Abstract
Suicide is a major public health concern; however, its neurobiology is unclear. Post-mortem brain tissue obtained from suicide victims and normal controls offers a useful method for studying the neurobiology of suicide. Despite several limitations, these studies have offered important leads in the neurobiology of suicide. In this article, we discuss some important findings resulting from these studies, focusing on serotonergic mechanisms, signal transduction systems, neuroendocrine studies and immune function abnormalities in suicide. These studies suggest that abnormalities of certain receptor subtypes, components of signaling systems such as protein kinase C and protein kinase A, transcription factors such as cyclic AMP response element-binding protein and neurotrophins may play an important role in the pathophysiology of suicide. These studies also suggest abnormalities of hypothalamic–pituitary–adrenal axis system components, feedback mechanisms and cytokines, which are chemical mediators of the immune functions. Post-mortem brain tissue offers an opportunity for future studies, such as genetic and epigenetic studies.
Keywords: brain-derived neurotrophic factor, CREB, cytokine, depression, hypothalamic–pituitary–adrenal axis, phospholipase C, post-mortem brain, protein kinase A, protein kinase C, suicide
Suicide is a major public health concern world-wide. Approximately 30,000 people commit suicide in the USA alone each year [1–3]. The suicide rate in the USA was 10.7 per 100,000 in 1999 [4]. Suicide is also a major contributor to mortality in some patients suffering from mood disorders, schizophrenia and alcoholism [5,6]. In the USA, suicide is the third leading cause of death for adolescents (after motor vehicle accidents and homicide) [7]. More than 90% of suicides in the USA are associated with mental illness, including alcohol and/or substance abuse disorders [8]. Other risk factors that may trigger suicide among both adolescents and adults include impulsive-aggressive behavior [9–11], stress [12] and hopelessness [13]. Stress related to relationship loss is common in both adults [14] and adolescents [15,16].
A clinical model of risk factors for suicidal behavior has recently been proposed by Mann et al. [17]. There is evidence to suggest that some factors associated with adolescent suicide may be different from those of adult suicide, or at least they may appear to be different. Although impulsive-aggressive behavior is a risk factor for both adult and teenage suicide, adolescent suicide completers may be more prone to impulsive-aggressive behavior. Brent et al. have reported that adolescent suicide completers had more impulsive-aggressive personality disorders and higher aggression ratings than control subjects [9]. Apter et al. have also shown that adolescents with aggression and conduct disorders may be suicidal even in the absence of depression [18]. Hopelessness is another important risk and predictive factor for adult suicide that is also associated with suicidality in adolescents. Finally, neurodevelopmental factors may be different in adolescents compared with adults. It is reported that while the serotonergic system appears to be well developed in adolescents, the noradrenergic system is not yet fully developed [19,20], and this may be the reason adolescent depression is more responsive to treatment with selective serotonin (5-hydroxytryptamine [5HT]) reuptake inhibitors than with noradrenergic antidepressants [21–24].
Although the risk factors associated with suicide may be different in some respects in teenage and adult suicide, it is not yet known whether these factors have any impact on the neurobiological mechanisms associated with teenage suicide.
Whereas there is some understanding of the psychosocial factors associated with suicide, the neurobiology of suicide is less clear. The availability of well characterized post-mortem brain samples from suicide victims and normal controls has greatly enhanced our understanding of suicide. In this article, we review some of the important findings resulting from these studies, emphasizing the importance and use of post-mortem brain samples in understanding the neurobiology of suicide. We also emphasize that suicide and suicidal behavior have their own neuropathophysiological mechanisms rather than being a consequence of associated neuropsychiatric disorders, as discussed later.
Since the majority of subjects who commit suicide have some form of psychopathology, it is quite logical that some of the neurobiological abnormalities associated with psychiatric disorders, particularly with mood disorders, have been associated and studied in suicide. However, there are several other risk factors in addition to the presence of mental disorders associated with suicide, such as impulsive-aggressive behavior, stress and hopelessness. The biology of these risk factors are different in some respect. Furthermore, only a small percentage of patients with mental disorders commit suicide. Although the etiology of suicide is likely to overlap with that of other psychiatric disorders because of the high percentage of suicide in these disorders, some studies have suggested that the neurobiological abnormalities associated with suicide may be independent of psychiatric diagnoses and may be a common feature of suicidal behavior [25]. One of the important factors in studies of the neurobiology of suicide is to examine if these abnormalities are specific to suicide and independent of psychiatric diagnosis or related to suicide with specific mental disorders such as depression. These observations thus suggest that suicide and suicidal behavior may have their own neuropathological mechanisms that are independent of the neurobiology associated with these disorders, emphasizing that neurobiological mechanisms in suicide need to be studied independently of psychiatric disorders.
Biological factors associated with suicidal behavior
Initial studies of the biological factors associated with suicide were primarily conducted in patients with suicidal behavior, which is defined as the presence of serious suicidal thoughts or ideation or previous suicide attempts. These studies were primarily performed in tissues such as blood cells, cerebrospinal fluid (CSF) and plasma obtained from depressed patients and normal control subjects. As stated previously, the majority of depressed patients exhibit suicidal behavior. Therefore, it was logical to study biological abnormalities associated with suicidal, depressed patients. The major initial findings of the biological abnormalities observed in depressed patients related to abnormal hypothalamic–pituitary–adrenocortical (HPA) axis function and abnormalities of serotonergic mechanisms. Therefore, it was not surprising that the initial studies into suicidal behavior focused on these two biological mechanisms.
Studies of the serotonin system in suicidal behavior initially focused on the determination of a metabolite of serotonin known as 5-hydroxindoleacidic acid in the CSF of depressed and suicidal depressed patients [26–31]. An important observation was made that depressed patients with suicidal behavior had a significantly lower level of 5-hydroxindoleacidic acid in the CSF compared with depressed patients with non-suicidal behavior in control subjects [26,28,29,31–33]. This observation implicated an abnormal serotonergic system in suicidal behavior. Other evidence involving serotonergic mechanisms in suicidal behavior is derived from studies of serotonin uptake and of the serotonin transporter, which mediates the uptake of 5HT, in the platelets of suicidal patients, and it was reported by several investigators that serotonin uptake and serotonin transporter levels are decreased in the platelets of patients exhibiting suicidal behavior [34–39]. Further evidence implicating serotonergic mechanisms in suicidal behavior has been derived from neuroendocrine studies. For example, fenfluramine-induced prolactin response was found to be decreased in patients with depression compared with normal subjects [40,41].
Several subtypes of serotonin receptors have been identified – approximately 13 to date. Of these serotonin receptor subtypes, 5HT2A receptors have been shown to be present in human platelets. It was shown by Pandey et al. that the Bmax of 5HT2A receptors was higher in depressed patients [42]; furthermore, when the depressed patients were divided into suicidal and nonsuicidal patients, it was observed that the 5HT2A receptor levels were still much higher in suicidal patients compared with nonsuicidal depressed patients. This observation suggested that increased 5HT2A receptors may be specific to suicidal patients. To further evaluate this, some other studies also reported an increase in 5HT2A receptors in suicidal patients relative to normal control subjects. To further examine the specificity of increased 5HT2A receptors in suicidal patients, Pandey et al. determined 5HT2A receptor levels in the platelets of suicidal patients across diagnostic groups, including bipolar, schizophrenic and schizoaffective patients [25]. They reported that the platelet Bmax of the 5HT2A receptor was significantly higher in all suicidal patients irrespective of diagnosis when compared with normal controls and nonsuicidal subjects. These studies provided convincing evidence that increased 5HT2A receptors may be associated with suicidal behavior.
The second line of studies was conducted by determining the dexamethasone (DEX) suppression test (DST) and levels of cortisol in depressed and suicidal patients. It was reported by some investigators that suicidal patients exhibited DST nonsuppression and that DST nonsuppression may be a good predictor of the eventual suicide that some patients committed.
Abnormalities of noradrenergic function have been implicated in the etiology of depressive illness; however, noradrenergic studies in suicidal behavior are fewer in number relative to studies of serotonin function. This may be primarily due to the involvement of serotonin in impulsive-aggressive behavior.
Although these studies provided initial evidence of abnormalities of serotonin function and the HPA axis in suicide, it was not clear if the abnormalities observed in the periphery were also present in the brain of suicidal subjects. These studies were initially difficult because of the lack of availability of appropriate post-mortem brain samples and because of the inaccessibility of the living human brain. Subsequently, as adequate post-mortem brain samples became available, some of these studies were conducted in post-mortem brain samples obtained from suicide victims and normal control subjects.
In this article, it is not our intention to review all biological studies in the post-mortem samples of suicide victims; instead, we will focus on the main findings from studies of serotonin function, serotonin-mediated signal transduction mechanisms and HPA axis abnormalities in suicide. Recently, abnormalities of immune function have also been implicated in depression and to a certain extent in suicidal behavior. Accordingly, we will also include a brief discussion of the studies of immune function in suicide. We will review important results from some of the previous studies conducted in the post-mortem brains of suicide victims with regard to these measures.
Post-mortem brain studies
Post-mortem brain samples obtained from suicide victims and controls offer enormous opportunities to study molecular mechanisms associated with suicide. Earlier studies of molecular mechanisms studied in particular cells such as platelets, lymphocytes or CSF and always raised the question of whether such abnormalities reflect similar changes in the brain or have any relevance to the neurobiology of suicide. The availability of appropriate brain samples from well established brain collection programs has not only addressed these issues, but has also resulted in several neurobiological studies related to suicide and established the significance of these abnormalities in the brain. However, post-mortem brain studies need to be performed by taking into account the following criteria.
In order to obtain useful information from post-mortem brain studies, it is important that the quality of the post-mortem brain samples conform to certain standards. For example, one of the main problems in studies of post-mortem brain samples is the prolonged post-mortem interval. Recent post-mortem brain collection programs have minimized this limitation, and post-mortem tissue, in most cases, is obtained at relatively shorter intervals, not exceeding 24 h. An important requisite for post-mortem brain studies is careful neuropathological examination for abnormalities.
Another limitation of post-mortem brain samples is that most patients have been on several medications, often including psychoactive medications. It is difficult to address this issue; however, it is important to obtain good toxicological screening of these post-mortem samples either by determining medications in the post-mortem samples themselves or in the blood of the subjects.
The post-mortem brain samples should be adequate enough for performing the studies for determining the levels of protein and gene expression (mRNA). Most of the investigators using the post-mortem brain samples examine the quality of the tissue by determining the pH of the samples and the RNA integrity number.
The psychological autopsy is a complex experimental approach with several methodological issues. It offers an opportunity to obtain information on various domains linked to suicidal behavior, such as psychiatric diagnosis, physical disorders, childhood history, early life trauma and life events, among others. If psychological autopsy includes normal controls, it also helps us to assess the risk factors for suicide. Much of this information is important in post-mortem brain studies, especially accurate diagnosis, effect of early life trauma and evaluation of a proper control group, which is also of utmost importance in post-mortem brain research into suicide.
Careful psychological autopsy is another important aspect of post-mortem brain studies. Subjects need to be diagnosed carefully and accurately. Since it is a post-mortem diagnosis, it is primarily performed using the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria on the evidence received from medical records and interviews with family and friends.
The psychological autopsy method has been in existence for the last 40–50 years [43] and has been used by many investigators. The psychological autopsy studies in suicide have been reviewed by Cavanagh et al. [44]. Pouliot and De Leo have discussed in great detail the critical issues in psychological autopsy studies [45]. In a recent report, Snider et al. have discussed the issue of standardizing the psychological autopsy [46].
Serotonin receptor studies in suicide
Serotonin1A receptors in suicide
On the basis of both animal and human studies, abnormalities in 5HT1A receptors have been implicated in depression and anxiety [47]. Thus, it is not surprising that because of the involvement of the serotonergic system in suicidal behavior, 5HT1A receptors have been studied in the post-mortem brains of suicide victims by several investigators [48–54]. 5HT1A receptors were studied either by autoradiographic methods or by homogenate binding methods using 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) as the ligand (Table 1).
Table 1.
Summary of 5HT1A-receptor binding studies in the post-mortem brains of suicide victims.
| Radioligand | Type of binding study | Brain region | Result | Ref. |
|---|---|---|---|---|
| [3H]8-OH-DPAT | Homogenate | PFC 8 and 9 | ↑ Bmax in nonviolent suicides | [56] |
| [3H]8-OH-DPAT | Homogenate and sections | Various PFC regions and hippocampus | No change | [58] |
| [3H]8-OH-DPAT | Sections | Temporal and entorhinal cortex and hippocampus | ↑ in cortical areas and ↑ in CA1 and CA3 | [57] |
| [3H]8-OH-DPAT | Homogenate | PFC 9, 10 and 11 | No change in violent or nonviolent suicides | [51] |
| [3H]8-OH-DPAT | Sections | PFC 8, 9, 11, 12, 24, 32, 45, 46 and 47 | ↑ PFC 45 and 46 | [50] |
| [3H]8-OH-DPAT | Homogenate | Frontal cortex and hippocampus | No change | [55] |
| [3H]8-OH-DPAT | Sections | PFC 10 and hippocampus | No change | [52] |
| [3H]8-OH-DPAT | Sections | Midbrain dorsal raphe | ↑ in dorsal and ventrolateral subnuclei | [53] |
| [3H]8-OH-DPAT | Sections | Brainstem dorsal raphe | No change, but ↓ in dorsal raphe volume | [49] |
| [3H]8-OH-DPAT | Sections | Dorsal raphe nucleus | No change | [49] |
| [3H]8-OH-DPAT | Sections | Dorsal raphe nucleus | Binding capacity decreased | [49] |
| [3H]8-OH-DPAT | Sections | Rostral orbitofrontal cortex | No change | [54] |
| [3H]MPPF | Sections | Rostral orbitofrontal cortex | Decrease | [54] |
8-OH-DPAT: 8-hydroxy-2-(di-n-propylamino) tetralin; MPPF: 2′-methoxyphenyl-(N-2′-pyridinyl)-p-fluoro-benzamidoethyipiperazine; PFC: Prefrontal cortex.
Data adapted from [62].
Arango and colleagues, who determined 5HT1A-receptor binding sites in Brodmann’s areas 8 and 9 in suicide victims and normal control subjects, did not find any differences in either the Bmax or KD of [3H]8-OH-DPAT binding between normal control subjects and suicide victims [50]. However, they found that the nonviolent suicide group had significantly higher Bmax values when compared with normal control subjects, whereas the violent suicide subjects and the controls were not significantly different from each other in terms of the Bmax or the [3H]8-OH-DPAT binding. Another interesting observation was that this increase in the Bmax of [3H]8-OH-DPAT binding was observed only in the male suicide victims but not in the female suicide victims. 5HT1A receptors have also been studied in the prefrontal cortex (PFC) [51,52,55]; however, none of these groups – Arranz et al., Stockmeier et al. or Lowther et al. [51,52,55] – found any changes in 5HT1A receptors in the PFCs of suicide victims compared with control subjects. However, Matsubara et al. found an increase in 5HT1A receptor Bmax in the PFC of nonviolent suicide victims [56]. In another study, Stockmeier et al. found an increase in 5HT1A receptors in the midbrain dorsal raphe of suicide victims compared with controls [53]. However, in a recent study, Stockmeier et al. reported that while there was no difference in the agonist-binding to 5HT1A receptors between depressed and control subjects, the antagonist-binding was significantly decreased in outer layers of the orbitofrontal cortex obtained from subjects with major depressive disorder [54]. Joyce et al. found an increase in 5HT1A-receptor binding sites in the CA1 area of the hippocampus of suicide victims compared with control subjects [57]. Several other studies did not find any differences in 5HT1A-receptor binding in the hippocampus of suicide victims compared with control subjects. [52,55,58]
In summary, although the results of 5HT1A receptor studies in suicide victims seem inconsistent and mixed, it appears that there may be an increase in 5HT1A receptors in some cortical areas, as reported by Joyce et al. [57], Arango et al. [50] and Stockmeier et al. [53], but not by other investigators.
Serotonin2A receptors in suicide
Initial studies of serotonin receptor subtypes, such as 5HT1A and 5HT2A, were carried out by radiolabeled ligand binding studies. 5HT2A receptors can be labeled by several ligands, such as ketanserin, spiperone and lysergic acid diethyl amide (LSD), among others. However, none of these ligands specifically label 5HT2A receptors, since they will also label some other serotonin receptor subtypes.
The first studies of 5HT2A receptors were reported by Stanley and Mann [59] and Mann et al. [60]. Stanley and Mann used [3H]spiperone as the ligand and used the homogenate from PFC Brodmann’s areas 8 and 9 for their studies and found that the Bmax of 5HT2A receptors was increased in the post-mortem brains of suicide victims compared with normal control subjects [59]. Subsequent to these studies, approximately 20 studies of 5HT2A-receptor binding have been reported in the post-mortem brains of suicide victims and normal control subjects [61–63]. Of these, nine studies using different ligands report an increase in the 5HT2A-receptor binding, primarily in the PFCs of suicide victims compared with normal control subjects [34,48,59,60,64–68]. The other ten studies did not find any differences in 5HT2A-receptor binding between suicide victims and normal control subjects [35,51,52,57,69–74]. Only one study found a decrease in 5HT2A receptors using [3H]ketanserin (Table 2) [75].
Table 2.
Summary of 5HT2A-receptor binding studies in the post-mortem brains of suicide victims.
| Radioligand | Type of binding study | Brain region | Result | Ref. |
|---|---|---|---|---|
| [3H]spiperone | Homogenate | PFC 8 and 9 | ↑ Bmax | [59] |
| [3H]ketanserin | Homogenate | PFC 8, 9 and | No change | [73] |
| [3H]ketanserin | Homogenate | PFC 10 | No change | [35] |
| [3H]ketanserin | Homogenate | Frontal cortex | No change | [69] |
| [3H]spiperone | Homogenate | PFC 8 and 9 | ↑ Bmax | [60] |
| [3H]ketanserin | Homogenate | PFC 10 | No change | [72] |
| [3H]ketanserin | Homogenate | PFC 10 and hippocampus | No change in PFC and ↓ in hippocampus | [70] |
| [3H]spiperone | Homogenate | PFC 8 and 9 | ↑ Bmax only in violent suicides | [34] |
| [125I]LSD | Homogenate and sections | PFC 9 | ↑ Bmax and ↑ in sections | [48] |
| [3H]ketanserin | Homogenate and sections | PFC and hippocampus | ↓ Bmax and ↓ in sections in PFC only | [75] |
| [3H]ketanserin | Sections | PFC 9 | ↑ midlayers | [68] |
| [3H]ketanserin | Homogenate | PFC 10 | ↑ Bmax | [65] |
| [3H]ketanserin | Homogenate | PFC 9 and amygdala | ↑ Bmax | [64] |
| [125I]LSD | Sections | Temporal and entorhinal cortex and hippocampus | No change | [57] |
| [3H]ketanserin | Homogenate | PFC 9, 10 and 11 | No change in violent or nonviolent suicides | [51] |
| [3H]ketanserin/[3H]spiperone | Homogenate | PFC 10 and hippocampus | No change | [71] |
| [3H]ketanserin | Sections | PFC 10 and hippocampus | No change | [52] |
| [3H]ketanserin | Homogenate | PFC 8 and 9 | ↑ Bmax | [67] |
| [3H]ketanserin | Homogenate | PFC 9, 10, 11 and hippocampus | No change in PFC and ↓ Bmax in hippocampus | [74] |
| [3H]LSD | Homogenate | PFC 8 and 9 | ↑ Bmax | [66] |
LSD: Lysergic acid diethylamide; PFC: Prefrontal cortex.
Data adapted from [62].
Although it is generally believed that 5HT2A-receptor binding is increased in the post-mortem brain of suicide victims, the reasons for these inconsistent results are not clear. Besides other methodological issues, one factor that could explain this discrepancy is the use of non specific ligands in these studies, such as ketanserin and LSD, which label other receptors besides 5HT2A receptors.
The nonspecificity of the radioligands used for these 5HT2A receptor studies may be a variable that caused these discrepancies. Thus, it was important to examine either the protein or the mRNA levels of 5HT2A receptors in the suicide brain. Pandey et al. examined 5HT2A-receptor binding, as well as protein and mRNA expression of 5HT2A receptors in several regions of the post-mortem brains of teenage suicide victims [66]. The binding studies indicated a significant increase in [125I]LSD binding in the PFCs of suicide victims compared with normal control subjects. They also observed that protein expression levels of 5HT2A receptors were significantly increased in both the PFC and hippocampus of suicide victims compared with normal control subjects; however, no significant differences in protein expression were observed in the nucleus accumbens (NA) between suicide victims and normal control subjects. The immunogold labeling technique was used to examine the cellular localization of 5HT2A receptors. It was found that the expression of 5HT2A receptors was most dense in pyramidal neurons (layers III, V and VI) and their atypical dendrites. The mean expression levels of 5HT2A receptors were significantly greater in the pyramidal cells of layer V of the teenage suicide victims than in normal control subjects, whereas no changes in the expression levels of 5HT2A receptors were found in the pyramidal cells of other cortical layers (layers III and VI) or in the surrounding neuropil (layers IV, V and VI).
It was also found that the mean 5HT2A mRNA levels were significantly greater in the PFC and hippocampus of teenage suicide victims compared with normal control subjects. However, no significant differences in mRNA levels between suicide victims and normal control subjects were found in the NA [66].
This study by Pandey et al. suggests an increase in 5HT2A receptors using a binding technique, in protein expression levels using the western blot technique and in mRNA levels using the reverse transcriptase PCR technique [66]. These results are further supported by the immunohistochemical technique, which showed that the increase in 5HT2A receptors observed by the western blot technique was localized in the pyramidal cells in layer V of the PFC [66].
More recently, Escriba et al. determined the mRNA levels for 5HT2A receptors in the post-mortem brains of suicide victims [76]. Similar to the results reported by Pandey et al. [66], they also found a significant increase in the expression of mRNA for the 5HT2A receptors in the PFCs of 12 suicide victims compared with the control subjects.
More recently, Shelton et al. determined 5HT2A-receptor protein expression in Brodmann area 10 obtained from major depressive disorder subjects with or without suicide and observed increased protein expression of 5HT2A receptors compared with controls [77].
In summary, 5HT2A receptor studies, especially those carried out with the radioligand binding techniques, are mixed, with some studies showing an increase and some studies showing no changes. Stockmeier et al. summarized these studies (see Table 2) [62]. The studies of protein and mRNA expression, although few, appear to be more consistent.
Serotonin2C receptors in suicide
One of the serotonin receptor subtypes, known as the 5HT2C receptor, is widely distributed in the CNS and is thought to play a role in regulating mood, appetite and sexual behavior [78]. The 5HT2C receptor is also the only known G protein-coupled receptor whose mRNA undergoes post-transcriptional editing into two different receptor isoforms [79]. The 5HT2C receptor is a substrate for deaminating editing enzymes that attack five closely spaced adenosine residues located within sequences encoding the putative second intracellular domain of the receptor. The 5HT2C-receptor mRNA editing leads to receptor isoforms with small functional differences, which suggests that this editing function is a fine-tuning mechanism that adjusts receptor function to changes in synaptic activity [79–81].
Because of the receptor’s role in mood and anxiety disorders and because, as observed, this may be the only G protein-coupled receptor that undergoes pre-mRNA editing, its pre-mRNA editing has been examined in suicide and other mental disorders.
Gurevich et al. found that in suicide victims who had a history of major depression, the pre-mRNA editing of the 5HT2C receptors at the C′ site was significantly increased, the editing at the D site was significantly decreased and the C site showed a trend towards increased editing in the suicide victims compared with control subjects [82].
In another study, Dracheva et al. determined 5HT2C-mRNA editing in dorsolateral PFCs from subjects with bipolar disorder or schizophrenia who died of suicide or natural causes [83]. They found that 5HT2C-mRNA editing differences were present only in those subjects with bipolar disorder or schizophrenia who died by suicide, but not with comorbid psychiatric diagnoses. These studies in the postmortem brains of suicide victims suggest that altered pre-mRNA editing of the 5HT2C receptors may be involved in the pathophysiology of suicidal behavior.
Pandey et al. determined the protein and mRNA expression of 5HT2C receptors in the PFC (Brodmann area 9), hippocampus and choroid plexus of suicide victims and normal control subjects [84]. They found higher protein expression of 5HT2C receptors in the PFC but not the hippocampus or choroid plexus of suicide victims compared with controls. No significant difference was observed in the mRNA expression of 5HT2C receptors in any of these areas compared with control subjects.
In summary, the studies of 5HT2C receptors in the brains of suicide victims suggest changes in protein expression of 5HT2C receptors only in the PFC but not in other areas of the brains of suicide victims compared with controls. However, some studies suggest altered pre-mRNA editing of 5HT2C receptors in suicide victims compared with controls. It was also shown that serotonin agonists are less potent at the edited receptors, which probably couple less efficiently to G proteins [80]. These abnormalities suggest that 5HT2C-receptor-coupled signaling is altered in suicide victims compared with controls, due to altered G protein-coupling as a result of mRNA editing [81].
Receptor-linked signal transduction in suicide
The functional role of receptors lie in their ability to activate a signal transduction system causing not only a functional and behavioral response but also the transcription of some important genes. The responses of the altered receptors may result in an altered functional response and altered sensitivity or response of the signaling cascade to which these receptors are linked. It is also possible that the altered function of this signaling cascade may be related to alterations in the levels and the sensitivity of the individual components of the signaling cascade. Therefore, it is unsurprising to note that not only receptors but also their signaling systems have been recently studied in post-mortem brains obtained from suicide victims and control subjects [85–87].
Phosphoinositide & adenylyl cyclase signaling systems
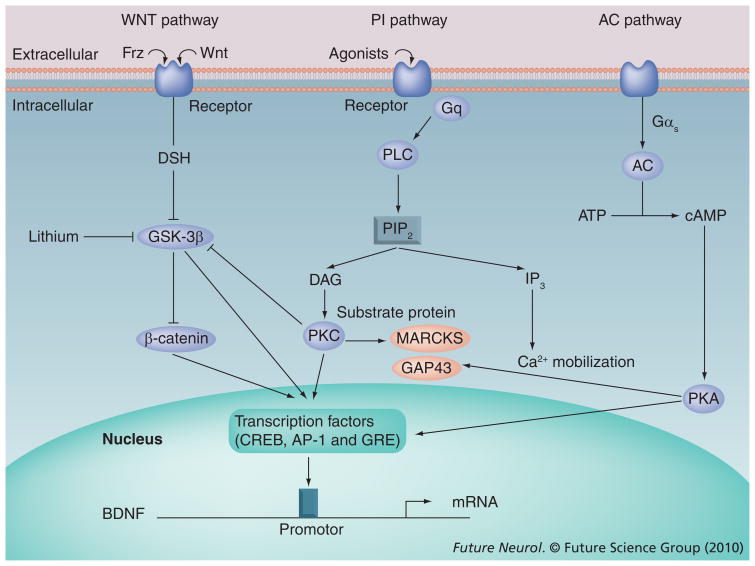
The activation of receptors by neurotransmitters or agonists initiates a series of events, causing the transfer of signals from cell surface receptors to the nucleus. Several signaling mechanisms have been identified and different receptors and receptor subtypes are linked to different signaling systems. Among these signaling systems, the phosphoinositide (PI) signaling system and the adenylyl cyclase (AC) signaling system have been widely studied and implicated in the pathophysiology of mood disorders and suicide [86,88]. Some of the receptors (e.g., 5HT2A receptors [89] and muscarinic receptor subtypes [90]) are linked to the PI signaling system. Agonist-induced activation of these G protein-coupled receptors causes the hydrolysis of phosphatidylinositol 4,5-biphosphate by the PI-specific enzyme phospholipase C, resulting in the formation of two secondary messengers, diacylglycerol and inositol 1,4,5-trisphosphate. Diacylglycerol activates the phospholipid- and calcium-dependent enzyme protein kinase C (PKC) and increases its affinity for calcium [91–93]. PKC, a phosphorylating enzyme, subsequently activates several transcription factors, such as CREB [94] and GSK-3β [95]. Activation of transcription factors by PKC results in the transcription of several important genes, such as brain-derived neurotrophic factor (BDNF) [96–99]. PI, AC and Wnt signaling pathways and the interaction of their components are shown in Figure 1.
Figure 1. Phosphoinositide, adenylyl cyclase and Wnt signaling pathways, showing interactions between components.
AC: Adenylyl cyclase; BDNF: Brain-derived neurotrophic factor; cAMP: Cyclic AMP; IP3: 1,4,5-triphosphate; PI: Phosphoinositide; PIP2: 4,5-biphosphate; PKA: Protein kinase A; PKC: Protein kinase C; PLC: Phospholipase C.
Various components of these cascades have been studied in the post-mortem brains of suicide victims; however, our focus will be studies on PKC, protein kinase A (PKA), CREB and BDNF.
Protein kinase C in suicide
Protein kinase C is a key regulatory enzyme that is present in various tissues [100–102]. PKC has been shown to be a family of at least 12 structurally related isozymes [103–105]. On the basis of molecular structure and enzymatic characterization, the PKC family has been subgrouped into three classes: conventional (α, βI, βII and γ) [106,107], novel (δ, ε, η and θ) [108] and atypical (ι, κ. λ and τ) isozymes [101,109,110].
The conventional isozymes are phospholipid- and calcium-dependent [108], whereas the novel isozymes do not require calcium for that activation [108]. The atypical isozymes are unresponsive to phorbol esters but can be activated by phosphatidyl serine [109,110]. Marked differences occur in the distribution of PKC isozymes. Most PKC isozymes are present in the brain [104,111,112], while in platelets only α, β and δ isozymes have been reported [113,114].
The biochemical properties of each isozyme have been identified with respect to activation and to phosphorylation, proteolytic activation/degradation and substrate specificity. It has been suggested that an association of PKC with the membrane is required for the subsequent physiological response. The majority of the nonactivated PKC is located in the cytosolic fraction and tends to relocate upon activation [115]. PKC is involved in the modulation of many neuronal and cellular functions, such as neurotransmitter synthesis and release, regulation of receptors and ion channels, neuronal excitability, gene expression, secretion and cell proliferation [93]. Activation of PKC also causes the activation of transcription factors involved in the transcription of important genes [94,99,116].
Pandey et al. examined the role of PKC in the post-mortem brains of teenage suicide victims and normal control subjects [87]. They found that the Bmax of [3H]phorbol 12,13-dibutyrate (PDBu) binding was significantly decreased in both membrane and cytosol fractions obtained from the PFCs of teenage suicide victims compared with control subjects [87]. They also determined the catalytic activity of PKC and the protein levels of various isozymes in the membrane and cytosol fractions of the PFC and in the hippocampus obtained from post-mortem brain samples of teenage suicide victims and non-psychiatric control subjects. To further examine whether any changes in isozymes are related to altered transcription, the mRNA levels of these isozymes were also determined.
In addition, Pandey et al. found that PKC activity was significantly decreased in the membrane and the cytosol fractions of the PFC and hippocampus of teenage suicide victims were comparable with control subjects [87]. They also found that the protein expression levels of PKC-α, -βI, -βII and -γ were significantly decreased in membrane and cytosol fractions of the PFC and hippocampus of teenage suicide victims compared with control subjects. They then examined whether decreased PKC isozyme levels were related to altered transcription of their respective mRNAs and found that the mRNA levels of PKC-α, -β and -γ were also significantly decreased both in the PFC and the hippocampus of suicide victims compared with control subjects.
Coull et al. determined [3H]PDBu binding in the PFC and hippocampus of antidepressant-treated and antidepressant-free adult depressed suicide victims [117]. They did not find any significant differences in [3H]PDBu binding between antidepressant-treated suicide victims and control subjects. On the other hand, they found a significant increase in the Bmax of [3H]PDBu binding in the soluble fractions of antidepressant-free suicide victims compared with control subjects.
Protein kinase A in the post-mortem brains of teenage suicide victims
Protein kinase A, a key component of the AC signaling systems, is activated by cyclic AMP (cAMP), and the activated PKA then phosphorylates several intracellular proteins and activates transcription factors such as CREB. In the absence of cAMP, the PKA holoenzyme exists as an inactive tetramer composed of two catalytic subunits bound to a regulatory subunit dimer. On the basis of elution patterns, two different PKA isozymes, known as PKA I and PKA II, have been identified. These two isozymes have been shown to be comprised of two different R subunits, known as RI and RII, which are further comprised of subunits known as RIα, RIβ, RIIα and RIIβ. In addition, three catalytic subunits, known as Cα, Cβ and Cγ, have also been identified. Each R subunit has two cAMP binding sites and after activation and binding with cAMP, each R subunit dissociates into a dimeric R subunit complex and two monomeric active C subunits [118].
The role of PKA in mood disorders has been studied by many investigators (for a review, see Dwivedi and Pandey [119]). However, studies of PKA in post-mortem brains of suicide victims are limited. In a study of post-mortem brain samples obtained from suicide victims, Dwivedi et al. reported that 3H-cAMP binding and PKA activity was significantly decreased in the PFCs of suicide victims [120]. In a subsequent study, Dwivedi et al. also observed that the protein and mRNA expression of PKA subunits RIIβ and Cβ were significantly decreased in the PFCs of suicide subjects relative to normal controls [85].
In order to examine whether abnormalities of PKA are also involved in the pathophysiology of teenage suicide, Pandey et al. determined the level of cAMP binding to PKA, PKA activity and the protein and mRNA expression of different subunits of PKA in cytosol and membrane fractions obtained from the PFC, hippocampus and NA of post-mortem brains from teenage suicide victims and nonpsychiatric control subjects. They found that PKA activity was significantly decreased in the PFC but not the hippocampus of teenage suicide victims compared with control subjects [121]. However, the protein and mRNA expression of only two PKA subunits (i.e., PKA RIα and RIβ) but not any other subunits, such as Cα, Cβ, RIIα or RIIβ, was observed to be decreased in the PFCs of teenage suicide victims compared with control subjects. These results in the teenage suicide victims, although similar in some respects to those observed in adult suicide victims by Dwivedi et al. [85,120], were also dissimilar in some other respects. For example, decreased cAMP binding and PKA activity was observed in both adult and teenage suicide victims and decreased expression of RIIα and Cβ was found in the adult suicide victims, whereas it was the RIα and RIβ subunits that were abnormal in the teenage suicide victims. The significance and implications of these observations with regard to the pathophysiology of teenage and adult suicide are unclear at this time.
Transcription factor CREB in suicide
Activation of transcription factors is the final step in a signal transduction pathway, which is mediated by the binding of a cell surface receptor with an agonist. Transcription factors can alter the expression of specific genes. Activation of PKC, as well as PKA, causes the phosphorylation of several transcription factors, such as the AP-1 family (Jun-B and Jun-D) and CREB [122]. CREB is one of the important transcription factors and has been recently implicated in the pathophysiology of depression and suicide. The evidence that CREB could possibly be involved in such disorders as these is evident from studies showing an increased expression of CREB in the post-mortem brains of depressed patients treated with antidepressants [123] and from the observation that treatment with almost all antidepressants caused an increase in CREB in the brains of rats [124]. Studies of CREB in suicide seem to be very limited, although Yamada et al. determined CREB protein and its phosphorylated form in the orbital frontal cortex of antidepressant-free patients with major depression and found that the immunoreactivity of both CREB and its phosphorylated form were significantly decreased in depressed subjects compared with normal control subjects [125].
Dwivedi et al. determined mRNA and protein expression and functional characteristics of CREB by examining cAMP response element DNA (CRE-DNA) binding activity in the PFC and hippocampus of suicide victims [126]. It was found that the protein expression of CREB was significantly decreased in the nuclear fractions of both the PFC and hippocampus obtained from suicide victims compared with normal control subjects. It was also observed that this decrease in protein expression levels was associated with a significant decrease in the mRNA levels of CREB in both the PFC and hippocampus of suicide victims compared with normal control subjects. Since a decrease in the mRNA and protein expression of CREB in the PFC and hippocampus of suicide victims was observed, the CRE-DNA binding activity was significantly decreased in the nuclear fractions of both the PFC and hippocampus of suicide victims compared with normal control subjects [126]. The results of this study thus indicate a decrease not only in the activity of CRE-DNA binding of CREB but also in the protein and mRNA expression of CREB in the PFC and hippocampus of depressed suicide victims [126].
Pandey et al. determined CREB levels in the post-mortem brains obtained from teenage suicide victims and normal control subjects [127]. They found a significant decrease in the protein and mRNA expression levels of CREB in the PFCs of teenage suicide victims compared with controls. They also found that the decrease in the protein and mRNA expression of CREB was associated with a significant decrease in CRE-DNA binding in teenage suicide victims relative to controls. However, they did not find any significant difference in protein or mRNA expression or in CRE-DNA binding between teenage suicide victims and normal controls in the hippo campus. These observations suggest some differences in the expression of CREB between adult and teenage suicide victims. While CREB expression was found to be decreased in the PFCs of both adult and teenage suicide, CREB expression was significantly decreased in the hippo campus of adult but not teenage suicide victims. These observations indicate another subtle difference in the neurobiology of teenage and adult suicides.
Brain-derived neurotrophic factor & TrkB receptors in suicide
As described in the previous section, CREB, which is a transcription factor, plays an important role in the regulation of several genes, including BDNF. Activation of CREB increases BDNF transcription through Ca2+ and the cAMP response element within exon 3 of BDNF [128]. BDNF is a member of the neurotrophin family, which includes nerve growth factor and neurotrophins [129]. Neurotrophins promote the growth and development of immature neurons and enhance the survival and function of specific neuronal populations, including neuronal growth, plasticity, phenotype maturation, synthesis of proteins and synaptic functioning [130–132]. The suggestion that BDNF may play a role in the pathophysiology of suicide is derived from many studies. Besides its regulation by CREB, which is an important transcription factor that has been shown to be decreased in the post-mortem brain of suicide victims [126], another important line of evidence is the observation that treatment with antidepressants caused an increase in BDNF in the rat brain [133], which suggests that depression and/or suicide may be associated with decreased levels of BDNF. These observations suggest that increasing the expression of BDNF may be the mechanism through which most antidepressants may produce their common therapeutic effects.
Dwivedi et al. determined the protein and mRNA expression levels of BDNF in the PFC and hippocampus of 27 suicide victims and 27 normal control subjects [134]. Immunolabeling of the brain samples obtained from suicide victims and normal control subjects indicated that the protein expression level of BDNF was significantly decreased both in the PFC and hippo campus of suicide victims compared with normal control subjects. Similarly, when the mRNA expression level was determined in these subjects, it was found that the mRNA level of BDNF was significantly decreased both in the PFC and hippocampus of suicide victims compared with normal control subjects.
Brain-derived neurotrophic factor produces its physiological effects by binding with the TrkB receptors, which exist as truncated and full-length isoforms, both of which are important in mediating the functions of BDNF [135–137]. Therefore, the protein and mRNA expression of TrkB receptors in the PFC and hippocampus of suicide victims and normal control subjects was also studied [134]. It was found that the protein expression levels of full-length TrkB receptors, but not of the truncated isoform, were significantly decreased in the PFC and hippocampus of suicide victims compared with control subjects. Similarly, the mRNA levels of TrkB receptors were significantly decreased in the PFC and hippocampus of suicide victims compared with normal control subjects. Although BDNF has not been studied in the post-mortem brains of suicide victims by other investigators, a recent study indicated that the protein expression of BDNF was increased in the post-mortem brains of patients with depression who were treated with antidepressants [138]. Another important observation of the study was the finding that the protein and mRNA expression of the full-length TrkB receptor was significantly decreased in the PFC and hippocampus of suicide victims [134]. Importantly, it is the full-length or catalytic form of TrkB receptors that mediates the biological functions of BDNF. The observation that both BDNF levels and TrkB receptor levels are decreased in the post-mortem brain of suicide victims may have important implications. The structural abnormalities in the brains of patients with depression and during stress could be associated with a decrease in BDNF and in the TrkB receptors.
Hypothalamic–pituitary–adrenal axis function in depression & suicide
Depression and stress are major risk factors for suicide. An abnormal HPA axis in depression is one of the most consistent findings in biological psychiatry [139–141]. Most patients with depression have been shown to have increased concentrations of cortisol in the plasma and CSF, increased cortisol response to adrenocorticotropic hormone (ACTH) and a deficient feedback mechanism, as evidenced by an abnormal DST [139–143] and enlarged pituitary and adrenal glands [139].
A strong association between HPA axis dysfunction and suicide is also evident. Yerevanian et al. found that DST nonsuppressors were significantly more likely to commit and complete suicide than DST suppressors [144]. Other investigators have also found an association between DST nonsuppression and suicide [144–148]. A meta-analysis found that suicide completions but not attempts were associated with DST nonsuppression [149]. The findings regarding DST nonsuppression and suicide attempts are inconsistent. Some find an association between suicide attempts and DST nonsuppression [150,151], while others do not [152–154]. Thus, it appears that HPA axis abnormalities may be more strongly related to suicide completers rather than attempters, suggesting the importance of studying HPA axis abnormalities in the brains of suicide victims.
The release of corticotropin-releasing factor (CRF) from the paraventricular nucleus (PVN) of the hypothalamus causes the release of ACTH from the pituitary, which stimulates the production of glucocorticoids (cortisol in humans and corticosterone in animals) from the adrenals. Glucocorticoids regulate the HPA axis through a negative feedback mechanism while binding to soluble glucocorticoid receptors (GRs) in the pituitary and the hypothalamus and inhibiting the release of CRF and ACTH [155,156].
Depressed patients also exhibit higher levels of CRF and CSF and a blunted ACTH response to a CRF challenge. Increased CRF has been related at least in part to the failure of cortisol to suppress CRF production through negative feedback regulation [139]. This failure, sometimes referred to as glucocorticoid resistance, is believed to be regulated by GRs.
Corticotropin-releasing factor is the primary chemical regulator of the stress-induced HPA axis and it has been suggested that the increased cortisol levels consistently observed in depressed patients may be related to the excessive secretion of CRF [140,157,158]. The evidence suggesting an abnormality of CRF in depression is derived from the observations of a blunted ACTH response to exogenous administration of CRF [159], increased levels of CRF in the CSF of depressed patients [140,160] and increased numbers of CRF-immunoreactive and CRF mRNA-containing PVNs in hypothalamic neurons [158,161]. More recently, Austin and coworkers reported that CRF immunoreactivity is increased in the locus coeruleus and other brain areas of depressed suicide subjects compared with controls [162,163].
Nemeroff et al. have reported a significant decrease in the number of CRF-receptor binding sites in the frontal cortex of suicide victims compared with controls [164]. A shift in the ratio of CRF-R1:R2 has also been reported in the pituitaries of suicide victims [165]. CRF mRNA levels have been found to be increased in the PVNs of depressed patients [161]. Although there is preliminary evidence to suggest alterations of CRF receptors in suicide, it is not clear which receptor subtypes are altered in depression or in suicide.
Merali et al. found increased levels of CRF and CRF immunoreactivity in the frontopolar cortex of suicide victims compared with control subjects [166,167]. This was associated with decreased levels of CRF-R1 receptor mRNA but not CRF-R2 mRNA [166]. Taken together, these studies in the brains of adult suicide victims suggest an increase in CRF levels and a decrease in CRF-R1 but no change in CRF-R2 receptors.
The reasons for increased glucocorticoid levels in depressed patients remain unclear, but it is believed that glucocorticoid-mediated feedback inhibition is impaired in major depression since administration of the synthetic glucocorticoid DEX does not cause suppression of cortisol in these patients [141,168]. The feedback regulation of the HPA axis by glucocorticoids is mediated through two different intracellular receptor subtypes, known as mineralocorticoid receptors (MRs) and GRs [156]. It has been observed that MRs have a high affinity for endogenous cortisol and that stress plays a role in the diurnal regulation of these hormones. However, GRs have a high affinity for DEX and a lower affinity for endogenous cortisol. Therefore, it is believed that GRs play a more important role in the regulation of the stress response when endogenous levels of glucocorticoids are high. Corticoid receptors may play an important role in depression and in the dysregulation of the HPA axis. These receptors have been widely studied in the peripheral tissues of depressed patients and to a lesser degree in the post-mortem brains of patients with mood disorders [141,169,170].
Both GRs and MRs are present in high concentrations in different areas of the human brain, such as the PFC, hippocampus, amygdala, locus coeruleus and hypothalamus, as shown by in situ hybridization and autoradiographic techniques. However, the studies into GRs and MRs in the post-mortem brain are limited. Perlman et al. and Webster et al. have observed decreased levels of GR mRNA in the PFC and hippocampus of unipolar, bipolar or schizophrenic subjects, providing preliminary evidence for the alteration of GRs in those patients [169,170]; however, this has not been studied in suicidal patients.
Cytokines & suicide
There are many interactions between neural, immune and neuroendocrine systems and this has led to the question of whether the immune system may also be involved in some brain-related disorders such as depression [171–174]. In recent years, it has been suggested that depression, which is one of the major psychiatric disorders known to be related to changes in the neuroendocrine system, may also be related to or caused by changes in the immune system.
Cytokines are a diverse group of proteins that can be considered to be the hormones of the immune system. These small molecules are secreted by various cells and act as signals between the cells to regulate the immune responses to injury and infection. The responses of cytokines are mediated through cytokine receptors. As is the case with other receptors, specific cytokine receptors respond to the presence of specific cytokines and thus produce their physiological responses. Cytokine receptors are present both in soluble forms and forms that are associated with the membranes.
One of the two major lines of evidence suggesting that cytokines play an important role in the pathophysiology of depression is derived from the observation that the administration of some cytokines, such as IFN-α, to patients with hepatitis or cancer patients with melanoma produced a symptom known as ‘sickness behavior’, which is very similar to depression [175–186]. The other major evidence for a role of cytokines in the pathophysiology of depression is derived from the observations that the levels of pro- and/or anti-inflammatory cytokines are altered in the serum [187–201] or the CSF of depressed patients [202,203]. Other indirect evidence suggesting a role for cytokines in depression comes from the observation that stress, which is a major risk factor for depressive illness, alters not only the immune system but also the levels of several cytokines [203–208].
Although the role of cytokines and immune dysregulation has been studied in great detail in patients with mood disorders and schizophrenia, their role in suicide is less clear. Since both depression and stress are major risk factors for suicide, it is quite likely that abnormalities of proinflammatory cytokines may be associated with the pathophysiology of suicide. There is also some direct and indirect evidence suggesting a relationship between immune dysregulation and suicide. Steiner et al. have found increased microgliosis in the post-mortem brains of suicide victims with affective disorders and schizophrenia compared with normal control subjects [209]. Goodwin and Eaton found a significant association between asthma and increased suicidal ideation and suicide attempts among adults in the community [210]. Goodwin et al. also found that youths who are hospitalized for asthma have higher than expected levels of suicidal ideation [211]. The notion that an abnormality in cytokines may be associated with suicidal behavior is further supported by a recent report by Tonelli et al. that found increased mRNA expression of IL-4 and IL-3 in the PFCs of female suicide victims and IL-13 in male suicide victims compared with normal control subjects [212]. Taken together, these studies suggest that cytokines may be abnormal in suicide. Lindqvist et al. have observed increased levels of IL-6 in the CSF of suicide attempters [213].
Although abnormal levels of cytokines are observed in the serum of patients with depression, it is not clear if there are also abnormal levels of cytokines in the brain. The immunological aspects of the neurobiology of suicide have been reported by Steiner et al., but the cytokines in the brain of suicide victims or subjects with depression have not been systematically studied [209].
Conclusion
Although there are some limitations in the studies of post-mortem brain samples in suicide, these studies have nonetheless provided important and useful insights into the pathophysiology of suicide, often extending and confirming the results from studies of peripheral tissues in suicidal patients.
The earlier findings of increased 5HT2A receptors in the platelets of suicidal patients were confirmed by the finding that the expression of 5HT2A receptors is increased in certain relevant brain areas of suicide victims. The observation that neurotrophins in general and BDNF in particular are decreased in the post-mortem brains of suicide victims conform with the suggested hypothesis that structural abnormalities observed in the brain of patients with depression or suicide are related to the decreased expression of BDNF in these patients.
The studies of post-mortem brain samples also indicate that the BDNF decrease in the brains of suicide victims may be related to an abnormal cellular signaling initiated by 5HT2A receptors or other receptors, which initiate the PI and AC signaling cascades, resulting in altered expression of PKC, phospholipase C and CREB. Decreased CREB expression may be responsible for decreased BDNF gene expression, a target gene for CREB. These studies also suggest that abnormalities of the signaling systems may be important in understanding the pathophysiology of suicide.
Because space limitations in this article, we focused on the biological systems outlined previously, but other biological systems, such as noradrenergic, γ-aminobutyric acid and glutamate systems, may also be important in understanding the neurobiology of suicide.
Future perspective
Earlier studies on the neurobiology of suicide have been conducted primarily on peripheral tissues obtained from patients with suicidal behavior. Suicidal behavior is defined as having suicidal thoughts or suicide attempts; however, only a small number of suicidal patients eventually commit suicide. These studies provided important initial information on the neurobiology of suicide but did not differentiate the neurobiological abnormalities between suicidal patients and suicide completers. Post-mortem brain studies offer a useful method of discriminating between the two groups and are indicative of the neurobiological abnormalities also observed in the brain.
Although studies of post-mortem brain samples have several limitations, including longer post-mortem interval, prior treatment with psycho active drugs and sometimes inadequate psychological autopsy, they have provided pertinent and important leads related to the pathophysiology of suicide, such as abnormal serotonergic mechanisms, decreased neurotrophin expression and altered signaling systems. There are some studies that suggest a role for immune functions, HPA axis, γ-aminobutyric acid and glutamate in suicide. It is likely that these systems will be studied in a more comprehensive fashion and their role in suicide delineated.
Because of small sample sizes, genetic and association studies in post-mortem brain samples have been limited. With an increase and improvement in brain collection programs, larger samples of post-mortem brain tissue may be available for genetic and association studies. It has been shown that epigenetics plays a role in depression, schizophrenia and, to some extent, suicide. This area is likely to be studied more comprehensively in the post-mortem brains of suicide victims.
A limitation of some psychological autopsies is the absence of data on early life trauma in suicide victims. Early life trauma has been shown to play a role not only in stress but also in the resulting suicidal behavior. It will be important to obtain early life trauma data while performing the psychological autopsy of suicide victims.
Suicide victims generally suffer from a variety of psychiatric disorders, such as depression, bipolar illness, schizophrenia and substance abuse, among others. Therefore, it is important to examine whether the biological abnormalities associated with suicide are independent of psychiatric diagnosis or if they are related to a specific diagnosis, such as depression.
Another limitation of the neurobiology of suicide studies is the absence of post-mortem brain samples from psychiatric patients who die of natural causes. For example, it will be important to examine if the neurobiological abnormalities in the post-mortem brains of suicide victims are related to suicide or to the psychiatric diagnosis, and hence it may be important to study post-mortem brain samples obtained from suicide victims with a specific diagnosis and post-mortem brain samples from subjects with that particular diagnosis who died of natural causes. This may determine whether the abnormalities are related to suicide or to a particular psychiatric illness.
Post-mortem brain studies provide ample opportunities and insights into the neurobiology of suicide, and these studies are likely to grow in the future.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
Funding support in the production of this manuscript was provided by grants RO1 MH 048153 (Ghanshyam N Pandey) and RO1 MH 068777 and RO1 MH082802 (Yogesh Dwivedi) from the NIH and by the Distinguished Investigators grant (Ghanshyam N Pandey) and Research grant (Yogesh Dwivedi) from the American Foundation for Suicide Prevention. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Botsis AF, Soldatos CR, Costas SN. Suicide: Biopsychosocial Approaches. Elsevier; The Netherlands: 1997. [Google Scholar]
- 2.Goldsmith SK, Pellmar TC, Kleinman J, Bunney WE, editors. Committee on Pathophysiology and Prevention of Adolescent and Adult Suicide; Board on Neuroscience and Behavioral Health; Institute of Medicine of the National Academies. Reducing Suicide, A National Imperative. The National Academies Press; DC, USA: 2002. [PubMed] [Google Scholar]
- 3.Moscicki EK, O’Carroll P, Rae DS, et al. Suicide attempts in the Epidemiologic Catchment Area Study. Yale J Biol Med. 1988;61(3):259–268. [PMC free article] [PubMed] [Google Scholar]
- 4.Minino AM, Smith BL. Deaths: preliminary data for 2000. Natl Vital Stat Rep. 2001;49(12):1–40. [PubMed] [Google Scholar]
- 5.Conwell Y, Duberstein PR, Cox C, et al. Relationships of age and axis I diagnoses in victims of completed suicide: a psychological autopsy study. Am J Psychiatry. 1996;153(8):1001–1008. doi: 10.1176/ajp.153.8.1001. [DOI] [PubMed] [Google Scholar]
- 6.Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205–228. doi: 10.1192/bjp.170.3.205. [DOI] [PubMed] [Google Scholar]
- 7.National Center for Health Statistics. Advance Report of Final Mortality Statistics, 1994. NICHS Monthly Vital Statistics; MD, USA: 1992. [Google Scholar]
- 8.Moscicki EK. Identification of suicide risk factors using epidemiologic studies. Psychiatr Clin North Am. 1997;20(3):499–517. doi: 10.1016/s0193-953x(05)70327-0. [DOI] [PubMed] [Google Scholar]
- 9.Brent DA, Baugher M, Bridge J, Chen T, Chiappetta L. Age- and sex-related risk factors for adolescent suicide. J Am Acad Child Adolesc Psychiatry. 1999;38(12):1497–1505. doi: 10.1097/00004583-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Brent DA, Kolko DJ, Wartella ME, et al. Adolescent psychiatric inpatients’ risk of suicide attempt at 6-month follow-up. J Am Acad Child Adolesc Psychiatry. 1993;32(1):95–105. doi: 10.1097/00004583-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Linnoila VM, Virkkunen M. Aggression, suicidality, and serotonin. J Clin Psychiatry. 1992;53(Suppl):46–51. [PubMed] [Google Scholar]
- 12.Westrin A. Stress system alterations and mood disorders in suicidal patients. A review. Biomed Pharmacother. 2000;54(3):142–145. doi: 10.1016/S0753-3322(00)89047-2. [DOI] [PubMed] [Google Scholar]
- 13.Beck AT, Steer RA, Beck JS, Newman CF. Hopelessness, depression, suicidal ideation, and clinical diagnosis of depression. Suicide Life Threat Behav. 1993;23(2):139–145. [PubMed] [Google Scholar]
- 14.Welte JW, Abel EL, Wieczorek W. The role of alcohol in suicides in Erie County, NY, 1972–84. Public Health Rep. 1988;103(6):648–652. [PMC free article] [PubMed] [Google Scholar]
- 15.Brent DA, Kolko DJ, Allan MJ, Brown RV. Suicidality in affectively disordered adolescent inpatients. J Am Acad Child Adolesc Psychiatry. 1990;29(4):586–593. doi: 10.1097/00004583-199007000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Weishaar ME, Beck AT. Cognitive approaches to understanding and treating suicidal behavior. In: Blumenthal SJ, Kupfer DJ, editors. Suicide Over the Life Cycle: Risk Factors, Assessment, and Treatment of Suicidal Patients. American Psychiatric Press; DC, USA: 1990. pp. 469–498. [Google Scholar]
- 17.Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am J Psychiatry. 1999;156(2):181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- 18.Apter A, Gothelf D, Orbach I, et al. Correlation of suicidal and violent behavior in different diagnostic categories in hospitalized adolescent patients. J Am Acad Child Adolesc Psychiatry. 1995;34(7):912–918. doi: 10.1097/00004583-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Black IB, Hendry IA, Iversen LL. Trans-synaptic regulation of growth and development of adrenergic neurones in a mouse sympathetic ganglion. Brain Res. 1971;34(2):229–240. doi: 10.1016/0006-8993(71)90278-2. [DOI] [PubMed] [Google Scholar]
- 20.Goldman-Rakic PS, Brown RM. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Brain Res. 1982;256(3):339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- 21.Emslie GJ, Rush AJ, Weinberg WA, et al. A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry. 1997;54(11):1031–1037. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- 22.Hazell P, O’Connell D, Heathcote D, Robertson J, Henry D. Efficacy of tricyclic drugs in treating child and adolescent depression: a meta-analysis. BMJ. 1995;310(6984):897–901. doi: 10.1136/bmj.310.6984.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman J, Martin A, King RA, Charney D. Are child-, adolescent-, and adult-onset depression one and the same disorder? Biol Psychiatry. 2001;49(12):980–1001. doi: 10.1016/s0006-3223(01)01127-1. [DOI] [PubMed] [Google Scholar]
- 24.Keller MB, Ryan ND, Strober M, et al. Efficacy of paroxetine in the treatment of adolescent major depression: a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2001;40(7):762–772. doi: 10.1097/00004583-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Pandey GN, Pandey SC, Dwivedi Y, et al. Platelet serotonin-2A receptors: a potential biological marker for suicidal behavior. Am J Psychiatry. 1995;152(6):850–855. doi: 10.1176/ajp.152.6.850. [DOI] [PubMed] [Google Scholar]
- 26.Agren H. Symptom patterns in unipolar and bipolar depression correlating with monoamine metabolites in the cerebrospinal fluid: I. General patterns. Psychiatry Res. 1980;3(2):211–223. doi: 10.1016/0165-1781(80)90038-4. [DOI] [PubMed] [Google Scholar]
- 27.Asberg M, Bertilsson L, Martensson B, et al. CSF monoamine metabolites in melancholia. Acta Psychiatr Scand. 1984;69(3):201–219. doi: 10.1111/j.1600-0447.1984.tb02488.x. [DOI] [PubMed] [Google Scholar]
- 28▪.Asberg M, Traskman L, Thoren P. 5-HIAA in the cerebrospinal fluid. A biochemical suicide predictor? Arch Gen Psychiatry. 1976;33(10):1193–1197. doi: 10.1001/archpsyc.1976.01770100055005. Major initial finding that linked abnormalities of serotonin (5HT) function with suicide. They reported that the level of 5-hydroxyindole acetic acid in cerebrospinal fluid was significantly decreased in patients who attempted suicide. [DOI] [PubMed] [Google Scholar]
- 29.Meltzer HY, Lowy MT. The serotonin hypothesis of depression. In: Meltzer H, editor. Psychopharmacology: The Third Generation of Progress. Raven Press; NY, USA: 1987. pp. 513–526. [Google Scholar]
- 30.Roy A, Pickar D, Linnoila M, et al. Cerebrospinal fluid monoamine and monoamine metabolite concentrations in melancholia. Psychiatry Res. 1985;15(4):281–292. doi: 10.1016/0165-1781(85)90065-4. [DOI] [PubMed] [Google Scholar]
- 31.van Praag HM. CSF 5-HIAA and suicide in non-depressed schizophrenics. Lancet. 1983;2(8356):977–978. doi: 10.1016/s0140-6736(83)90501-9. [DOI] [PubMed] [Google Scholar]
- 32.Nordstrom P, Samuelsson M, Asberg M, et al. CSF 5-HIAA predicts suicide risk after attempted suicide. Suicide Life Threat Behav. 1994;24(1):1–9. [PubMed] [Google Scholar]
- 33.van Praag HM. Depression, suicide and the metabolism of serotonin in the brain. J Affect Disord. 1982;4(4):275–290. doi: 10.1016/0165-0327(82)90025-8. [DOI] [PubMed] [Google Scholar]
- 34.Arora RC, Meltzer HY. 3H-imipramine binding in the frontal cortex of suicides. Psychiatry Res. 1989;30(2):125–135. doi: 10.1016/0165-1781(89)90154-6. [DOI] [PubMed] [Google Scholar]
- 35.Crow TJ, Cross AJ, Cooper SJ, et al. Neurotransmitter receptors and monoamine metabolites in the brains of patients with Alzheimer-type dementia and depression, and suicides. Neuropharmacology. 1984;23(12B):1561–1569. doi: 10.1016/0028-3908(84)90100-x. [DOI] [PubMed] [Google Scholar]
- 36.Meltzer HY, Arora RC, Baber RT, Ricou BJ. Serotonin uptake in blood platelets of psychiatric patients. Arch Gen Psychiatry. 1981;38(12):1322–1326. doi: 10.1001/archpsyc.1981.01780370024002. [DOI] [PubMed] [Google Scholar]
- 37.Perry EK, Marshall EF, Blessed G, Tomlinson BE, Perry RH. Decreased imipramine binding in the brains of patients with depressive illness. Br J Psychiatry. 1983;142:188–192. doi: 10.1192/bjp.142.2.188. [DOI] [PubMed] [Google Scholar]
- 38.Rausch JL, Janowsky DS, Risch SC, Huey LY. A kinetic analysis and replication of decreased platelet serotonin uptake in depressed patients. Psychiatry Res. 1986;19(2):105–112. doi: 10.1016/0165-1781(86)90003-x. [DOI] [PubMed] [Google Scholar]
- 39▪.Stanley M, Virgilio J, Gershon S. Tritiated imipramine binding sites are decreased in the frontal cortex of suicides. Science. 1982;216(4552):1337–1339. doi: 10.1126/science.7079769. First report suggesting abnormalities of the 5HT system in the post-mortem brains of suicide victims. They observed decreased tritiated imipramine binding in the frontal cortex of suicides, a finding consistent with earlier reports of decreased tritiated imipramine binding in the platelets of depressed patients. [DOI] [PubMed] [Google Scholar]
- 40.Lichtenberg P, Shapira B, Gillon D, et al. Hormone responses to fenfluramine and placebo challenge in endogenous depression. Psychiatry Res. 1992;43(2):137–146. doi: 10.1016/0165-1781(92)90128-p. [DOI] [PubMed] [Google Scholar]
- 41.Mann JJ, McBride PA, Brown RP, et al. Relationship between central and peripheral serotonin indexes in depressed and suicidal psychiatric inpatients. Arch Gen Psychiatry. 1992;49(6):442–446. doi: 10.1001/archpsyc.1992.01820060022003. [DOI] [PubMed] [Google Scholar]
- 42.Pandey GN, Pandey SC, Janicak PG, Marks RC, Davis JM. Platelet serotonin-2 receptor binding sites in depression and suicide. Biol Psychiatry. 1990;28(3):215–222. doi: 10.1016/0006-3223(90)90576-n. [DOI] [PubMed] [Google Scholar]
- 43.Shneidman ES, Farberow NLL. Clues to Suicide. McGraw-Hill; NY, USA: 1957. [Google Scholar]
- 44.Cavanagh JT, Carson AJ, Sharpe M, Lawrie SM. Psychological autopsy studies of suicide: a systematic review. Psychol Med. 2003;33(3):395–405. doi: 10.1017/s0033291702006943. [DOI] [PubMed] [Google Scholar]
- 45.Pouliot L, De Leo D. Critical issues in psychological autopsy studies. Suicide Life Threat Behav. 2006;36(5):491–510. doi: 10.1521/suli.2006.36.5.491. [DOI] [PubMed] [Google Scholar]
- 46.Snider JE, Hane S, Berman AL. Standardizing the psychological autopsy: addressing the Daubert standard. Suicide Life Threat Behav. 2006;36(5):511–518. doi: 10.1521/suli.2006.36.5.511. [DOI] [PubMed] [Google Scholar]
- 47.Coplan JD, Wolk SI, Klein DF. Anxiety and the serotonin1a receptor. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; NY, USA: 1995. pp. 1301–1310. [Google Scholar]
- 48.Arango V, Ernsberger P, Marzuk PM, et al. Autoradiographic demonstration of increased serotonin 5-HT2 and β-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry. 1990;47(11):1038–1047. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- 49.Arango V, Underwood MD, Boldrini M, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25(6):892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 50▪▪.Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688(1–2):121–133. doi: 10.1016/0006-8993(95)00523-s. Links 5HT1A receptors with suicide. Using quantitative radiography in coronal sections of the prefrontal cortex, the authors reported that 5HT1A-receptor binding was higher in the suicide group. [DOI] [PubMed] [Google Scholar]
- 51.Arranz B, Eriksson A, Mellerup E, Plenge P, Marcusson J. Brain 5-HT1A, 5-HT1D, and 5-HT2 receptors in suicide victims. Biol Psychiatry. 1994;35(7):457–463. doi: 10.1016/0006-3223(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 52▪▪.Stockmeier CA, Dilley GE, Shapiro LA, et al. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16(2):162–173. doi: 10.1016/S0893-133X(96)00170-4. Major study of 5HT receptors in suicide. The authors studied 5HT1A and 5HT2A receptors in brain samples from suicide victims with major depression and matched comparison subjects. [DOI] [PubMed] [Google Scholar]
- 53.Stockmeier CA, Shapiro LA, Dilley GE, et al. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18(18):7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stockmeier CA, Howley E, Shi X, et al. Antagonist but not agonist labeling of serotonin-1A receptors is decreased in major depressive disorder. J Psychiatr Res. 2009;43(10):887–894. doi: 10.1016/j.jpsychires.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lowther S, De Paermentier F, Cheetham SC, et al. 5-HT1A receptor binding sites in post-mortem brain samples from depressed suicides and controls. J Affect Disord. 1997;42(2–3):199–207. doi: 10.1016/s0165-0327(96)01413-9. [DOI] [PubMed] [Google Scholar]
- 56.Matsubara S, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: 5-HT1A binding sites in frontal cortex of suicide victims. J Neural Transm Gen Sect. 1991;85(3):181–194. doi: 10.1007/BF01244944. [DOI] [PubMed] [Google Scholar]
- 57.Joyce JN, Shane A, Lexow N, et al. Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics. Neuropsychopharmacology. 1993;8(4):315–336. doi: 10.1038/npp.1993.32. [DOI] [PubMed] [Google Scholar]
- 58.Dillon KA, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain Res. 1991;554(1–2):56–64. doi: 10.1016/0006-8993(91)90171-q. [DOI] [PubMed] [Google Scholar]
- 59.Stanley M, Mann JJ. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1(8318):214–216. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- 60.Mann JJ, Stanley M, McBride PA, McEwen BS. Increased serotonin2 and β-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry. 1986;43(10):954–959. doi: 10.1001/archpsyc.1986.01800100048007. [DOI] [PubMed] [Google Scholar]
- 61.Gross-Isseroff R, Biegon A, Voet H, Weizman A. The suicide brain: a review of postmortem receptor/transporter binding studies. Neurosci Biobehav Rev. 1998;22(5):653–661. doi: 10.1016/s0149-7634(97)00061-4. [DOI] [PubMed] [Google Scholar]
- 62.Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003;37(5):357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 63.Turecki G. Suicidal behavior: is there a genetic predisposition? Bipolar Disord. 2001;3(6):335–349. doi: 10.1034/j.1399-5618.2001.30608.x. [DOI] [PubMed] [Google Scholar]
- 64.Hrdina PD, Demeter E, Vu TB, Sotonyi P, Palkovits M. 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res. 1993;614(1–2):37–44. doi: 10.1016/0006-8993(93)91015-k. [DOI] [PubMed] [Google Scholar]
- 65.Laruelle M, Abi-Dargham A, Casanova MF, et al. Selective abnormalities of prefrontal serotonergic receptors in schizophrenia A postmortem study. Arch Gen Psychiatry. 1993;50(10):810–818. doi: 10.1001/archpsyc.1993.01820220066007. [DOI] [PubMed] [Google Scholar]
- 66▪▪.Pandey GN, Dwivedi Y, Rizavi HS, et al. Higher expression of serotonin 5-HT2A receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry. 2002;159(3):419–429. doi: 10.1176/appi.ajp.159.3.419. Comprehensive study of increased protein and mRNA levels in different brain areas of suicide victims, providing specific localization of these abnormalities. [DOI] [PubMed] [Google Scholar]
- 67.Turecki G, Briere R, Dewar K, et al. Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am J Psychiatry. 1999;156(9):1456–1458. doi: 10.1176/ajp.156.9.1456. [DOI] [PubMed] [Google Scholar]
- 68.Yates M, Leake A, Candy JM, et al. 5HT2 receptor changes in major depression. Biol Psychiatry. 1990;27(5):489–496. doi: 10.1016/0006-3223(90)90440-d. [DOI] [PubMed] [Google Scholar]
- 69.Owen F, Chambers DR, Cooper SJ, et al. Serotonergic mechanisms in brains of suicide victims. Brain Res. 1986;362(1):185–188. doi: 10.1016/0006-8993(86)91415-0. [DOI] [PubMed] [Google Scholar]
- 70.Cheetham SC, Crompton MR, Katona CL, Horton RW. Brain 5-HT2 receptor binding sites in depressed suicide victims. Brain Res. 1988;443(1–2):272–280. doi: 10.1016/0006-8993(88)91621-6. [DOI] [PubMed] [Google Scholar]
- 71.Lowther S, De Paermentier F, Crompton MR, Katona CL, Horton RW. Brain 5-HT2 receptors in suicide victims: violence of death, depression and effects of antidepressant treatment. Brain Res. 1994;642(1–2):281–289. doi: 10.1016/0006-8993(94)90932-6. [DOI] [PubMed] [Google Scholar]
- 72.McKeith IG, Marshall EF, Ferrier IN, et al. 5-HT receptor binding in post-mortem brain from patients with affective disorder. J Affect Disord. 1987;13(1):67–74. doi: 10.1016/0165-0327(87)90075-9. [DOI] [PubMed] [Google Scholar]
- 73.Owen F, Cross AJ, Crow TJ, et al. Brain 5-HT-2 receptors and suicide. Lancet. 1983;2(8361):1256. doi: 10.1016/s0140-6736(83)91310-7. [DOI] [PubMed] [Google Scholar]
- 74.Rosel P, Arranz B, San L, et al. Altered 5-HT2A binding sites and second messenger inositol trisphosphate (IP3) levels in hippocampus but not in frontal cortex from depressed suicide victims. Psychiatry Res. 2000;99(3):173–181. doi: 10.1016/s0925-4927(00)00076-7. [DOI] [PubMed] [Google Scholar]
- 75.Gross-Isseroff R, Salama D, Israeli M, Biegon A. Autoradiographic analysis of [3H] ketanserin binding in the human brain postmortem: effect of suicide. Brain Res. 1990;507(2):208–215. doi: 10.1016/0006-8993(90)90274-f. [DOI] [PubMed] [Google Scholar]
- 76.Escriba PV, Ozaita A, Garcia-Sevilla JA. Increased mRNA expression of α2A-adrenoceptors, serotonin receptors and μ-opioid receptors in the brains of suicide victims. Neuropsychopharmacology. 2004;29(8):1512–1521. doi: 10.1038/sj.npp.1300459. [DOI] [PubMed] [Google Scholar]
- 77.Shelton RC, Sanders-Bush E, Manier DH, Lewis DA. Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience. 2009;158(4):1406–1415. doi: 10.1016/j.neuroscience.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giorgetti M, Tecott LH. Contributions of 5-HT2C receptors to multiple actions of central serotonin systems. Eur J Pharmacol. 2004;488(1–3):1–9. doi: 10.1016/j.ejphar.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 79.Burns CM, Chu H, Rueter SM, et al. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387(6630):303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- 80.Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J Biol Chem. 1999;274(14):9472–9478. doi: 10.1074/jbc.274.14.9472. [DOI] [PubMed] [Google Scholar]
- 81.Wang Q, O’Brien PJ, Chen CX, et al. Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin2C receptors. J Neurochem. 2000;74(3):1290–1300. doi: 10.1046/j.1471-4159.2000.741290.x. [DOI] [PubMed] [Google Scholar]
- 82.Gurevich I, Tamir H, Arango V, et al. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002;34(3):349–356. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- 83.Dracheva S, Chin B, Haroutunian V. Altered serotonin 2C receptor RNA splicing in suicide: association with editing. Neuroreport. 2008;19(3):379–382. doi: 10.1097/WNR.0b013e3282f556d2. [DOI] [PubMed] [Google Scholar]
- 84.Pandey GN, Dwivedi Y, Ren X, et al. Regional distribution and relative abundance of serotonin2C receptors in human brain: effect of suicide. Neurochem Res. 2006;31(2):167–176. doi: 10.1007/s11064-005-9006-6. [DOI] [PubMed] [Google Scholar]
- 85.Dwivedi Y, Rizavi HS, Shukla PK, et al. Protein kinase A in postmortem brain of depressed suicide victims: altered expression of specific regulatory and catalytic subunits. Biol Psychiatry. 2004;55(3):234–243. doi: 10.1016/j.biopsych.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 86.Pacheco MA, Stockmeier C, Meltzer HY, et al. Alterations in phosphoinositide signaling and G-protein levels in depressed suicide brain. Brain Res. 1996;723(1–2):37–45. doi: 10.1016/0006-8993(96)00207-7. [DOI] [PubMed] [Google Scholar]
- 87.Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Conley RR. Decreased catalytic activity and expression of protein kinase C isozymes in teenage suicide victims: a postmortem brain study. Arch Gen Psychiatry. 2004;61(7):685–693. doi: 10.1001/archpsyc.61.7.685. [DOI] [PubMed] [Google Scholar]
- 88.Pandey GN, Dysken MW, Garver DL, Davis JM. β-adrenergic receptor function in affective illness. Am J Psychiatry. 1979;136(5):675–678. doi: 10.1176/ajp.136.5.675. [DOI] [PubMed] [Google Scholar]
- 89.Sanders-Bush E. 5-HT receptors coupled to phosphoinositide hydrolysis. In: Sanders-Bush E, editor. The Serotonin Receptors. Humana Press; NJ, USA: 1988. pp. 181–198. [Google Scholar]
- 90.Garro MA, Lopez de Jesus M, Ruiz de Azua I, et al. Regulation of phospholipase Cβ activity by muscarinic acetylcholine and 5-HT2 receptors in crude and synaptosomal membranes from human cerebral cortex. Neuropharmacology. 2001;40(5):686–695. doi: 10.1016/s0028-3908(00)00206-9. [DOI] [PubMed] [Google Scholar]
- 91.Abdel-Latif AA. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986;38(3):227–272. [PubMed] [Google Scholar]
- 92.Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 93.Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- 94.Xie H, Rothstein TL. Protein kinase C mediates activation of nuclear cAMP response element-binding protein (CREB) in B lymphocytes stimulated through surface Ig. J Immunol. 1995;154(4):1717–1723. [PubMed] [Google Scholar]
- 95.Grimes CA, Jope RS. CREB DNA binding activity is inhibited by glycogen synthase kinase-3 β and facilitated by lithium. J Neurochem. 2001;78(6):1219–1232. doi: 10.1046/j.1471-4159.2001.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borrelli E, Montmayeur JP, Foulkes NS, Sassone-Corsi P. Signal transduction and gene control: the cAMP pathway. Crit Rev Oncog. 1992;3(4):321–338. [PubMed] [Google Scholar]
- 97.Jackson SP. Regulating transcription factor activity by phosphorylation. Trends Cell Biol. 1992;2(4):104–108. doi: 10.1016/0962-8924(92)90014-e. [DOI] [PubMed] [Google Scholar]
- 98.Karin M, Smeal T. Control of transcription factors by signal transduction pathways: the beginning of the end. Trends Biochem Sci. 1992;17(10):418–422. doi: 10.1016/0968-0004(92)90012-x. [DOI] [PubMed] [Google Scholar]
- 99.Montminy MR, Gonzalez GA, Yamamoto KK. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990;13(5):184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- 100.Garcia-Sevilla JA, Escriba PV, Guimon J. Imidazoline receptors and human brain disorders. Ann NY Acad Sci. 1999;881:392–409. doi: 10.1111/j.1749-6632.1999.tb09388.x. [DOI] [PubMed] [Google Scholar]
- 101.Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- 102.Shearman MS, Naor Z, Kikkawa U, Nishizuka Y. Differential expression of multiple protein kinase C subspecies in rat central nervous tissue. Biochem Biophys Res Commun. 1987;147(3):911–919. doi: 10.1016/s0006-291x(87)80157-2. [DOI] [PubMed] [Google Scholar]
- 103.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270(48):28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 104.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9(7):484–496. [PubMed] [Google Scholar]
- 105.Stabel S, Parker PJ. Protein kinase C. Pharmacol Ther. 1991;51(1):71–95. doi: 10.1016/0163-7258(91)90042-k. [DOI] [PubMed] [Google Scholar]
- 106.Hug H, Sarre TF. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291(Pt 2):329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kiley SC, Jaken S. Protein kinase C: interactions and consequences. Trends Cell Biol. 1994;4(6):223–227. doi: 10.1016/0962-8924(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 108.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 109.Akimoto K, Mizuno K, Osada S, et al. A new member of the third class in the protein kinase C family, PKC λ, expressed dominantly in an undifferentiated mouse embryonal carcinoma cell line and also in many tissues and cells. J Biol Chem. 1994;269(17):12677–12683. [PubMed] [Google Scholar]
- 110.Ono Y, Fujii T, Ogita K, et al. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci USA. 1989;86(9):3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ono Y, Fujii T, Ogita K, et al. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988;263(14):6927–6932. [PubMed] [Google Scholar]
- 112.Shearman MS, Ase K, Saito N, et al. Molecular heterogeneity and differential expression of multiple protein kinase C subspecies in central nervous tissue. Biochem Soc Trans. 1988;16(4):460–463. doi: 10.1042/bst0160460. [DOI] [PubMed] [Google Scholar]
- 113.Baldassare JJ, Henderson PA, Burns D, Loomis C, Fisher GJ. Translocation of protein kinase C isozymes in thrombin-stimulated human platelets Correlation with 1,2-diacylglycerol levels. J Biol Chem. 1992;267(22):15585–15590. [PubMed] [Google Scholar]
- 114.Grabarek J, Raychowdhury M, Ravid K, et al. Identification and functional characterization of protein kinase C isozymes in platelets and HEL cells. J Biol Chem. 1992;267(14):10011–10017. [PubMed] [Google Scholar]
- 115.Kaczmarek LK. The role of protein kinase C in the regulation of ion channels and neurotransmitter release. Trends Neurosci. 1987;10(1):30–34. [Google Scholar]
- 116.Nichols M, Weih F, Schmid W, et al. Phosphorylation of CREB affects its binding to high and low affinity sites: implications for cAMP induced gene transcription. EMBO J. 1992;11(9):3337–3346. doi: 10.1002/j.1460-2075.1992.tb05412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Coull MA, Lowther S, Katona CL, Horton RW. Altered brain protein kinase C in depression: a post-mortem study. Eur Neuropsychopharmacol. 2000;10(4):283–288. doi: 10.1016/s0924-977x(00)00084-5. [DOI] [PubMed] [Google Scholar]
- 118.Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci. 2000;5:D678–D693. doi: 10.2741/skalhegg. [DOI] [PubMed] [Google Scholar]
- 119.Dwivedi Y, Pandey GN. Adenylyl cyclase-cyclic AMP signaling in mood disorders: role of the crucial phosphorylating enzyme protein kinase A. Neuropsychiatr Dis Treat. 2008;4(1):161–176. doi: 10.2147/ndt.s2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dwivedi Y, Conley RR, Roberts RC, Tamminga CA, Pandey GN. [3H]cAMP binding sites and protein kinase a activity in the prefrontal cortex of suicide victims. Am J Psychiatry. 2002;159(1):66–73. doi: 10.1176/appi.ajp.159.1.66. [DOI] [PubMed] [Google Scholar]
- 121.Pandey GN, Dwivedi Y, Ren X, et al. Brain region specific alterations in the protein and mRNA levels of protein kinase A subunits in the post-mortem brain of teenage suicide victims. Neuropsychopharmacology. 2005;30(8):1548–1556. doi: 10.1038/sj.npp.1300765. [DOI] [PubMed] [Google Scholar]
- 122.Holian O, Kumar R, Attar B. Apoprotein A-1 is a cofactor independent substrate of protein kinase C. Biochem Biophys Res Commun. 1991;179(1):599–604. doi: 10.1016/0006-291x(91)91413-7. [DOI] [PubMed] [Google Scholar]
- 123.Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased temporal cortex CREB concentrations and antidepressant treatment in major depression. Lancet. 1998;352(9142):1754–1755. doi: 10.1016/S0140-6736(05)79827-5. [DOI] [PubMed] [Google Scholar]
- 124.Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16(7):2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamada S, Yamamoto M, Ozawa H, Riederer P, Saito T. Reduced phosphorylation of cyclic AMP-responsive element binding protein in the postmortem orbitofrontal cortex of patients with major depressive disorder. J Neural Transm. 2003;110(6):671–680. doi: 10.1007/s00702-002-0810-8. [DOI] [PubMed] [Google Scholar]
- 126.Dwivedi Y, Rao JS, Rizavi HS, et al. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60(3):273–282. doi: 10.1001/archpsyc.60.3.273. [DOI] [PubMed] [Google Scholar]
- 127.Pandey GN, Dwivedi Y, Ren X, et al. Cyclic AMP response element-binding protein in post-mortem brain of teenage suicide victims: specific decrease in the prefrontal cortex but not the hippocampus. Int J Neuropsychopharmacol. 2007;10(5):621–629. doi: 10.1017/S1461145706007231. [DOI] [PubMed] [Google Scholar]
- 128.Finkbeiner S. Calcium regulation of the brain-derived neurotrophic factor gene. Cell Mol Life Sci. 2000;57(3):394–401. doi: 10.1007/PL00000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Altar CA, Cai N, Bliven T, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389(6653):856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 131.Bartrup JT, Moorman JM, Newberry NR. BDNF enhances neuronal growth and synaptic activity in hippocampal cell cultures. Neuroreport. 1997;8(17):3791–3794. doi: 10.1097/00001756-199712010-00027. [DOI] [PubMed] [Google Scholar]
- 132.Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270(5236):593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 133.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and TrkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134▪▪.Dwivedi Y, Rizavi HS, Conley RR, et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. Demonstrates the abnormalities in brain-derived neurotrophic factor in the brains of suicide victims. The authors found that mRNA and protein levels of brain-derived neurotrophic factor were significantly reduced in the prefrontal cortex and hippocampus of suicide victims. [DOI] [PubMed] [Google Scholar]
- 135.Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25(11):1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 136.Dechant G, Rodriguez-Tebar A, Barde YA. Neurotrophin receptors. Prog Neurobiol. 1994;42(2):347–352. doi: 10.1016/0301-0082(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 137.Middlemas DS, Lindberg RA, Hunter T. TrkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11(1):143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50(4):260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 139.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 140.Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996;1(4):336–342. [PubMed] [Google Scholar]
- 141.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49(5):391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 142.Carroll BJ. The dexamethasone suppression test for melancholia. Br J Psychiatry. 1982;140:292–304. doi: 10.1192/bjp.140.3.292. [DOI] [PubMed] [Google Scholar]
- 143.Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression Relation to the neurobiology of stress (1) N Engl J Med. 1988;319(6):348–353. doi: 10.1056/NEJM198808113190606. [DOI] [PubMed] [Google Scholar]
- 144.Yerevanian BI, Feusner JD, Koek RJ, Mintz J. The dexamethasone suppression test as a predictor of suicidal behavior in unipolar depression. J Affect Disord. 2004;83(2–3):103–108. doi: 10.1016/j.jad.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 145.Coryell W, Schlesser MA. Suicide and the dexamethasone suppression test in unipolar depression. Am J Psychiatry. 1981;138(8):1120–1121. doi: 10.1176/ajp.138.8.1120. [DOI] [PubMed] [Google Scholar]
- 146.Lester D. The dexamethasone suppression test as an indicator of suicide: a meta-analysis. Pharmacopsychiatry. 1992;25(6):265–270. doi: 10.1055/s-2007-1014419. [DOI] [PubMed] [Google Scholar]
- 147.Norman WH, Brown WA, Miller IW, Keitner GI, Overholser JC. The dexamethasone suppression test and completed suicide. Acta Psychiatr Scand. 1990;81(2):120–125. doi: 10.1111/j.1600-0447.1990.tb06463.x. [DOI] [PubMed] [Google Scholar]
- 148.Yerevanian BI, Olafsdottir H, Milanese E, et al. Normalization of the dexamethasone suppression test at discharge from hospital Its prognostic value. J Affect Disord. 1983;5(3):191–197. doi: 10.1016/0165-0327(83)90041-1. [DOI] [PubMed] [Google Scholar]
- 149▪.Coryell W, Schlesser M. The dexamethasone suppression test and suicide prediction. Am J Psychiatry. 2001;158(5):748–753. doi: 10.1176/appi.ajp.158.5.748. Demonstrates abnormal hypothalamic– pituitary–adrenocortical function with suicide. The authors found that patients with abnormal dexamethasone suppression tests had higher risks for eventual suicide. [DOI] [PubMed] [Google Scholar]
- 150.Banki CM, Arato M, Papp Z, Kurcz M. Biochemical markers in suicidal patients. Investigations with cerebrospinal fluid amine metabolites and neuroendocrine tests. J Affect Disord. 1984;6(3–4):341–350. doi: 10.1016/s0165-0327(84)80012-9. [DOI] [PubMed] [Google Scholar]
- 151.Targum SD, Rosen L, Capodanno AE. The dexamethasone suppression test in suicidal patients with unipolar depression. Am J Psychiatry. 1983;140(7):877–879. doi: 10.1176/ajp.140.7.877. [DOI] [PubMed] [Google Scholar]
- 152.Black DW, Monahan PO, Winokur G. The relationship between DST results and suicidal behavior. Ann Clin Psychiatry. 2002;14(2):83–88. doi: 10.1023/a:1016839404032. [DOI] [PubMed] [Google Scholar]
- 153.Brown RP, Mason B, Stoll P, et al. Adrenocortical function and suicidal behavior in depressive disorders. Psychiatry Res. 1986;17(4):317–323. doi: 10.1016/0165-1781(86)90079-x. [DOI] [PubMed] [Google Scholar]
- 154.Robbins DR, Alessi NE. Suicide and the dexamethasone suppression test in adolescence. Biol Psychiatry. 1985;20(1):107–110. doi: 10.1016/0006-3223(85)90144-1. [DOI] [PubMed] [Google Scholar]
- 155.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43(4):425–473. [PubMed] [Google Scholar]
- 156.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117(6):2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 157.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 158.Claes SJ. Corticotropin-releasing hormone (CRH) in psychiatry: from stress to psychopathology. Ann Med. 2004;36(1):50–61. doi: 10.1080/07853890310017044. [DOI] [PubMed] [Google Scholar]
- 159.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158(4):575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 160.Nemeroff CB, Widerlov E, Bissette G, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 161.Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60(4):436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 162.Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry. 2003;8(3):324–332. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- 163.Austin MC, Rice PM, Mann JJ, Arango V. Localization of corticotropin-releasing hormone in the human locus coeruleus and pedunculopontine tegmental nucleus: an immunocytochemical and in situ hybridization study. Neuroscience. 1995;64(3):713–727. doi: 10.1016/0306-4522(94)00420-a. [DOI] [PubMed] [Google Scholar]
- 164▪▪.Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45(6):577–579. doi: 10.1001/archpsyc.1988.01800300075009. Demonstrates abnormal corticotropin-releasing factor in the brains of suicide victims. Reports a marked reduction in the number of corticotropin-releasing factor binding sites in the frontal cortex of suicide victims compared with the controls. [DOI] [PubMed] [Google Scholar]
- 165.Hiroi N, Wong ML, Licinio J, et al. Expression of corticotropin releasing hormone receptors type I and type II mRNA in suicide victims and controls. Mol Psychiatry. 2001;6(5):540–546. doi: 10.1038/sj.mp.4000908. [DOI] [PubMed] [Google Scholar]
- 166.Merali Z, Du L, Hrdina P, et al. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABAA receptor subunits in frontal cortical brain region. J Neurosci. 2004;24(6):1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Merali Z, Kent P, Du L, et al. Corticotropin-releasing hormone, arginine vasopressin, gastrin-releasing peptide, and neuromedin B alterations in stress-relevant brain regions of suicides and control subjects. Biol Psychiatry. 2006;59(7):594–602. doi: 10.1016/j.biopsych.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 168.Owens MJ, Nemeroff CB. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Found Symp. 1993;172:296–308. doi: 10.1002/9780470514368.ch15. discussion 308–216 (1993) [DOI] [PubMed] [Google Scholar]
- 169.Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor α messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biol Psychiatry. 2004;56(11):844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 170.Webster MJ, Knable MB, O’Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry. 2002;7(9):985–994. 924. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- 171.Muller N, Ackenheil M. Psychoneuroimmunology and the cytokine action in the CNS: implications for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22(1):1–33. doi: 10.1016/s0278-5846(97)00179-6. [DOI] [PubMed] [Google Scholar]
- 172.Anisman H, Kokkinidis L, Merali Z. Further evidence for the depressive effects of cytokines: anhedonia and neurochemical changes. Brain Behav Immun. 2002;16(5):544–556. doi: 10.1016/s0889-1591(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 173.Hopkins SJ, Rothwell NJ. Cytokines and the nervous system I: expression and recognition. Trends Neurosci. 1995;18(2):83–88. [PubMed] [Google Scholar]
- 174.Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. 2000;157(5):683–694. doi: 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- 175.Bonaccorso S, Marino V, Biondi M, et al. Depression induced by treatment with interferon-α in patients affected by hepatitis C virus. J Affect Disord. 2002;72(3):237–241. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 176.Bonaccorso S, Marino V, Puzella A, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-α-based immunotherapy are related to interferon-α-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22(1):86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 177.Bonaccorso S, Puzella A, Marino V, et al. Immunotherapy with interferon-α in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Res. 2001;105(1–2):45–55. doi: 10.1016/s0165-1781(01)00315-8. [DOI] [PubMed] [Google Scholar]
- 178.Pariante CM, Orru MG, Baita A, Farci MG, Carpiniello B. Treatment with interferon-α in patients with chronic hepatitis and mood or anxiety disorders. Lancet. 1999;354(9173):131–132. doi: 10.1016/S0140-6736(98)04793-X. [DOI] [PubMed] [Google Scholar]
- 179.Collier J, Chapman R. Combination therapy with interferon-α and ribavirin for hepatitis C: practical treatment issues. BioDrugs. 2001;15(4):225–238. doi: 10.2165/00063030-200115040-00003. [DOI] [PubMed] [Google Scholar]
- 180.Hunt CM, Dominitz JA, Bute BP, et al. Effect of interferon-α treatment of chronic hepatitis C on health-related quality of life. Dig Dis Sci. 1997;42(12):2482–2486. doi: 10.1023/a:1018852309885. [DOI] [PubMed] [Google Scholar]
- 181.Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Disord. 2004;82(2):175–190. doi: 10.1016/j.jad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 182.Malaguarnera M, Di Fazio I, Restuccia S, et al. Interferon α-induced depression in chronic hepatitis C patients: comparison between different types of interferon α. Neuropsychobiology. 1998;37(2):93–97. doi: 10.1159/000026485. [DOI] [PubMed] [Google Scholar]
- 183.Pavol MA, Meyers CA, Rexer JL, et al. Pattern of neurobehavioral deficits associated with interferon alfa therapy for leukemia. Neurology. 1995;45(5):947–950. doi: 10.1212/wnl.45.5.947. [DOI] [PubMed] [Google Scholar]
- 184.Renault PF, Hoofnagle JH, Park Y, et al. Psychiatric complications of long-term interferon α therapy. Arch Intern Med. 1987;147(9):1577–1580. [PubMed] [Google Scholar]
- 185.Valentine AD, Meyers CA, Kling MA, Richelson E, Hauser P. Mood and cognitive side effects of interferon-α therapy. Semin Oncol. 1998;25(1 Suppl 1):39–47. [PubMed] [Google Scholar]
- 186.Yirmiya R, Weidenfeld J, Pollak Y, et al. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Adv Exp Med Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
- 187.Anisman H, Merali Z. Anhedonic and anxiogenic effects of cytokine exposure. Adv Exp Med Biol. 1999;461:199–233. doi: 10.1007/978-0-585-37970-8_12. [DOI] [PubMed] [Google Scholar]
- 188.Berk M, Wadee AA, Kuschke RH, O’Neill-Kerr A. Acute phase proteins in major depression. J Psychosom Res. 1997;43(5):529–534. doi: 10.1016/s0022-3999(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 189.Kim YK, Suh IB, Kim H, et al. The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: effects of psychotropic drugs. Mol Psychiatry. 2002;7(10):1107–1114. doi: 10.1038/sj.mp.4001084. [DOI] [PubMed] [Google Scholar]
- 190.Kubera M, Kenis G, Bosmans E, et al. Plasma levels of interleukin-6, interleukin-10, and interleukin-1 receptor antagonist in depression: comparison between the acute state and after remission. Pol J Pharmacol. 2000;52(3):237–241. [PubMed] [Google Scholar]
- 191▪.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(1):11–38. doi: 10.1016/0278-5846(94)00101-m. Links abnormal immune function with depression. Demonstrates that major depression may be accompanied by systemic immune activation or an inflammatory response. [DOI] [PubMed] [Google Scholar]
- 192.Maes M, Bosmans E, De Jongh R, et al. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 193.Maes M, Meltzer HY, Bosmans E, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34(4):301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- 194.Musselman DL, Miller AH, Porter MR, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158(8):1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 195.Nassberger L, Traskman-Bendz L. Increased soluble interleukin-2 receptor concentrations in suicide attempters. Acta Psychiatr Scand. 1993;88(1):48–52. doi: 10.1111/j.1600-0447.1993.tb03412.x. [DOI] [PubMed] [Google Scholar]
- 196.O’Brien SM, Scott LV, Dinan TG. Cytokines: abnormalities in major depression and implications for pharmacological treatment. Hum Psychopharmacol. 2004;19(6):397–403. doi: 10.1002/hup.609. [DOI] [PubMed] [Google Scholar]
- 197.Sluzewska A, Rybakowski JK, Laciak M, et al. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann NY Acad Sci. 1995;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [DOI] [PubMed] [Google Scholar]
- 198.Song C, Dinan T, Leonard BE. Changes in immunoglobulin, complement and acute phase protein levels in the depressed patients and normal controls. J Affect Disord. 1994;30(4):283–288. doi: 10.1016/0165-0327(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 199.Thomas AJ, Davis S, Morris C, et al. Increase in interleukin-1β in late-life depression. Am J Psychiatry. 2005;162(1):175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- 200.Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E. Increased serum tumor necrosis factor-α levels and treatment response in major depressive disorder. Psychopharmacology. 2003;170(4):429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- 201.Weisse CS. Depression and immunocompetence: a review of the literature. Psychol Bull. 1992;111(3):475–489. doi: 10.1037/0033-2909.111.3.475. [DOI] [PubMed] [Google Scholar]
- 202.Carpenter LL, Heninger GR, Malison RT, Tyrka AR, Price LH. Cerebrospinal fluid interleukin (IL)-6 in unipolar major depression. J Affect Disord. 2004;79(1–3):285–289. doi: 10.1016/S0165-0327(02)00460-3. [DOI] [PubMed] [Google Scholar]
- 203.Minami M, Kuraishi Y, Yamaguchi T, et al. Immobilization stress induces interleukin-1 β mRNA in the rat hypothalamus. Neurosci Lett. 1991;123(2):254–256. doi: 10.1016/0304-3940(91)90944-o. [DOI] [PubMed] [Google Scholar]
- 204.Anisman H, Merali Z. Cytokines, stress, and depressive illness. Brain Behav Immun. 2002;16(5):513–524. doi: 10.1016/s0889-1591(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 205.Connor TJ, Leonard BE. Depression, stress and immunological activation: the role of cytokines in depressive disorders. Life Sci. 1998;62(7):583–606. doi: 10.1016/s0024-3205(97)00990-9. [DOI] [PubMed] [Google Scholar]
- 206.Leonard BE, Song C. Stress, depression, and the role of cytokines. Adv Exp Med Biol. 1999;461:251–265. doi: 10.1007/978-0-585-37970-8_14. [DOI] [PubMed] [Google Scholar]
- 207.Merali Z, Lacosta S, Anisman H. Effects of interleukin-1β and mild stress on alterations of norepinephrine, dopamine and serotonin neurotransmission: a regional microdialysis study. Brain Res. 1997;761(2):225–235. doi: 10.1016/s0006-8993(97)00312-0. [DOI] [PubMed] [Google Scholar]
- 208.Tilders FJ, Schmidt ED. Cross-sensitization between immune and non-immune stressors A role in the etiology of depression? Adv Exp Med Biol. 1999;461:179–197. doi: 10.1007/978-0-585-37970-8_11. [DOI] [PubMed] [Google Scholar]
- 209.Steiner J, Bielau H, Brisch R, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psych Res. 2008;42(2):151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 210.Goodwin RD, Eaton WW. Asthma, suicidal ideation, and suicide attempts: findings from the Baltimore epidemiologic catchment area follow-up. Am J Pub Health. 2005;95(4):717–722. doi: 10.2105/AJPH.2003.019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Goodwin RD, Messineo K, Bregante A, Hoven CW, Kairam R. Prevalence of probable mental disorders among pediatric asthma patients in an inner-city clinic. J Asthma. 2005;42(8):643–647. doi: 10.1080/02770900500264770. [DOI] [PubMed] [Google Scholar]
- 212.Tonelli LH, Stiller J, Rujescu D, et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatrica Scandinavica. 2008;117(3):198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lindqvist D, Janelidze S, Hagell P, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66(3):287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]