Abstract
The tumor suppressor oncoprotein, p53, is a critical regulator of stress-induced growth arrest and apoptosis. p53 activity is regulated through the ubiquitin proteasome system (UPS) with stress-induced disruption leading to increased accumulation of p53, resulting in growth arrest. In the present study, we investigate the role of p53 to determine sensitivity to cadmium (Cd) and whether induction of stress signaling responses and perturbation of the UPS are involved in Cd-induced cytotoxicity and apoptosis. We treated synchronously cultured p53 transgenic mouse embryonic fibroblasts, both wild-type p53+/+ and knockout p53−/− cells, with cadmium chloride (Cd, 0.5–20μM) for 24 h. Cd-induced cytotoxicity was assessed by cellular morphology disruption and neutral red dye uptake assay. Proteins in the stress signaling pathway, including p38 mitogen-activated protein kinase (MAPK) and stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK); ubiquitination, such as high-molecular weight of polyubiquitinated proteins (HMW-polyUb); and apoptotic pathways, were all measured. We found that Cd induced p53-dependent cytotoxicity in the p53+/+ cells, which exhibited a twofold greater sensitivity. We observed a dose-dependent stimulation of p38 MAPK and SAPK/JNK phosphorylation that corresponded to accumulation of HMW-polyUb conjugates and lead to the induction of apoptosis, as evidenced by the elevation of cleaved caspase-3. Our study suggests that Cd-mediated cytotoxicity and induction of stress signaling responses, elevated accumulation of HMW-polyUb conjugates, and resulting apoptosis are all dependent on p53 status.
Keywords: cadmium, p53, cell cycle arrest, apoptosis, ubiquitination, stress signaling
Cadmium (Cd) is an industrial and environmental pollutant that has been associated with various toxic insults, including carcinogenicity and neurotoxicity (Chang, 1996). Cd exposure exhibits an extensive biological half-life (> 20 years) and can impact numerous tissues, including the liver, kidney, lung, gastrointestinal tract, central nervous system, ovaries, and testis (Goering et al., 1994). At the molecular level, Cd exposure has been linked to increased cytotoxicity, cell cycle arrest, and apoptosis (Robinson et al., 2009; Watjen et al., 2002). However, the overall mechanism of Cd toxicity has yet to be fully defined. Several studies have suggested that Cd-associated toxicity may occur through a metal-induced disruption of the ubiquitin proteasome system (UPS) (Figueiredo-Pereira et al., 2002; Stewart et al., 2003; Yu et al., 2008a, 2010). UPS-dependent degradation is a critical regulatory process that allows cells to rapidly respond to changing environmental conditions and intracellular signals by adjusting the levels of key effector proteins (Vabulas, 2007). This highly conserved UPS plays a critical role in the cellular mechanism for protein catabolism and is important in both housekeeping and the turnover of many regulatory proteins involved in DNA repair, cell cycle control, oncogenesis, antigen processing, transcription, neural and muscular degeneration, cellular differentiation, stress response, and apoptosis (Hamer et al., 2010; Salomons et al., 2010; Vabulas, 2007). The UPS pathway is highly conserved and plays a critical role in common environmental stress pathways. Significantly, more investigation is needed to determine the role of this pathway in defining environmental susceptibility. Our recent integrative analysis of genome-wide gene expression and pathway mapping in mouse embryonic fibroblast (MEF) cells exposed to Cd, MeHg, and arsenic demonstrated an induction of oxidative stress, in addition to the disruption of the UPS and cell cycle regulation (Yu et al., 2010). Cd treatment in MEF cells induced significant alteration of UPS pathway genes, including significant decreases in ubiquitin-conjugating enzyme Ube2c, Ubce8, and proteasome subunit Psmb9, and upregulation of DUB enzyme Uchl1, Usp29, and Usp12.
The tumor suppressor protein, p53, plays a central role in the regulation of the cell cycle and apoptosis via its transcription of downstream targets, such as p21, cdc2/cyclin B1, and GADD45 (Morrison et al., 2003; Taylor and Stark, 2001; Vairapandi et al., 2002). Under stress, p53 is stabilized by phosphorylation via the mitogen-activated protein kinases (MAPKs), p38, and SAPK/JNK (Sionov and Haupt, 1999; Zhu et al., 2002). This allows p53 to promote cell cycle arrest and apoptosis. It has been demonstrated that Cd treatment results in stabilization of p53 through phosphorylation. Furthermore, the Cd-mediated induction of p53 has been associated with subsequent cell cycle arrest as well as apoptosis (Cao et al., 2007; Chatterjee et al., 2009; Chen and Shaikh, 2009), suggesting the involvement of p53-dependent mechanisms for Cd toxicity. The level of p53 is controlled through the UPS, where it is degraded under nonstressed conditions, allowing the cells to undergo proliferation through the completion of the cell cycle (Tasdemir et al., 2008; Worrall et al., 2009). Studies have demonstrated that a dysfunctional proteasome can result in the accumulation of high-molecular weight polyubiquitinated protein (HMW-polyUb) conjugates, leading to aberrant events, such as apoptosis (Rideout et al., 2003; Young and Heikkila, 2010). In the present study, we examine whether cadmium treatment induces the activation of stress signaling pathways and if the induction of UPS is associated with differential sensitivity between p53 wild-type (p53+/+) and p53 knockout cells.
MATERIALS AND METHODS
Cell culture materials and chemicals.
Dulbecco’s modified Eagle’s medium (DMEM): F12 media, penicillin–streptomycin, L-glutamine, trypsin-EDTA, balanced salt solutions, and fetal bovine serum (FBS) were obtained from Invitrogen Life Technologies (Grand Island, NY). Nu-Serum was obtained form Becton Dickinson (Bedford, MA). Tissue culture–treated dishes and flasks were obtained from Corning (Corning, NY). Cadmium chloride (Cd) and neutral red (NR) were obtained from Sigma (St Louis, MO). MG132, lactacystin, protease, and phosphatase inhibitor cocktails were obtained form Calbiochem (La Jolla, CA). Anisomycin and caspase-specific fluorogenic substrates were obtained from Axxora (San Diego, CA). Cell lysis buffer and all phospho-specific antibodies were obtained from Cell Signaling (Beverly, MA). All other antibodies used in this study were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The enhanced chemiluminescence–based Western blotting detection reagent was obtained from Amersham Pharmacia Life Sciences (Piscataway, NJ).
Isolation and culture of MEFs.
p53 transgenic mice were prepared as previously described (Yu et al., 2008b). Fourteen days after a successful mating, gravid uteri were removed from pregnant mice. Isolated embryos were washed separately several times in Earl’s balanced salt solution. Tail DNA was obtained for genotyping, and subsequent passages of cells were genotyped with each experiment. Tissue dissociation of torso and limbs was performed overnight at 4°C using 0.25% (wt/vol) trypsin (DIFCO, Detroit, MI) in calcium and magnesium free Hank's balanced salt solution (CMF-EBSS). Single-cell fibroblast suspensions were then plated in 100-mm tissue culture dishes and maintained in DMEM-F12, containing 10% (vol/vol) FBS, 2mM L-glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. At passages 6–7, appropriate numbers of cells were plated in 100-mm tissue culture dishes with DMEM-F12 containing 10% (vol/vol) Nu-Serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 2mM L-glutamine in order to achieve uniform confluence prior to treatment. Culture stocks were frozen at −80°C in DMEM-F12 containing 20% (vol/vol) FBS and 10% (vol/vol) dimethyl sulfoxide (DMSO; Sigma).
Chemical treatments.
A 100mM stock solution of cadmium chloride (Cd) was prepared in sterile molecular biology grade water (Gibco) and was filter sterilized. Further serial dilutions (10 and 1mM) of this stock solution were prepared with sterile water and stored at −20°C. MEFs were grown to near confluency (85–90%) in a humidified atmosphere of 5% CO2/95% air at 37°C. They were then synchronized by removing the growth medium and washed twice with calcium-magnesium–free CMF-PBS. Lastly, DMEM-F12 plus 0.05% Nu-Serum, Pen-Strep, and L-glutamine were added. Cells were maintained in this medium for a further 24 h before Cd treatments were initiated. Cd treatments were prepared from the relevant stock solutions in complete cell culture medium (DMEM-F12 plus 10% Nu-Serum, Pen-Strep, and L-glutamine) at the stated working concentrations (0.5–20μM). Cells were treated continuously for 24 h. Separate treatments were conducted with MG132, lactacystin, and anisomycin. Stock solutions of these agents were prepared in DMSO at 5mM (MG and lactacystin) and 50mM (anisomycin). Synchronized cells were treated for 24 h with 0.5μM MG132, 1μM lactacystin, or 10μM anisomycin, similarly to the Cd treatments.
Assessment of cell morphology.
Following chemical treatment, cellular morphology was examined at the stated time points with a Nikon inverted microscope equipped with phase-contrast optics (Nikon, Tokyo, Japan). All images were captured and digitized using a Cool Snap Camera (Roper Scientific, Inc.), and these images were processed using Photoshop 5.5 software.
Cytotoxicity/cell viability.
Cell viability was determined using the NR assay based on lysosomal accumulation of NR in viable cells (Borenfreund and Puerner, 1985; Yu et al., 2009). Briefly, cells were treated in 12-well tissue culture plates with increasing concentrations of Cd (0, 0.5, 1, 2.5, 5, 10, and 20μM) for 24 h. At the desired end point, the growth medium plus treatment was aspirated. Cells were washed once with 1× CMF-PBS. Fresh media containing 50 μg/ml NR was added to each well, including a media only–background control. After 3 h of incubation at 37°C and 5% CO2, the cells were washed again and the NR was eluted with 2 ml of 0.5% acetic acid/50% ethanol solution. The resulting NR solution was allowed to incubate for a further 1 h at room temperature to allow proper mixing. Two hundred microliters of NR solution was added to a microtiter plate in replicates of four per well. After rapid agitation by the plate shaker, absorbance was measured at 540 nm. The average of each set was established, and from this, the media background mean was subtracted to permit the calculation of the total percent viability by comparing each value to the untreated control (0μM).
Western analysis and immunoprecipitation.
At the stated time points, cells were washed twice with 5 ml ice-cold CMF-PBS followed by the addition of 0.5 ml of cell lysis buffer (Cell Signaling Technology Inc., Beverly, MA), containing additional phosphatase (cocktails I and II) and protease inhibitor cocktail (Calbiochem). Cells were harvested by scraping in cell lysis buffer and were then placed on ice. After storage at −80°C, all extracts were homogenized by sonication and then centrifuged (16,000 × g, 10 min, 4°C) to remove insoluble material. The resulting supernatant (cell extract) was gently removed, and total protein was determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA).
Western blot analysis for the selected proteins was performed according to the previously described method (Yu et al., 2005, 2008a). Gels were transferred to polyvinylidene fluoride membranes (Millipore) using a vertical transfer apparatus. Membranes were rinsed briefly in Tris-buffered saline (TBS), pH 7.6, blocked with 5% non-fat–dried milk in TBS with 0.1% Tweens-20 (T-TBS) for 20 min and rinsed again with T-TBS. Membranes were then incubated overnight with primary antibody for 1.5 h with a secondary antibody. Following antibody incubation, the membrane was washed four times for 5 min with T-TBS. The primary antibodies included phospho-SAPK/JNK, phospho-p38 MARP, caspase-3 (Cell Signaling, Inc.), and ubiquitin (Santa Cruz Biotechnology). β-Actin (Santa Cruz Biotechnology) was used as an internal standard for protein loading. After hybridization with secondary antibodies conjugated to horseradish peroxidase, the immunocomplex was detected with the ECL detection reagent (Amersham Pharmacia Biotech) and exposed to x-ray films. Quantification of band intensities was achieved using the NIH ImageJ (http://rsb.info.nih.gov/ij/index.html).
Assessment of apoptosis.
Apoptosis-associated end points were determined in cell extracts by Western analysis using a caspase-3 antibody, which detects the cleaved form of caspase-3, or by measuring functional activities associated with various caspases using caspase-specific fluorogenic substrates (Yu et al., 2008a). The activities of caspase-3/7 and -8 were measured in a microtiter plate by a fluorometric assay using DEVD-AMC (Ac-Asp-Glu-Val-Asp-AMC) and IETD-AMC (Ac-Ile-Glu-Thr-Asp-AMC) as the respective caspase-specific substrates. Ten microliters of cell extract was added in duplicate to a 96-well plate. Reaction buffer containing the fluorogenic substrate was added to initiate the reaction, which was incubated at 37°C for 2 h, and an enzyme-catalyzed release of 7-amino-4-methylcoumarin (AMC) was measured by a fluorescence microplate reader at excitation 360 nm and emission 460 nm. Fluorescent units were converted to picomole of AMC released per microgram of protein and incubation time (2 h) using a standard curve generated with known serial dilutions of AMC. The absolute activities of the metal treatments relative to untreated controls were converted by expressing the former as a percentage of the control.
Fluorogenic peptide substrate assay for proteasome activity.
Using the fluorogenic substrates, Suc-Leu-Leu-Val-Tyr-AMC (Suc-LLVY-AMC, 50μM) and Z-Leu-Leu-Glu-AMC (Z-LLE-AMC, 200μM), two different proteasome activities were measured as previously described (Bobba et al., 2002; Rodgers and Dean, 2003). These substrates are used to measure chymotryptic and peptidyl-glutamyl peptide-hydrolyzing (PGPH) activities associated with the proteasome. Hydrolysis of these substrates was independent of the ubiquitin system. Lysates (25 μg) were incubated at 37°C with the fluorogenic substrates in 100 μl of 50mM HEPES (pH 8) and 5mM EGTA, for 4 h, respectively. Enzyme-catalyzed release of AMC was measured by a fluorescence microplate reader at excitation 360 nm and emission 460 nm. Fluorescent units were converted to picomole of AMC released using a standard curve generated with known serial dilutions of AMC.
Statistical analysis.
The results of the quantitative densitometry analyses of Western blot data, cleaved caspase activities, cell viability, and proteasome activity after normalization to % of control are expressed as mean ± SEM. Differences between treatments (doses) and genotypes were examined for statistical significance using two-way ANOVA. A p value of ≤ 0.05 denoted the presence of a statistically significant difference (ANOVA ≤ 0.05) among the genotypes or doses. Tukey-Kramer multiple comparison tests were conducted to compare the individual treatments with the control. *Indicates significance at p ≤ 0.05 and **indicates significance at p ≤ 0.01 of the individual treatment versus control in Tukey-Kramer multiple comparison tests. Statistical analysis was conducted with the JMP program (SAS Inc).
RESULTS
Cd Exposure Induced p53-Dependent alterations in MEF Morphology and Cell Viability
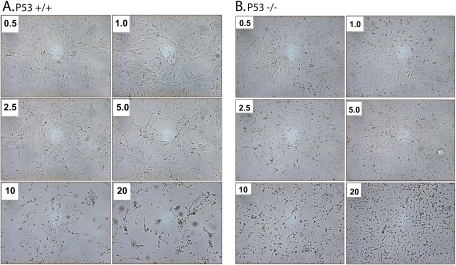
We examined the effects of Cd on cellular morphological integrity by utilizing synchronous p53 transgenic MEFs and investigated whether this correlated with a corresponding compromise of functional integrity. As shown in Figure 1 (A, p53+/+; B, p53−/−), we observed a consistent concentration-dependent compromise of the morphological integrity of p53 MEFs exposed to Cd (micromolar). Morphological changes were observed that were characteristic of apoptosis. These changes included irregular cell shape, marked cytoplasmic condensation, and cell loss. Significant alterations were not observed in either genotype at a Cd concentration below 5μM. However, significant morphological changes were observed in both genotypes at a concentration of ≥10μM. Cd-induced disruption of morphological integrity appeared to correlate with increased cytotoxicity, as shown in Figure 2. Assessment of cell viability using the NR dye uptake assay demonstrated a concentration-dependent loss in cell viability in both p53 genotypes upon Cd exposure (ANOVA, p < 0.001). Furthermore, we observed an approximately twofold greater decrease in cell viability to Cd exposure in the p53+/+ cells as compared with the p53−/− MEF cells (ANOVA, p < 0.001). We also observed a ∼45–50% reduction in cell viability in the p53+/+ cells at 5μM compared with ∼10% level in the p53−/− cells. These results suggest that cytotoxicity is dependent on p53 status. Furthermore, cells with functional p53 are more sensitive to Cd-mediated cytotoxicity.
FIG. 1.
Cadmium (Cd) treatment induced dose-dependent morphological changes in p53+/+ (A) and p53−/− (B) cells. Synchronous cultures of cells were treated with various concentrations of Cd (micromolar) for 24 h as stated in the “Materials and Methods” section. Representative micrographs are shown at ×200 magnification for both genotypes. A concentration-dependent perturbation of morphology is evident with severe disruption at concentrations ≥10μM of Cd.
FIG. 2.
Cadmium (Cd) treatment induced dose-dependent changes in cell viability in p53+/+ and p53−/− cells. Synchronous cultures of p53 MEFs were exposed to various concentrations of Cd (micromolar) for 24 h. After this exposure, cell viability was assessed by the NR dye uptake assay. Each point represents the mean ± SD of three to five independent experiments. Statistical significance was determined by two-way ANOVA followed by Tukey-Kramer multiple comparison (*p ≤ 0.05, **p ≤ 0.01) as compared with the control for each dose.
Cd Exposure Induced Accumulation of HMW-polyUb conjugates in a p53-Dependent Manner
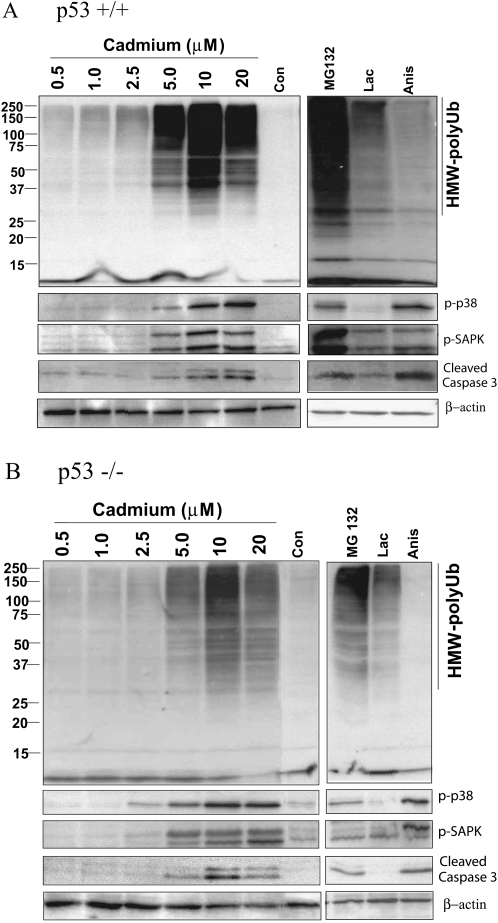
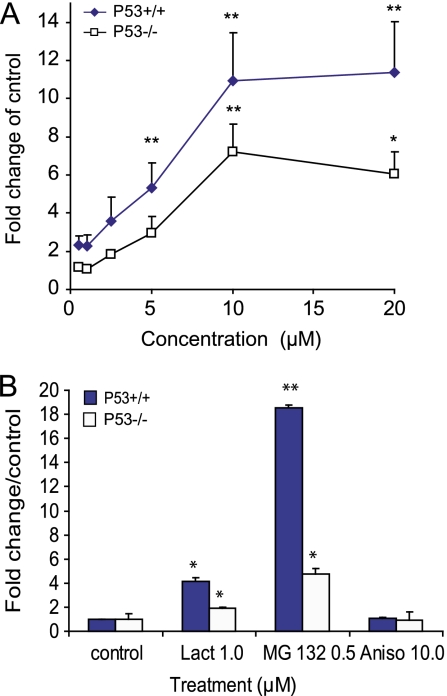
We next determined whether Cd-induced perturbation of morphological integrity and the resultant reduction of cell viability were associated with the accumulation of HMW-polyUb conjugates and if such changes were associated with p53 status. We measured the changes of HMW-polyUb by Western blot analysis after Cd treatments. The representative Western blot images (Figs. 3A and 3B), as well as the quantitative densitometry analyses (Fig. 4A), show that Cd exposure resulted in a concentration-dependent accumulation of HMW-polyUb conjugates, which appeared to be dependent on p53 status (Figs. 3 and 4; ANOVA, p ≤ 0.05). The accumulation of these HMW-polyUb conjugates, evident at 5μM Cd in both genotypes, was statistically more pronounced in the p53+/+ cells (Figs. 3A and 4, *p ≤ 0.05 vs. control). In the p53−/− cells at 5μM Cd, the response was not significantly relative to the control (Fig. 4). In both genotypes, the peak response—with the p53+/+ cells exhibiting a statistically greater response—was observed at a Cd concentration of 10μM (**p ≤ 0.01). A flat response was observed at 20μM, likely as a response to overt cytotoxicity. Overt cytotoxicity is supportive of morphological deterioration and is depicted in the photomicrographs (Figs. 1A and 1B). As a result of this aberrant cytotoxicity, we did not examine Cd concentrations >20μM in either genotype. Two prototypic inhibitors of proteasomal function, MG132 and lactacystin, induced significant accumulation of HMW-polyUb conjugates in both p53+/+ and p53−/− cells (Figs. 3 and 4B, *p ≤ 0.01, **p ≤ 0.01 vs. control). However, more significant changes were observed in the p53+/+ cells (ANOVA, p ≤ 0.05). Aisomycin, a stress inducer, did not induce the accumulation of HMW-polyUb in either genotype.
FIG. 3.
Cadmium treatment induced cellular signaling responses in p53+/+ (A) and p53−/− (B) in comparison with proteasomal inhibitors and anisomycin. Synchronous cultures of p53 MEFs were exposed to various concentrations of Cd (micromolar), MG132 (0.5μM), lactacystin (Lac; 1μM), or anisomycin (Anis; 10μM) for 24 h. Cell extracts were prepared and subjected to Western blot analysis to examine the ubiquitination (directed against ubiquitin mouse monoclonal) and phosphorylation status using phospho-specific antibodies against p38 MAPK and SAPK/JNK as stated under the “Materials and Methods” section. In addition, the expression of cleaved caspase-3 was examined using an antibody that recognizes only the cleaved form. Gel loading was normalized against β-actin expression.
FIG. 4.
Cadmium-induced accumulation of HMW-polyUb in p53+/+ and p53−/− cells after exposed to cadmium (A) and proteasomal inhibitors and anisomycin (B). Synchronous p53 MEFs were exposed to various concentrations of Cd and proteasomal inhibitors and anisomycin for 24 h. Quantification of Western blot bands of HMW-polyUb was conducted in NIH ImageJ and expressed as the percentage of the corresponded control after normalization to β-actin. Statistical significance was determined using two-way ANOVA followed by Tukey-Kramer multiple comparison tests. A p value ≤ 0.05 or 0.01 denoted the presence of a statistically significant difference. *Indicates significance at p ≤ 0.05 and **indicates significance at p ≤ 0.01 of the individual treatment versus control in Tukey-Kramer multiple comparison tests.
Cd Exposure Activated the Stress Signaling in MEF in a p53-Dependent Manner
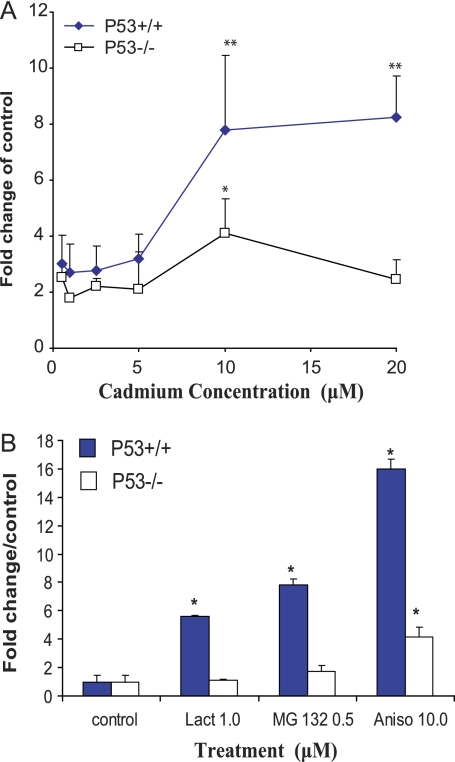
We further examined the possible relationship between the accumulation of HMW-polyUb and activation of cellular stress signaling by assessing the phosphorylation status of several key intracellular signaling intermediates that are involved in mediating chemical stress. Cd exposure resulted in a robust phosphorylation of both these classic markers of cellular stress, p38 MAPK and SAPK/JNK (ANOVA p ≤ 0.05), evident at a Cd concentration >5μM (Figs. 3 and 5) in both genotypes (*p ≤ 0.05). In Figure 5A, it is evident that there was no statistical difference between the p53+/+ and p53−/− cells with respect to the dose-dependent phosphorylation of p38 MAPK (ANOVA, p > 0.05). In contrast, the parallel phosphorylations of SAPK/JNK (p46/54 JNK) were highly significant in the p53+/+ cells exhibiting a nearly ninefold difference at a Cd concentration of 10μM (Fig. 5C, *p ≤ 0.05). This response was further amplified in the p53+/+ cells at 20μM (p ≤ 0.01). Proteasomal inhibitor MG132 induced significant activation of p-SAPK/JNK and p38 MAPK in both genotypes (ANOVA, p ≤ 0.05), whereas lactacystin only statistically activated SAPK/JNK but not p38 MAPK in the p53+/+ cells (Figs. 3 and 5B and 5D). The dose-dependent stimulation of p38 MAPK and SAPK/JNK phosphorylation induced by Cd as well as proteasome inhibitor MG132 corresponded to accumulation of HMW-polyUb conjugates in both genotypes. To further clarify the relationship between stress signaling and accumulation of HMW-polyUb induced by Cd, we included anisomycin, a very effective agent in inducing stress signaling in both p38 MAPK and SAPK/JNK. As shown in Figures 3 and 5B and 5D, anisomycin treatment induced significant activation of p38 MAPK and SAPK/JNK in both genotypes (Figs. 5B and 5D, *p ≤ 0.05, **p ≤ 0.01). However, in contrast to Cd, no accumulation of HMW-polyUb conjugates was observed. These results suggest that Cd-induced accumulation of HMW-polyUb is not necessarily the subsequent effect of Cd-induced activation of the stress signaling pathway.
FIG. 5.
Cd-induced upregulation of phosphorylation of p38 MAPK (A and B), SAPK/JNK (C and D). Synchronous p53 MEFs were exposed to various concentrations of Cd and proteasomal inhibitors and anisomycin for 24 h. Quantification of Western blot bands of phosphorylation of p38 MAPK and SAPK/JNK was conducted in NIH ImageJ and expressed as the percentage of the corresponded control after normalization to β-actin. Statistical significance was determined using two-way ANOVA followed by Tukey-Kramer multiple comparison tests. A p value ≤ 0.05 or 0.01 denoted the presence of a statistically significant difference. *Indicates significance at p ≤ 0.05 and **indicates significance at p ≤ 0.01 of the individual treatment versus control in Tukey-Kramer multiple comparison tests.
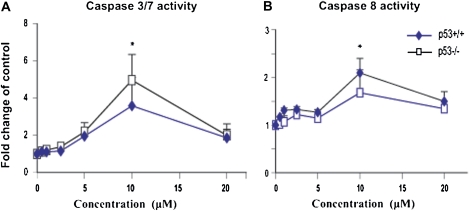
Cd Induces Apoptosis through Activation of Caspase-3/7 and -8 Cleavages
The caspase family of proteases plays a crucial role in apoptotic cell death. After demonstrating that Cd induces p53-depedent cytotoxicity, we further assessed whether Cd-induced cytotoxicity was associated with an apoptotic response. Evidence for this was obtained from both Western blot detection of cleaved caspase-3 expression (Figs. 3A and 3B and 6) as well as the functional assessment of the corresponding activity (Fig. 7) using specific fluorogenic substrates. These substrates distinguish between cleaved caspase-3/7 and -8 activities. Cd induced the activation of cleaved caspase-3 in both genotypes (ANOVA, p ≤ 0.05) and was detected using Western blot (Figs. 3 and 6A). Significant increases were observed in the p53+/+ cells (ANOVA, p ≤ 0.05). The increase of cleaved caspase-3 appeared to parallel the accumulation of HMW-polyUb (Fig. 4) and concomitant activation of the stress signaling response (Fig. 5). In addition, we measured the activity of caspase-3/7 and -8 with specific substrates, as shown in Figures 7A and 7B. The Cd-mediated activation of caspase-3/7 activity was observed only at a concentration of 10μM where caspase-8 activity was also evident (Fig. 7, p ≤ 0.05), but there were no significant differences between either of the genotypes. The induction of cleaved caspase-3, and hence apoptosis, was significantly attenuated in both genotypes at the elevated concentration of 20μM and is consistent with the overt cytotoxicity.
FIG. 6.
Dose-dependent increase in cleaved caspase-3 after exposure to Cd (A) proteasomal inhibitors and anisomycin (B). Synchronous p53 MEFs were exposed to various concentrations of Cd (A) and proteasomal inhibitors and anisomycin (B) for 24 h. Western blot analysis of cleaved caspase-3 was conducted as indicated in Figure 3. Quantification of Western blot bands of caspses-3 was conducted in NIH ImageJ and expressed as the percentage of the corresponded control after normalization to β-actin. Statistical significance was determined by ANOVA followed by Tukey-Kramer multiple comparison as compared with the control for each dose. *Indicates significance at p ≤ 0.05 and **indicates significance at p ≤ 0.01 of the individual treatment versus control in Tukey-Kramer multiple comparison tests.
FIG. 7.
Dose-dependent increase in caspase-3/7-like (A) and -8 (B) activities in response to cadmium (Cd) treatment. Synchronous p53 MEFs were exposed to various concentrations of Cd (micromolar) for 24 h. Cell extracts were prepared and equal amounts (10 μg) of total lysates subjected to analysis of caspase activities (caspase-3/7 and -8) using specific fluorogenic substrates. The resulting caspase activities were normalized to control values, and data represented graphically as % activity relative to control. Statistical significance was determined by ANOVA followed by Tukey-Kramer multiple comparison as compared with the control for each dose. *Indicates significance at p ≤ 0.05 and **indicates significance at p ≤ 0.01 of the individual treatment versus control in Tukey-Kramer multiple comparison tests.
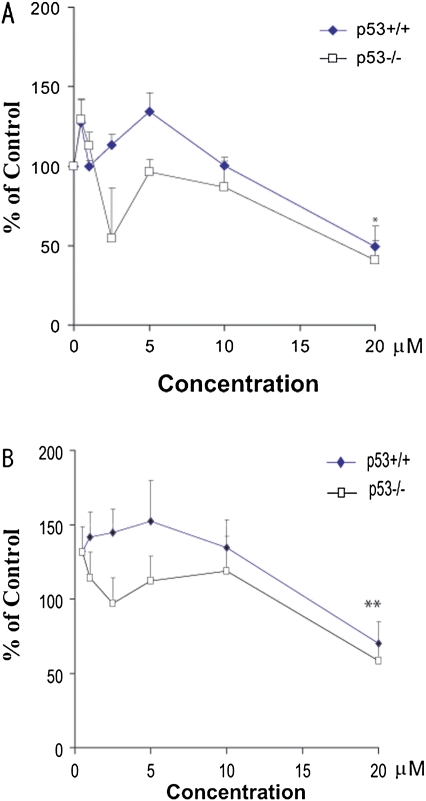
Cd-Induced Alteration of the Proteasome Activity
Steady-state levels of HMW-polyUb can theoretically be influenced by conjugation and ubiquitination loss rates, either through degradation by the proteasome or by deubiquitination through isopeptidase. To clarify whether the inhibition of the proteasomal activity by Cd was the main mechanism leading to the accumulation of HMW-polyUb proteins, we measured two different proteasome activities, as previously reported (Bobba et al., 2002; Rodgers and Dean, 2003). After Cd treatments for 24 h, PGPH (Fig. 8A) and chymotrypsin-like (Fig. 8B) proteasomal activities were measured. Dose-dependent inhibitions of PGPH and chymotrypsin-like activities were observed following Cd treatment in both genotypes. The maximum effect was observed at 20μM (*p ≤ 0.05, **p ≤ 0.01). MG132 and lactacystin significantly inhibited the chymotrypsin-like activity of the proteasome throughout the 24-h treatment in both genotypes but exerted only marginal effects on the inhibition of PGPH activity (data not shown). This initial observation suggests that at relatively high concentrations, the inhibition of the proteasomal activity by Cd may contribute to the accumulation of HMW-polyUb, a response not significant at low exposure levels.
FIG. 8.
Cadmium (Cd) induced changes in the proteasome activity of chymotrypsin (A) and PGPH (B). Proteasomal activities including chymotrypsin like (A) and PGPH (B) after Cd treatments for 24 h were measured with the fluorogenic substrates Z-Leu-Leu-Glu-AMC (A) and Suc-Leu-Leu-Val-Tyr-AMC (B). Enzyme-catalyzed release of AMC was measured by fluorescence. Data are expressed as the mean and SE from at least three separate experiments for both p53+/+ and p53−/− cells. Statistical significance was determined by ANOVA (p ≤ 0.05) followed by Tukey-Kramer multiple comparison as compared with the control for each dose. *Indicates significance at p ≤ 0.05 and **indicates significance at p ≤ 0.01 of the individual treatment versus control in Tukey-Kramer multiple comparison tests. Significant inhibition of PGPH and chymotrypsin by Cd was only observed at high concentrations.
DISCUSSION
In the present study, we investigate the role of p53 in determining cadmium sensitivity and if the induction of stress signaling responses and perturbation of the UPS are involved in mediating Cd-induced cytotoxicity and apoptosis. It has been demonstrated that a dysfunctional proteasome results in the accumulation of HMW-polyUb, which, in turn, leads to subsequent aberrant events (Rideout et al., 2003; Young and Heikkila, 2010). Inhibition of the proteasome, as observed in the classic inhibitors lactacystin and MG132, has been associated with alterations to the cell cycle, the activation of caspase-3, and the resulting induction of apoptosis (Young and Heikkila, 2010). Several proteasome-specific protease inhibitors have been essential as model chemicals for investigating the UPS-dependent degradation pathway (Bianchi et al., 2009; Kesarwala et al., 2009). In this study, we selected MG132 (a broad UPS inhibitor) and lactacystin (a more specific UPS inhibitor) to serve as model chemicals in a comparison with metals. This methodology allowed us to test our hypothesis that the disruption of the UPS pathway is an important mechanism in Cd-induced toxicity. In addition, to examine the association of the activation of the stress signaling pathway and the accumulation of HMW-polyUb, we also included anisomycin, a classical inducer of chemical stress.
As reported previously, we observed a sensitization in p53 status upon exposure to Cd. p53−/− cells demonstrated enhanced resistance to Cd treatment, exhibiting cytotoxicity and morphological perturbation indicative of an apoptotic response at 10μM versus a similar response at 5μM in the p53+/+ cells (Cao et al., 2007; Chatterjee et al., 2009; Chen and Shaikh, 2009). Alternate studies have previously demonstrated Cd-mediated cytotoxicity and apoptosis within the dose range of 5–10μM (Iryo et al., 2000; Poliandri et al., 2003). We demonstrate dose-dependent increases in both cytotoxicity and apoptosis, evidenced by the cleaved caspase-3 dependency on p53 status. Both measurements of cleaved caspase-3 and activities of caspase-3/7 or caspase-8 have been linked to apoptosis. However, we only observed statistically significant differences between the genotypes in the cleaved caspase-3, possibly due to the specificity of the substrates used. Although the induction of p53 has previously been associated with low-level Cd exposure and subsequent apoptosis (Achanzar et al., 2000), our study demonstrates a p53-dependent sensitivity to Cd. In addition to p53-dependent pathways, p53-indpendent pathways, which lead to both apoptotic and cytotoxic responses, also play a critical role in Cd-mediated toxicity.
Oxidative stress has been shown to induce reactive oxygen species, which in turn can mediate the induction of the stress signaling cascade (Pan et al., 2009). Key players in this cascade are the stress-activated kinases, p38 MAPK, and SAPK/JNK, whose expression modulates cell growth and apoptosis in response to stress (Pan et al., 2009; Ranawat and Bansal, 2009). Recent evidence highlights their critical role in mediating Cd-induced apoptosis, cell cycle arrest, and cytotoxicity (Kalariya et al., 2009; Yu et al., 2008a). In the present study, we observed a parallel stimulation of p38 MAPK and SAPK/JNK in the 5–10μM range with a nearly ninefold enhanced sensitivity in the p53+/+ cells. The activation of p38 MAPK and SAPK/JNK phosphorylation at the critical dose of 10μM in the p53−/− cells versus a more pronounced response in the p53+/+ cells at 5μM was associated with increased apoptotic and cytotoxic responses. Both p38 MAPK and SAPK/JNK stabilize p53 in response to stress, leading to subsequent impacts on cell proliferation and apoptosis (Martindale and Holbrook, 2002). These MAPKs also stabilize the cell cycle checkpoint protein, p21, resulting in a p53-independent response to oxidative stress (Barnouin et al., 2002). Barnouin et al. (2002) have demonstrated the induction of p21 in response to H2O2 in the absence of p53, illustrating the potential for a p53-independent/p21-dependent cellular response to stress, as suggested in the Cd-mediated response. Therefore, cadmium-mediated stress response and resulting apoptosis and cytotoxicity may act in both a p53-dependent and -independent nature, with p53 dependence resulting in increased sensitivity.
The results of the present study demonstrate that doses (≤10μM) of Cd lead to a robust stress response in conjunction with cytotoxicity, dependent on p53. In addition, stress response parallels the accumulation of HMW-polyUb. Cadmium exhibited similar properties to the classic proteasomal inhibitor, MG132, and to a lesser degree lactacystin. This resulted in the activation of stress proteins, as well as the accumulation of HMW-polyUb and apoptosis. The Cd-mediated accumulation of HMW-polyUb did not correlate with the marginal inhibition of proteasomal activity. Cd-induced accumulation of HMW-polyUb conjugates, activation of stress signaling cascades, and the resultant induction of apoptosis were dependent on p53 status. There was a statistically significant p53 dependency on overall cytotoxicity, and this was observed to parallel the magnitude of stress and accumulation of HMW-polyUb responses. In general, these responses occurred at concentrations that were lower than or paralleled what was required to see the activation of stress proteins and apoptosis. It is also very interesting to note that though the non-metal stress-inducing agent anisomycin induced stress and apoptosis, it did not impact UPS function. This suggests that the UPS might be the primary target of Cd with a secondary activation of cellular stress signaling resulting in cell death. This finding needs to be further confirmed by applying pharmacological stress inhibitors. Our findings provide a novel mechanistic understanding of metal-induced cytotoxicity, especially regarding the understanding of UPS dysfunction.
These observations suggest that exposure to Cd could increase its toxicity through its effect on the UPS. The UPS regulates numerous critical proteins, such as p53 and p21, which are involved in the cell cycle, cellular stress responses, and apoptosis (Naujokat and Hoffman, 2002). Previous studies have highlighted the involvement of the UPS in Cd-mediated oxidative stress response, demonstrated by the Cd-induced accumulation of HMW-polyUb (Figueiredo-Pereira et al., 1997). A dysfunctional UPS may cause proteins, usually turned over, to aggregate, leading to cytotoxicity. Previous studies have demonstrated that the ubiquitin system offers protection against Cd toxicity, whereby defects in the UPS sensitized cells resulting from Cd-mediated impacts (Tsirigotis et al., 2001), and mutants in specific ubiquitin-conjugating enzymes are hypersensitive to Cd (Jungmann et al., 1993). The accumulation of p53 following proteasomal inhibition has been linked with the p53-dependent induction of p21, G1 arrest, and apoptosis (Chen et al., 2000). Alternatively, some cell lines exhibit a p53-independent apoptosis in response to proteasomal inhibitors (Wagenknecht et al., 1999). The activation of a number of stress signaling responses as well as the accumulation of HMW-polyUb conjugates is partially dependent on the p53 status due to an obvious increase in the magnitude of the p53+/+ cells.
Our findings provide a more thorough mechanistic understanding of Cd-induced cytotoxicity, especially from the involvement of UPS perturbation. Future studies will address additional molecular targets other than p53, which could be implicated in Cd-associated cytotoxicity. Future targets may also include other stress response proteins, such as HO-1 and Nrf-2, which have been associated with Cd-induced expression and oxidative stress responses (Chen and Shaikh, 2009; Liu et al., 2009).
In summary, we investigated the dose-dependent induction by Cd of stress signaling pathways and the disruption of the UPS, as well as whether or not these resulted in the induction of apoptosis in the context of p53 in a comparison to two known proteasomal inhibitors (MG132 and lactacystin) and a prototypic inducer of chemical stress (anisomycin). Cd treatment resulted in a dose-dependent accumulation of HMW-polyUb levels, in addition to the increased phosphorylation of p38 MAPK and SAPK/JNK and induction of cleaved caspase-3 and its activities. The p53+/+ cells displayed increased sensitivity that paralleled cytotoxicity. Our study suggests that Cd-mediated cytotoxicity and induction of stress signaling responses, elevated accumulation of HMW-polyUb conjugates, and resulting apoptosis are dependent on p53 status.
FUNDING
This work was supported in part by the National Institute of Environmental Health Sciences (NIEHS), the Environmental Protection Agency (EPA), and the National Science Foundation (NSF) through the following grants: The Toxicogenomics Consortium (NIEHS: U10 ES 11387 and R01-ES10613), the Center for Children’s Environmental Health Risks Research (NIEHS: 5-P01-ES009601 and EPA: RD-83170901), the Pacific Northwest Center for Human Health and Ocean Studies (NIH/NIEHS: P50 ES012762 and NSF: OCE-0434087 and OCE-0910624), and the UW NIEHS Center for Ecogenetics and Environmental Health (NIEHS: 5 P30 ES07033). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH, NSF, or EPA.
References
- Achanzar WE, Achanzar KB, Lewis J, Webber M, Waalkes MP. Cadmium induces c-myc, p53 and c-jun expression in normal human prostate epithelial cells as a prelude to apoptosis. Toxicol. Appl. Pharmacol. 2000;164:291–300. doi: 10.1006/taap.1999.8907. [DOI] [PubMed] [Google Scholar]
- Barnouin K, Dubuisson M, Child E, Fernandez de Mattos S, Glassford J, Medema R, Mann D, Lam E. H2O2 induces a transient multi-phase cell cycle arrest in mouse fibroblasts through modulating cyclin D and p21Cip1 expression. J. Biol. Chem. 2002;277:13761–13770. doi: 10.1074/jbc.M111123200. [DOI] [PubMed] [Google Scholar]
- Bianchi G, Oliva L, Cascio P, Pengo N, Fontana F, Cerruti F, Orsi A, Pasqualetto E, Mezghrani A, Calbi V, et al. The proteasome load versus capacity balance determines apoptotic sensitivity of multiple myeloma cells to proteasome inhibition. Blood. 2009;113:3040–3049. doi: 10.1182/blood-2008-08-172734. [DOI] [PubMed] [Google Scholar]
- Bobba A, Canu N, Atlante A, Petragallo V, Calissano P, Marra E. Proteasome inhibitors prevent cytochrome c release during apoptosis but not in excitotoxic death of cerebellar granule neurons. FEBS Lett. 2002;515:8–12. doi: 10.1016/s0014-5793(02)02231-7. [DOI] [PubMed] [Google Scholar]
- Borenfreund E, Puerner J. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985;24:119–124. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Cao F, Zhou T, Simpson D, Zhou Y, Boyer J, Chen B, Jin T, Cordeiro-Stone M, Kaufmann W. p53-Dependent but ATM-independent inhibition of DNA synthesis and G2 arrest in cadmium-treated human fibroblasts. Toxicol. Appl. Pharmacol. 2007;218:174–185. doi: 10.1016/j.taap.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. Toxicology of Metals. Boca Raton, FL: CRC Press; 1996. [Google Scholar]
- Chatterjee S, Kundu S, Sengupta S, Bhattacharyya A. Divergence to apoptosis from ROS induced cell cycle arrest: effect of cadmium. Mutat. Res. 2009;663:22–31. doi: 10.1016/j.mrfmmm.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Chen F, Chang D, Goh M, Klibanov S, Ljungman M. Role of p53 in cell cycle regulation and apoptosis following exposure to proteasome inhibitors. Cell Growth Differ. 2000;11:239–246. [PubMed] [Google Scholar]
- Chen J, Shaikh ZA. Activation of Nrf2 by cadmium and its role in protection against cadmium-induced apoptosis in rat kidney cells. Toxicol. Appl. Pharmacol. 2009;241:81–89. doi: 10.1016/j.taap.2009.07.038. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira ME, Li Z, Jansen M, Rockwell P. N-acetylcysteine and celecoxib lessen cadmium cytotoxicity which is associated with cyclooxygenase-2 up-regulation in mouse neuronal cells. J. Biol. Chem. 2002;277:25283–25289. doi: 10.1074/jbc.M109145200. [DOI] [PubMed] [Google Scholar]
- Figueiredo-Pereira ME, Yakushin S, Cohen G. Accumulation of ubiquitinated proteins in mouse neuronal cells induced by oxidative stress. Mol. Biol. Rep. 1997;24:35–38. doi: 10.1023/a:1006848405975. [DOI] [PubMed] [Google Scholar]
- Goering P, Waalkes M, Klaassen C. Cadmium toxicity. In: Goyer R, Cherian M, editors. Handbook of Experimental Pharmacology; Toxicology of Metals, Biochemical Effects. New York, NY: Springer-Verlag; 1994. pp. 189–214. [Google Scholar]
- Hamer G, Matilainen O, Holmberg CI. A photoconvertible reporter of the ubiquitin-proteasome system in vivo. Nat. Methods. 2010;7:473–478. doi: 10.1038/nmeth.1460. [DOI] [PubMed] [Google Scholar]
- Iryo Y, Matsuoka M, Wispriyono B, Sugiura T, Igisu H. Involvement of the extracellular signal-regulated protein kinase (ERK) pathway in the induction of apoptosis by cadmium chloride in CCRF-CEM cells. Biochem. Pharmacol. 2000;60:1875–1882. doi: 10.1016/s0006-2952(00)00510-4. [DOI] [PubMed] [Google Scholar]
- Jungmann J, Reins H, Schobert C, Jentsch S. Resistance to cadmium mediated by ubiquitin-dependent proteolysis. Nature. 1993;361:369–371. doi: 10.1038/361369a0. [DOI] [PubMed] [Google Scholar]
- Kalariya NM, Wills NK, Ramana KV, Srivastava SK, van Kuijk FJ. Cadmium-induced apoptotic death of human retinal pigment epithelial cells is mediated by MAPK pathway. Exp. Eye Res. 2009;89:494–502. doi: 10.1016/j.exer.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Kesarwala AH, Samrakandi MM, Piwnica-Worms D. Proteasome inhibition blocks ligand-induced dynamic processing and internalization of epidermal growth factor receptor via altered receptor ubiquitination and phosphorylation. Cancer Res. 2009;69:976–983. doi: 10.1158/0008-5472.CAN-08-2938. [DOI] [PubMed] [Google Scholar]
- Liu J, Qu W, Kadiiska MB. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009;238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale J, Holbrook N. Cellular response to oxidative stress: signaling for suicide and survival. J. Cell. Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- Morrison RS, Kinoshita Y, Johnson MD, Guo W, Garden GA. p53-dependent cell death signaling in neurons. Neurochem. Res. 2003;28:15–27. doi: 10.1023/a:1021687810103. [DOI] [PubMed] [Google Scholar]
- Naujokat C, Hoffman S. Role and function of the 26S proteasome in proliferation and apoptosis. Lab. Invest. 2002;82:965–980. doi: 10.1097/01.lab.0000022226.23741.37. [DOI] [PubMed] [Google Scholar]
- Pan JS, Hong MZ, Ren JL. Reactive oxygen species: a double-edged sword in oncogenesis. World J. Gastroenterol. 2009;15:1702–1707. doi: 10.3748/wjg.15.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliandri A, Cabilla J, Velardez M, Bodo C, Duvilanski B. Cadmium induces apoptosis in anterior pituitary cells that can be reversed by treatment with antioxidants. Toxicol. Appl. Pharmacol. 2003;190:17–24. doi: 10.1016/s0041-008x(03)00191-1. [DOI] [PubMed] [Google Scholar]
- Ranawat P, Bansal MP. Apoptosis induced by modulation in selenium status involves p38 MAPK and ROS: implications in spermatogenesis. Mol. Cell. Biochem. 2009;330:83–95. doi: 10.1007/s11010-009-0103-8. [DOI] [PubMed] [Google Scholar]
- Rideout H, Wang Q, Park D, Stefanis L. Cyclin-dependent kinsase activity is required for apoptotic death but not inclusion formation in cortical neurons after proteasomal inhibition. J. Neurosci. 2003;23:1237–1245. doi: 10.1523/JNEUROSCI.23-04-01237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JF, Yu X, Hong S, Griffith WC, Beyer R, Kim E, Faustman EM. Cadmium-induced differential toxicogenomic response in resistant and sensitive mouse strains undergoing neurulation. Toxicol. Sci. 2009;107:206–219. doi: 10.1093/toxsci/kfn221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers KJ, Dean RT. Assessment of proteasome activity in cell lysates and tissue homogenates using peptide substrates. Int. J. Biochem. Cell Biol. 2003;35:716–727. doi: 10.1016/s1357-2725(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Salomons FA, Acs K, Dantuma NP. Illuminating the ubiquitin/proteasome system. Exp. Cell Res. 2010;316:1289–1295. doi: 10.1016/j.yexcr.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Sionov RV, Haupt Y. The cellular response to p53: the decision between life and death. Oncogene. 1999;18:6145–6157. doi: 10.1038/sj.onc.1203130. [DOI] [PubMed] [Google Scholar]
- Stewart D, Killeen E, Naquin R, Alam S, Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J. Biol. Chem. 2003;278:2396–2402. doi: 10.1074/jbc.M209195200. [DOI] [PubMed] [Google Scholar]
- Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D'Amelio M, Criollo A, Morselli E, Zhu C, Harper F, et al. Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 2008;10:676–687. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W, Stark G. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1851. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- Tsirigotis M, Zhang M, Chiu R, Wouters B, Gray D. Sensitivity of mammalian cells expressing mutant ubiquitin to protein-damaging agents. J. Biol. Chem. 2001;276:46073–46078. doi: 10.1074/jbc.M109023200. [DOI] [PubMed] [Google Scholar]
- Vabulas RM. Proteasome function and protein biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:24–31. doi: 10.1097/MCO.0b013e328011645b. [DOI] [PubMed] [Google Scholar]
- Vairapandi M, Balliet AG, Hoffman B, Liebermann DA. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotixic stress. J. Cell. Physiol. 2002;192:327–338. doi: 10.1002/jcp.10140. [DOI] [PubMed] [Google Scholar]
- Wagenknecht B, Hermisson M, Eitel K, Weller M. Proteasome inhibitors induce p53/p21-independent apoptosis in human glioma cells. Cell. Physiol. Biochem. 1999;9:117–125. doi: 10.1159/000016308. [DOI] [PubMed] [Google Scholar]
- Watjen W, Haase H, Biagioli M, Beyersmann D. Induction of apoptosis in mammalian cells by cadmium and zinc. Environ. Health Perspect. 2002;110:865–867. doi: 10.1289/ehp.110-1241262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall EG, Wawrzynow B, Worrall L, Walkinshaw M, Ball KL, Hupp TR. Regulation of the E3 ubiquitin ligase activity of MDM2 by an N-terminal pseudo-substrate motif. J. Chem. Biol. 2009;2:113–129. doi: 10.1007/s12154-009-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JT, Heikkila JJ. Proteasome inhibition induces hsp30 and hsp70 gene expression as well as the acquisition of thermotolerance in Xenopus laevis A6 cells. Cell Stress Chaperones. 2010;15:323–334. doi: 10.1007/s12192-009-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Hong S, Faustman EM. Cadmium-induced activation of stress signaling pathways, disruption of ubiquitin-dependent protein degradation and apoptosis in primary rat Sertoli cell-gonocyte cocultures. Toxicol. Sci. 2008a;104:385–396. doi: 10.1093/toxsci/kfn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Hong S, Moreira EG, Faustman EM. Improving in vitro Sertoli cell/gonocyte co-culture model for assessing male reproductive toxicity: Lessons learned from comparisons of cytotoxicity versus genomic responses to phthalates. Toxicol. Appl. Pharmacol. 2009;239:325–336. doi: 10.1016/j.taap.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Robinson JF, Gribble E, Hong SW, Sidhu JS, Faustman EM. Gene expression profiling analysis reveals arsenic-induced cell cycle arrest and apoptosis in p53-proficient and p53-deficient cells through differential gene pathways. Toxicol. Appl. Pharmacol. 2008b;233:389–403. doi: 10.1016/j.taap.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Robinson JF, Sidhu JS, Hong S, Faustman EM. A system-based comparison of gene expression reveals alterations in oxidative stress, disruption of ubiquitin-proteasome system and altered cell cycle regulation after exposure to cadmium and methylmercury in mouse embryonic fibroblast. Toxicol. Sci. 2010;114:356–377. doi: 10.1093/toxsci/kfq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Sidhu JS, Hong S, Faustman EM. Essential role of extracellular matrix (ECM) overlay in establishing the functional integrity of primary neonatal rat Sertoli cell/gonocyte co-cultures: an improved in vitro model for assessment of male reproductive toxicity. Toxicol. Sci. 2005;84:378–393. doi: 10.1093/toxsci/kfi085. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Mao XO, Sun Y, Xai Z, Greenburg DA. p38 mitogen-activated protein kinase mediates hypoxic regulation of mdm2 and p53 in neurons. J. Biol. Chem. 2002;277:22909–22914. doi: 10.1074/jbc.M200042200. [DOI] [PubMed] [Google Scholar]