Abstract
Methoxychlor (MXC), an organochlorine pesticide, and its metabolites, mono-hydroxy MXC (MOH) and bis-hydroxy MXC (HPTE) are known ovarian toxicants and can cause inhibition of antral follicle growth. Since these chemicals bind to estrogen receptor alpha (ESR1), we hypothesized that ovaries overexpressing ESR1 (ESR1 OE) would be more susceptible to toxicity induced by MXC and its metabolites because the chemicals can bind to more ESR1 in the antral follicles. We cultured antral follicles from controls and ESR1 OE mouse ovaries with either the vehicle dimethylsulfoxide (DMSO), MXC, MOH, or HPTE. The data show that at 96 h, the cultured antral follicles from ESR1 OE antral follicles are more susceptible to toxicity induced by MXC, MOH, and HPTE because low doses of these chemicals cause follicle growth inhibition in ESR1 OE mice but not in control mice. On comparing gene expression levels of nuclear receptors in the cultured antral follicles of ESR1 OE and control follicles, we found differential messenger RNA (mRNA) expression of Esr1, estrogen receptor beta (Esr2), androgen receptor (Ar), progesterone receptor (Pr), and aryl hydrocarbon receptor (Ahr) between the genotypes. We also analyzed mRNA levels of Cyp3a41a, the enzyme metabolizing MOH and HPTE, in the cultured follicles and found that Cyp3a41a was significantly lower in DMSO-treated ESR1 OE follicles compared with controls. In ESR1 OE livers, we found that Cyp3a41a levels were significantly lower compared with control livers. Collectively, these data suggest that MXC and its metabolites cause differential gene expression in ESR1 OE mice compared with controls. The results also suggest that the increased sensitivity of ESR1 OE mouse ovaries to toxicity induced by MXC and its metabolites is due to low clearance of the metabolites by the liver and ovary.
Keywords: methoxychlor; mono-hydroxy methoxychlor; HPTE; pesticide; metabolizing enzymes; nuclear receptors; estrogen receptor alpha; ovary, antral follicles
MXC (1,1-trichloro-2,2-bis-(4-methoxyphenyl)-ethane) is a broad spectrum organochlorine pesticide and is a model compound for environmental estrogens because it has been shown to have pro- and antiestrogenic activity (Cummings, 1997). Although MXC has a short half-life in mammals, it can persist in the environment for a considerable amount of time because it binds strongly to soil particles (Golovleva et al., 1984). Hence, decades of MXC usage can result in considerable amounts of the pesticide persisting in the environment even in areas where direct exposure is minimal. The estrogenic activity of MXC has drawn attention because of its undesirable effects on mammalian female reproduction (Bulger et al., 1978; Gray et al., 1988). Previous studies have demonstrated that MXC treatment causes adverse ovarian effects such as increased atresia (programmed cell death) of antral follicles and proliferation of ovarian surface epithelium in mice (Borgeest et al., 2002).
Mammalian female reproduction is entirely dependent on the proper development and functioning of antral follicles (Hirshfield, 1991). Being the only follicle type that can release the oocyte for fertilization, the antral follicle plays a crucial role in reproduction. Besides this, the antral follicle can synthesize and secrete sex steroid hormones such as estrogens, which are critical for the growth of the follicles (Hsueh et al., 1984). The estrogens produced by antral follicles bind to estrogen receptors alpha and beta (ESR1 and 2) located in the ovary to bring about normal menstrual or estrous cyclicity and maintenance of the female reproductive tract (Matthews and Gustafsson, 2003). In the ovary, ESR1 is predominantly expressed in the ovarian surface epithelium and theca cells, whereas ESR2 is largely localized in the granulosa cells (Couse et al., 1997b). Although estrogen binding to ESRs is important for normal physiological functions, it is critical that the levels of ESRs in the body be normal (Couse et al., 1997a). However, in certain cases, genetic polymorphisms may cause abnormalities in gene expression levels resulting in an overexpression of estrogen receptors (Tanaka et al., 2003). Overexpression of estrogen receptors may also be caused by exposure to estrogenic chemicals that bind to ESRs and block endogenous estrogen from binding to the receptors (Danzo, 1997). Binding of estrogenic chemicals to ESRs may hinder the normal functioning of the receptor as well as cause aberrant gene transcription causing an induction of ESR gene expression in the ovary (Newbold et al., 2007). Overexpression of ESRs may make the ovary more susceptible to further toxic insult because of an increased number of binding sites for estrogenic chemicals.
In this study, we focused on whether overexpression of ESR1 in the ovary results in an increased susceptibility of the antral follicles to toxicity induced by MXC or its metabolites. MXC is metabolized predominantly to 1,1,1-trichloro-2-(4-hydroxyphenyl)-2-(4-methoxyphenyl)ethane (MOH) and the bisphenolic compound 1,1,1-trichloro-2,2-bis(4-hydroxyphenyl)ethane (HPTE) by cytochrome P450 (Cyp) enzymes (Stresser and Kupfer 1997, 1998). Cyp1a2 and Cyp2c29 convert MXC to MOH and HPTE, whereas Cyp3a41a converts MOH and HPTE to water-soluble compounds that can be excreted from the body (Stresser and Kupfer, 1997).
Overexpression of ESR1 in the mouse ovary was achieved by transgenic techniques as previously described (Tomic et al., 2007). Previous results have shown that administration of MXC in ESR1 OE in vivo caused an increase in the percentage of atretic antral follicles compared with control animals (Tomic et al., 2006). MXC and its metabolites are also known to cause growth inhibition of antral follicles in vitro (Gupta et al., 2006; Miller et al., 2005, 2006) and hence, this study was designed to determine whether overexpression of ESR1 increases the susceptibility of antral follicles to growth inhibition induced by MXC and its metabolites compared with controls. Moreover, MXC and its metabolites have also been shown to cause downstream gene transcription/alterations in several species and cell types (Gaido et al., 1999, 2000; Waters et al., 2001). However, little information is available on the effects of MXC, MOH, and HPTE separately on gene expression levels of receptors such as Esr1, Esr2, Ar, Pr, and Ahr and the metabolizing enzymes Cyp1a2, Cyp2c29, and Cyp3a41a in mouse antral follicles. Hence, we analyzed whether the estrogenic compounds MXC or its metabolites are able to cause changes in gene transcription in ESR1 OE and control antral follicles after in vitro treatment. We hypothesized that MXC and its metabolites may cause differential gene expression in ESR1 OE antral follicles compared with controls because of the increased number of ESR1 receptors in ESR1 OE that can bind MXC and its metabolites in the ovaries.
MATERIALS AND METHODS
Generation of ESR1 OE and control mice.
The ESR1 OE and control mice used in this study were generated using C57BL6 and FVB mice as previously described (Tomic et al., 2007). Briefly, transgenic mice carrying a transgene composed of a coding sequence for murine ESR alpha (ESR1) placed under the regulatory control of a tet-op promoter (tet-op-ESR1 mice) (Hruska et al., 2002) were mated to tet-op-tTA/tet-op-luciferase mice (Shockett et al., 1995) to produce triple-transgenic mice tet-op-tTA/tet-op-luciferase/tet-op-ESR1. The transcription of the ESR1 transgene is only achieved in the presence of a tetracycline-responsive transactivator (tTA) protein. In the triple-transgenic mice, the initial transcription of tTA protein occurs through a leaky transcription from the tet-op promoter, producing only small amounts of tTA protein. These small amounts then positively feedback on the tet-op promoter and further increase tTA protein production (Shockett et al., 1995). The tTA protein binds to the tet-op promoter linked to the ESR1 transgene as well, driving the expression of transgenic ESR1 and luciferase.
Animals.
ESR1 OE and control mice that were 32–35 days old were used for all experiments. The mice were housed in the University of Illinois core animal facility under a 12:12 dark:light cycle and provided food and water ad libitum. Only triple-transgenic ESR1 OE (tet-op-ESR1/tet-op-tTA/tet-op-luciferase) and double transgenic control (tet-op-tTA/tet-op-luciferase) mice were used for the experiments. All animal procedures were approved by the University of Illinois Institutional Animal Care and Use Committee.
Screening/genotyping ESR1 overexpressing and control mice.
Mice were genotyped using PCR-based assays. Briefly, ear punch tissues from pups were lysed in 8 μl of buffer (1M Tris pH 8.0, 5M NaCl, 0.5M EDTA, and 20% SDS) containing 2 μl of 20 mg/ml proteinase K (Qiagen Inc., Valencia, CA). Digestion was carried out at 55°C for 1 h followed by enzyme inactivation at 100°C for 3 min. The lysate was then subjected to PCR using primers (1) 5′-CGAGCTCGGTACCCGGGTCG-3′ and (2) 5′-GAACACAGTGGGCTTGCTGTTG-3′ for tet-op-ESR1 and primers (1) 5′-CGAGCTCGGTACCCGGGTCG-3′, and (2) 5′-GCAAAAGTGAGTATGGTGCC- 3′ for tet-op-tTA. The conditions for tet-op-tTA PCR were 94°C for 3 min of initial denaturation followed by 35 cycles at 94°C for 60 s, 61°C for 60 s, 72°C for 180 s, and final extension at 72°C for 10 min. The conditions for tet-op-ESR1 were 94°C for 3 min of initial denaturation followed by 35 cycles at 94°C for 60 s, 57°C for 90 s, and 72° for 120 s. PCR products then were subjected to agarose gel electrophoresis. The presence of a 372-bp fragment indicated that the mice were controls (tet-op-tTA/tet-op/luciferase), and the presence of both 372- and 369-bp bands indicated that the mice were ESR1 overexpressors (tet-op-tTA/tet-op-luciferase/tet-op-ESR1).
Immunohistochemical staining.
The levels of ESR1 expression were compared in ESR1 OE and control mouse ovaries by immunostaining using mouse monoclonal anti-ESR1 antibody (NCL-L-ER-6F11; Leica Microsystems, IL). The cyclicity of the mice was determined by vaginal smears, and only animals in estrous were used for immunostaining. The mice were deeply anesthetized by administering ketamine/xylazine (150/10 mg/kg body weight) and perfused intracardially with heparinized phosphate-buffered saline followed by ice-cold 10% neutral buffered formalin. The ovaries were removed and put in 4% paraformaldehyde fixative. The fixed ovaries were embedded in paraffin blocks within 24 h, sectioned at 5 μm and mounted on slides. For immunohistochemical staining, the slides were first deparaffinized and rehydrated followed by steam antigen retrieval using a 10mM sodium citrate buffer at pH 6. The staining was carried out in Shandon sequenza coverplate assembly (Thermo Fisher Scientific Inc., MA) using ready-to-use reagents from the HistoMouse-Plus Kit (Invitrogen Corporation, CA) according to the manufacturer’s protocol. Incubation with ESR1 antibody (1:40) was carried out for 2 h at room temperature followed by washes and incubation with monoclonal secondary antibody for 1 h.
The levels of ESR1 staining in MXC-treated mice were quantified using published methods (Barnett et al., 2007). Specifically, images from stained sections obtained from at least three different animals per treatment group were digitally captured using a Leica DFC 290 camera and analyzed using the ImageJ software (http://rsb.info.nih.gov/nih-image/). Digital images were initially converted to eight-bit grayscale images and then converted to pseudo colored images. Colors were based on relative stain intensity, as defined digitally. Areas with no staining appeared dark blue, whereas areas with the most intense staining appeared deep orange to red. The ESR1 labeling index, expressed as a percentage of positively stained area per follicle, was determined for all follicles in each section (two to three follicles per section from at least three separate animals per group). Values for ESR1 labeling indices were generated by dividing the ESR1 positively stained area per follicle by the total area per follicle and multiplying the values by 100.
Chemicals.
MXC (99% pure) was purchased from Chemservice (West Chester, PA). MOH and HPTE (99% pure) were synthesized in Dr Vincent Njar’s laboratory (University of Maryland, Baltimore now at Thomas Jefferson University, Philadelphia, PA). Stock solutions of MXC, MOH, and HPTE for in vitro dosing were prepared using dimethylsulfoxide (DMSO) (Sigma, St Louis MO) as a solvent.
Follicle culture.
Antral follicles (determined by appearance and relative size) were isolated from ESR1 OE and control ovaries using fine watchmaker forceps. About 75–80 antral follicles (300–400 μm) were obtained from at least two mice of each genotype per experiment. The follicles were then randomly divided into five groups—nontreatment (NT), vehicle control (DMSO), and three chemical treatments of MXC, MOH, or HPTE. Increasing concentrations (1.33, 13.3, and 133 mg/ml) of MXC and (0.133, 1.33, and 13.3 mg/ml) of MOH and HPTE were made to allow an equal volume to be added to each of the treatment groups in the 96-well culture plate to control the solvent concentration. The final concentrations of MXC in each well of the culture were 1, 10, and 100 μg/ml. Similarly, final concentrations of MOH and HPTE in culture were 0.1, 1, and 10 μg/ml. MOH and HPTE were used at 10-fold lower concentrations than MXC because the metabolites of MXC are thought to be more toxic than the parent compound. Moreover, the amount of the metabolites reaching the follicles after MXC has been metabolized is likely to be lower than the parent compound. In the vehicle control treatment group, DMSO was used at 0.075%, which is not toxic to cultured follicles. The doses selected for in vitro studies were based on previously published studies showing that these concentrations of MXC, MOH, and HPTE induce toxicity in antral follicles and granulosa cell culture models (Gupta et al., 2006; Miller et al., 2005). A nontreatment group was included to control for culture conditions and follicles in this group were placed only in supplemented Minimum Essential Medium alpha (α-MEM) devoid of either DMSO or chemicals.
The follicles were cultured in supplemented α-MEM as described previously. Briefly, supplemented α-MEM was prepared using 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium), 100 U/ml penicillin, 100 mg/ml streptomycin, 5 IU/ml human recombinant follicle-stimulating hormone (Dr A. F. Parlow, National Hormone and Peptide Program, Harbor- UCLA Medical Center, Torrance, CA), and 5% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA). Follicles were incubated for 96 h at 37°C in 95% air and 5% CO2.
Growth analysis of follicles in culture.
Antral follicles were cultured for 96 h and follicle growth was examined at 24-h intervals using published methods (Gupta et al., 2006). Briefly, starting on the day of culture (0 h), follicles in the culture wells were measured every 24 h using an inverted microscope equipped with a calibrated ocular micrometer. Follicle diameters were measured in perpendicular axes, averaged among treatment groups and plotted to compare the effects of chemical treatments on growth over time. Data were analyzed as percent change of diameter from the 0-h time point.
Gene analysis.
Gene expression analysis was carried out on liver samples as well as cultured antral follicles. Liver samples were taken from adult female ESR1 OE and control mice. Total RNA from liver samples was extracted using RNeasy Mini Kit (Qiagen, Inc.) per the manufacturer’s protocol. Cultured antral follicles were collected from 96-well plates and snap-frozen in liquid nitrogen. Total RNA was extracted from follicles using the RNeasy Micro Kit (Qiagen, Inc.) by homogenizing the follicles in buffer according to the manufacturer’s protocol. Total RNA (100–500 ng) from liver and follicles were then reverse transcribed to obtain complementary DNA (cDNA) using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules CA) according to the manufacturer’s protocol. Quantitative real-time (q-PCR) was conducted using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) and accompanying software according to the manufacturer's instructions. The machine quantifies the amount of q-PCR product generated by measuring the dye (SsoFast EvaGreen from Bio-Rad Laboratories, Inc.) that fluoresces when bound to double-stranded DNA. A standard curve was generated from five serial dilutions of purified q-PCR product. To detect genomic DNA contamination, a well containing total RNA was included each time. Gene-specific primers (synthesized by Integrated DNA Technologies, Inc., Coralville, Iowa) were used for the q-PCR (Table 1) and mouse β-actin was used as the reference gene. An initial incubation of 95°C for 10 min was followed by 40–50 cycles of 94°C for 10 s (denaturation step), 55– 60°C (annealing step) for 10 s, and 72°C for 10 s (extension step), along with a final extension at 72°C for 10 min. Starting quantity (SQ), an arbitrary term generated by CFX96, denotes the relative gene expression level in the experimental samples. These values are calculated based on the standard curve and are read at the cycle at which the gene begins amplifying exponentially. For each gene, a melting curve was performed at 55–90°C to monitor the generation of a single product. SQs from ESR1 OE and control samples were normalized by obtaining the ratio of gene:β-actin. The specificity of the primers was verified by the lack of amplification from genomic DNA and by a single melting curve. For liver samples, fold changes were calculated as a ratio of normalized values of ESR1 OE samples and mean normalized values of controls. All experiments were performed in triplicate.
TABLE 1.
Sequences of Primer Sets Used for Analysis of Gene Expression
| Accession number | Gene name | Gene nomenclature | Forward | Reverse |
| NM_007956 | Estrogen receptor alpha | Esr1 | 5′-GTGCTTCAACATTCTCCCTCCTC-3′ | 5′-CCGTGTGCAATGACTATGCC-3′ |
| NM_207707 | Estrogen receptor beta | Esr2 | 5′-GGAATCTCTTCCCAGCAGCA-3′ | 5′-GGGACCACATTTTTGCACTT-3′ |
| NM_013476 | Androgen receptor | Ar | 5′-GGCGGTCCTTCACTAATGTCAACT-3′ | 5′-GAGACTTGTGCATGCGGTACTCAT-3′ |
| NM_008829 | Progesterone receptor | Pr | 5′-GCTTGCATGATCTTGTGAAACAGC-3′ | 5′-GGAAATTCCACAGCCAGTGTCC-3′ |
| NM_013464 | Aryl hydrocarbon receptor | Ahr | 5′-TTCTTAGGCTCAGCGTCAGCTA-3′ | 5′-GCAAATCCTGCCAGTCTCTGAT-3′ |
| NM_009993 | Cytochrome P450 1a2 | Cyp1a2 | 5′-TGTTCCGCCCAGAGCGGTTT-3′ | 5′-ACTTCCCACTTGGCCGGGAT-3′ |
| NM_017396 | Cytochrome P450 3a41a | Cyp3a41a | 5′-TGAGCTTTCTCAGTGTCTGTACAGGTT-3′ | 5′-TGAGATGTGGATGGAGATGGTCCC-3′ |
| NM_007815 | Cytochrome P450 2c29 | Cyp2c29 | 5′-TTTCTGGCCCCTGCTGTGATGC-3′ | 5′-TGCTGGGTCTTGAGAGAAGAGGGT-3′ |
| NM_007393 | Mouse Beta-actin | β-Actn | 5′-GGGCACAGTGTGGGTGAC-3′ | 5′-CTGGCACCACACCTTCTAC-3′ |
Statistical analysis.
All data were analyzed using SPSS statistical software (SPSS, Inc., Chicago, IL). Data were expressed as means ± SEM from at least three separate experiments. Multiple comparisons between experimental groups were done using ANOVA followed by Tukey’s post hoc comparison. Comparison between two groups was done using Student’s t-test. Statistical significance was assigned at p ≤ 0.05.
RESULTS
ESR1 is overexpressed in ESR1 OE mouse ovaries
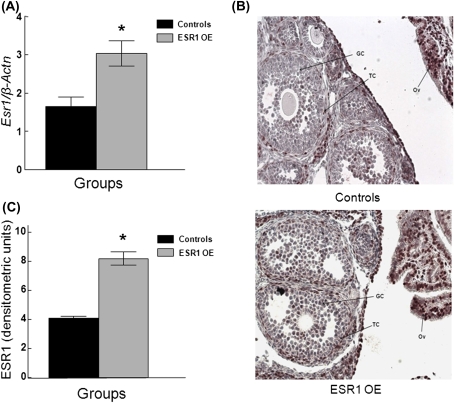
To confirm that ESR1 was overexpressed in ESR1 OE and control mouse ovaries, q-PCR was carried out using Esr1-specific primers (Fig. 1A). As previously shown (Tomic et al., 2007), ESR1 OE mouse ovaries had significantly higher expression of Esr1 messenger RNA (mRNA) than ovaries collected from control animals (Controls: 1.7 ± 0.3 SQ; ESR1 OE: 3.0 ± 0.6 SQ; p ≤ 0.05; n = 4).
FIG. 1.
ESR1 expression in ESR1 OE and control mouse ovaries. Ovaries were isolated from adult cycling ESR1 OE and control mice and subjected to gene or protein analysis. (A) The gene expression of Esr1 was measured by q-PCR techniques and normalized to the reference gene β-actin (β-Actn). (B) Protein expression of ESR1 was analyzed by immunohistochemical staining of ESR1 in ovarian sections (×20 magnification) of control (top panel) and ESR1 OE (bottom panel) mice. Red, ESR1 staining; Blue, counterstain. GC, granulosa cells; TC, theca cells; Ov, oviduct. (C) Immunohistochemical staining was quantified as described in the “Materials and methods” section and presented as a bar graph. Each bar represents means ± SE from three separate experiments. *Indicate statistically significant differences from controls (n = 3, p ≤ 0.05).
To further validate the transgenic ESR1 OE mice, protein levels of ESR1 were compared in the ovaries of control and ESR1 OE mice using immunohistochemical analysis (Fig. 1B; top panel: controls and bottom panel: ESR1 OE). The results show that ESR1 staining (red) is predominantly localized in the theca as well as interstitial cells of both genotypes, but is higher in ESR1 OE than controls. The staining in the theca cells was quantified by densitometry and indicates statistically significant higher levels in ESR1 OE ovaries compared with controls (Fig 1C; controls: 4.1 ± 0.2 densitometric units; ESR1 OE: 8.2 ± 0.1 densitometric units; p ≤ 0.05; n = 4–5 follicles from three different ovaries).
MXC and its metabolites cause increased growth inhibition in antral follicles of ESR1 OE compared with controls
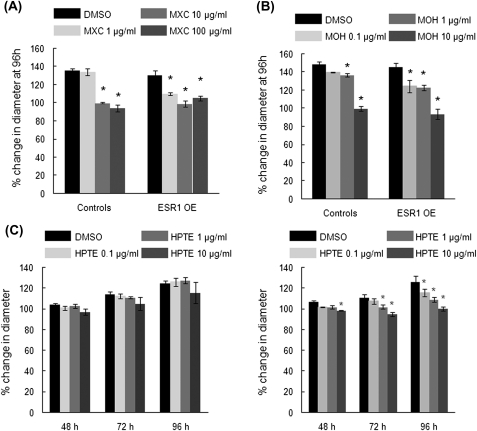
Growth analysis of ESR1 OE and control antral follicles treated with MXC shows that after 96 h of culture, 10 and 100 μg/ml MXC inhibited growth of follicles compared with DMSO-treated follicles (Fig. 2A). However, in ESR1 OE mice, follicles treated with even the lowest dose of 1 μg/ml MXC had growth inhibition compared with control follicles (controls: DMSO = 135.9 μm ± 1.8; 1 μg/ml MXC = 133.9 μm ± 3.73; 10 μg/ml MXC = 99.9 μm ± 0.8; 100 μg/ml MXC = 94.2 μm ± 4.0 ESR1 OE: DMSO = 130.7 μm ± 2.8; 1 μg/ml MXC = 109.7 μm ± 1.4; 10 μg/ml MXC = 98.7 μm ± 3.3; 100 μg/ml MXC = 105.4 μm ± 2.7; n = 13–15 follicles from five separate experiments; p ≤ 0.05). MXC did not affect growth of antral follicles at 0–72 h (data not shown).
FIG. 2.
Effect of MXC, MOH, and HPTE on the growth of antral follicles from controls and ESR1 OE mice. Antral follicles were cultured with either the chemical or the vehicle DMSO. Growth of follicles was measured in micrometers at 0, 24, 48, 72, and 96 h of culture and plotted as percent change from time 0 h. (A) 1–100 μg/ml MXC at 96 h; (B) 0.1–10 μg/ml MOH at 96 h; (C) 0.1–10 μg/ml HPTE treatments on control (left panel) and ESR1 OE (right panel) antral follicles at 48, 72, and 96 h. Each bar represents means ± SE. *Indicate statistically significant differences from controls (n = 13–15 follicles per treatment from three separate experiments; p ≤ 0.05).
With MOH treatment, 1 and 10 μg/ml MOH inhibited growth of control follicles compared with DMSO-treated follicles. However, in ESR1 OE mice, all three doses of MOH (0.1, 1, and 10 μg/ml) inhibited growth of antral follicles (Fig. 2B; controls: DMSO = 148.7 μm ± 2.9; 0.1 μg/ml MOH = 139.8 μm ± 0.7; 1 μg/ml MOH = 136.7 μm ± 1.9; 10 μg/ml MOH = 99.7 μm ± 2.6; ESR1 OE: DMSO = 145.8 μm ± 4.1; 0.1 μg/ml MOH = 124.5 μm ± 6.7; 1 μg/ml MOH = 123.1 μm ± 3.0; 10 μg/ml MOH = 93.9 μm ± 5.7; n = 13–15 follicles from three separate experiments; p ≤ 0.05). MOH did not affect growth of antral follicles at 0–72 h (data not shown).
HPTE treatment also caused differences in antral follicle growth between ESR1 OE and control mice (Fig. 2C). In control animals, the growth of antral follicles was not inhibited with any of the three doses of HPTE compared with DMSO-treated follicles (Fig 2C, left panel). However, in ESR1 OE antral follicles, 10 μg/ml HPTE significantly inhibited follicle growth as early as 48 h compared with DMSO treatment (Fig 2C, right panel). Significant growth inhibition by 1 μg/ml HPTE was seen by 72 h compared with DMSO treatment, and by 96 h even the lowest dose caused a significant inhibition of antral follicle growth compared with DMSO-treated antral follicles (Fig2C, right panel; at 96h—controls: DMSO = 124.7 μm ± 2.3; 0.1 μg/ml HPTE = 126.1 μm ± 3.9; 1 μg/ml HPTE = 127.5 μm ± 2.9; 10 μg/ml HPTE = 115.5 μm ± 10.2; ESR1 OE: DMSO = 126.1 μm ± 5.8; 0.1 μg/ml HPTE = 115.7 μm ± 3.8; 1 μg/ml HPTE = 108.9 μm ± 2.1; 10 μg/ml HPTE = 100.5 μm ± 2.0; n = 13–15 follicles from three separate experiments; p ≤ 0.05).
MXC and its metabolites cause differential gene expression of nuclear receptors in antral follicles of ESR1 OE and controls
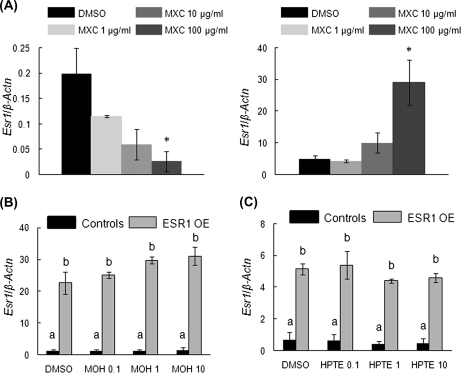
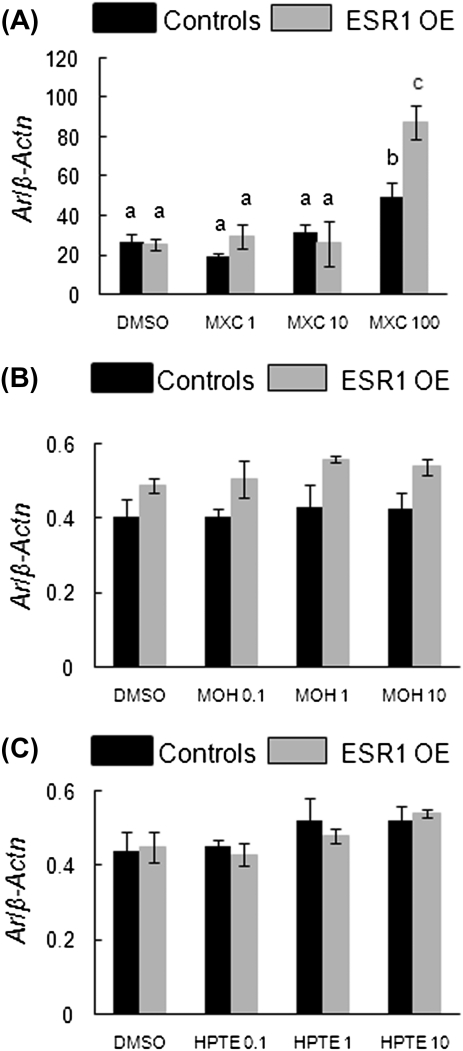
Since ESR1 is a nuclear transcription factor, which upon ligand binding, can cause gene transcription of various genes including nuclear receptors (Murdoch and Gorski, 1991), ESR1 OE and control antral follicles treated with MXC, MOH, and HPTE in vitro were subjected to q-PCR to analyze mRNA levels of nuclear receptors including Esr1, Esr2, Ar, Ahr, and Pr. The results show that MXC treatment caused differential gene expression of Esr1 in controls and ESR1 OE (Fig. 3A). In controls, MXC treatment caused a dose-dependent decrease in Esr1 mRNA and was significantly reduced at the highest dose of treatment compared with DMSO-treated follicles (Fig. 3A, left panel; controls: DMSO = 0.2 SQ ± 0.1; 1 μg/ml MXC = 0.1 SQ ± 0.002; 10 μg/ml MXC = 0.06 SQ ± 0.02; 100 μg/ml MXC = 0.03 SQ ± 0.02; n = 13–15 follicles from five separate experiments; p ≤ 0.05). In ESR1 OE mice, the mRNA levels of Esr1 increased in a dose-dependent manner and were significantly higher than controls in follicles treated with 100 μg/ml MXC compared with DMSO-treated follicles (Fig. 3A, right panel; ESR1 OE: DMSO = 5.0 SQ ± 0.9; 1 μg/ml MXC = 4.4 SQ ± 0.4; 10 μg/ml MXC = 10.0 SQ ± 3.1; 100 μg/ml MXC = 29.0 SQ ± 7.0; n = 13–15 follicles from five separate experiments; ; p ≤ 0.05).
FIG. 3.
Effect of MXC, MOH, and HPTE on gene expression levels of Esr1 in antral follicles of control and ESR1 OE mice. Antral follicles were cultured with MXC (1–100 μg/ml), MOH (0.1–10 μg/ml), HPTE (0.1–10 μg/ml), or the vehicle DMSO for 96 h and then subjected to q-PCR with Esr1-specific primers. The relative gene expression quantities of Esr1 were normalized to β-Actn. (A) MXC-treated control follicles (left panel) and ESR1 OE follicles (right panel); (B) MOH-treated follicles; (C) HPTE-treated follicles. Each bar represents means ± SEM. * Indicate significant differences from DMSO controls within genotype. Bars with different letters are significantly different from each other (n = 13–15 follicles per treatment from three separate experiments; p ≤ 0.05).
In follicles treated with MOH and HPTE, mRNA levels of Esr1 were significantly higher in ESR1 OE antral follicles compared with controls (Figs. 3B and 3C). However, gene expression levels did not differ between the treatment groups in either genotype when compared with DMSO-treated follicles.
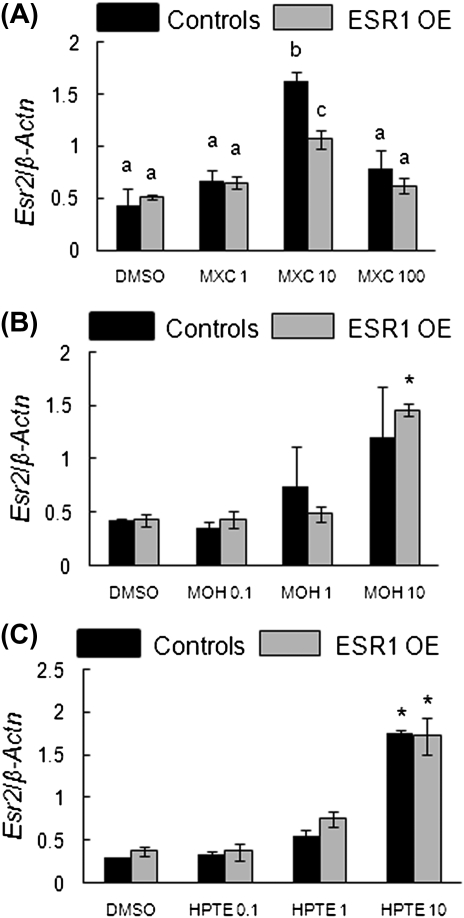
MXC (10 μg/ml) significantly upregulated Esr2 levels in controls and ESR1 OE antral follicles. However, Esr2 levels in controls were significantly higher than ESR1 OE antral follicles at this concentration (Fig. 4A; controls: DMSO = 0.4 SQ ± 0.2; 10 μg/ml MXC = 1.6 SQ ± 0.1; ESR1 OE: DMSO = 0.5 SQ ± 0.02; 10 μg/ml MXC = 1.07 SQ ± 0.1; n = 13–15 follicles from three separate experiments; p ≤ 0.05).
FIG. 4.
Effect of MXC, MOH, and HPTE on gene expression levels of Esr2 in antral follicles of control and ESR1 OE mice. Antral follicles were cultured with MXC (1–100 μg/ml), MOH (0.1–10 μg/ml), HPTE (0.1–10 μg/ml), or the vehicle DMSO for 96 h and then subjected to q-PCR with Esr2-specific primers. The relative gene expression quantities of Esr2 were normalized to β-Actn. (A) MXC-treated follicles; (B) MOH-treated follicles; (C) HPTE-treated follicles. Each bar represents means ± SEM. * Indicate significant differences from DMSO controls within genotype. Bars with different letters are significantly different from each other (n = 13–15 follicles per treatment from three separate experiments; p ≤ 0.05).
With MOH treatment, Esr2 was upregulated significantly at 10 μg/ml in ESR1 OE mice compared with controls; whereas in control follicles, levels of Esr2 were not different between treatment groups (Fig. 4B; controls: DMSO = 0.4 SQ ± 0.02; 10 μg/ml MOH = 1.21 SQ ± 0.5; ESR1 OE: DMSO = 0.43 SQ ± 0.06; 10 μg/ml MOH = 1.46 SQ ± 0.06; n = 13–15 follicles from three separate experiments). HPTE treatment of antral follicles significantly upregulated Esr2 levels at 10 μg/ml in both genotypes (Fig. 4C; controls: DMSO = 0.3 SQ ± 0.01; 10 μg/ml HPTE = 1.77 SQ ± 0.02; ESR1 OE: DMSO = 0.37 SQ ± 0.05; 10 μg/ml HPTE = 1.72 SQ ± 0.22; n = 13–15 follicles from three separate experiments; p ≤ 0.05).
MXC treatment caused a dose-dependent increase in the levels of Ar in ESR1 OE and control antral follicles (Fig. 5A). In both genotypes, 100 μg/ml MXC caused a significant increase in the levels of Ar compared with DMSO-treated follicles. However, the levels of Ar were significantly higher in ESR1 OE antral follicles compared with control antral follicles with 100 μg/ml MXC treatment (controls: DMSO = 26.9 SQ ± 4.3; 100 μg/ml MXC = 49.6 SQ ± 7.1; ESR1 OE: DMSO = 25.5 SQ ± 2.6; 100 μg/ml MXC = 87.5 SQ ± 8.9; n = 13–15 follicles from three separate experiments; p ≤ 0.05). MOH and HPTE treatment did not have any effect on Ar levels in ESR1 OE or control antral follicles (Figs. 5B and 5C).
FIG. 5.
Effect of MXC, MOH, and HPTE on gene expression levels of Ar in antral follicles of control and ESR1 OE mice. Antral follicles were cultured with MXC (1–100 μg/ml), MOH (0.1–10 μg/ml), HPTE (0.1–10 μg/ml) or the vehicle DMSO for 96 h and then subjected to q-PCR with Ar-specific primers. The relative gene expression quantities of Ar were normalized to β-Actn. (A) MXC-treated follicles; (B) MOH-treated follicles; (C) HPTE-treated follicles. Each bar represents means ± SEM. Bars with different letters are significantly different from each other (n = 13–15 follicles per treatment from three separate experiments; p ≤ 0.05).
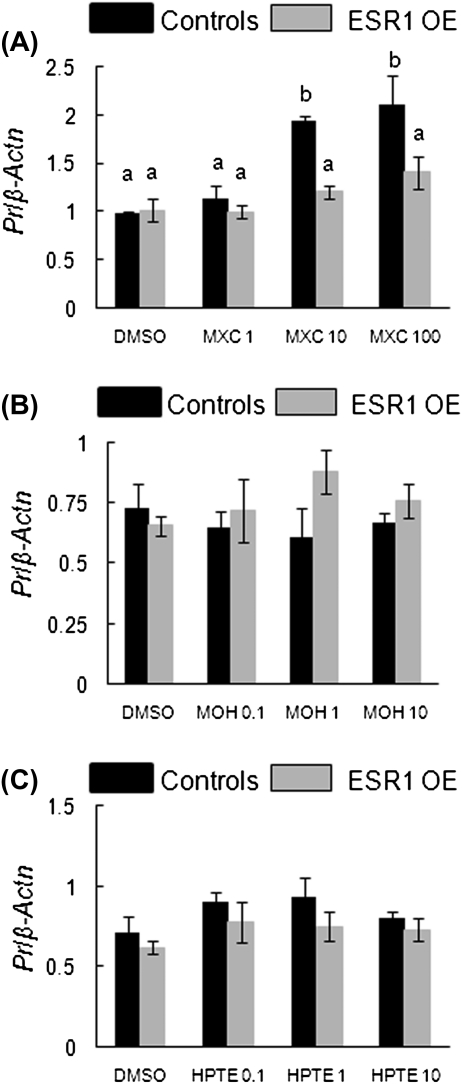
MXC treatment did not cause any change in Pr levels in ESR1 OE-treated antral follicles. However, in control follicles, Pr levels at 10 and 100 μg/ml MXC were significantly increased compared with DMSO-treated antral follicles (Fig 6A). At these doses of MXC, Pr levels in control follicles were significantly higher than ESR1 OE follicles as well (controls: DMSO = 0.9 SQ ± 0.02; 10 μg/ml MXC = 1.9 SQ ± 0.1; 100 μg/ml MXC = 2.1 SQ ± 0.3; ESR1 OE: DMSO = 1.0 SQ ± 0.1; 1 μg/ml MXC = 1 SQ ± 0.1; 10 μg/ml MXC = 1.2 SQ ± 0.1; 100 μg/ml MXC = 1.4 SQ ± 0.2; n = 13–15 follicles from three separate experiments; p ≤ 0.05). MOH or HPTE treatments did not alter Pr levels in either genotype (Figs. 6B and 6C).
FIG. 6.
Effect of MXC, MOH, and HPTE on gene expression levels of Pr in antral follicles of control and ESR1 OE mice. Antral follicles were cultured with MXC (1–100 μg/ml), MOH (0.1–10 μg/ml), HPTE (0.1–10 μg/ml), or the vehicle DMSO for 96 h and then subjected to q-PCR with Pr-specific primers. The relative gene expression quantities of Pr were normalized to β-Actn. (A) MXC-treated follicles; (B) MOH-treated follicles; (C) HPTE-treated follicles. Each bar represents means ± SEM. Bars with different letters are significantly different from each other (n = 13–15 follicles per treatment from three separate experiments; p ≤ 0.05).
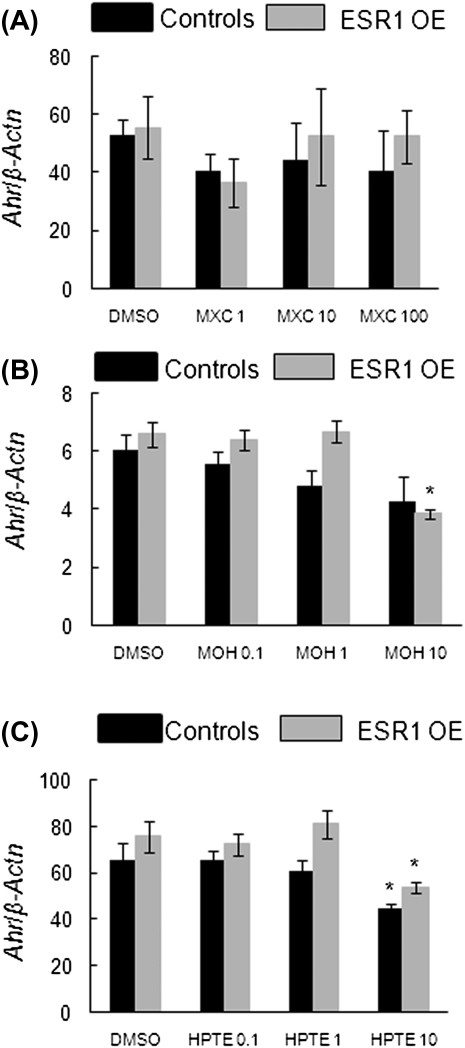
MXC treatments did not affect levels of Ahr in either genotype (Fig 7A). In MOH- and HPTE-treated antral follicles, 10 μg/ml treatment significantly decreased the levels of Ahr in ESR1 OE mice compared with DMSO, 0.1 and 1 μg/ml treatments (Figs. 7B and 7C). However, MOH treatment had no effect on the levels of Ahr in control follicles (Figs 7B, controls: DMSO = 6.1 SQ ± 0.5; 10 μg/ml MOH = 4.3 SQ ± 0.8; ESR1 OE: DMSO = 6.6 SQ ± 0.4; 10 μg/ml MOH = 3.84 SQ ± 0.2; n = 13–15 follicles from three separate experiments; Fig. 7C, controls: DMSO = 65.8 SQ ± 7; 10 μg/ml HPTE = 44.9 SQ ± 2.1; ESR1 OE: DMSO = 75.6 SQ ± 6.6; 10 μg/ml HPTE = 53.9 SQ ± 2.4; n = 13–15 follicles from three separate experiments; p ≤ 0.05).
FIG. 7.
Effect of MXC, MOH, and HPTE on gene expression levels of Ahr in antral follicles of control and ESR1 OE mice. Antral follicles were cultured with MXC (1–100 μg/ml), MOH (0.1–10 μg/ml), HPTE (0.1–10 μg/ml), or the vehicle DMSO for 96h and then subjected to q-PCR with Ahr-specific primers. The relative gene expression quantities of Ahr were normalized to β-Actn. (A) MXC-treated follicles; (B) MOH-treated follicles; (C) HPTE-treated follicles. Each bar represents means ± SEM. * Indicate significant differences from DMSO controls within genotype (n = 13–15 follicles per treatment from three separate experiments; p ≤ 0.05).
MXC and its metabolites cause differential gene expression of metabolizing enzymes in antral follicles of ESR1 OE and controls
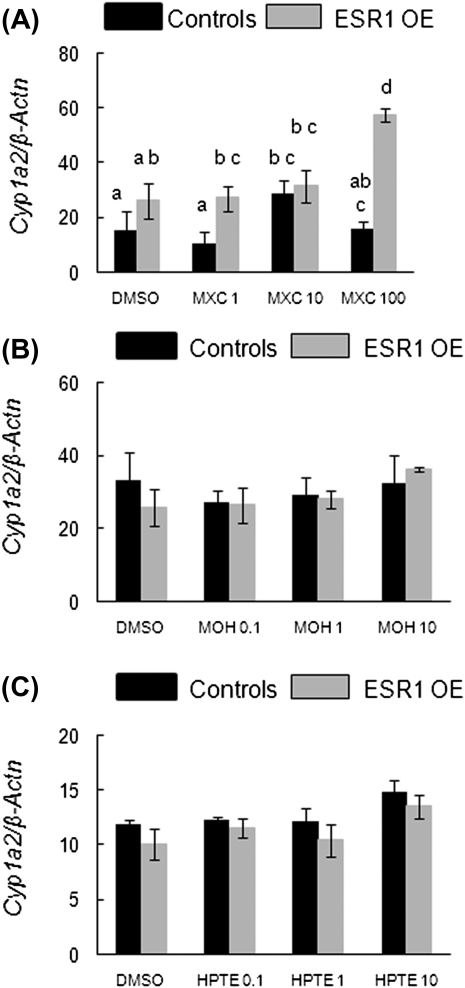
Since Cyp1a2 and Cyp2c29 are involved in the metabolism of MXC and its metabolites (Stresser and Kupfer, 1998), we analyzed the mRNA levels of these enzymes in antral follicles cultured with MXC and its metabolites. MXC treatment significantly induced Cyp1a2 in control follicles at 10 μg/ml compared with the DMSO, 1 and 100 μg/ml treated follicles. However, in ESR1 OE follicles, MXC treatment significantly induced Cyp1a2 at the 100 μg/ml concentration compared with DMSO, 1 and 10 μg/ml treatment groups. Interestingly, at 100 μg/ml MXC, Cyp1a2 induction was significantly greater in ESR1 OE follicles compared with controls (Fig 8A; controls: DMSO = 15.5 SQ ± 3.0; 100 μg/ml MXC = 16.3 SQ ± 2.3; ESR1 OE: DMSO = 26.2 SQ ± 5; 100 μg/ml MXC = 57.7 SQ ± 7.8; n = 13–15 follicles from three separate experiments; p ≤ 0.05). MOH or HPTE did not have any effect on Cyp1a2 in any of the treatment groups compared with DMSO treatment (Figs. 8B and 8C).
FIG. 8.
Effect of MXC, MOH, and HPTE on gene expression levels of Cyp1a2 (MXC metabolizing enzyme) in antral follicles of control and ESR1 OE mice. Antral follicles were cultured with MXC (1–100 μg/ml), MOH (0.1–10 μg/ml), HPTE (0.1–10 μg/ml), or the vehicle DMSO for 96 h and then subjected to q-PCR with Cyp1a2-specific primers. The relative gene expression quantities of Cyp1a2 were normalized to β-Actn. (A) MXC-treated follicles; (B) MOH-treated follicles; (C) HPTE-treated follicles. Each bar represents means ± SEM. Bars with different letters are significantly different from each other (n = 13–15 follicles per treatment from three separate experiments; p ≤ 0.05).
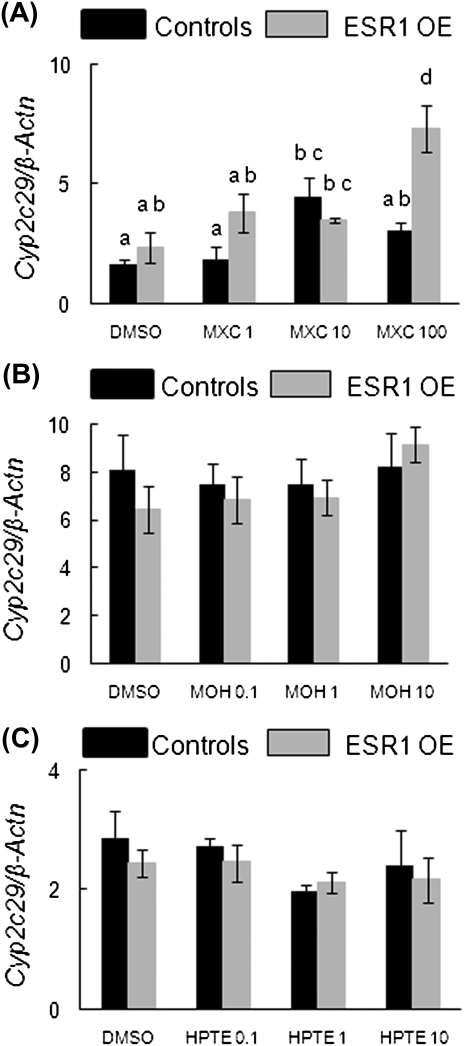
MXC treatment significantly induced Cyp2c29 in control follicles at 10 μg/ml compared with DMSO-treated follicles, whereas in ESR1 OE follicles, MXC treatment significantly induced Cyp2c29 at the 100 μg/ml concentration compared with DMSO-treated follicles. Similar to Cyp1a2, at 100 μg/ml MXC treatment, Cyp2c29 induction was significantly greater in ESR1 OE follicles compared with controls (Fig. 9A; controls: DMSO = 01.7 SQ ± 0.1; 100 μg/ml MXC = 3.1 SQ ± 0.3; ESR1 OE: DMSO = 2.36 SQ ± 0.63; 100 μg/ml MXC = 7.34 SQ ± 1; n = 13–15 follicles from three separate experiments; p ≤ 0.05). MOH or HPTE did not affect Cyp2c29 in any of the treatment groups compared with DMSO treatment (Figs. 9B and 9C).
FIG. 9.
Effect of MXC, MOH, and HPTE on gene expression levels of Cyp2c29 (MXC metabolizing enzyme) in antral follicles of control and ESR1 OE mice. Antral follicles were cultured with MXC (1–100 μg/ml), MOH (0.1–10 μg/ml), HPTE (0.1–10 μg/ml), or the vehicle DMSO for 96 h and then subjected to q-PCR with Cyp2c29-specific primers. The relative gene expression quantities of Cyp2c29 were normalized to β-Actn. (A) MXC-treated follicles; (B) MOH-treated follicles; (C) HPTE-treated follicles. Each bar represents means ± SEM. Bars with different letters are significantly different from each other (n = 13–15 follicles per treatment from three separate experiments; p ≤ 0.05).
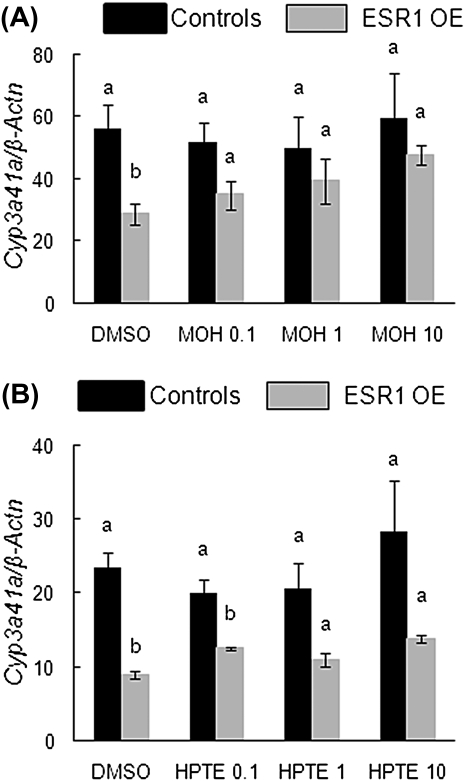
Because Cyp3a41a is involved in the metabolism of MOH and HPTE (Stresser and Kupfer, 1997), we analyzed the levels of Cyp3a41a in follicles treated with these chemicals. With MOH treatment, there was a significant induction of the Cyp3a41a gene in all treatment groups compared with DMSO treatment in ESR1 OE antral follicles. However, 1 and 10 μg/ml HPTE treatments significantly downregulated Cyp3a41a compared with DMSO and 0.1 μg/ml in the ESR1 OE antral follicles. Between the genotypes, Cyp3a41a was significantly lower in DMSO-treated follicles of ESR1 OE compared with controls (Figs. 10A, controls: DMSO = 56.2 SQ ± 7.8; ESR1 OE: DMSO = 28.9 SQ ± 3.3; Fig. 10B, controls: DMSO = 23.5 SQ ± 2.1; ESR1 OE: DMSO = 8.9 SQ ± 0.5; n = 13–15 follicles from three separate experiments; p ≤ 0.05).
FIG. 10.
Effect of MXC, MOH, and HPTE on gene expression levels of Cyp3a41a (MOH and HPTE metabolizing enzyme) in antral follicles of control and ESR1 OE mice. Antral follicles were cultured with MOH (0.1–10 μg/ml), HPTE (0.1–10 μg/ml), or the vehicle DMSO for 96 h and then subjected to q-PCR with Cyp3a41a-specific primers. The relative gene expression quantities of Cyp3a41a were normalized to β-Actn. (A) MOH-treated follicles; (B) HPTE-treated follicles. Each bar represents means ± SEM. Bars with different letters are significantly different from each other (n = 13–15 follicles per treatment from three separate experiments; p ≤ 0.05).
Gene expression of Cyp3a41a is lower in ESR1 OE livers compared to controls
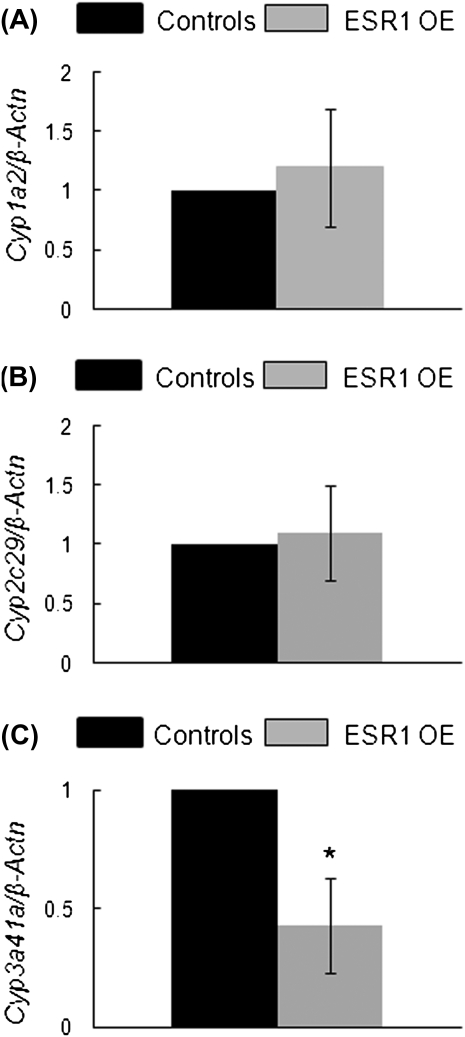
Because we found significantly low levels of Cyp3a41a in DMSO-treated follicles in vitro, we analyzed the fold change in gene expression levels of Cyp1a2, Cyp2c29, and Cyp3a41a between liver tissues of ESR1 OE and control mice. Although there was no difference in the fold-change levels of Cyp1a2 and Cyp2c29 between ESR1 OE and control livers (Figs. 11A and 11B) the fold-change level of Cyp3a41a was significantly lower in ESR1 OE livers compared with controls (Fig. 11C; controls = 1 SQ; ESR1 OE = 0.43 SQ ± 0.2; n = 5; p ≤ 0.05).
FIG. 11.
Gene expression levels of Cyp1a2, Cyp2c29, and Cyp3a41a (enzymes metabolizing MXC, MOH, and HPTE) in livers of ESR1 OE and control mice. Sections of the lower lobe of the livers from adult female ESR1 OE and control mice were isolated and subjected to real-time PCR using specific primers for Cyp1a2, Cyp2c29, and Cyp3a41a. (A) Cyp1a2; (B) Cyp2c29; (C) Cyp3a41a mRNA levels. Each bar represents means ± SEM. * Indicate significant differences from DMSO controls within genotype (n = 3; p ≤ 0.05).
DISCUSSION
Our present study shows that ESR1 OE antral follicles treated with MXC have increased growth inhibition compared with controls, indicating that ESR1 OE antral follicles are more sensitive to MXC-induced growth inhibition than controls. This is consistent with previous studies in which in vivo MXC treatment resulted in a higher percentage of atretic antral follicles in ESR1 OE mice compared with controls (Tomic et al., 2006). When we analyzed growth in follicles treated with the metabolites of MXC, we found that the lower dose of MOH caused a significant growth inhibition in ESR1 OE follicles compared with control follicles. We also found that although HPTE did not inhibit growth in control follicles, it inhibited growth in ESR1 OE antral follicles. This concurs with previous findings that MOH is more toxic to antral follicles than HPTE (Miller et al., 2006). However, ESR1 OE follicles treated with HPTE have growth inhibition by 48 h, whereas MOH-treated follicles (0.1 and 1 μg/ml) have growth inhibition only by 96 h, which suggests that ESR1 OE follicles are more sensitive to toxicity induced by HPTE than MOH. This could be because HPTE has a greater affinity for ESR1 than MXC (Gaido et al., 1999) and possibly MOH and hence can bind to the higher numbers of ESR1 in ESR1 OE follicles at a faster rate inducing toxicity at an earlier time point compared with controls. Additionally, the ratio of ESR1:ESR2 in any tissue is critical for determining the specificity and end point of ligand binding (Chang et al., 2008; Hall and McDonnell, 1999). In tissues where ESR2 is higher, such as the control antral follicles in this study, it is possible that MXC, HPTE, and MOH have a completely different effect than in tissues where ESR1 expression is higher such as ESR1 OE antral follicles of this study.
ESR1 belongs to a large superfamily of nuclear hormone receptors that share a common structural organization comprising domains that are responsible for specific functions such as ligand binding, dimerization, DNA binding, and transactivation (Murdoch and Gorski, 1991). In this study, we determined whether MXC and its metabolites cause differential gene expression of nuclear receptors Esr1, Esr2, Ar, Pr, and Ahr in ESR1 OE mice compared with controls. Our results show that, with MXC treatment, there is a dose-dependent downregulation of Esr1 in control antral follicles, whereas in ESR1 OE follicles, there is a dose-dependent induction of Esr1. As mentioned earlier, these data suggest that MXC is acting differentially in ESR1 OE and control follicles because of the ratio of ESR1 and ESR2 expressed in these tissues. In control antral follicles, ESR2 is higher because granulosa cells predominantly express ESR2, whereas theca cells predominantly express ESR1. However, in ESR1 OE follicles, ESR1 is higher than ESR2 because of the transgenic overexpression of Esr1 in the theca and interstitium of ESR1 OE ovaries. The relative levels of ESR1 and ESR2 are critical for determining the sensitivity and specificity of responses of tissues to estrogens and estrogenic chemicals such as MXC and its metabolites (Chang et al., 2008; Hall and McDonnell, 1999). Studies have indicated that various estrogenic chemicals will cause varied phenotypes in tissues that express predominantly ESR1 compared with those expressing ESR2 by differential gene transcription (Tee et al., 2004; Waters et al., 2001). Moreover, studies done on the structure activity relationships of ESRs with its ligands show that the ligand determines the specificity of coregulators binding to response elements (Tee et al., 2004). Hence, it is possible that in tissues overexpressing ESR1, MXC binds predominantly to the overexpressed ESR1 and recruiting coactivators to auto-induce gene transcription of Esr1. However, in control tissues, MXC interacts predominantly with ESR2, downregulating Esr1 levels because ESR2 is known to significantly reduce the stimulatory effect of ESR1, thus inhibiting ESR1-mediated gene transcription (Hall and McDonnell, 1999; Lindberg et al., 2003).
We also found that Esr2 mRNA levels were induced significantly in controls compared with ESR1 OE antral follicles treated with 10 μg/ml MXC, but not 100 μg/ml MXC. Studies have shown that the ability of ESR2 to function as a transcriptional inhibitor or activator depends on the agonist concentration, implying that, at various concentrations, there may be completely different patterns of gene expression (Hall and McDonnell, 1999). It is possible that at 100 μg/ml, the binding of MXC to ESR2 results in an unstable ESR2 dimer–DNA complex that results in inhibition of gene transcription, whereas at 10 μg/ml, MXC results in Esr2 gene transcription. Additionally, studies have shown that estrogenic chemicals exhibit differential effects on the two ESRs because of the differences in recruiting coactivators (Kuiper et al., 1998). ESR2 has been shown to retain a better ability to associate with co-activators when bound to xenoestrogens than when bound to estradiol (Kuiper et al., 1998; Lemaire et al., 2006), thus leading to increased gene transcription when exposed to environmental estrogens such as MXC. In our study, because control follicles have higher levels of ESR2 than ESR1, MXC could be binding predominantly to ESR2 and causing an increase in gene transcription resulting in higher levels of Esr2 mRNA. Our results showing that HPTE induces Esr2 expression in controls and ESR1 OE antral follicles are consistent with previous findings showing that HPTE treatment induces Esr2 levels in the ovary attributing it to an estrogen-like response (Waters et al., 2001). We think that MOH induces Esr2 in ESR1 OE antral follicles in a similar way.
The dose-dependent increase in Ar expression levels in ESR1 OE and control follicles could be because MXC can bind to AR (Gaido et al., 2000), and may be inducing gene transcription increasingly with dose. Interestingly, 100 μg/ml MXC induced Ar significantly in ESR1 OE antral follicles compared with controls. This could be because in ESR1 OE antral follicles treated with 100 μg/ml MXC, there is also a considerable induction of Esr1 that could possibly result in cross talk and subsequent higher expression of Ar in ESR1 OE follicles compared with control follicles.
In ESR1 OE antral follicles, MXC treatment does not cause an alteration in Pr expression levels, whereas in control antral follicles, there is a significant induction of Pr at higher concentrations of MXC. It is possible that MXC may be directly binding to PR and inducing gene transcription at higher concentrations (Scippo et al., 2004). This may also explain the low levels of Esr1 and Esr2 mRNA at 100 μg/ml MXC in control antral follicles because there is evidence that activation of PR has an inhibitory effect on gene transcription by ESRs (Kraus et al., 1995).
Both MOH and HPTE downregulate Ahr at their highest dose (10 μg/ml) in control and ESR1 OE antral follicles. There is substantial evidence of cross talk between ESRs and AhR in various tissues including the ovary (Safe and Wormke, 2003). Studies have shown that 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced activation of AhR results in down-regulation of ESRs (Krishnan et al., 1995). Hence, it is possible that the upregulation of Esr2 by MOH and HPTE may be downregulating Ahr in control and ESR1 OE antral follicles.
Previous studies have shown that MXC treatment alters various CYP450 enzymes including Cyp1a2 and Cyp2c29 in the liver and ovary (Oropeza-Hernandez et al., 2003; Symonds et al., 2006). Moreover, ESR1 expression can also regulate certain CYP450 enzymes (Mwinyi et al., 2010) and hence it is possible that overexpression of ESR1 in ESR1 OE antral follicles is altering gene transcription of Cyp1a2 and Cyp2c29 as well. Interestingly, when we analyzed Cyp3a41a, which is the enzyme responsible for metabolizing MOH and HPTE in ESR1 OE and control follicles treated with the chemicals, we observed a significant decrease of Cyp3a41a enzyme in DMSO-treated ESR1 OE follicles compared with control follicles. This led us to analyze the levels of the metabolizing enzymes in the liver of adult cycling ESR1 OE and control female mice. Although there were no changes in gene expression levels of Cyp1a2 and Cyp2c29 in the liver, we observed a significant decrease in Cyp3a41a mRNA in ESR1 OE antral follicles but not in control follicles. This suggests that the metabolism of MOH and HPTE in ESR1 OE mice is slower than controls, which could also explain the increased sensitivity of ESR1 OE mouse ovaries to MXC compared with controls (Tomic et al., 2006).
In conclusion, several factors play a role in the interaction of ESR1 and ESR2 with different ligands and response elements as well as recruitment of distinct co-regulators, all of which collectively account for the differences in gene expression in control and ESR1 OE ovaries (Sanchez et al., 2002). Our present study shows that MXC, HPTE, and MOH affect growth and gene transcription differentially in control and ESR1 OE antral follicles. Furthermore, our findings show that MXC, which is less estrogenic than HPTE and MOH (Gaido et al., 2000) causes dramatic changes in gene transcription compared to the metabolites. Collectively, these results suggest that the levels of ESR1 in the ovary are vital for determining the response of the ovaries to environmental chemicals. Any disruption in the equilibrium between ESR1 and ESR2 in the ovaries may result in a considerable change in the way the ovaries respond to estrogenic chemicals. This differential gene expression may account for the increased sensitivity of ESR1 OE antral follicles to toxicity induced by MXC and its metabolites.
FUNDING
National Institute of Environmental Health Sciences (R21ES13061, R01ES012893, and R01ES019178 to J.A.F.); Eli Lilly Fellowship in Toxicology/Pharmacology for graduate students (T.P.).
Acknowledgments
The authors thank Dr Zelieann R. Craig, Bethany Karman, Dr Rupesh Gupta, Liying Gao, and Sharon Meachum for technical help and thank Lalji Gedia (Dr Vincent Njar laboratory) for the synthesis of MOH.
References
- Barnett KR, Tomic D, Gupta RK, Miller KP, Meachum S, Paulose T, Flaws JA. The aryl hydrocarbon receptor affects mouse ovarian follicle growth via mechanisms involving estradiol regulation and responsiveness. Biol. Reprod. 2007;76:1062–1070. doi: 10.1095/biolreprod.106.057687. [DOI] [PubMed] [Google Scholar]
- Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicol. Sci. 2002;68:473–478. doi: 10.1093/toxsci/68.2.473. [DOI] [PubMed] [Google Scholar]
- Bulger WH, Muccitelli RM, Kupfer D. Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem. Pharmacol. 1978;27:2417–2423. doi: 10.1016/0006-2952(78)90354-4. [DOI] [PubMed] [Google Scholar]
- Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen receptors alpha and beta as determinants of gene expression: Influence of ligand, dose, and chromatin binding. Mol. Endocrinol. 2008;22:1032–1043. doi: 10.1210/me.2007-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Davis VL, Hanson RB, Jefferson WN, McLachlan JA, Bullock BC, Newbold RR, Korach KS. Accelerated onset of uterine tumors in transgenic mice with aberrant expression of the estrogen receptor after neonatal exposure to diethylstilbestrol. Mol. Carcinog. 1997a;19:236–242. doi: 10.1002/(sici)1098-2744(199708)19:4<236::aid-mc4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor alpha and estrogen receptor beta messenger ribonucleic acid in the wild-type and ER alpha knockout mouse. Endocrinology. 1997b;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- Cummings AM. Methoxychlor as a model for environmental estrogens. Crit. Rev. Toxicol. 1997;27:367–379. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- Danzo BJ. Environmental xenobiotics may disrupt normal endocrine function by interfering with the binding of physiological ligands to steroid receptors and binding proteins. Environ. Health Perspect. 1997;105:294–301. doi: 10.1289/ehp.97105294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaido KW, Leonard LS, Maness SC, Hall JM, McDonnell DP, Saville B, Safe S. Differential interaction of the methoxychlor metabolite 2,2-Bis-(p-hydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors α and β. Endocrinology. 1999;140:5746–5753. doi: 10.1210/endo.140.12.7191. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. Interaction of methoxychlor and related compounds with estrogen receptor α and β, and androgen receptor: structure-activity studies. Mol. Pharmacol. 2000;58:852–858. [PubMed] [Google Scholar]
- Golovleva LA, Polyakova AB, Pertsova RN, Finkelshtein ZI. The fate of methoxychlor in soils and transformation by soil microorganisms. J. Environ. Health Sci. B. 1984;19:523–538. doi: 10.1080/03601238409372448. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby JS, Ferrell JM, Sigmon CR, Goldman JM. Methoxychlor induces estrogen-like alterations of behavior and the reproductive tract in the female rat and hamster: effects on sex behavior, running wheel activity, and uterine morphology. Tox. Appl. Pharm. 1988;96:525–540. doi: 10.1016/0041-008x(88)90012-9. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol. Sci. 2006;93:382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor b-isoform (ERb) of the human estrogen receptor modulates ERa transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Hirshfield AN. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991;124:43–101. doi: 10.1016/s0074-7696(08)61524-7. [DOI] [PubMed] [Google Scholar]
- Hruska KS, Tilli MT, Ren S, Cotarla I, Kwong T, Li M, Fondell JD, Hewitt JA, Koos RD, Furth PA, et al. Conditional over-expression of estrogen receptor alpha in a transgenic mouse model. Transgenic Res. 2002;11:361–372. doi: 10.1023/a:1016376100186. [DOI] [PubMed] [Google Scholar]
- Hsueh AJ, Adashi EY, Jones PB, Welsh TH., Jr Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr. Rev. 1984;5:76–127. doi: 10.1210/edrv-5-1-76. [DOI] [PubMed] [Google Scholar]
- Kraus WL, Weis KE, Katzenellenbogen BS. Inhibitory cross-talk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist- and antagonist-occupied progestin receptors. Mol. Cell. Biol. 1995;15:1847–1857. doi: 10.1128/mcb.15.4.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Porter W, Santostefano M, Wang X, Safe S. Molecular mechanism of inhibition of estrogen-induced cathepsin D gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in MCF-7 cells. Mol. Cell. Biol. 1995;15:6710–6719. doi: 10.1128/mcb.15.12.6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper G, Lemmen JG, Carlsson B, Corton JC, Safe S, Van Der Saag PT, Van Der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor b. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lemaire G, Mnif W, Mauvais P, Balaguer P, Rahmani R. Activation of a and b estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. 2006;79:1160–1169. doi: 10.1016/j.lfs.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor (ER)-beta reduces ER(alpha)-regulated gene transcription supporting a “Ying-Yang” relationship between ER alpha and ER beta in mice. Mol. Endocrinol. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: A subtle balance between ER alpha and ER beta. Mol. Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Flaws JA. Methoxychlor metabolites may cause ovarian toxicity through estrogen-regulated pathwats. Toxicol. Sci. 2006;93:180–188. doi: 10.1093/toxsci/kfl034. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol. Sci. 2005;88:213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- Murdoch FE, Gorski J. The role of ligand in estrogen receptor regulation of gene expression. Mol. Cell. Endocrinol. 1991;78:C103–C108. doi: 10.1016/0303-7207(91)90114-8. [DOI] [PubMed] [Google Scholar]
- Mwinyi J, Cavaco I, Pederson RS, Persson A, Burkhardt S, Mkrtchian S, Ingelman-Sundberg M. Regulation of CYP2C19 expression by estrogen receptor alpha: implications for estrogen-dependent inhibition of drug metabolism. Mol. Pharmacol. 2010;78:886–894. doi: 10.1124/mol.110.065540. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod. Toxicol. 2007;24:253–258. doi: 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza-Hernandez LF, Lopez-Romero R, Albores A. Hepatic CYP1A, 2B, 2C, 2E and 3A regulation by methoxychlor in male and female rats. Toxicol. Lett. 2003;144:93–103. doi: 10.1016/s0378-4274(03)00230-3. [DOI] [PubMed] [Google Scholar]
- Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem. Res. Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- Sanchez R, Nguyen D, Rocha W, White JH, Mader S. Diversity in the mechanism of gene regulation by estrogen receptors. Bioessays. 2002;24:244–254. doi: 10.1002/bies.10066. [DOI] [PubMed] [Google Scholar]
- Scippo M-L, Argiris C, Van de Weerdt C, Muller M, Willemsen P, Martial J, Maghuin-Rogister G. Recombinant human estrogen, androgen and progesterone receptors for detection of potential endocrine disruptors. Anal. Bioanal. Chem. 2004;378:664–669. doi: 10.1007/s00216-003-2251-0. [DOI] [PubMed] [Google Scholar]
- Shockett P, Difilippantonio M, Hellman N, Schatz DG. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc. Natl Acad. Sci. U S A. 1995;92:6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stresser DM, Kupfer D. Catalytic characteristics of CYP3A4: requirement for a phenolic function in ortho hydroxylation of estradiol and mono-O-demethylated methoxychlor. Biochemistry. 1997;36:2203–2210. doi: 10.1021/bi962129k. [DOI] [PubMed] [Google Scholar]
- Stresser DM, Kupfer D. Human cytochrome P450-catalyzed conversion of the proestrogenic pesticide methoxychlor into an estrogen. Role of CYP2C19 and CYP1A2 in O-demethylation. Drug Metab. Dispos. 1998;26:868–875. [PubMed] [Google Scholar]
- Symonds DA, Miller KP, Tomic D, Flaws JA. Effect of methoxychlor and estradiol on cytochrome p450 enzymes in the mouse ovarian surface epithelium. Toxicol. Sci. 2006;89:510–514. doi: 10.1093/toxsci/kfj044. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sasaki M, Kaneuchi M, Fujimoto S, Dahiya R. Estrogen receptor alpha polymorphisms and renal cell carcinoma - a possible risk. Mol. Cell. Endocrinol. 2003;202:109–116. doi: 10.1016/s0303-7207(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Tee MK, Rogatsky I, Tzagaraski-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol. Biol. Cell. 2004;15:1262–1272. doi: 10.1091/mbc.E03-06-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic D, Frech MS, Babus JK, Gupta RK, Furth PA, Flaws JA. Methoxychlor induces atresia of antral follicles in ERalpha-overexpressing mice. Toxicol. Sci. 2006;93:196–204. doi: 10.1093/toxsci/kfl040. [DOI] [PubMed] [Google Scholar]
- Tomic D, Frech MS, Babus JK, Symonds D, Furth PA, Koos RD, Flaws JA. Effects of ERalpha overexpression on female reproduction in mice. Reprod. Toxicol. 2007;23:317–325. doi: 10.1016/j.reprotox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Waters KM, Safe S, Gaido KW. Differential gene expression in response to methoxychlor and estradiol through ERα, ERβ, and AR in reproductive tissues of female mice. Toxicol. Sci. 2001;63:47–56. doi: 10.1093/toxsci/63.1.47. [DOI] [PubMed] [Google Scholar]