 5.7 | 2.9 ± 7.6 | 0.15 | 0.06 |
| Current smoking | 69 (15.4) | 44 (12.7) | 110(11.6) | 0.07 | 0.29 |
| Physical activity (MET/week) | 13.2 ± 16.7 | 13.6 ± 17.5 | 12.5 ± 14.4 | 0.42 | 0.30 |
| Postmenopausal status | 372 (86.7) | 294 (88.6) | 660 (72.5) | <0.01 | <0.01 |
| HbA1c (%) | 7.2 ± 1.7 | 7.2 ± 1.8 | 6.7 ± 1.7 | <0.01 | <0.01 |
| Duration of diabetes (years) | 7.0 ± 8.3 | 6.8 ± 8.1 | 3.2 ± 6.7 | <0.01 | <0.01 |
Data are means ± SD or n (%).
Test of differences between cases and controls: chi-square test for categorical and student’s t tests for continuous variables.
Obesity gene variants and CVD risk
Of the nine SNPs examined, rs9939609 (FTO), rs17782313 (MC4R), rs7498665 (SH2B1) and rs10838738 (MTCH2) showed positive associations with BMI, whereas the associations between other SNPs rs17700633 (MC4R), rs6548238 (TMEM18), rs11084753 (KCTD15), rs2815752 (NEGR1), and rs10938397 (GNPDA2) and BMI were not statistically significant; however, the direction of effect of these SNPs on BMI was consistent with the published data3, 4. In sensitivity analysis, we obtained similar results when we used BMI in 1990 (data not shown). Obesity risk variants of rs9939609 (FTO), rs11084753 (KCTD15), rs10838738 (MTCH2), and rs10938397 (GNPDA2) showed nominally significant associations with increased CVD risk even after adjustment for BMI and other potential covariates. Similar results were observed for rs9939609 (FTO) and rs10938397 (GNPDA2) with CHD risk. (All P < 0.05; supplementary Table 1).
Genetic risk score and CVD risk
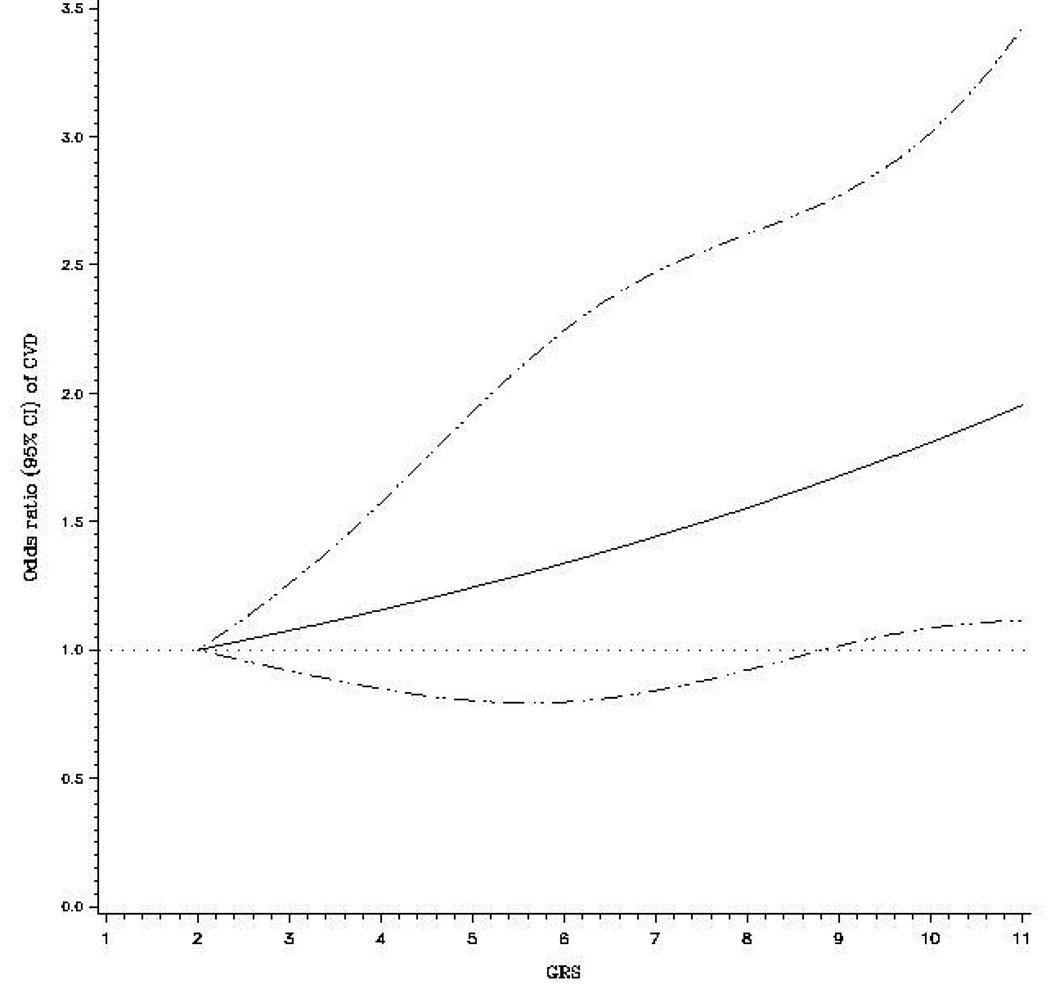
The GRS was positively associated with baseline (in 1976) BMI (P trend = 0.04); but not associated with other factors including age, blood pressure, smoking, alcohol consumption, and physical activity (data not shown). Women who developed CVD had a significantly higher GRS (mean: 6.4; SE: 0.10) than those who were free of CVD (mean: 6.1; SE: 0.07) (P = 0.02). The positive association between the GRS and CVD risk was dose-dependent (P for trend = 0.013) after adjustment for age, BMI, smoking, alcohol consumption, physical activity, postmenopausal status, hypertension, HbA1c, and duration of diabetes. The OR (95% CI) for CVD associated with each point scored, corresponding to one risk allele, was 1.08 (1.02–1.15) (Table 2). Women in the highest GRS quartile (GRS > 8.0) were at 53% greater risk of developing CVD (OR = 1.53, 95% CI = 1.06–2.22; P = 0.024) than those within the lowest GRS quartile (GRS < 5.0) (Table2). In the sensitivity analysis, we removed the SNP that deviated from HWE (rs7498665) and obtained the similar results. After adjustment for conventional risk factors, the OR (95% CI) for CVD associated with each point scored was 1.10 (95% CI: 1.04–1.18; P = 0.003). Results were similar when the outcome was restricted to CHD. In addition, we used a restricted cubic spline regressions model to investigate the associations continuously. The regression splines demonstrated a linear relationship between GRS and the risk of CVD among women with diabetes (Figure 1).
Table 2.
Associations between quartiles of GRS, and CVD and CHD risks in women with diabetes*
| Quartile of GRS* median (range) |
CVD | CHD | Controls | CVD; OR (95% CI) | CHD; OR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | Crude | P | Adjusted† | P | Crude | P | Adjusted† | P | |
| Q1: 4.0 (< 5.0) | 86(19.2) | 64 (18.4) | 218 (23.0) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Q2: 5.6 (5.0–5.9) | 139(31.0) | 112(32.3) | 306 (32.4) | 1.15(0.84–1.59) | 0.39 | 1.19(0.83–1.69) | 0.35 | 1.25(0.88–1.77) | 0.22 | 1.28(0.87–1.90) | 0.21 |
| Q3: 7.0 (6.0–7.9) | 99 (22.0) | 72 (20.7) | 198 (20.9) | 1.27(0.90–1.79) | 0.18 | 1.46(1.00–2.16) | 0.05 | 1.24(0.84–1.83) | 0.28 | 1.43(0.92–2.20) | 0.11 |
| Q4: 9.0(> 8.0) | 125 (27.8) | 99 (28.5) | 224 (23.7) | 1.42(1.02–1.97) | 0.041 | 1.53(1.06–2.22) | 0.024 | 1.51(1.05–2.17) | 0.028 | 1.65(1.10–2.47) | 0.016 |
| P trend | 0.033 | 0.013 | 0.039 | 0.016 | |||||||
| Continuous GRS | 1.07(1.01–1.12) | 0.02 | 1.08(1.02–1.15) | 0.01 | 1.07(1.01–1.14) | 0.02 | 1.09(1.02–1.16) | 0.01 | |||
See methods for GRS (genetic risk score) calculation.
Adjusted for age, BMI, smoking, alcohol consumption, physical activity, postmenopausal status, hypertension, HbA1c, and duration of diabetes.
Figure 1. Odds ratio of CVD among women with diabetes according to genetic risk score.
Dashed lines are 95% CI. Relative risks were estimated using unconditional logistic regression controlled for age, BMI, smoking, alcohol consumption, physical activity, postmenopausal status, and duration of diabetes.
Associations of GRS with plasma biomarkers
To elucidate the potential mechanisms underlying the observed associations, we further examined the associations between the GRS and biochemical risk factors for CVD, including adipocytokines, lipids, and markers of inflammation and endothelial dysfunction (Table 3). In the single SNP association analysis, the SNPs rs9939609 in FTO and rs11084753 in KCTD15 were significantly associated with adiponectin levels; SNP rs17700633 in MC4R was associated with HDL levels after adjustment for other conventional risk factors. The GRS (in quartiles) was significantly associated with lower plasma adiponectin levels (means: 8.01, 7.57, 7.67, and 6.53 ug/ml for the lowest to the highest quartiles of GRS, respectively; P = 0.02). Adjustment for age, smoking, alcohol consumption, physical activity, postmenopausal status, HbA1c and duration of diabetes did not change results materially (P = 0.005), nor did further adjustment for BMI (P = 0.006). In addition, the GRS was significantly associated with lower HDL levels (P = 0.01). Adjustment for covariates except BMI did not change the association (P = 0.009), and further adjustment for BMI attenuated the association (P = 0.05). No significant associations were observed with other biomarkers. However, in the models further adjusting for adiponectin and HDL, the associations between GRS and CVD/CHD remained significant with modest attenuation (data not shown).
Table 3.
Associations between GRS and biochemical measures in diabetic women
| GRS | Crude | Adjusted | Adjusted | ||||
|---|---|---|---|---|---|---|---|
| Variables | Q1 | Q2 | Q3 | Q4 | P | P* | P† |
| Adipocytokines | |||||||
| Adiponectin (ug/ml) | 8.01 | 7.57 | 7.67 | 6.53 | 0.02 | 0.005 | 0.006 |
| Leptin (ng/ml) | 43.4 | 49.7 | 52.2 | 49.1 | 0.08 | 0.057 | 0.22 |
| Inflammation | |||||||
| TNF-R2 (pg/ml) | 2576 | 2603 | 2658 | 2603 | 0.63 | 0.65 | 0.79 |
| CRP (ng/ml) | 5.63 | 5.93 | 5.98 | 6.33 | 0.45 | 0.48 | 0.93 |
| Lipids | |||||||
| Total cholesterol (mg/dl) | 227.5 | 230.5 | 230.7 | 228.2 | 0.81 | 0.67 | 0.82 |
| LDL (mg/dl) | 138.2 | 142.0 | 140.3 | 142.4 | 0.62 | 0.51 | 0.59 |
| HDL (mg/dl) | 53.5 | 50.0 | 50.9 | 49.5 | 0.01 | 0.009 | 0.05 |
| Triglycerides (mg/dl) | 195.4 | 213.1 | 217.0 | 208.5 | 0.39 | 0.47 | 0.76 |
| Endothelial dysfunction | |||||||
| ICAM-1 (ng/ml) | 318.0 | 312.6 | 325.4 | 314.0 | 0.63 | 0.97 | 0.94 |
| E-selectin (ng/ml) | 63.6 | 65.0 | 68.1 | 69.2 | 0.69 | 0.95 | 0.96 |
Data are means.
P value obtained in GLM test in the comparison across quartile of GRS (genetic risk score) adjusting for age, smoking, alcohol consumption, physical activity, postmenopausal status, HbA1c and duration of diabetes.
P value obtained by further adjusting for BMI.
Discussion
In this study we examined the joint effects of previously reported obesity-predisposing loci derived from GWASs on the risk of CVD in diabetic women. Our data indicate that the accumulation of obesity risk alleles significantly increases CVD risk independently of BMI and other conventional CVD risk factors.
People with diabetes are more obese and have a 2–3 fold higher risk of CVD than the general population19. Our data indicate that the obesity-predisposing genetic variants may increase CVD risk among people with diabetes. Because gene variants generally uncorrelated with environmental factors, the observed associations are unlikely to be owing to confounding factors. Of note, the associations are independent of BMI, suggesting the genetic effects may be largely mediated by the changes in fat accumulation that are not fully captured by BMI, or that the gene variants act pleiotropically, influencing obesity and CVD risk through distinct pathways.
As the individual genetic effects on CVD are moderate, we combined the variants by computing GRS, which congregates information from multiple genetic variants. We found that persons in the highest quartile of the GRS had a 53% greater risk of developing CVD than those in the lowest quartile. We chose this approach because it accounts to some extent for an individual’s genetic background and provides a broader characterization of their risk profile. Although the GRS we computed did not explicitly account for the individual effect sizes for each SNP, previous studies that have compared a weighted and unweighted GRSs reported similar effects for both models20. This may be because the effects for each allele tend to be normally distributed in most populations studied to date; thus, alleles with larger effects are counterbalanced by those with smaller effects, and when summing these effects the weighted mean approximates that of the unweighted score.
Obesity may influence CVD-risk through systemic inflammation, insulin resistance, endothelial dysfunction, hypertension and dyslipidemia2, 21–25. In the present study, the GRS was inversely associated with the plasma adiponectin levels. Adiponectin is secreted by adipose tissue and can improve insulin action and glucose and lipid metabolism26, 27 and has been related to lower CVD risk in epidemiology studies11, 14, 28, 29. The association between the GRS and adiponectin was not mediated by BMI in the present study, which is consistent with our observations for CVD risk. We also observed an inverse and significant association of GRS with HDL. HDL is an established protective factor for CVD30, 31, through preventing cholesterol accumulation in the artery wall30, blocking inflammation32, 33, antioxidation34, and antithrombotic actions35. Further studies are warranted to investigate the mechanisms underlying the associations between obesity genes and the changes in adiponectin and HDL concentrations. Further adjustment for adiponectin and HDL did not fully abolish the association between GRS and CVD risk, suggesting that other biological mechanisms may be also involved.
Our study sample was ethnically homogeneous (Whites of European ancestry). Therefore, the associations are unlikely to be confounded by population stratification. The magnitude of the associations of the individual gene variants and CVD risk are generally modest. We acknowledged that our study may be underpowered to detect such modest effects, which may explain the lack of statistical association for some of these variants. In addition, our study includes only women and it remains to be determined whether the effects observed here are similar in men with diabetes and in non-Whites.
In summary, we found that a genetic risk score, comprised of nine obesity-predisposing variants, is significantly associated with an increased risk of CVD in women with type 2 diabetes. The genetic effects are not fully mediated by the changes in biochemical risk factors such as adiponectin and HDL. Future studies are needed to investigate the potential mechanisms underlying the links among obesity genes, diabetes, and CVD risk.
Acknowledgments
This work was supported by the National Institutes of Health (DK58845, HL34594, and HL71981), American Heart Association Scientist Development Award and the Boston Obesity Nutrition Research Center (DK46200 to LQ). Dr. Zhang C. was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health. Dr. Franks was support by the Swedish Research Council, the Royal Physiological Society of Lund, and Västerbotten’s regional health authority (strategic appointment 2006–09).
Footnotes
Disclosure
None.
References
- 1.Hu FB. Obesity epidemiology. New York: Oxford University Press; 2008. [Google Scholar]
- 2.Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207:928–934. doi: 10.1016/j.jamcollsurg.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, Coin L, Collins FS, Connell JM, Cooper C, Smith GD, Dennison EM, Deodhar P, Elliott P, Erdos MR, Estrada K, Evans DM, Gianniny L, Gieger C, Gillson CJ, Guiducci C, Hackett R, Hadley D, Hall AS, Havulinna AS, Hebebrand J, Hofman A, Isomaa B, Jacobs KB, Johnson T, Jousilahti P, Jovanovic Z, Khaw KT, Kraft P, Kuokkanen M, Kuusisto J, Laitinen J, Lakatta EG, Luan J, Luben RN, Mangino M, McArdle WL, Meitinger T, Mulas A, Munroe PB, Narisu N, Ness AR, Northstone K, O'Rahilly S, Purmann C, Rees MG, Ridderstrale M, Ring SM, Rivadeneira F, Ruokonen A, Sandhu MS, Saramies J, Scott LJ, Scuteri A, Silander K, Sims MA, Song K, Stephens J, Stevens S, Stringham HM, Tung YC, Valle TT, Van Duijn CM, Vimaleswaran KS, Vollenweider P, Waeber G, Wallace C, Watanabe RM, Waterworth DM, Watkins N, Witteman JC, Zeggini E, Zhai G, Zillikens MC, Altshuler D, Caulfield MJ, Chanock SJ, Farooqi IS, Ferrucci L, Guralnik JM, Hattersley AT, Hu FB, Jarvelin MR, Laakso M, Mooser V, Ong KK, Ouwehand WH, Salomaa V, Samani NJ, Spector TD, Tuomi T, Tuomilehto J, Uda M, Uitterlinden AG, Wareham NJ, Deloukas P, Frayling TM, Groop LC, Hayes RB, Hunter DJ, Mohlke KL, Peltonen L, Schlessinger D, Strachan DP, Wichmann HE, McCarthy MI, Boehnke M, Barroso I, Abecasis GR, Hirschhorn JN. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, Berndt SI, Jacobs KB, Chanock SJ, Hayes RB, Bergmann S, Bennett AJ, Bingham SA, Bochud M, Brown M, Cauchi S, Connell JM, Cooper C, Smith GD, Day I, Dina C, De S, Dermitzakis ET, Doney AS, Elliott KS, Elliott P, Evans DM, Sadaf Farooqi I, Froguel P, Ghori J, Groves CJ, Gwilliam R, Hadley D, Hall AS, Hattersley AT, Hebebrand J, Heid IM, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Herrera B, Hinney A, Hunt SE, Jarvelin MR, Johnson T, Jolley JD, Karpe F, Keniry A, Khaw KT, Luben RN, Mangino M, Marchini J, McArdle WL, McGinnis R, Meyre D, Munroe PB, Morris AD, Ness AR, Neville MJ, Nica AC, Ong KK, O'Rahilly S, Owen KR, Palmer CN, Papadakis K, Potter S, Pouta A, Qi L, Randall JC, Rayner NW, Ring SM, Sandhu MS, Scherag A, Sims MA, Song K, Soranzo N, Speliotes EK, Syddall HE, Teichmann SA, Timpson NJ, Tobias JH, Uda M, Vogel CI, Wallace C, Waterworth DM, Weedon MN, Willer CJ, Wraight, Yuan X, Zeggini E, Hirschhorn JN, Strachan DP, Ouwehand WH, Caulfield MJ, Samani NJ, Frayling TM, Vollenweider P, Waeber G, Mooser V, Deloukas P, McCarthy MI, Wareham NJ, Barroso I, Jacobs KB, Chanock SJ, Hayes RB, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Kraft P, Hankinson SE, Hunter DJ, Hu FB, Lyon HN, Voight BF, Ridderstrale M, Groop L, Scheet P, Sanna S, Abecasis GR, Albai G, Nagaraja R, Schlessinger D, Jackson AU, Tuomilehto J, Collins FS, Boehnke M, Mohlke KL. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers JC, Elliott P, Zabaneh D, Zhang W, Li Y, Froguel P, Balding D, Scott J, Kooner JS. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet. 2008;40:716–718. doi: 10.1038/ng.156. [DOI] [PubMed] [Google Scholar]
- 7.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 8.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 9.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Kroleswki AS, Rosner B, Arky RA, Speizer FE, Hennekens CH. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med. 1991;151:1141–1147. [PubMed] [Google Scholar]
- 10.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. diabetes care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 11.Qi L, Li T, Rimm E, Zhang C, Rifai N, Hunter D, Doria A, Hu FB. The +276 polymorphism of the APM1 gene, plasma adiponectin concentration, and cardiovascular risk in diabetic men. Diabetes. 2005;54:1607–1610. doi: 10.2337/diabetes.54.5.1607. [DOI] [PubMed] [Google Scholar]
- 12.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 13.Qi L, van Dam RM, Liu S, Franz M, Mantzoros C, Hu FB. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 2006;29:207–211. doi: 10.2337/diacare.29.02.06.dc05-1903. [DOI] [PubMed] [Google Scholar]
- 14.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. Jama. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 15.Qi L, Rifai N, Hu FB. Interleukin-6 receptor gene, plasma C-reactive protein, and diabetes risk in women. Diabetes. 2009;58:275–278. doi: 10.2337/db08-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colditz GA, Hankinson SE. The Nurses' Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 17.Cornelis MC, Qi L, Zhang C, Kraft P, Manson J, Cai T, Hunter DJ, Hu FB. Joint effects of common genetic variants on the risk for type 2 diabetes in U.S. men and women of European ancestry. Ann Intern Med. 2009;150:541–550. doi: 10.7326/0003-4819-150-8-200904210-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 19.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–1426. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 20.Renstrom F, Payne F, Nordstrom A, Brito EC, Rolandsson O, Hallmans G, Barroso I, Nordstrom P, Franks PW. Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum Mol Genet. 2009;18:1489–1496. doi: 10.1093/hmg/ddp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 22.Superko HR, King S., 3rd Lipid management to reduce cardiovascular risk: a new strategy is required. Circulation. 2008;117:560–568. doi: 10.1161/CIRCULATIONAHA.106.667428. discussion 568. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 24.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219–4225. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 25.Shibata R, Ouchi N, Murohara T. Adiponectin and cardiovascular disease. Circ J. 2009;73:608–614. doi: 10.1253/circj.cj-09-0057. [DOI] [PubMed] [Google Scholar]
- 26.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 28.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 29.Koenig W, Khuseyinova N, Baumert J, Meisinger C, Lowel H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men: results from the 18-year follow-up of a large cohort from southern Germany. J Am Coll Cardiol. 2006;48:1369–1377. doi: 10.1016/j.jacc.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 30.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 31.Gordon DJ, Rifkind BM. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 32.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 33.Rye KA, Barter PJ. Antiinflammatory actions of HDL: a new insight. Arterioscler Thromb Vasc Biol. 2008;28:1890–1891. doi: 10.1161/ATVBAHA.108.173575. [DOI] [PubMed] [Google Scholar]
- 34.Negre-Salvayre A, Dousset N, Ferretti G, Bacchetti T, Curatola G, Salvayre R. Antioxidant and cytoprotective properties of high-density lipoproteins in vascular cells. Free Radic Biol Med. 2006;41:1031–1040. doi: 10.1016/j.freeradbiomed.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]