Abstract
There is growing interest in assessing the neurotoxic and endocrine disrupting potential of perfluoroalkyl compounds (PFCs). Several studies have reported in vitro and in vivo effects related to neuronal development, neural cell differentiation, prenatal and postnatal development and behavior. PFC exposure altered hormone levels and the expression of hormone-responsive genes in mammalian and aquatic species. This study is the first to assess the effects of PFCs on messenger RNA (mRNA) expression in primary cultures of neuronal cells in two avian species: the domestic chicken (Gallus domesticus) and herring gull (Larus argentatus). The following thyroid hormone (TH)–responsive genes were examined using real-time reverse transcription-PCR: type II iodothyronine 5′-deiodinase (D2), D3, transthyretin (TTR), neurogranin (RC3), octamer motif–binding factor (Oct-1), and myelin basic protein. Several PFCs altered the mRNA expression levels of genes associated with the TH pathway in avian neuronal cells. Short-chained PFCs (less than eight carbons) altered the expression of TH-responsive genes (D2, D3, TTR, and RC3) in chicken embryonic neuronal cells to a greater extent than long-chained PFCs (more than or equal to eight carbons). Variable transcriptional changes were observed in herring gull embryonic neuronal cells exposed to short-chained PFCs; mRNA levels of Oct-1 and RC3 were upregulated. This is the first study to report that PFC exposure alters mRNA expression in primary cultures of avian neuronal cells and may provide insight into the possible mechanisms of action of PFCs in the avian brain.
Keywords: perfluoroalkyl compounds, avian, brain, thyroid hormone, in vitro, mRNA expression
Perfluoroalkyl compounds (PFCs) are a class of ubiquitous synthetic chemicals found in the environment that are used in many industrial and commercial products, including the lining of nonstick cookware, food wraps and containers, popcorn bags, aqueous firefighting foams, and industrial lubricating agents (Jensen and Leffers, 2008). PFCs consist of a carbon chain (4–14 carbons) completely saturated by fluorine atoms that is attached to a charged functional group. This molecular arrangement produces highly stable compounds with extremely low surface tension and gives PFCs their desirable water-, oil-, and soil-resistant properties. The hydrophobic fluorine-carbon backbone and the hydrophilic charged functional group are responsible for the lipophilic and hydrophilic properties of these compounds, respectively.
PFCs have been detected in plasma, tissues, and eggs of wild avian populations; examples include bald eagles, albatrosses, cormorants, and herring gulls (Gebbink et al., 2009; Giesy and Kannan, 2001; Holmstrom and Berger, 2008; Houde et al., 2006). Temporal monitoring studies have indicated increases in PFC concentrations in wild avian species. For example, perfluorooctane sulfonate (PFOS), the predominant PFC measured in the environment, increased twofold in herring gull eggs in Northern Norway from 1983 to 2003 (21 ng/g wet weight [ww] to 42 ng/g ww, respectively) (Verreault et al., 2007). Furthermore, a wide range of PFCs were detected at relatively high concentrations in herring gull eggs from colonies spanning the Great Lakes of North America (∑PFCs of up to 600 ± 56 ng/g ww) (Gebbink et al., 2009). PFC exposure has been associated with many detrimental effects in mammalian species, including mortality, reduced body weight, and increased liver weight (Lau et al., 2007). The hepatotoxic effects of PFCs have been well characterized but less is known about their effects in the brain. A few studies have shown that PFCs alter thyroid hormone (TH) levels and interfere with brain development in mammals (Johansson et al., 2008, 2009; Lau et al., 2003; Thibodeaux et al., 2003; Yu et al., 2009).
Although the toxic and biochemical effects of PFCs are becoming increasingly characterized in mammals, there are limited studies on their effects in avian species. Previous chicken egg injection studies reported reduced hatching and higher incidences of physical deformities (e.g., splayed legs and abnormal pigmentation) in perfluorooctanoic acid (PFOA)-treated groups (Yanai et al., 2008). Other studies reported reduced hatchability and embryo pippability following PFOS exposure (Molina et al., 2006; O'Brien et al. 2009). Pinkas et al. (2009) determined that in ovo exposure to PFOS and PFOA interfered with posthatch cognitive behavior by altering the concentration of cytosolic protein kinase C (PKC), which is involved in controlling imprinting behavior. Reduced hatching and pippability may be consequences of poor motor skills associated with altered brain development. Immune alterations, brain asymmetry, and cognitive behavioral changes have also been observed following in ovo PFC exposure (Peden-Adams et al., 2008; Pinkas et al., 2009). The results from these studies highlight a need to study the effects of PFCs in the avian brain.
The objectives of this study were to utilize an in vitro screening method to determine the effects of PFC exposure on the messenger RNA (mRNA) expression of TH-responsive genes in primary cultures of chicken embryonic neuronal (CEN) and herring gull embryonic neuronal (HGEN) cells. Several short- and long-chained PFCs (less than eight carbons and more than or equal to eight carbons, respectively) were assessed to determine if differences in transcriptional responses were related to PFC chain length.
MATERIALS AND METHODS
Chemicals
Perfluoroalkyl sulfonates (PFSAs) and perfluorocarboxylates (PFCAs) were obtained from Wellington Laboratories (Guelph, ON, Canada). The PFCs were perfluorobutanoic acid (PFBA) (Lot: PFBAGA06; molecular weight [MW]: 236.02 g/mol), perfluorobutanesulfonate (PFBS) (Lot: 24318BB; MW: 338.19 g/mol), perfluorohexanoic acid (PFHxA) (Lot: PFHxAGA06; MW: 336.04 g/mol), perfluorohexanesulfonate (PFHxS) (Lot: LPFHxSAM05; MW: 422.10 g/mol), perfluoroheptanoic acid (PFHpA) (Lot: NaPFHpARM07; MW: 386.04 g/mol), perfluoroheptanesulfonate (PFHpS) (Lot: PFHpSM06; MW: 472.10 g/mol), PFOA (Lot: PFOAGA05; MW: 414.07 g/mol), PFOS (Lot: TPFOS0907; MW: 538.22 g/mol), perfluorononanoic acid (PFNA) (Lot: 01479; MW: 464.08 g/mol), perfluoroundecanoic acid (PFuDA) (Lot: PFuDARM07; MW: 564.09 g/mol), and perfluorododecanoic acid (PFDoA) (Lot: PFDoANR07; MW: 614.10 g/mol). Triiodothyronine (T3) was purchased from Sigma-Aldrich (Oakville, ON, Canada). Stock solutions and serial dilutions of PFCs and T3 were prepared in dimethyl sulfoxide (DMSO).
Source of Eggs and Incubation Conditions
Twenty-four fertile unincubated white leghorn chicken (Gallus domesticus) eggs were obtained from the Canadian Food Inspection Agency (Ottawa, ON, Canada) and incubated at 37°C and 60% relative humidity in Curfew Model RX250 incubators (Althorne, Essex, UK) for 11 days. This corresponds to midincubation for chickens as the time to hatch is 20–21 days (Hamburger and Hamilton, 1951). Chicken embryos were euthanized by decapitation.
Fifteen fertile unincubated herring gull eggs were collected from Chantry Island, ON, Canada (44°29′22″N and 81°24′7″W) in April 2009. Herring gull eggs were transported to the laboratory in a padded cooler at ambient temperature and then incubated at 37°C and 60% relative humidity in Curfew Model RX250 incubators (Althorne) for 14 days. This corresponds to midincubation for herring gulls as the time to hatch is 28–30 days. Herring gull embryos were euthanized at midincubation by decapitation. All procedures were carried out using methods approved by the Animal Care Committee of the National Wildlife Research Centre.
Preparation and Dosing of Avian Neuronal Cell Cultures
Chicken.
Primary cultures of CEN cells were prepared from the cerebral cortices of day 11 chicken embryos as described previously (Crump et al., 2008b). Briefly, cerebral cortices were dissected from chicken embryos, pooled in neuronal isolation medium (2.5mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 128.5mM NaCl, 5.4mM KCl, 5.5mM D-glucose, 51.8mM sucrose, and 0.1% bovine serum albumin), and minced into small pieces using a scalpel. The mixture was incubated at room temperature for ∼120 min and further dissociated by pipetting up and down against the glass bottom. The dissociated cells were vacuum filtered through a series of nylon sieves of decreasing pore sizes (∼200, 120, 45, and 22 μm), and the filtrate was centrifuged for 13 min at ×60 g at 14°C. The resulting pellet was resuspended in isolation medium and vacuum filtered/centrifuged a second time. The cell pellet was resuspended in Neurobasal medium containing 0.5mM L-glutamine, 25μM glutamate, and 2% B27 supplement (Invitrogen, Burlington, ON, Canada). Cells were plated at a density of 3.5 mg cells/ml in 48-well plates that were precoated with 3.3 μg/ml poly-D-lysine (Sigma-Aldrich). Neuronal cultures were incubated at 37°C and 5% CO2 for 24 h prior to chemical administration.
Each PFC was administered at six concentrations: 0.01, 0.1, 1, 3, 10, and 50μM. A DMSO vehicle and an untreated (UT) control were also included. T3 was included as a positive control to determine how the TH-responsive genes responded to their natural agonist. T3 was administered at four concentrations (0.03, 0.3, 3, and 30nM) plus a DMSO vehicle control. The volume of PFC and T3 solutions added to each well was 1.75 μl per well and 2.5 μl per well, respectively. All reported concentrations are final in-well concentrations. Four replicate wells were included for each treatment group. Exposure to each compound was repeated on two separate plates—one plate was used for RNA isolation and the other plate was used to assess cell viability. Following chemical administration, cells were incubated for another 24 h at 37°C and 5% CO2. After 24 h, the medium was aspirated from all wells and plates were either immediately frozen at −80°C for subsequent RNA isolation or assessed for cell viability.
Herring gull.
Primary cultures of HGEN cells were prepared from the cerebral cortices of day 14 embryos following the same protocol with minor modifications. HGEN cells were treated with PFBA, PFBS, PFHxA, PFHxS, PFHpA, and PFHpS at five concentrations: 0.01, 0.1, 1, 3, and 10μM. T3 was administered at five concentrations: 0.03, 0.3, 3, 30, and 300nM. A DMSO vehicle group was included for both T3- and PFC-treated cells. Exposure to each compound was repeated on two separate plates. Following chemical administration, PFC-treated cells were incubated for 24 h at 37°C and 5% CO2. T3-treated cells were incubated at 37°C and 5% CO2 for 3, 6, 12, 18, 24, and 36 h. After exposure, the medium was aspirated from all wells and the plates were immediately frozen at −80°C or assessed for cell viability.
Cell Viability
In CEN cells, viability of PFC-treated cells (three wells per treatment group) was compared with a DMSO vehicle, UT control, and 100% ethanol (negative control). In HGEN cells, viability was only determined for T3-treated cells at 3 and 24 h and compared with the same controls as above (three to four wells per treatment group). Cell viability was estimated using the Calcein-AM assay according to the manufacturer's instructions (Molecular Probes, Eugene, OR). A working solution was prepared by adding 3 μl of Calcein-AM to 10 ml of PBS-EDTA. The medium in each well was aspirated, replaced with 200 μl of the Calcein-AM solution, and incubated for 45 min. Fluorescence was measured using a fluorescence plate reader (Millipore, Billerica, MA) with an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Statistically significant differences between experimental and DMSO vehicle control groups were determined using a one-way ANOVA followed by Bonferroni's t-test for multiple comparisons versus control. Changes were considered statistically different if p < 0.05.
RNA Isolation and Complementary DNA Synthesis
Total RNA was extracted using Qiagen RNeasy 96 kits (Qiagen, Mississauga, ON, Canada) as described by the manufacturer. The only modification was that the on-column DNase treatment was not performed in this experiment. Following the extraction of total RNA, a DNase treatment was performed using the Ambion DNA-free kit (Ambion, Austin, TX), and the DNase-treated RNA was quantified using the Nanodrop 2000 spectrometer (Thermo Scientific).
Complementary DNA (cDNA) was synthesized using random hexamers and Superscript II reverse transcriptase (RT) (Invitrogen) according to the manufacturer's instructions. Reactions without reverse transcriptase (no-RT control) were included to verify the absence of genomic DNA. Aliquots of cDNA were prepared by diluting stocks 1:5 in diethyl pyrocarbonate-treated H2O for real-time reverse transcription (RT)-PCR.
Real-Time RT-PCR
All real-time RT-PCR assays were performed using the Strategene Mx3000P or Mx3005P instruments (Strategene, La Jolla, CA). β-Actin was used as a normalizer gene because its expression was found to be invariable in all treatment groups for which data are presented. The mRNA levels were quantified using TaqMan probes and Brilliant Quantitative-PCR Core Reagent kits (Stratagene). Taqman reactions (25 μl) contained 1× Core RT-PCR buffer, 5mM MgCl2, 0.8mM dNTPs, 0.08% vol/vol glycerol, 60nM ROX reference dye, 0.05 U Surestart Taq DNA polymerase, primers and probes for β-actin and target genes, and 5 μl of 1:5 diluted cDNA. The thermal profile for all reactions was 10 min at 95°C, followed by 40 cycles of 30 s at 95°C, and 1 min at 60°C.
Chicken primers and probes for several genes were previously designed for β-actin, TH receptor alpha and beta (TR-α and TR-β), transthyretin (TTR), type II iodothyronine 5′-deiodinase (D2), and myelin basic protein (MBP) (Crump et al., 2008a,b). Primers and probes for chicken D3, neurogranin (RC3), and octamer motif–binding factor-1 (Oct-1) were designed using partial chicken mRNA sequences and are shown in Table 1. The herring gull TR-α and TR-β primers and probes were designed by Crump et al. (2008c). Herring gull primer pairs and Taqman fluorogenic probes were designed using partial herring gull sequences obtained by homology cloning for D2, RC3, and Oct-1 (accession numbers GQ281790 and GQ281789 for RC3 and Oct-1, respectively) (Table 1).
TABLE 1.
Primer and Probe Nucleotide Sequences, Final Concentrations, and Accession Numbers for the Transcripts That Were Assessed Using Real-time RT-PCR Analysis in Two Different Avian Species
| Gene | Sequence (5′–3′) | Final concentrations (nM) | Accession number | |
| Chicken | Type III iodothyronine 5′-deiodinase (D3) | F: CCTCCTCTTTCCCCGCTTCC | 900 | NM_001122648.1 |
| R: GTGGGCATCGTCAGCATCTTC | 300 | |||
| P: CCGCTGTGATGCTCTGGCTCCTGG | 200 | |||
| Neurogranin (RC3) | F: ACGAAGCTGGATGAGGACATC | 900 | XM_001232109.1 | |
| R: GAAGCTGGCCTGGATCTTGG | 900 | |||
| P: CCCCTGGACGACCCCGACGCC | 200 | |||
| Octamer motif–binding factor 1 (Oct-1) | F: CTGCTGGTGCCACCATCTCG | 900 | NM_205472.1 | |
| R: AGGTTGAGATTCTGCTGCTGAAG | 900 | |||
| P: CCTCTGCTGCAACGCCGATGACGC | 200 | |||
| Herring Gull | Type II iodothyronine 5′-deiodinase (D2) | F: CGCATCGGTCTTCTTGGTTCC | 300 | Not Available |
| R: GCTGACTTTCTGTTGGTCTACATC | 900 | |||
| P: CACCCGTCAGATGGCTGGGCTGCC | 200 | |||
| Neurogranin (RC3) | F: TGGATGAGGACATCCTGGACATC | 900 | GQ281790 | |
| R: TTGATCTTCTTGCGGGTCATATGG | 900 | |||
| P: CTTGGACGATCCCGACGCCAACGC | 200 | |||
| Octamer motif–binding factor 1 (Oct-1) | F: GCCGGAGGACAGATCACTGG | 300 | GQ281789 | |
| R: GCTGCTGACTGGCTGAATGC | 900 | |||
| P: CAGCCGCCAGCAACTGTGCCTGC | 200 |
Note. F, forward primers; R, reverse primers; P, probe.
Standard curves were generated for all genes from a 1:2 dilution series of cDNA. Primer concentrations were optimized so that PCR reaction efficiencies for all target genes were within ±10% of the amplification efficiency of β-actin. No-RT and no-template controls (NTCs) were included in all assays to verify the absence of contamination. Each treatment group consisted of four technical replicates (four wells per treatment group), and each sample was analyzed in duplicate by real-time RT-PCR.
Statistical Analysis
Real-time RT-PCR data were analyzed using MxPro v3.00 software (Strategene). Relative quantity values were normalized to β-actin, and fold changes were calculated relative to the DMSO-treated cells using the equation (Livak and Schmittgen, 2001). Statistically significant differences between experimental and DMSO vehicle control groups were determined using a one-way ANOVA followed by Bonferroni's t-test for multiple comparisons versus control. Changes were considered statistically different if p< 0.05.
RESULTS
Cell Viability
There were no significant changes in cell viability in T3-treated CEN or HGEN cells up to the maximum concentration of 30 and 300nM, respectively (data not shown). Ethanol-killed cells (negative control), as expected, exhibited a significant decrease in viability. Among all the PFCs studied, only PFHxA treatment significantly decreased cell viability at 30 and 50μM in CEN cells (data not shown). Based on these results, 10μM was the highest PFC concentration used for subsequent mRNA expression analysis in CEN and HGEN cells.
Chicken mRNA Expression
T3—positive control.
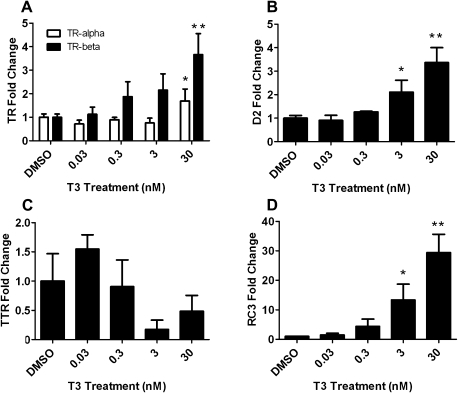
Following T3 exposure, there was upregulation of TR-α and TR-β mRNA expression (Fig. 1A), indicating that CEN cells responded to treatment with the natural ligand. T3 treatment also significantly increased D2 mRNA levels and decreased TTR mRNA expression in CEN cells (Figs. 1B and 1C). RC3 mRNA expression increased to ∼14- and 30-fold following T3 administration (3 and 30nM, respectively) (Fig. 1D). There were no significant changes in the other TH-responsive genes examined (D3, Oct-1, and MBP; data not shown).
FIG. 1.
The relative expression of TR-α and TR-β (A), D2 (B), TTR (C), and RC3 (D) mRNA in CEN cells following 24 h of T3 treatment. Error bars represent the SD of the mean, and a one-way ANOVA was used to determine significant differences (N = 3–4 wells per treatment; *p < 0.05 and **p < 0.001 comparing DMSO- and T3-treated groups).
Perfluoroalkyl compounds.
The fold changes for the TH-responsive genes following PFC treatment in CEN cells are summarized in Table 2. Results for PFCs that caused significant concentration-dependent effects are presented in more detail in Figure 2.
TABLE 2.
Mean Fold Change ± SD of Target Gene mRNA Expression Following Exposure of CEN Cells to 11 PFCs. (SD Was Calculated From Three to Four Replicate Wells on a Single 48-Well Plate)
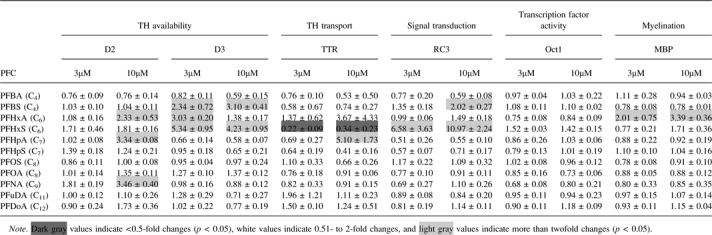 |
FIG. 2.
The relative expression of D2 (A), D3 (B), TTR (C), RC3 (D), and MBP (E) mRNA in CEN cells following treatment with several PFCs. Error bars represent the SD of the mean, and a one-way ANOVA was used to determine significant differences (N = 3–4 wells per treatment; *p < 0.05 and **p < 0.001 comparing DMSO- and PFC-treated groups).
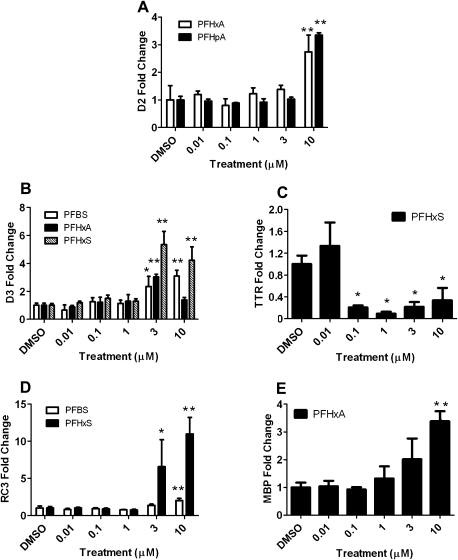
D2 mRNA was upregulated following exposure to PFHxA, PFHpA, and PFNA (Fig. 2A and Table 2), which was similar to the trend observed in T3-treated CEN cells (Fig. 1B). The complete concentration-dependent figure for PFNA was not included due to an insufficient yield of RNA in some of the treatment groups. D3 mRNA expression increased twofold to fivefold following exposure to several PFCs (PFBS, PFHxA, and PFHxS) (Fig. 2B). PFHxS treatment significantly decreased TTR mRNA expression (by as much as 10-fold at 1μM; Fig. 2C), which was similar to that observed following T3 exposure (Fig. 1C). In contrast to the results observed for PFHxS, PFHpA exposure increased TTR mRNA levels by fourfold at 10μM (Table 2). The complete concentration-dependent figure for PFHpA was not obtained due to an insufficient yield of RNA in some of the treatment groups.
PFBS and PFHxS treatment increased RC3 mRNA expression (Fig. 2D), which was similar to the trend observed in T3-treated cells (Fig. 1D). RC3 mRNA expression was upregulated 2- and 11-fold following PFBS and PFHxS treatment at 10μM, respectively. There were no changes to Oct-1 mRNA levels following treatment with any of the PFCs examined, which was similar to the response observed in T3-treated CEN cells. Finally, MBP mRNA expression was upregulated at 3 and 10μM following PFHxA treatment relative to the control (Fig. 2E). There were no significant changes in MBP mRNA expression following T3 exposure or treatment with the other PFCs.
General observations in CEN cells.
Of the 11 PFCs studied, five elicited significant mRNA expression changes in the genes that were examined (Table 2). The shorter-chained PFCs (less than eight carbons) altered mRNA expression to a greater extent than the longer-chained compounds. Of the short-chained PFCs, PFBS, PFHxA, PFHxS, and PFHpA altered the expression of at least two or more TH-responsive genes in a concentration-dependent manner (Fig. 2). With the exception of PFNA, there were no significant gene expression changes following exposure to the long-chained PFCs.
Herring Gull mRNA Expression
T3—positive control.
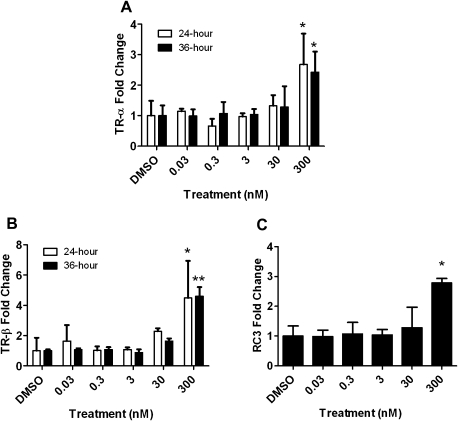
T3 treatment for 24 and 36 h induced TR-α and TR-β mRNA expression up to twofold and fourfold, respectively (Figs. 3A and 3B). T3 treatment for 36 h also increased RC3 mRNA expression more than twofold (Fig. 3C). There were no significant changes in the other TH-responsive genes examined (D2 and Oct-1; data not shown). Additionally, no significant changes were observed in the TH-responsive genes at any of the earlier time points (i.e., 3, 6, 12, or 18 h).
FIG. 3.
The relative expression of TR-α (A), TR-β (B), and RC3 (C) mRNA in HGEN cells following T3 treatment for 24 h (white bars) or 36 h (black bars). Error bars represent the SD of the mean, and a one-way ANOVA was used to determine significant differences (N = 3–4 wells per treatment; *p < 0.05 and **p < 0.001 comparing DMSO- and T3-treated groups).
Perfluoroalkyl compounds.
The fold changes for the TH-responsive genes following PFC treatment in HGEN cells are summarized in Table 3. Only PFCs that demonstrated significant concentration-dependent effects on the TH-responsive genes are presented in more detail in Figure 4.
TABLE 3.
Mean Fold Change ± SD of Target Gene mRNA Expression in PFC-Treated HGEN Cells. (SD Was Calculated From Three to Four Replicate Wells on a Single 48-Well Plate)
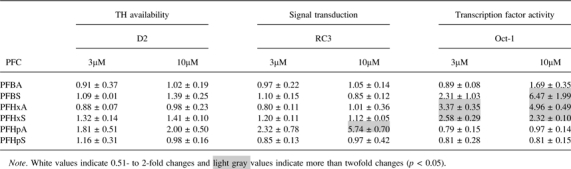 |
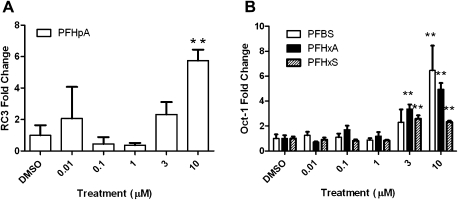
FIG. 4.
The relative mRNA expression of RC3 (A) and Oct-1 (B) in HGEN cells following treatment with several PFCs. Error bars represent the SD of the mean, and a one-way ANOVA was used to determine significant differences (N = 3–4 wells per treatment; *p < 0.05 and **p < 0.001 comparing DMSO- and PFC-treated groups).
Similar to T3-treated HGEN cells, there were no significant changes in D2 mRNA levels following treatment with any of the PFCs that were examined. There was an upregulation in RC3 mRNA expression (Fig. 4A) following PFHpA exposure, which was similar to the pattern observed in T3-treated HGEN cells (Fig. 3C); however, RC3 mRNA expression did not change following treatment with the other PFCs examined. There was an increase in Oct-1 mRNA following treatment with PFBS, PFHxA, and PFHxS (Fig. 4B) but not following T3 exposure.
DISCUSSION
To our knowledge, this is the first study to assess the effects of PFCs on mRNA expression in cultured avian neuronal cells. Specific molecular endpoints were responsive to PFC exposure in CEN and HGEN cells, helping to elucidate the possible mechanisms of action of PFCs in the avian brain. To ensure that transcriptional changes were related to mRNA expression and not cell viability, the mRNA data reported are from treatment groups that had no significant effect on cell viability. The highest PFC concentration used for mRNA expression analysis was 10μM. T3 was administered at maximum concentrations of 30 and 300nM in CEN and HGEN cells, respectively.
The optimal incubation period required to observe mRNA expression effects was determined in HGEN cells exposed to T3 for 3, 6, 12, 18, 24, and 36 h. The minimum time required to demonstrate significant mRNA expression changes in the TH-responsive genes was 24 h as no alterations were observed at 3, 6, 12, or 18 h. T3 upregulated TR-α and TR-β mRNA expression (at 24 and 36 h), which indicated that primary cultures of CEN and HGEN cells responded to treatment with the natural ligand. During chicken development, maximal concentrations of TRs and high T3 levels are detected in the avian brain at midincubation (McNabb, 2007), the developmental stage assessed in this study. PFC effects were compared with those of T3, which was included as a positive control for TH pathway activation in this study.
Exposure to T3 and several PFCAs (PFHxA, PFHpA, and PFNA) induced D2 mRNA expression in CEN cells. D2 converts T4 to its active form, T3, via 5′ outer ring deiodination (Bianco and Kim, 2006). Increased D2 mRNA levels following T3 administration could be partially explained by the developmental time period at which the neuronal cells were harvested (i.e., day 11 embryos). TH concentrations and D2 mRNA levels are elevated at day 11 and, in fact, D2 mRNA increases up to 14-fold from day 10 to day 17 (Gereben et al., 2004). This may be a means to increase T3 bioavailability in preparation for pipping, hatching, and growth at later stages (McNabb, 2007). The concordance of D2 induction following T3, PFHxA, PFHpA, and PFNA treatment suggests a similar mode of action.
PFHxA, PFHxS, and PFBS induced D3 mRNA expression twofold to fivefold in CEN cells. D3 catalyzes inner ring 5′-deiodination, which degrades both T4 and T3 to their inactive derivatives, reverse T3 (rT3) and 3,3′-diiodothyronine (T2), respectively (Hernandez, 2005). D3 is found primarily in the cerebral cortex (Hernandez, 2005), the brain region from which neuronal cells were harvested in this experiment. Elevated D3 mRNA levels would be expected when TH concentrations are over abundant as this would provide a mechanism to eliminate excess TH and maintain homeostasis. Hernandez (2005) suggested that D3 upregulation may occur as a means to protect the brain from elevated TH levels.
TTR mRNA expression was significantly downregulated following PFHxS exposure at concentrations as low as 0.1μM; at 1μM TTR mRNA levels were diminished by ∼10-fold. T3 also decreased TTR mRNA levels indicating a potential shared mechanism of action. Previous studies in our laboratory measured TTR mRNA expression following T3 administration in primary cultures of CEN (36 h exposure as opposed to 24 h in this study) and HGEN cells (24 h exposure). As in the present study, T3 decreased TTR expression in CEN (Crump, unpublished results) and HGEN cells (twofold to fivefold; Crump et al., 2008c). TTR, a major TH-binding protein in the bloodstream of birds, is synthesized by the choroid plexus and transports T4 into the central nervous system where it is converted to its more active T3 form by deiodination (review Schreiber, 2002; review McNabb, 2007). A reduction of TTR expression could result in decreased TH availability to target tissues, which could affect TH-dependent processes.
Exposure to the four- and six-carbon PFSAs induced RC3 mRNA expression up to 2- and 11-fold, respectively. Similarly, T3 administration increased RC3 mRNA expression up to ∼30-fold. Iniguez et al. (1993) reported that TH altered RC3 mRNA expression in brain regions of rodents, including the cerebral cortex, striatum, and hippocampus. Furthermore, a commercial mixture of polychlorinated biphenyls, Aroclor 1254 (A1254), induced RC3 mRNA expression in the cortex of rodents (Gauger et al., 2004). The transcriptional response of RC3 following exposure to T3, PFCs, and other anthropogenic chemicals makes it a useful marker to examine disturbances in TH homeostasis. RC3 is a calmodulin-binding PKC substrate that is involved in the Ca2+ signal transduction system and implicated in events that lead to long-term potentiation (Gerendasy, 1999; Iniguez et al., 1993, 1996). Changes in RC3 expression could have functional consequences in synaptic plasticity, associative learning, and memory (Iniguez et al., 1993, 1996).
PFHxA treatment induced MBP mRNA expression in CEN cells (at 3 and 10μM). MBP is essential in the process of nerve myelination, which facilitates the conduction of nerve impulses. MBP contains a thyroid-responsive element in its promoter region, and in vitro binding studies demonstrated that both TH and TRs are required to positively regulate MBP expression (Farsetti et al., 1991). T3 administration did not alter MBP mRNA expression in this study, which may have been due to differences in experimental designs (e.g., T3 concentrations, experimental approach [binding study vs. gene expression study], and species differences).
Although many significant changes were observed in TH-responsive genes following PFC exposure in CEN cells, this was not the case in PFC-treated HGEN cells. The mRNA levels of three TH-responsive genes (D2, Oct-1, and RC3) were examined in HGEN cells treated with short-chained PFCs because, based on the results in CEN cells, short-chained PFCs were more transcriptionally active. TR-α and TR-β mRNA levels were also measured in HGEN cells following T3 treatment and were induced at 300nM. A previous study by Crump et al. (2008c) also reported an induction of TR-α and TR-β in HGEN cells, albeit at lower T3 concentrations. Although differences were observed between the Crump et al. (2008c) study and the present study, the important finding was that HGEN cells from both studies were responsive to a TR agonist. Variability in response of HGEN cells to T3 between the two studies, conducted with herring gull eggs collected 2 years apart, could be attributed to factors including: (1) maternal influences (e.g., variability in hormone and contaminant deposition to the developing embryo during ovogenesis), (ii) interannual variation in colony dynamics (e.g., stress, influence of predators, weather conditions), (iii) genetic variability—especially compared with chicken embryos that come from a managed flock, and (iv) variation in the stage of embryonic development at which neurons were obtained—ideally, midincubation (i.e., day 14)—due to incubation prior to collection. The use of a wild avian species for such an in vitro screening approach poses several challenges not present in a domestic species; however, it is important to generate data for species exposed naturally to environmental contaminants.
It was determined that exposure to short-chained PFCs elicited variable transcriptional responses when comparing primary cultures of herring gull and chicken neuronal cells (refer to Table 4). Differences in the transcriptional responses between species may be related to variable levels of baseline contaminant exposure and/or unknown factors that determine species-specific differences in sensitivity. For example, the chicken embryos were obtained from a managed breeding facility where the exposure to environmental contaminants is minimal. Herring gull eggs were collected from Chantry Island, a Great Lakes colony that is used as a reference (i.e., clean) site; however, detectable levels of a wide range of chemical contaminants are found in egg homogenates (Gauthier et al. 2009; Gebbink et al. 2009; Jeremyn-Gee et al. 2005). ∑PFC concentrations in herring gull eggs collected from Chantry Island were 192 ng/g ww (Gebbink et al., 2009). In order to put these environmentally relevant levels in the context of in vitro concentrations (micromolar) used in this study, calculations were made based on the weight of the neuronal cells in each well and the administered PFC concentration. The maximum possible uptake rate into neuronal cells was 100%, and based on this, the lowest effective in vitro concentration (3μM for Oct-1 mRNA expression) would be approximately three orders of magnitude greater than levels detected in herring gull eggs collected from Chantry Island (∼340 μg/g vs. 0.192 μg/g ww). It is important to note that actual PFC concentrations in neuronal cells were not determined, and this is a key consideration for future in vitro studies in order to determine if the 100% uptake rate assumption, that forms the basis of the comparative calculation, is accurate.
TABLE 4.
Summary of the Overall Significant mRNA Expression Changes in CEN and HGEN Cells Following Exposure to T3 and Several PFCs
| Species | Compound | TH availability | TH transport | Signal transduction | Transcription factor activity | Myelination | ||
| D2 | D3 | TTR | RC3 | Oct-1 | MBP | |||
| Chicken | T3 | ↑ | — | ↓ | ↑ | — | — | |
| PFBS | — | ↑ | — | ↑ | — | — | ||
| PFHxA | ↑ | ↑ | — | — | — | ↑ | ||
| PFHxS | — | ↑ | ↓ | ↑ | — | — | ||
| PFHpA | ↑ | — | ↑ | — | — | — | ||
| PFNA | ↑ | — | — | — | — | — | ||
| Herring Gull | T3 | — | nd | nd | ↑ | — | nd | |
| PFBS | — | nd | nd | — | ↑ | nd | ||
| PFHxA | — | nd | nd | — | ↑ | nd | ||
| PFHxS | — | nd | nd | — | ↑ | nd | ||
| PFHpA | — | nd | nd | ↑ | — | nd |
Note. —, no change; nd, not determined.
T3- and PFHpA-treated HGEN cells induced RC3 mRNA expression up to twofold and sixfold, respectively. This was similar to the transcriptional response observed in CEN cells treated with short-chained PFCs (PFBS and PFHxS). Similar transcriptional responses in RC3 following exposure to T3 and PFCs in the neuronal cells of two different avian species make this gene a useful marker to examine disturbances in TH homeostasis.
Oct-1 mRNA expression was induced following exposure to PFBS, PFHxA, and PFHxS in HGEN cells but was invariable in CEN cells. Oct-1, a DNA-binding protein, has been associated with DNA replication and the transcription of histone 2B genes, small nuclear RNAs, and immunoglobulins (Fletcher et al., 1987; Mittal et al., 1996; Sive and Roeder, 1986; Verrijzer et al., 1990). Oct-1 may be important in regulating the growth of eukaryotic cells because reduced expression was associated with cell cycle arrest and morphological differentiation (Lakin et al., 1995). Oct-1 is considered to be a TH-responsive gene for several reasons. Dowling et al. (2000) reported that a single T4 administration increased Oct-1 mRNA in the cortex of rat pups exposed at gestation day 16 (athyroid state). Furthermore, Oct-1 expression was regulated by T3 from fetal life until adulthood in rodents. Not only has Oct-1 demonstrated TH responsiveness but also it has shown responsiveness following exposure to environmental contaminants; A1254 induced Oct-1 expression in the cortex of rodents (Gauger et al., 2004).
This study reported significant gene expression changes (up to 12-fold) in PFC-treated avian neuronal cells. It was determined that PFCs differ substantially in their effects on mRNA expression in avian neuronal cells. The six-carbon PFHxA and PFHxS were the most transcriptionally active compounds in CEN cells, altering the expression of three TH-responsive genes. The results suggest that PFBS, PFHxS, and PFHxA may share similar modes of action with T3 given the similar patterns in transcriptional responses. These findings are important given the persistence of newer, replacement PFCs in tissues, eggs, and blood of avian species worldwide. Short-chained PFCs are being manufactured as replacement alternatives to PFOS and PFOA and the concentrations of short-chained PFCs are expected to rise due to increasing usage and production.
Studies that have assessed PFC chain length effects have reported that the degree of fluorination, carbon chain length, and functional group are key factors that influence PFC effects (e.g., binding potency and neural functions) (Liao et al., 2009; Upham et al., 1998; Weiss et al., 2009). Long-chained PFCs bind more strongly to protein than short-chained PFCs (Jones et al., 2003), which may have caused these compounds to bind to the protein in the medium, the walls of the plastic wells, or to nonspecific proteins on the outside of neuronal cells rendering them less available for uptake into neuronal cells. Therefore, the augmented transcriptional response elicited by short-chained PFCs in this study may be due to their bioavailability. In other words, they could have entered neuronal cells more readily due to their lower binding affinities to extracellular proteins.
The TH-responsive genes appear to be useful endpoints for in vitro screening of effects of PFCs in primary cultures of avian neuronal cells. This technique permits an initial assessment of PFC effects on various avian species and provides more insight into the mechanisms of action of these contaminants. It is important to validate these findings in ovo to determine how predictive the in vitro approach is in terms of identifying whole animal effects. In addition, future work should examine alterations of the proteins encoded by these transcripts. This study has contributed to our understanding of PFC effects on the TH pathway and has not only provided evidence that the brain may be a target organ for PFC effects but that these contaminants have the potential to alter TH homeostasis in birds.
FUNDING
Chemicals Management Plan; Environment Canada's Strategic Technology Applications of Genomics for the Environment.
Acknowledgments
The authors wish to thank Jason O'Brien for providing suggestions to improve this manuscript and Drs Robert Letcher and Jonathan Martin for their expert advice on PFC chemistry.
References
- Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J. Clin. Invest. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump D, Chiu S, Egloff C, Kennedy SW. Effects of hexabromocyclododecane and polybrominated diphenyl ethers on mRNA expression in chicken (Gallus domesticus) hepatocytes. Toxicol. Sci. 2008a;106:479–487. doi: 10.1093/toxsci/kfn196. [DOI] [PubMed] [Google Scholar]
- Crump D, Jagla MM, Chiu S, Kennedy SW. Detection of PBDE effects on mRNA expression in chicken (Gallus domesticus) neuronal cells using real-time RT-PCR and a new differential display method. Toxicol. In Vitro. 2008b;22:1337–1343. doi: 10.1016/j.tiv.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Crump D, Jagla MM, Kehoe A, Kennedy SW. Detection of polybrominated diphenyl ethers in herring gull (Larus argentatus) brains: effects on mRNA expression in cultured neuronal cells. Environ. Sci. Technol. 2008c;42:7715–7721. doi: 10.1021/es801145j. [DOI] [PubMed] [Google Scholar]
- Dowling AL, Martz GU, Leonard JL, Zoeller RT. Acute changes in maternal thyroid hormone induce rapid and transient changes in gene expression in fetal rat brain. J. Neurosci. 2000;20:2255–2265. doi: 10.1523/JNEUROSCI.20-06-02255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsetti A, Mitsuhashi T, Desvergne B, Robbins J, Nikodem VM. Molecular basis of thyroid hormone regulation of myelin basic protein gene expression in rodent brain. J. Biol. Chem. 1991;266:23226–23232. [PubMed] [Google Scholar]
- Fletcher C, Heintz N, Roeder RG. Purification and characterization of OTF-1, a transcription factor regulating cell cycle expression of a human histone H2b gene. Cell. 1987;51:773–781. doi: 10.1016/0092-8674(87)90100-0. [DOI] [PubMed] [Google Scholar]
- Gauger KJ, Kato Y, Haraguchi K, Lehmler HJ, Robertson LW, Bansal R, Zoeller RT. Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ. Health Perspect. 2004;112:516–523. doi: 10.1289/ehp.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier LT, Potter D, Hebert CE, Letcher RJ. Temporal trends and spatial distribution of non-polybrominated diphenyl ether flame retardants in the eggs of colonial populations of Great Lakes herring gulls. Environ. Sci. Technol. 2009;43:312–317. doi: 10.1021/es801687d. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, Hebert CE, Letcher RJ. Perfluorinated carboxylates and sulfonates and precursor compounds in herring gull eggs from colonies spanning the Laurentian Great Lakes of North America. Environ. Sci. Technol. 2009;43:7443–7449. doi: 10.1021/es901755q. [DOI] [PubMed] [Google Scholar]
- Gereben B, Pachucki J, Kollar A, Liposits Z, Fekete C. Ontogenic redistribution of type 2 deiodinase messenger ribonucleic acid in the brain of chicken. Endocrinology. 2004;145:3619–3625. doi: 10.1210/en.2004-0229. [DOI] [PubMed] [Google Scholar]
- Gerendasy D. Homeostatic tuning of Ca2+ signal transduction by members of the calpacitin protein family. J. Neurosci. Res. 1999;58:107–119. [PubMed] [Google Scholar]
- Giesy JP, Kannan K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001;35:1339–1342. doi: 10.1021/es001834k. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hernandez A. Structure and function of the type 3 deiodinase gene. Thyroid. 2005;15:865–874. doi: 10.1089/thy.2005.15.865. [DOI] [PubMed] [Google Scholar]
- Holmstrom KE, Berger U. Tissue distribution of perfluorinated surfactants in common guillemot (Uria aalge) from the Baltic Sea. Environ. Sci. Technol. 2008;42:5879–5884. doi: 10.1021/es800529h. [DOI] [PubMed] [Google Scholar]
- Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. Biological monitoring of polyfluoroalkyl substances: a review. Environ. Sci. Technol. 2006;40:3463–3473. doi: 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- Iniguez MA, De Lecea L, Guadano-Ferraz A, Morte B, Gerendasy D, Sutcliffe JG, Bernal J. Cell-specific effects of thyroid hormone on RC3/neurogranin expression in rat brain. Endocrinology. 1996;137:1032–1041. doi: 10.1210/endo.137.3.8603571. [DOI] [PubMed] [Google Scholar]
- Iniguez MA, Rodriguez-Pena A, Ibarrola N, Aguilera M, Munoz A, Bernal J. Thyroid hormone regulation of RC3, a brain-specific gene encoding a protein kinase-C substrate. Endocrinology. 1993;133:467–473. doi: 10.1210/endo.133.2.8344193. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Leffers H. Emerging endocrine disrupters: perfluoroalkylated substances. Int. J. Androl. 2008;31:161–169. doi: 10.1111/j.1365-2605.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- Jeremyn-Gee K, Pekarik C, Havelka T, Barrett G, Weseloh DV. An Atlas of Contaminants in Eggs of Fish-Eating Colonial Birds of the Great Lakes (1998–2001) Vol I and II (reports 416 and 417) Gatineau, PQ, Canada: Canadian Wildlife Service. Environment Canada; 2005. [Google Scholar]
- Johansson N, Eriksson P, Viberg H. Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicol. Sci. 2009;108:412–418. doi: 10.1093/toxsci/kfp029. [DOI] [PubMed] [Google Scholar]
- Johansson N, Fredriksson A, Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008;29:160–169. doi: 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Jones PD, Hu W, De CW, Newsted JL, Giesy JP. Binding of perfluorinated fatty acids to serum proteins. Environ. Toxicol. Chem. 2003;22:2639–2649. doi: 10.1897/02-553. [DOI] [PubMed] [Google Scholar]
- Lakin ND, Palmer R, Lillycrop KA, Howard MK, Burke LC, Thomas NS, Latchman DS. Down regulation of the octamer binding protein Oct-1 during growth arrest and differentiation of a neuronal cell line. Brain Res. Mol. Brain Res. 1995;28:47–54. doi: 10.1016/0169-328x(94)00183-f. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol. Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol. Sci. 2003;74:382–392. doi: 10.1093/toxsci/kfg122. [DOI] [PubMed] [Google Scholar]
- Liao C, Wang T, Cui L, Zhou Q, Duan S, Jiang G. Changes in synaptic transmission, calcium current, and neurite growth by perfluorinated compounds are dependent on the chain length and functional group. Environ. Sci. Technol. 2009;43:2099–2104. doi: 10.1021/es802985e. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McNabb FM. The hypothalamic-pituitary-thyroid (HPT) axis in birds and its role in bird development and reproduction. Crit. Rev. Toxicol. 2007;37:163–193. doi: 10.1080/10408440601123552. [DOI] [PubMed] [Google Scholar]
- Mittal V, Cleary MA, Herr W, Hernandez N. The Oct-1 POU-specific domain can stimulate small nuclear RNA gene transcription by stabilizing the basal transcription complex SNAPc. Mol. Cell Biol. 1996;16:1955–1965. doi: 10.1128/mcb.16.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina ED, Balander R, Fitzgerald SD, Giesy JP, Kannan K, Mitchell R, Bursian SJ. Effects of air cell injection of perfluorooctane sulfonate before incubation on development of the white leghorn chicken (Gallus domesticus) embryo. Environ. Toxicol. Chem. 2006;25:227–232. doi: 10.1897/04-414r.1. [DOI] [PubMed] [Google Scholar]
- O'Brien JM, Carew AC, Chu S, Letcher RJ, Kennedy SW. Perfluorooctane sulfonate (PFOS) toxicity in domestic chicken (Gallus gallus domesticus) embryos in the absence of effects on peroxisome proliferator activated receptor alpha (PPARalpha)-regulated genes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009;149:524–530. doi: 10.1016/j.cbpc.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Peden-Adams MM, Stuckey JE, Gaworecki KM, Berger-Ritchie J, Bryant K, Jodice PG, Scott TR, Ferrario JB, Guan B, Vigo C, et al. Developmental toxicity in white leghorn chickens following in ovo exposure to perfluorooctane sulfonate (PFOS) Reprod. Toxicol. 2008;27:307–318. doi: 10.1016/j.reprotox.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Pinkas A, Slotkin TA, Brick-Turin Y, Van der Zee EA, Yanai J. Neurobehavioral teratogenicity of perfluorinated alkyls in an avian model. Neurotoxicol. Teratol. 2009;32:182–186. doi: 10.1016/j.ntt.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G. The evolutionary and integrative roles of transthyretin in thyroid hormone homeostasis. J. Endocrinol. 2002;175:61–73. doi: 10.1677/joe.0.1750061. [DOI] [PubMed] [Google Scholar]
- Sive HL, Roeder RG. Interaction of a common factor with conserved promoter and enhancer sequences in histone H2B, immunoglobulin, and U2 small nuclear RNA (snRNA) genes. Proc. Natl. Acad. Sci. U.S.A. 1986;83:6382–6386. doi: 10.1073/pnas.83.17.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA, Lau C. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol. Sci. 2003;74:369–381. doi: 10.1093/toxsci/kfg121. [DOI] [PubMed] [Google Scholar]
- Upham BL, Deocampo ND, Wurl B, Trosko JE. Inhibition of gap junctional intercellular communication by perfluorinated fatty acids is dependent on the chain length of the fluorinated tail. Int. J. Cancer. 1998;78:491–495. doi: 10.1002/(sici)1097-0215(19981109)78:4<491::aid-ijc16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Verreault J, Berger U, Gabrielsen GW. Trends of perfluorinated alkyl substances in herring gull eggs from two coastal colonies in northern Norway: 1983-2003. Environ. Sci. Technol. 2007;41:6671–6677. doi: 10.1021/es070723j. [DOI] [PubMed] [Google Scholar]
- Verrijzer CP, Kal AJ, Van der Vliet PC. The DNA binding domain (POU domain) of transcription factor oct-1 suffices for stimulation of DNA replication. EMBO J. 1990;9:1883–1888. doi: 10.1002/j.1460-2075.1990.tb08314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Andersson PL, Lamoree MH, Leonards PE, van Leeuwen SP, Hamers T. Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol. Sci. 2009;109:206–216. doi: 10.1093/toxsci/kfp055. [DOI] [PubMed] [Google Scholar]
- Yanai J, Dotan S, Goz R, Pinkas A, Seidler FJ, Slotkin TA, Zimmerman F. Exposure of developing chicks to perfluorooctanoic acid induces defects in prehatch and early posthatch development. J. Toxicol. Environ. Health A. 2008;71:131–133. doi: 10.1080/15287390701613280. [DOI] [PubMed] [Google Scholar]
- Yu WG, Liu W, Jin YH. Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environ. Toxicol. Chem. 2009;28:990–996. doi: 10.1897/08-345.1. [DOI] [PubMed] [Google Scholar]