Abstract
Arsenic, an environmental carcinogen, remains a major public health problem. Arsenic damages biological systems through multiple mechanisms, including the generation of reactive oxygen species. ABCB6 is an ATP-binding cassette transporter that is highly expressed in cells resistant to arsenic. We have recently demonstrated that ABCB6 expression protects against cellular stressors. In the present study, we evaluated the significance of ABCB6 expression to arsenic toxicity both in mice and in cell culture. We show that sodium arsenite induces ABCB6 expression in a dose-dependent manner both in mice fed sodium arsenite in drinking water and in cells exposed to sodium arsenite in vitro. Arsenite-induced ABCB6 expression was transcriptionally regulated, but this induction was not mediated by the redox-sensitive transcription factor nuclear factor-erythroid 2–related factor 2 (Nrf2). We demonstrate that, in HepG2 and Hep3B cells, knockdown of ABCB6 expression using ABCB6-specific small interfering RNA sensitized the cells to arsenite toxicity. In contrast, stable overexpression of ABCB6 conferred a strong survival advantage toward arsenite-induced oxidative stress. Collectively, these results, obtained by both loss of function and gain of function analysis, suggest that ABCB6 expression in response to sodium arsenite might be an endogenous protective mechanism activated to protect cells against arsenite-induced oxidative stress.
Keywords: arsenic, oxidative stress, ABC transporters
Arsenic is a ubiquitous environmental pollutant. Chronic exposure of humans to inorganic arsenic results in formation of tumors of the liver and kidney and probably skin, lung, and urinary bladder (IARC, 2004; Kurt et al., 2009). In addition to its carcinogenic effect, epidemiological studies have associated chronic arsenic exposure with an increased risk in many human nonmalignant diseases, such as peripheral vascular disease, diabetes, and chronic lung disease (ATSDR, 2007). Evidence suggests that arsenic exerts its chronic toxicity by interacting with sulfhydryl groups and generating reactive oxygen species (ROS) that cause damage to cellular macromolecules via oxidative stress (Gomez et al., 2005; Hei and Filipic, 2004; Kojima et al., 2009; Pi et al., 2002; Yamauchi et al., 2004). Of importance, exposure to pro-oxidants such as arsenic also results in the induction of a gene expression program whose primary function is to protect cells from oxidative stress (Liu et al., 2001a; Pi et al., 2003; Scandalios, 2005; Shinkai et al., 2006). Understanding this protective program of adaptation and acquired tolerance to arsenic could potentially open up new therapeutic avenues for prevention and intervention strategies against the toxic effects of arsenic.
The 97-kDa ABCB6 is a member of the ATP-binding cassette (ABC) superfamily of transport proteins that was originally identified while screening for novel drug-resistant genes in the liver (Furuya et al., 1997). Initial cloning and homology-modeling studies revealed that ABCB6 is related to the heavy metal tolerance factor 1 (HMT1) of Caenorhabditis elegans and Schizosaccharomyces pombe (Ortiz et al., 1992, 1995; Vatamaniuk et al., 2005). HMT1 is a half transporter that confers tolerance to heavy metal toxicity in S. pombe (Ortiz et al., 1992) and tolerance to cadmium in C. elegans (Vatamaniuk et al., 2005). These homology-modeling studies suggest a possible role for ABCB6 in metal tolerance. However, ABCB6 expression and metal tolerance in cell culture is conflicting. Recent studies by Annereau et al. (2004) and Paterson et al. (2007) suggest a direct correlation between arsenic resistance and ABCB6 expression in various human and mouse cell lines. They suggest that cell lines selected for resistance to arsenite have elevated levels of ABCB6 messenger RNA (mRNA) and protein. In contrast, studies by Gebel et al. (2002) demonstrate no increase in ABCB6 mRNA in HepG2 cells that were selected for arsenic resistance.
We recently characterized ABCB6 and found that this transporter localizes to the mitochondria and regulates de novo porphyrin biosynthesis (Krishnamurthy et al., 2006). ABCB6 overexpression increases the concentration and activity of hemoproteins, including the heme-dependent antioxidant defense enzyme catalase (Lynch et al., 2009). ABCB6’s ability to increase catalase activity protects cells against hydrogen peroxide (H2O2)–mediated oxidative stress (Lynch et al., 2009). Thus, ABCB6 expression protects against oxidative stress. However, based on the studies reported by Paterson et al. (2007) and Gebel et al. (2002), it is not clear whether ABCB6 can protect against arsenic-mediated oxidative stress.
Thus, the present study was conducted to determine whether arsenic can induce ABCB6 expression and, if so, whether its upregulation protects cells against arsenic-induced oxidative stress. We report that sodium arsenite induces upregulation of ABCB6. ABCB6 expression appears to be transcriptionally regulated, but this expression is not mediated by the redox-sensitive transcription factor Nrf2. More importantly, we demonstrate that loss of ABCB6 expression sensitizes cells to the toxic effects of arsenite. Finally, we provide convincing evidence that ABCB6’s ability to reduce arsenite-induced oxidative stress is a contributing mechanism by which ABCB6 protects against arsenite toxicity.
MATERIALS AND METHODS
Cell culture.
Human liver-derived cell lines Hep3B and HepG2 were from the American Type Culture Collection (Manassas, VA). Hep3B and HepG2 cells were cultured in modified Eagle's medium supplemented with 10% fetal bovine serum (FBS) and 100 units/ml penicillin. HepG2 and Hep3B cells were engineered to overexpress human ABCB6 as described (Krishnamurthy et al., 2006). ABCB6-overexpressing cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS, 100 units/ml penicillin, 2mM L-glutamine, and 500 μg/ml Geneticin (G418). All chemicals were from Sigma-Aldrich Corporation (St Louis, MO) unless otherwise stated.
ABCB6-specific and ABCB6 nonspecific (scrambled) small interfering RNA.
The small interfering RNA (siRNA) oligonucleotide and negative control scrambled oligonucleotide were custom synthesized by Dharmacon (Lafeyette, CO). Both siRNA and control oligonucleotides were used at a final concentration of 150nM and were added to cells using lipofectamine following the manufacturer's protocol.
siRNA 1: 5′ GCGCAUACUUUGUCACUGACA 3′
3′ UUCGCGUAUGAAACAGUGACUP 5′
siRNA 2: 5′ CCGAAUAGAUGGGCAGGACAU 3′
3′ UUGGCUUAUCUACCCGUCCUGP 5′
Scrambled oligo: 5′ UAGCGACUAAACACAUCAA 3′.
Real-time PCR and Western analysis.
Real-time PCR was performed as previously described (Krishnamurthy et al., 2006) by using primer sets specific for the human ABCB6 or mouse Abcb6 gene, human or mouse HO-1, and human or mouse Actin gene (Jiang et al., 2009). For Western analysis, cell lysates were prepared as described previously (Krishnamurthy et al., 2006) and 100 μg of total protein, mitochondrial protein, or microsomes was analyzed by polyacrylamide gel electrophoresis (PAGE). Blots were probed with mono- and/or polyclonal anti-ABCB6 antibody, monoclonal anti-HO-1 (Assaydesigns, Ann Arbor, MI), and monoclonal anti-porin (Mitosciences, Eugene, OR) antibodies. We detected the secondary antibody by using a chemiluminescence detection kit (Amersham Biosciences, Piscataway, NJ). ABCB6 antibodies were generated using a portion of the ABCB6 protein (aa 592–894) that is predicted to localize to the cytosol and is unique among the Abc transporters. The antibody was affinity purified and characterized for its ability to recognize the native ABCB6 protein.
Cell viability studies.
Trypan blue exclusion assay was used to determine cell viability (Krishnamurthy et al., 2004). Briefly, we exposed 106 HepG2 or Hep3B vector cells or ABCB6-expressing cells to increasing concentration of sodium arsenite in water for 24 or 48 h at 37°C and then counted the living cells (that were able to exclude the dye). Results are expressed as a percentage of cells surviving from the total plated.
Cytotoxicity assay.
In 96-well plates, we incubated Hep3B or HepG2 cells that express either an empty vector or an ABCB6-expressing vector at 37°C for 24 and 48 h and then added sodium arsenite in water in a dilution series and continued incubation for 2–4 days. We assessed cytotoxicity by using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide assay as described (Krishnamurthy et al., 2004).
Measurement of ROS.
Cells treated with sodium arsenite (25μM) for 24 h were incubated with 3μM 2′,7′-dichlorodihydrofluorescein diacetate (DCF; Molecular Probes, Eugene, OR) for 15 min at 37°C. After incubation, cells were washed with PBS, trypsinized, and resuspended in PBS solution. DCF fluorescence was measured using FACScan flow cytometer (excitation at 488 nm, emission at 515–545 nm). Data were analyzed with CellQuest software.
Isolation and purification of mitochondria.
Cells were pelleted in 1× Hanks buffered saline solution (Life Technologies, Carlsbad, CA), resuspended in buffer A (10 mmol/l NaCl, 1.5mM MgCl2, and 10 mmol/l Tris [pH 7.4]) containing 1× protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN), swollen on ice, and disrupted with a type B Dounce homogenizer. Buffer B (525 mmol/l mannitol, 175 mmol/l sucrose, 12.5 mmol/l Tris [pH7.4], and 2.5 mmol/l EDTA) was added in a ratio of 4:10 homogenate/buffer B. The supernatant was collected after centrifugation at 1500 × g for 10 min. The supernatant was centrifuged at 17,000 × g for 15 min to pellet mitochondria. The crude mitochondria were purified from the endoplasmic reticulum as previously described (Krishnamurthy et al., 2006; Lynch et al., 2009).
Catalase inhibition and catalase activity.
Catalase inhibition was achieved by using 3-aminotriazole (3-AT) as described (Wang et al., 1997). Briefly, cells were exposed to 20 mmol/l of 3-AT in PBS for the duration of the experiments. For catalase activity, cells (5 × 105) were washed twice with PBS, resuspended in PBS, sonicated for 10 s, and centrifuged at 14,000 revolutions per minute for 10 min. The supernatants from the centrifugation were subjected to the catalase activity assay. Catalase activity was determined by monitoring the rate of decomposition of H2O2, as assessed by the decrease in absorbance at 240 nm. One unit of activity represents the consumption of 1 mmol H2O2/min/mg protein. The assay mixture (1 ml) contained 19 mmol/l H2O2 and defined amounts of cell extract in 50 mmol/l phosphate buffer (pH 7) at 25°C.
Animals and treatments.
Nrf2 gene-deleted mice (Nrf2−/−) was a kind gift from Dr Curtis Klaassen (Professor and Chair, Department of Pharmacology, Toxicology and Therapeutics, University of Kansas Medical Center). Wild-type mice were C57BL/6J from Jackson's Laboratory. Mice were housed in polycarbonate cages (four per cage), provided normal diet and water ad libitum, and maintained on a 12–12 h light-dark cycle at 22 ± 5°C and 50 ± 20% relative humidity. At 8 weeks of age, Nrf2+/+ and Nrf2−/− mice (four mice per group) were fed sodium arsenite (10 ppm) in drinking water for 24 h. Animals were sacrificed at the end of treatment. RNA was isolated from livers of mice in Trizol (Invitrogen, Carlsbad, CA), and complementary DNA generated from the RNA was used for real-time PCR as described above.
Effect of sodium arsenite on Abcb6 expression was measured in 8-week-old C57BL/6J mice. Mice (four per group) were fed sodium arsenite (0, 1, 10, or 100 ppm; As(III); Sigma) in drinking water for 1 day or with 10 ppm of sodium arsenite for 1, 3, or 5 days. All mice survived sodium arsenite treatment. At the end of treatment, animals were sacrificed, tissues were harvested, and mitochondria or microsomes were isolated immediately as described above. Hundred micrograms of mitochondria or microsomes were analyzed by PAGE. Blots were probed for Abcb6, HO-1, and porin using anti-ABCB6, anti-HO-1, or anti-porin antibodies as described above.
Statistical analysis.
Statistical analysis of the observed values was performed using the Student's t-test. All calculations were performed with SPSS statistical software package (SPSS Inc., Chicago, IL). All values are expressed as mean ± SD. Significant differences between the groups were determined with SPSS 10.0 software (SPSS Inc.). A difference was considered significant at the p < 0.05 level. LC50 of sodium arsenite for HepG2 and Hep3B cells was calculated using the WATTOX.SAS statistical software package.
RESULTS
Abcb6 Expression in Mice Fed Sodium Arsenite
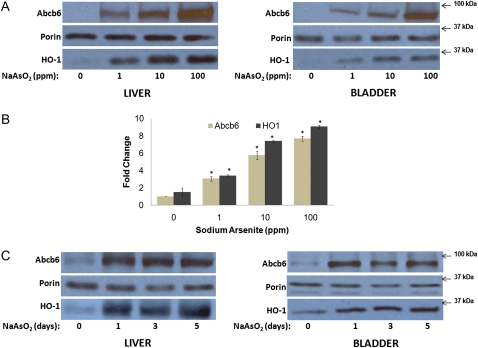
Abcb6 expression was measured in mice fed increasing concentrations of sodium arsenite (0, 1, 10, and 100 ppm) in drinking water for 24 h. Sodium arsenite induced both Abcb6 mRNA and protein levels in a dose-dependent manner (Figs. 1B and 1A, respectively). Highest induction of Abcb6 was seen in mice fed 100 ppm of sodium arsenite. Expression of heme oxygenase 1 (HO-1), a gene known to be induced in response to sodium arsenite, was used as positive control. HO-1 expression increased with increasing concentration of sodium arsenite, demonstrating treatment effectiveness (Figs. 1A and 1B). Mitochondrial protein porin whose expression is not altered in response to sodium arsenite was used as loading control in these experiments.
FIG. 1.
Abcb6 expression in mice fed sodium arsenite in drinking water. (A) Immunoblot analysis of Abcb6 expression in mice (C57BL/6J) fed increasing concentrations (0, 1, 10, and 100 ppm) of sodium arsenite for 24 h. Abcb6 expression was measured in isolated mitochondria (100 μg) using Abcb6-specific antibody. Results are representative of three independent experiments with n = 4 mice per treatment group. (B) Real-time PCR analysis of Abcb6 mRNA levels in livers of mice fed increasing concentrations (0, 1, 10, and 100 ppm) of sodium arsenite for 24 h. Abcb6 expression was measured using gene-specific primers. Values represent mean ± SD; n = 6. Results are representative of three independent experiments. “*” Significantly different from untreated controls. p < 0.01. (C) Immunoblot analysis of Abcb6 expression in mice fed sodium arsenite (10 ppm) for 1, 3, or 5 days. Abcb6 expression was measured in isolated mitochondria (100 μg) using Abcb6-specific antibody. Results are representative of three independent experiments with n = 4 mice per treatment group.
We next evaluated whether Abcb6 expression was regulated by sodium arsenite in a time-dependent manner. Mice (C57BL/6J) were exposed to 10 ppm of sodium arsenite for 1, 3, or 5 days. Sodium arsenite exposure induced Abcb6 expression throughout the 5-day treatment period (Fig. 1C). However, the magnitude of Abcb6 expression on day 3 and day 5 following sodium arsenite treatment was similar to expression levels seen on day 1 (Fig. 1C). As in the dose-dependent studies, expressions of HO-1 and porin were used as positive and negative controls, respectively. Cumulatively results presented in Figure 1 demonstrate that sodium arsenite induces Abcb6 expression in a dose-dependent manner in mice fed sodium arsenite in drinking water.
ABCB6 Expression in Human Hepatoma Cells Treated with Sodium Arsenite
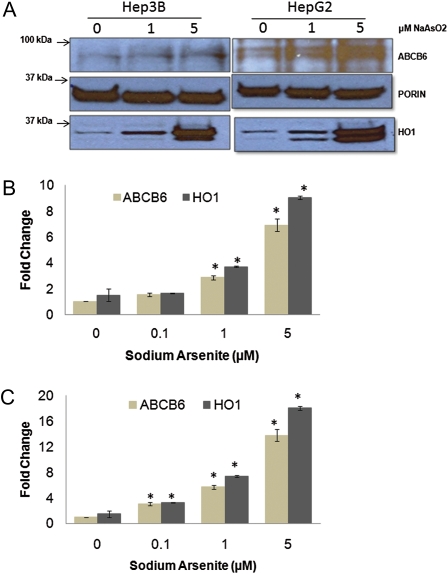
Human hepatoma cells (HepG2 and Hep3B) were treated with increasing concentration of sodium arsenite (0–5μM) for 24 h. These cells were selected because sodium arsenite elicits stress response, initiates ROS production, and induces apoptosis in these cells (Abiko et al.,2010; Chou et al., 2005; Gebel et al., 2002). Sodium arsenite treatment resulted in a dose-dependent induction of ABCB6 mRNA (Figs. 2B and 2C) and ABCB6 protein (Fig. 2A) in both HepG2 and Hep3B cells.
FIG. 2.
ABCB6 expression in hepatoma cells treated with sodium arsenite. (A) Immunoblot analysis of ABCB6 expression in HepG2 and Hep3B cells treated with increasing concentrations (0, 1, and 5μM) of sodium arsenite for 24 h. ABCB6 expression was measured in isolated mitochondria (100 μg) using ABCB6-specific antibody. Results are representative of three independent experiments. (B and C) Real-time PCR analysis of ABCB6 mRNA levels in (B) Hep3B and (C) HepG2 cells treated with increasing concentrations (0, 0.1, 1, and 5μM) of sodium arsenite for 24 h. ABCB6 expression was measured using gene-specific primers. HO-1 expression was used as positive control. Values represent mean ± SD. Results are representative of three independent experiments. “*” Significantly different from untreated controls. p < 0.01.
Abcb6 Expression in Nrf2+/+ and Nrf2−/− Mice Fed Sodium Arsenite in Drinking Water
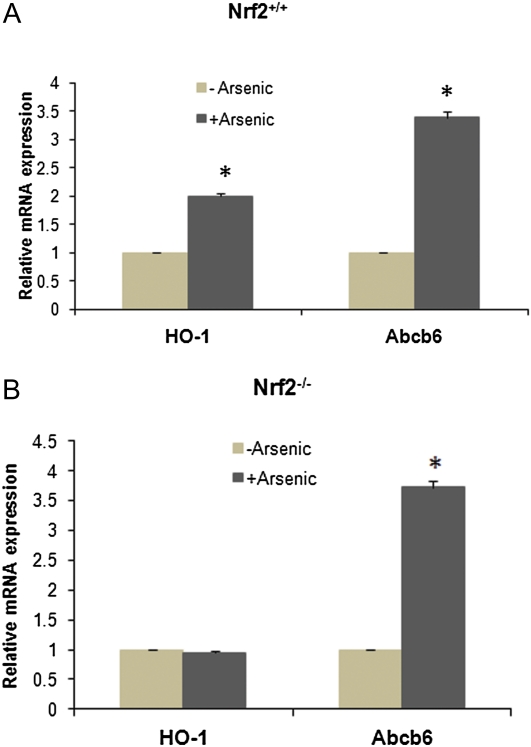
Arsenic-mediated regulation of gene transcription is believed to be governed by redox-sensitive transcription factors that are activated in response to oxidative stress. Among the many redox-sensitive transcription factors, nuclear factor-erythroid 2–related factor 2 (Nrf2) (Moi et al., 1994) appears to play a key role in arsenic-mediated activation of downstream genes (Jung and Kwak, 2010; Miyata et al., 2011; Shinkai et al., 2006). Because Abcb6 transcript was found in our studies to be upregulated by sodium arsenite, we tested whether this induction was mediated by Nrf2. We used the Nrf2 wild-type (Nrf2+/+) and Nrf2 gene-deleted (Nrf2−/−) mice for these studies. We did not see any difference in basal Abcb6 expression between Nrf2+/+ and Nrf2−/− mice (Supplementary fig. S1). Sodium arsenite treatment induced Abcb6 expression to a similar extent in both Nrf2+/+ and Nrf2−/− mice (Figs. 3A and 3B; ∼3.5-fold increase in Abcb6 mRNA in arsenite-treated vs. vehicle control mice), suggesting that sodium arsenite–dependent induction of Abcb6 is not mediated by Nrf2. HO-1, a gene whose expression is activated by Nrf2 (Alam et al., 1999), was used as a positive control (Fig. 3A). HO-1 expression was induced approximately twofold in arsenite-treated Nrf2+/+ mice but not in Nrf2−/− mice.
FIG. 3.
Abcb6 expression in the liver of Nrf2 wild-type (Nrf2+/+) and Nrf2 gene-deleted (Nrf2−/−) mice fed sodium arsenite in drinking water. (A) Nrf2 wild-type and (B) Nrf2 gene-deleted (Nrf2−/−) mice were treated with 10 ppm sodium arsenite for 24 h. Abcb6 expression was measured by real-time PCR using gene-specific primers. Expression of HO-1 was used as positive control in these studies. Values represent mean ± SD with n = 4 mice per treatment group. Results are representative of three independent experiments. “*” Significantly different from control mice (vehicle treated). p < 0.01.
Effect of Sodium Arsenite on Cell Survival in the Presence and Absence of ABCB6
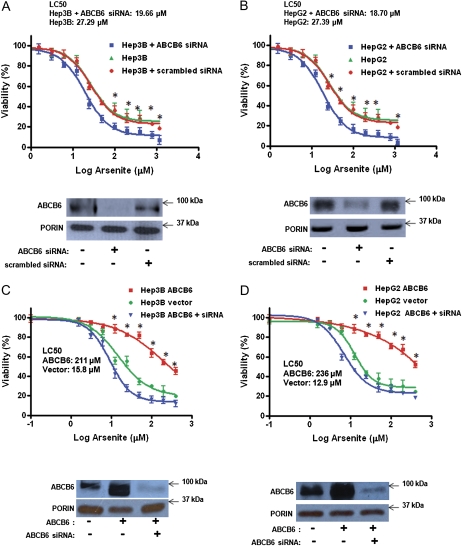
The functional importance of ABCB6 to arsenite toxicity was evaluated by blocking endogenous ABCB6 expression in two human cell lines (hepatoma cells Hep3B and HepG2) (Fig. 4). Reduced ABCB6 expression was achieved in these cells using ABCB6-specific siRNA, which resulted in greater than 70% reduction of endogenous ABCB6 (Figs. 4A and 4B, bottom panel) compared with scrambled siRNA. Arsenic toxicity was evaluated in these cells by measuring cell viability. Reduced ABCB6 expression in ABCB6-specific siRNA-treated cells sensitized both HepG2 and Hep3B cells to arsenite toxicity compared with vector control cells or cells transfected with the scrambled siRNA (inhibitor concentration [IC] 50 of 19.6 and 18.7μM in the presence of ABCB6-specific siRNA vs. 27.3 and 27.4μM in the absence of siRNA in Hep3B and HepG2 cells, respectively; Figs. 4A and 4B).
FIG. 4.
Effect of sodium arsenite on cell survival in ABCB6-expressing and ABCB6 knockdown cells. Survival of (A) Hep3B and (B) HepG2 cells treated with increasing concentrations of sodium arsenite for 48 h. Immunoblot analysis of ABCB6 expression in Hep3B and HepG2 mitochondria in cells transfected with ABCB6-specific and scrambled (nonspecific) siRNA is presented in the bottom panel (A and B). Survival of (C) ABCB6-overexpressing Hep3B and (D) ABCB6-overexpressing HepG2 cells treated with increasing concentrations of sodium arsenite for 48 h. Immunoblot analysis of ABCB6 expression in ABCB6-expressing and siRNA knockdown cells is presented in the bottom panel (C and D). Porin expression is used as loading control in these experiments. Values represent the mean ± SD; n = 5. Results representative of three independent experiments; “*” Significantly different from siRNA knockdown cells and vector control cells at the respective time points. p < 0.01.
Based on the results presented in Figures 4A and 4B, it is conceivable that ABCB6 overexpression is cytoprotective against arsenite toxicity. To test this hypothesis, we developed ABCB6-overexpressing cell lines using HepG2 and Hep3B cells as described (Krishnamurthy et al., 2006). ABCB6 expression in these cells was confirmed by immunoblot analysis using ABCB6-specific antibody (Figs. 4C and 4D, bottom panels). ABCB6’s ability to protect against arsenite toxicity was evaluated by measuring cell viability. We found that both HepG2 and Hep3B cells that overexpress ABCB6 demonstrate better survival in the presence of sodium arsenite compared with vector control cells (IC50 of 211μM vs. 15.8μM for ABCB6-expressing and empty vector Hep3B cells, respectively, and 236μM vs. 12.9μM for ABCB6-expressing vs. empty vector HepG2 cells, respectively; Figs. 4C and 4D). More importantly, knockdown of ABCB6 expression using ABCB6-specific siRNA resulted in sensitization of ABCB6-overexpressing HepG2 and Hep3B cells to arsenite toxicity (Figs. 4C and 4D).
Effect of Sodium Arsenite on Oxidative Stress in Cells Expressing ABCB6
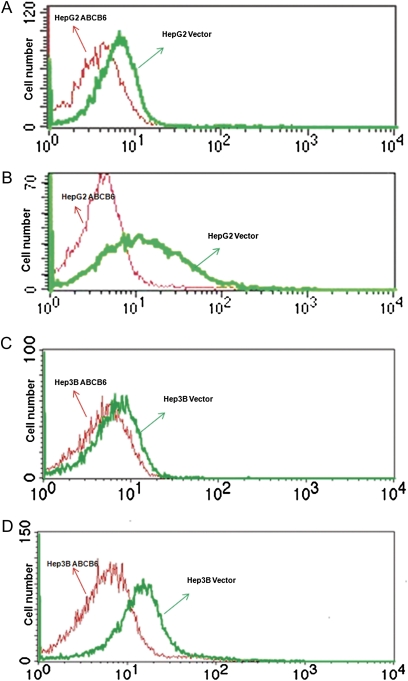
It has been reported that arsenic toxicity is in part attributed to increased oxidative stress (Gomez et al., 2005; Hei and Filipic, 2004; Kojima et al., 2009; Pi et al., 2002; Yamauchi et al., 2004). To test the hypothesis that ABCB6 protects against arsenite-induced oxidative stress, we measured ROS in ABCB6-overexpressing HepG2 and Hep3B cells in response to sodium arsenite. We first evaluated the basal level of ROS in ABCB6-overexpressing and vector control cells. We found reduced basal ROS in ABCB6-overexpressing cells compared with vector control cells (Figs. 5A and 5C). We next evaluated sodium arsenite–induced oxidative stress in the vector control and ABCB6-overexpressing cells. Sodium arsenite induced oxidative stress in both vector control and ABCB6-overexpressing cells. However, the magnitude of oxidative stress in ABCB6-overexpressing cells was significantly lower than that observed in vector control cells. (Figs. 4B and 4D). These results suggest that ABCB6 expression reduces arsenite-induced oxidative stress.
FIG. 5.
Sodium arsenite–induced oxidative stress in ABCB6-overexpressing and vector control cells. Fluorescence-activated cell-sorting analysis of DCF fluorescence as a measure of oxidative stress in (A) ABCB6-overexpressing HepG2 and (C) ABCB6-overexpressing Hep3B cells in the absence of sodium arsenite treatment. Arsenite-induced oxidative stress in (B) ABCB6-overexpressing HepG2 and (D) ABCB6-overexpressing Hep3B cells. Results are representative of three independent experiments.
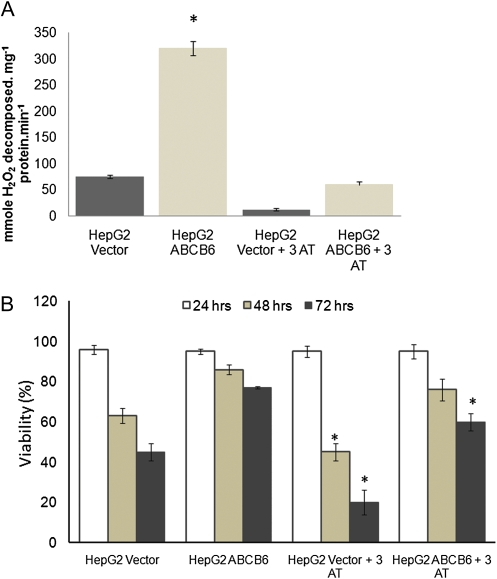
We have previously demonstrated that the expression and activity of catalase is increased in cells that overexpress ABCB6. Furthermore, this increased catalase activity in ABCB6-expressing cells protects these cells against H2O2-mediated oxidative stress (Lynch et al., 2009). Because arsenic toxicity is in part attributed to increased oxidative stress mediated by H2O2 (Biswas et al., 2010; Liu et al., 2001c; Wang et al., 2007), we tested the relative contribution of increased catalase activity in ABCB6-overexpressing cells in protecting against arsenite toxicity. In the studies reported here, we first evaluated the ability of 3-AT to inhibit catalase activity in ABCB6-expressing and nonexpressing cells. 3-AT at a concentration of 20 mmol/l was capable of inhibiting > 80% of ABCB6-induced catalase activity (Fig. 6A). We next evaluated the contribution of catalase to ABCB6-mediated protection against sodium arsenite. Following pretreatment with the catalase inhibitor (3-AT), ABCB6-overexpressing and nonexpressing cells were exposed to sodium arsenite for 48 h. Pretreatment with the catalase inhibitor was essential to eliminate most of the steady-state catalase protein that is induced in ABCB6-overexpressing cells (Lynch et al., 2009). Survival was evaluated at the end of 48 h by measuring cell viability. We found that, in vector control cells, blocking catalase activity increased arsenite-mediated cell death (63 and 45% at 48 and 72 h, respectively, in the absence of 3-AT compared with 45 and 20% in the presence of 3-AT; Fig. 6B), which was statistically significant (p < 0.05). In contrast, in ABCB6-expressing cells, blocking catalase activity increased significant (p < 0.05) arsenite-mediated cell death only after 72 h of treatment. ABCB6-overexpressing HepG2 cells showed 85 and 76% survival (48 and 72 h, respectively) in response to sodium arsenite in the absence of 3-AT compared with 76 and 60% survival in cells that were pretreated with 3-AT (Fig. 6B).
FIG. 6.
Effect of sodium arsenite on cell survival in ABCB6-expressing cells in the presence and absence of the catalase inhibitor 3-AT. (A) Effect of 3-AT (20 mmol/l) on ABCB6-induced catalase activation in human hepatoma cells HepG2 in the absence of sodium arsenite treatment. (B) Effect of 3-AT on cell death in ABCB6-expressing HepG2 cells treated with sodium arsenite (25μM) for 48 h. Values represent the mean ± SD; n = 5. Results are representative of three independent experiments. “*” Significantly different from their respective untreated groups. p < 0.05.
DISCUSSION
Inorganic arsenic is a ubiquitous environmental pollutant, and chronic exposure in humans has devastating health effects, including the formation of tumors of the liver, kidney, skin, lung, and bladder (IARC, 2004; Kurt et al., 2009). Its ubiquity in the environment has led to the evolution of arsenic defense mechanisms in all living organisms studied, from Escherichia coli to man (Rosen, 2002). These defense mechanisms work either alone or in concert to reduce cellular oxidative stress. In this study, we have investigated the role of ABCB6 to arsenic toxicity. We find that in vitro sodium arsenite induces ABCB6 expression in hepatoma cells at a concentration as low as 1μM. This low-dose induction suggests that ABCB6 expression is an adaptive response to arsenite toxicity, a phenomenon commonly termed as preconditioning that allows cells and tissues to resist future toxic insults to similar stressors (Mattson and Cheng, 2006). In vivo ABCB6 expression is induced in a dose-dependent manner within a day following arsenite treatment, which suggests that ABCB6 is an early response gene activated in response to injury.
As noted earlier, the redox-sensitive transcription factor Nrf2 provides a predominant pathway of oxidant-mediated induction of downstream genes. In our initial analysis, ABCB6 expression did not appear to be regulated by Nrf2. This suggests that pathways independent of Nrf2 might be involved in regulating ABCB6 expression. Alternatively, arsenite-induced ABCB6 expression might be regulated posttranscriptionally. Interestingly, recent studies by Hubner et al. (2009) demonstrated induction of ABCB6 mRNA in humans in response to cigarette smoking. In these studies, Nrf2 did not mediate ABCB6 expression induced by smoking-related oxidative stress. Our studies support these findings, demonstrating that Nrf2 does not regulate ABCB6 expression. In addition to Nrf2, arsenic exposure has been shown to activate several transcription factors including p53, Sp1, activator protein 1 (AP-1), and NF-kappaB (Drobna et al., 2003; Filippova and Duerksen-Hughes, 2003; Huang et al., 2001; Kumagai and Sumi, 2007; Simeonova et al., 2001; Wijeweera et al., 2001). Preliminary analysis of the human and mouse ABCB6/Abcb6 promoter using a pattern search program (nubiscan) reveals several putative cis-elements that may be capable of binding AP-1, NF-kB, and p53 (data not shown). Our future studies will evaluate the mechanism of sodium arsenite–mediated activation of ABCB6 and will address the role of these transcription factors in the activation of ABCB6.
In our studies, ABCB6 expression protected cells against acute arsenic toxicity. These results are in agreement with the studies reported by Paterson et al. (2007) but are in contrast to the studies reported by Gebel et al. (2002). Paterson et al. (2007) suggest a direct correlation between ABCB6 expression and arsenic resistance, whereas Gebel et al. (2002) demonstrate no increase in ABCB6 expression in HepG2 cells that were selected for arsenic resistance. One clear distinction between the studies reported in the manuscript and the studies reported by Gebel et al. (2002) is that Gebel et al. evaluated ABCB6 expression in arsenic-resistant and arsenic-susceptible cells, whereas studies presented in the manuscript use ABCB6 knockdown cells that could make these cells acutely susceptible to arsenic toxicity. Thus, taken together, the results from these studies suggest the possibility that ABCB6 expression is induced in response to short-term low-dose exposure to arsenic and that this increased expression could protect against short-term acute arsenic toxicity. In contrast, long-term exposure to acute arsenic toxicity might not induce ABCB6 expression. In this context, additional studies are required to evaluate if ABCB6 expression is induced in response to long-term acute arsenic toxicity and if ABCB6 expression can provide prolonged protection against acute arsenic toxicity.
Although our studies demonstrate protection against arsenic toxicity in in vitro cell culture, they do not provide any relevant information regarding ABCB6 expression to chronic arsenic–induced diseases in humans. The high dose of arsenic used in our study causes acute toxicity, whereas moderate doses of repetitive exposure are associated with human diseases such as cancer, diabetes, and cardiovascular diseases. Thus, the role of ABCB6 in arsenic-induced diseases of humans requires confirmation in vivo.
Arsenic is known to cause cellular injury by several mechanisms including the generation of ROS (Gomez et al., 2005; Hei and Filipic, 2004; Kojima et al., 2009; Pi et al., 2002), and the metabolism of arsenic has an important role in this process (Styblo et al., 2002; Vahter and Concha, 2001). Although high concentrations of inorganic arsenic can stimulate ROS production, most of the absorbed inorganic arsenic is biomethylated to its methylated species, which appear to stimulate ROS production more efficiently than inorganic arsenic (Kojima et al., 2009; Petrick et al., 2000; Styblo et al., 2000). Thus, one adaptive mechanism that protects against arsenic toxicity is increase in the expression of stress-response genes that reduce cellular oxidative stress (Liu et al., 2001b; Pi et al., 2003; Scandalios, 2005). Because we have previously demonstrated ABCB6-mediated protection against oxidative stress, we first evaluated whether ABCB6’s ability to protect against arsenic toxicity was associated with its ability to reduce oxidative stress. We found reduced arsenite-induced oxidative stress in ABCB6-expressing cells. Thus, one potential mechanism by which ABCB6 protects against arsenite toxicity is by reducing oxidative stress.
Results from both in vivo and in vitro studies show that both superoxide and H2O2 are produced in humans and animals exposed to arsenite (Biswas et al., 2010; Eblin et al., 2006; Pi et al., 2003). The production of H2O2 appears to be involved in the induction of apoptosis by arsenite in cell culture (Liu et al., 2001c; Shinkai et al., 2009; Wang et al., 2001). It is important to note that preincubation with catalase ameliorated arsenite-induced ROS in human bladder cell culture (Eblin et al., 2006), whereas treatment with 3-AT, an inhibitor of catalase, increased arsenite-induced micronuclei (Wang and Huang, 1994). We have previously demonstrated that ABCB6 expression protects cells against the toxic effects of H2O2, which is mediated by ABCB6’s ability to potentiate the expression and activity of catalase (Lynch et al., 2009). In the studies described in the manuscript, we tested whether ABCB6 protection against sodium arsenite is mediated by its ability to potentiate catalase activity. Treatment of cells with the catalase inhibitor 3-AT blocked ABCB6-mediated activation of catalase but had only a modest effect on the survival of cells exposed to sodium arsenite. Thus, although H2O2 is reported to be involved in arsenite toxicity, this does not appear to be the sole mechanism by which ABCB6 protects against arsenite toxicity.
Increased ABCB6 expression occurs in drug-resistant cell lines (Annereau et al., 2004; Szakacs et al., 2004; Yasui et al., 2004). Elevated ABCB6 mRNA levels were reported in adriamycin, camptothecin, paclitaxel, and 5-fluorouracil–resistant tumor cell lines (Park et al., 2006). Based on these observations, it was speculated that ABCB6 could be a multidrug resistance gene and that the multidrug resistance phenotype could be attributed to ABCB6’s ability to function as a transporter to reduce intracellular concentrations of the respective drug compounds. It is quite possible that a similar transport mechanism mediated by ABCB6 for inorganic arsenic or its metabolite could reduce cellular arsenic concentrations. Supporting this hypothesis in cells that are engineered to overexpress ABCB6, the transporter was shown to localize to the plasma membrane (Paterson et al., 2007). However, in our studies reported here, we were not able to detect endogenous ABCB6 expression at the plasma membrane. It is quite possible that either the concentration of arsenite used in these studies was not sufficient or the analytical techniques used were not sensitive enough to detect endogenous ABCB6 expression at the plasma membrane. Thus, additional studies are required to evaluate endogenous expression of ABCB6 at the plasma membrane and its potential contribution to arsenic toxicity. Alternatively, because most of the chemotherapeutic drugs used in the studies described by Annereau et al. (2004), Szakacs et al. (2004), and Yasui et al. (2004) cause cellular stress and based on the results presented in the manuscript, one could hypothesize that the mode of protection against drug-induced toxicity may be associated with ABCB6’s ability to protect against oxidative stress.
In summary, the present studies demonstrate that sodium arsenite induces ABCB6 expression both in vitro and in vivo. Moreover, this induction of ABCB6 is likely responsible for protection against arsenite toxicity in vitro. Future studies will focus on both the mechanisms by which arsenic causes induction of ABCB6 and the in vivo significance of ABCB6 expression to protection against oxidative stressors.
FUNDING
National Institutes of Health (P20RR021940, T32ES007079).
Supplementary Material
Acknowledgments
We thank Dr Curtis Klaassen for the Nrf2 gene-deleted animals. We thank Dr Lauren Aleksunes for help with the Nrf2 animal studies. We thank Dr Thomas Pazdernik for his constructive comments. We thank Dr Martha Montello (Director, Writing Consult Center, University of Kansas Medical Center) for her invaluable help in preparation of the manuscript.
References
- Abiko Y, Shinkai Y, Sumi D, Kumagai Y. Reduction of arsenic-induced cytotoxicity through Nrf2/HO-1 signaling in HepG2 cells. J. Toxicol. Sci. 2010;35:419–423. doi: 10.2131/jts.35.419. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Arsenic. Atlanta, GA: Public Health Service; 2007. [PubMed] [Google Scholar]
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- Annereau JP, Szakacs G, Tucker CJ, Arciello A, Cardarelli C, Collins J, Grissom S, Zeeberg BR, Reinhold W, Weinstein JN, et al. Analysis of ATP-binding cassette transporter expression in drug-selected cell lines by a microarray dedicated to multidrug resistance. Mol. Pharmacol. 2004;66:1397–1405. doi: 10.1124/mol.104.005009. [DOI] [PubMed] [Google Scholar]
- Biswas D, Sen G, Biswas T. Reduced cellular redox status induces 4 hydroxynonenal-mediated caspase 3 activation leading to erythrocyte death during chronic arsenic exposure in rats. Toxicol. Appl. Pharmacol. 2010;244:315–327. doi: 10.1016/j.taap.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Chou YH, Ho FM, Liu DZ, Lin SY, Tsai LH, Chen CH, Ho YS, Hung LF, Liang YC. The possible role of heat shock factor-1 in the negative regulation of heme oxygenase-1. Int. J. Biochem. Cell Biol. 2005;37:604–615. doi: 10.1016/j.biocel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Drobna Z, Jaspers I, Thomas DJ, Styblo M. Differential activation of AP-1 in human bladder epithelial cells by inorganic and methylated arsenicals. FASEB J. 2003;17:67–69. doi: 10.1096/fj.02-0287fje. [DOI] [PubMed] [Google Scholar]
- Eblin KE, Bowen ME, Cromey DW, Bredfeldt TG, Mash EA, Lau SS, Gandolfi AJ. Arsenite and monomethylarsonous acid generate oxidative stress response in human bladder cell culture. Toxicol. Appl. Pharmacol. 2006;217:7–14. doi: 10.1016/j.taap.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Filippova M, Duerksen-Hughes PJ. Inorganic and dimethylated arsenic species induce cellular p53. Chem. Res. Toxicol. 2003;16:423–431. doi: 10.1021/tx025606a. [DOI] [PubMed] [Google Scholar]
- Furuya KN, Bradley G, Sun D, Schuetz EG, Schuetz JD. Identification of a new P-glycoprotein-like ATP-binding cassette transporter gene that is overexpressed during hepatocarcinogenesis. Cancer Res. 1997;57:3708–3716. [PubMed] [Google Scholar]
- Gebel TW, Leister M, Schumann W, Hirsch-Ernst K. Low-level self tolerance to arsenite in human HepG2 cells is associated with a depressed induction of micronuclei. Mutat. Res. 2002;514:245–255. doi: 10.1016/s1383-5718(01)00343-6. [DOI] [PubMed] [Google Scholar]
- Gomez SE, del Razo LM, Munoz Sanchez JL. Induction of DNA damage by free radicals generated either by organic or inorganic arsenic (AsIII, MMAIII, and DMAIII) in cultures of B and T lymphocytes. Biol. Trace Elem. Res. 2005;108:115–126. doi: 10.1385/bter:108:1-3:115. [DOI] [PubMed] [Google Scholar]
- Hei TK, Filipic M. Role of oxidative damage in the genotoxicity of arsenic. Free Radic. Biol. Med. 2004;37:574–581. doi: 10.1016/j.freeradbiomed.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Huang C, Li J, Ding M, Wang L, Shi X, Castranova V, Vallyathan V, Ju G, Costa M. Arsenic-induced NFkappaB transactivation through Erks- and JNKs-dependent pathways in mouse epidermal JB6 cells. Mol. Cell. Biochem. 2001;222:29–34. [PubMed] [Google Scholar]
- Hubner RH, Schwartz JD, De Bishnu P, Ferris B, Omberg L, Mezey JG, Hackett NR, Crystal RG. Coordinate control of expression of Nrf2-modulated genes in the human small airway epithelium is highly responsive to cigarette smoking. Mol. Med. 2009;15:203–219. doi: 10.2119/molmed.2008.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) Arsenic in Drinking Water. International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risk to Humans. Vol. 84. Lyon, France: IARC Press; 2004. pp. 269–477. [Google Scholar]
- Jiang T, Huang Z, Chan JY, Zhang DD. Nrf2 protects against As(III) induced damage in mouse liver and bladder. Toxicol. Appl. Pharmacol. 2009;240:8–14. doi: 10.1016/j.taap.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KA, Kwak MK. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15:7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima C, Ramirez DC, Tokar EJ, Himeno S, Drobna Z, Styblo M, Mason RP, Waalkes MP. Requirement of arsenic biomethylation for oxidative DNA damage. J. Natl. Cancer Inst. 2009;101:1670–1681. doi: 10.1093/jnci/djp414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy P, Ross DD, Nakanishi T, Bailey-Dell K, Zhou S, Mercer KE, Sarkadi B, Sorrentino BP, Schuetz JD. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J. Biol. Chem. 2004;279:24218–24225. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG, Schuetz JD. Identification of a mammalian mitochondrial porphyrin transporter. Nature. 2006;443:586–589. doi: 10.1038/nature05125. [DOI] [PubMed] [Google Scholar]
- Kumagai Y, Sumi D. Arsenic: signal transduction, transcription factor, and biotransformation involved in cellular response and toxicity. Ann. Rev. Pharmacol. Toxicol. 2007;47:243–262. doi: 10.1146/annurev.pharmtox.47.120505.105144. [DOI] [PubMed] [Google Scholar]
- Kurt S, Lamia B-T, Robert B, Yann G, Béatrice S, Fatiha El G, Véronique B, Neela G, Crystal F, Laurent G, et al. A review of human carcinogens. Part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10:453–454. doi: 10.1016/s1470-2045(09)70134-2. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen H, Miller DS, Saavedra JE, Keefer LK, Johnson DR, Klaassen CD, Waalkes MP. Overexpression of glutathione S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. Mol. Pharmacol. 2001a;60:302–309. doi: 10.1124/mol.60.2.302. [DOI] [PubMed] [Google Scholar]
- Liu J, Kadiiska MB, Liu Y, Lu T, Qu W, Waalkes MP. Stress related gene expression in mice treated with inorganic arsenicals. Toxicol. Sci. 2001b;61:314–320. doi: 10.1093/toxsci/61.2.314. [DOI] [PubMed] [Google Scholar]
- Liu SX, Athar M, Lippai I, Waldren C, Hei TK. Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc. Natl. Acad. Sci. U.S.A. 2001c;98:1643–1648. doi: 10.1073/pnas.031482998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J, Fukuda Y, Krishnamurthy P, Du G, Schuetz JD. Cell survival under stress is enhanced by a mitochondrial ATP-binding cassette transporter that regulates hemoproteins. Cancer Res. 2009;69:5560–5567. doi: 10.1158/0008-5472.CAN-09-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng A. Neurohormetic phytochemicals: low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Miyata T, Takizawa S, van Ypersele de Strihou C. Intracellular sensors for oxygen and oxidative stress: novel therapeutic targets. Am. J. Physiol. Cell. Physiol. 2011;300:C226–C231. doi: 10.1152/ajpcell.00430.2010. [DOI] [PubMed] [Google Scholar]
- Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz DF, Kreppel L, Speiser DM, Scheel G, McDonald G, Ow DW. Heavy metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO J. 1992;11:3491–3499. doi: 10.1002/j.1460-2075.1992.tb05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz DF, Ruscitti T, McCue KF, Ow DW. Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J. Biol. Chem. 1995;270:4721–4728. doi: 10.1074/jbc.270.9.4721. [DOI] [PubMed] [Google Scholar]
- Park S, Shimizu C, Shimoyama T, Takeda M, Ando M, Kohno T, Katsumata N, Kang YK, Nishio K, Fujiwara Y. Gene expression profiling of ATP-binding cassette (ABC) transporters as a predictor of the pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res. Treat. 2006;99:9–17. doi: 10.1007/s10549-006-9175-2. [DOI] [PubMed] [Google Scholar]
- Paterson JK, Shukla S, Black CM, Tachiwada T, Garfield S, Wincovitch S, Ernst DN, Agadir A, Li X, Ambudkar SV, et al. Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane. Biochemistry. 2007;46:9443–9452. doi: 10.1021/bi700015m. [DOI] [PubMed] [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol. 2000;163:203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Pi J, Qu W, Reece JM, Kumagai Y, Waalkes MP. Transcription factor Nrf2 activation by inorganic arsenic in cultured keratinocytes: involvement of hydrogen peroxide. Exp. Cell Res. 2003;290:234–245. doi: 10.1016/s0014-4827(03)00341-0. [DOI] [PubMed] [Google Scholar]
- Pi J, Yamauchi H, Kumagai Y, Sun G, Yoshida T, Aikawa H, Hopenhayn-Rich C, Shimojo N. Evidence for induction of oxidative stress caused by chronic exposure of Chinese residents to arsenic contained in drinking water. Environ. Health Perspect. 2002;110:331–336. doi: 10.1289/ehp.02110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BP. Biochemistry of arsenic detoxification. FEBS Lett. 2002;529:86–92. doi: 10.1016/s0014-5793(02)03186-1. [DOI] [PubMed] [Google Scholar]
- Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005;38:995–1014. doi: 10.1590/s0100-879x2005000700003. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Sumi D, Fukami I, Ishii T, Kumagai Y. Sulforaphane, an activator of Nrf2, suppresses cellular accumulation of arsenic and its cytotoxicity in primary hepatocytes. FEBS Lett. 2006;580:1771–1774. doi: 10.1016/j.febslet.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Sumi D, Toyama T, Kaji T, Kumagai Y. Role of aquaporin in cellular accumulation of arsenic and its cytotoxicity in primary mouse hepatocytes. Toxicol. Appl. Pharmacol. 2009;237:232–236. doi: 10.1016/j.taap.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Simeonova PP, Wang S, Kashon ML, Kommineni C, Crecelius E, Luster MI. Quantitative relationship between arsenic exposure and AP-1 activity in mouse urinary bladder epithelium. Toxicol. Sci. 2001;60:279–284. doi: 10.1093/toxsci/60.2.279. [DOI] [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: a research update. Environ. Health Perspect. 2002;110:767–771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakacs G, Annereau JP, Lababidi S, Shankavaram U, Arciello A, Bussey KJ, Reinhold W, Guo Y, Kruh GD, Reimers M, et al. Predicting drug sensitivity and resistance: profiling ABC transporter genes in cancer cells. Cancer Cell. 2004;6:129–137. doi: 10.1016/j.ccr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Vahter M, Concha G. Role of metabolism in arsenic toxicity. Pharmacol. Toxicol. 2001;89:1–5. doi: 10.1034/j.1600-0773.2001.d01-128.x. [DOI] [PubMed] [Google Scholar]
- Vatamaniuk OK, Bucher EA, Sundaram MV, Rea PA. CeHMT-1, a putative phytochelatin transporter, is required for cadmium tolerance in Caenorhabditis elegans. J. Biol. Chem. 2005;280:23684–23690. doi: 10.1074/jbc.M503362200. [DOI] [PubMed] [Google Scholar]
- Wang TC, Jan KY, Wang AS, Gurr JR. Trivalent arsenicals induce lipid peroxidation, protein carbonylation, and oxidative DNA damage in human urothelial cells. Mutat. Res. 2007;615:75–86. doi: 10.1016/j.mrfmmm.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Wang TS, Hsu TY, Chung CH, Wang AS, Bau DT, Jan KY. Arsenite induces oxidative DNA adducts and DNA-protein cross-links in mammalian cells. Free Radic. Biol. Med. 2001;31:321–330. doi: 10.1016/s0891-5849(01)00581-0. [DOI] [PubMed] [Google Scholar]
- Wang TS, Huang H. Active oxygen species are involved in the induction of micronuclei by arsenite in XRS-5 cells. Mutagenesis. 1994;9:253–257. doi: 10.1093/mutage/9.3.253. [DOI] [PubMed] [Google Scholar]
- Wang TS, Shu YF, Liu YC, Jan KY, Huang H. Glutathione peroxidase and catalase modulate the genotoxicity of arsenite. Toxicology. 1997;121:229–237. doi: 10.1016/s0300-483x(97)00071-1. [DOI] [PubMed] [Google Scholar]
- Wijeweera JB, Gandolfi AJ, Parrish A, Lantz RC. Sodium arsenite enhances AP-1 and NFkappaB DNA binding and induces stress protein expression in precision-cut rat lung slices. Toxicol. Sci. 2001;61:283–294. doi: 10.1093/toxsci/61.2.283. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Aminaka Y, Yoshida K, Sun G, Pi J, Waalkes MP. Evaluation of DNA damage in patients with arsenic poisoning: urinary 8-hydroxydeoxyguanine. Toxicol. Appl. Pharmacol. 2004;198:291–296. doi: 10.1016/j.taap.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Yasui K, Mihara S, Zhao C, Okamoto H, Saito-Ohara F, Tomida A, Funato T, Yokomizo A, Naito S, Imoto I, et al. Alteration in copy numbers of genes as a mechanism for acquired drug resistance. Cancer Res. 2004;64:1403–1410. doi: 10.1158/0008-5472.can-3263-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.