Abstract
Inhibition of bile acid (BA) transport may contribute to the hepatotoxicity of troglitazone (TRO), a peroxisome proliferator–activated receptor gamma agonist. Typically, studies use taurocholic acid (TCA) as a model substrate to investigate effects of xenobiotics on BA disposition. However, TRO may differentially affect the transport of individual BAs, potentially causing hepatocyte accumulation of more cytotoxic BAs. The effects of TRO on the disposition of [14C]-labeled chenodeoxycholic acid ([14C]CDCA), an unconjugated cytotoxic BA, were determined in suspended hepatocytes and sandwich-cultured hepatocytes (SCH) from rats. (E)-3-[[[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl][[3-(dimethylamino)-3-oxopropyl]thio]methyl]thio]-propanoic acid (MK571), a multidrug resistance–associated protein (MRP) inhibitor, was included to evaluate involvement of MRPs in CDCA disposition. Accumulation in cells + bile of total [14C]CDCA species in SCH was sixfold greater than [3H]TCA and unaffected by 1 and 10μM TRO; 100μM TRO and 50μM MK571 ablated biliary excretion and significantly increased intracellular accumulation of total [14C]CDCA species. Results were similar in Mrp2-deficient TR− rat hepatocytes. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis revealed that taurine- and glycine-conjugated CDCA, in addition to unconjugated CDCA, accumulated in hepatocytes during the 10-min incubation. In suspended rat hepatocytes, initial [14C]CDCA uptake was primarily Na+-independent, whereas initial [3H]TCA uptake was primarily Na+-dependent; TRO and MK571 decreased [14C]CDCA uptake to a lesser extent than [3H]TCA. Unexpectedly, MK571 inhibited Na+-taurocholate cotransporting polypeptide and bile salt export pump. Differential effects on uptake and efflux of individual BAs may contribute to TRO hepatotoxicity. Although TCA is the prototypic BA used to investigate the effects of xenobiotics on BA transport, it may not be reflective of other BAs.
Keywords: troglitazone, bile acids, hepatotoxicity, transport, sandwich-cultured hepatocytes
Troglitazone (TRO) is a peroxisome proliferator–activated receptor gamma agonist used clinically to treat noninsulin-dependent type II diabetes until it was removed from the market following a number of cases of severe idiosyncratic hepapotoxicity. Much research has focused on determining the mechanisms of TRO-mediated hepatotoxicity. One hypothesis is that TRO inhibits hepatic bile acid (BA) transport; inhibition of the bile salt export pump (BSEP) may cause intracellular accumulation of BAs (reviewed in Masubuchi, 2006) and subsequent toxicity due to detergent effects (Delzenne et al., 1992; Pauli-Magnus et al., 2005). Impaired BSEP function is a significant medical issue (reviewed in Pauli-Magnus et al., 2010); however, drug-mediated inhibition of hepatic BA transport as a mechanism of drug-induced liver injury is poorly understood.
Normally, BA disposition is tightly controlled, and hepatic BA concentrations are governed by the rate of synthesis and by basolateral (sinusoidal) and apical (canalicular) transport proteins. On the basolateral membrane, the Na+-taurocholate cotransporting polypeptide (NTCP) mediates Na+-dependent uptake of BAs, whereas organic anion transporting polypeptide (OATP) isoforms mediate Na+-independent uptake of BAs, organic anions, some organic cations, and neutral species (Trauner and Boyer, 2003). On the apical membrane, BSEP is the major canalicular transport protein that mediates biliary excretion of monomeric BAs, whereas multidrug resistance–associated protein (MRP) 2 (MRP2/Mrp2) transports divalent BAs into the bile canaliculi (Kullak-Ublick et al., 2000). Together, NTCP and BSEP represent the major transport proteins responsible for the vectorial transport of BAs from blood to bile. The basolateral efflux transporters, MRP3, MRP4, and the organic solute transporter, OSTα/β, also may be involved in the excretion of BAs from hepatocytes back into the blood under cholestatic conditions (reviewed in Borst et al., 2007; Soroka et al., 2010).
Taurocholic acid (TCA) is the taurine conjugate of the primary BA cholic acid (CA) that is present in both rats and humans. TCA is utilized commonly as a prototypic BA to study the effect of xenobiotics and drugs, including TRO, on BA transport both in vivo and in vitro. TRO caused the accumulation of [14C]TCA in rat liver tissue (Funk et al., 2001b) and inhibited Bsep-mediated TCA transport in rat canalicular membrane vesicles (Funk et al., 2001a) and in membrane vesicles from Sf9 cells overexpressing Bsep from different species (Kis et al., 2009). TRO inhibited TCA uptake and biliary excretion in primary rat sandwich-cultured hepatocytes (SCH) (Kemp et al., 2005) and inhibited TCA transport in both basolateral and canalicular rat liver membrane vesicles (Snow and Moseley, 2006). Studies by our group in human SCH showed that TRO decreased TCA biliary excretion in a concentration-dependent manner, consistent with BSEP inhibition; Na+-dependent initial uptake of TCA also was inhibited in rat and human suspended hepatocytes (Marion et al., 2007). TRO also caused intracellular retention of preloaded [3H]TCA in human hepatocytes (Jemnitz et al., 2010).
BAs perform important physiological roles but are cytotoxic and may cause mitochondrial dysfunction and trigger apoptosis or necrosis if they accumulate to high intracellular concentrations. The hydrophobicity of individual BAs is inversely proportional to the number and orientation of hydroxyl groups on the steroid nucleus and is predictive of toxicity (reviewed in Thomas et al., 2008). Individual BAs also have different affinities for some BA transporters (Byrne et al., 2002; Gerloff et al., 1998; Noe et al., 2001); thus, perturbation of BA transport resulting in increased intracellular BAs may cause a disproportionate accumulation of more cytotoxic BAs through competition for transport.
The primary BA chenodeoxycholic acid (CDCA) comprises an estimated 10.5% (Tagliacozzi et al., 2003) to 37% (McRae et al., 2010) of the BAs in human plasma compared with 3% (Tagliacozzi et al., 2003) to 3.6% (McRae et al., 2010) for TCA. CDCA is more cytotoxic to hepatocytes than TCA (Miyazaki et al., 1984), and concentrations in human liver can reportedly increase 20-fold following extrahepatic biliary obstruction (Greim et al., 1973). Hepatic uptake of CDCA reportedly involves both Na+-dependent (NTCP-mediated) and Na+-independent (i.e., OATP1B1 and OATP1B3) transport mechanisms (Maglova et al., 1995; Van Dyke et al., 1982); OATP1B1 and OATP1B3 also can transport a fluorescent CDCA analog (Yamaguchi et al., 2006). The present study compared the effects of TRO on the disposition of CDCA versus TCA in rat SCH and suspended hepatocytes. Experiments were designed to determine whether TRO differentially affects the hepatobiliary disposition of individual BAs resulting in greater intracellular accumulation of CDCA compared with TCA, and to examine the mechanism(s) of potential alterations. (E)-3-[[[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl][[3-(dimethylamino)-3-oxopropyl]thio]methyl]thio]-propanoic acid (MK571), an MRP inhibitor, was included to investigate the potential involvement of MRPs in CDCA and TCA hepatobiliary disposition.
MATERIALS AND METHODS
Chemicals.
[14C]CDCA (50 mCi/mmol; purity >97%) and [14C]inulin (2.8 mCi/g; purity >97%) were purchased from American Radiolabeled Chemicals, Inc. (St Louis, MO), and [3H]TCA (5 Ci/mmol; purity >97%) was purchased from Perkin Elmer (Waltham, MA). TRO was purchased from Biomol (Plymouth Meeting, PA). MK571 sodium salt was obtained from Cayman Chemical (Ann Arbor, MI). Dexamethasone, Hanks’ balanced salt solution (HBSS) premix, and HBSS modified (without calcium chloride, magnesium sulfate, phenol red, and sodium bicarbonate) premix were purchased from Sigma-Aldrich (St Louis, MO). Collagenase (type I, class I) was obtained from Worthington Biochemical (Freehold, NJ), and dimethyl sulfoxide (DMSO) was purchased from Fisher Scientific (Fairlawn, NJ). GIBCO brand fetal bovine serum, recombinant human insulin, and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Invitrogen (Carlsbad, CA). ITS (insulin, transferrin, and selenium) Universal Culture Supplement Premix and Matrigel Basement Membrane Matrix were obtained from BD Biosciences (Palo Alto, CA). All other chemicals and reagents were of analytical grade and were readily available from commercial sources.
Hepatocyte isolation and culture.
Hepatocytes were isolated from wild-type (WT) male Wistar rats (250–300 g; Charles River Laboratories, Inc., Raleigh, NC) or male Mrp2-deficient TR− rats (250–300 g; in-house colony) using a two-step collagenase perfusion method previously described (LeCluyse et al., 1996). Rats were maintained on a 12-h light/dark cycle with free access to water and standard rodent chow and allowed to acclimate for at least 5 days before experimentation. The Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill approved all procedures.
Hepatocytes were seeded at a density of 1.75 × 106 cells per well on six-well BioCoat plates with collagen type I substratum in 1.5 ml DMEM supplemented with 5% (vol/vol) fetal bovine serum, 10μM insulin, 1μM dexamethasone, 2mM L-glutamine, 1% (vol/vol) Minimum Essential Medium (MEM) nonessential amino acids, 100 U penicillin G sodium, and 100 μg streptomycin sulfate. Cells were incubated at 37°C in a humidified incubator and allowed to attach for 2 h, after which time the medium was aspirated to remove unattached cells and replaced with fresh medium. Twenty-four hours later, on day 1 of culture, hepatocytes were overlaid with BD Matrigel basement membrane matrix at a concentration of 0.25 mg/ml in ice-cold DMEM supplemented with 1% (vol/vol) ITS + premix, 0.1μM dexamethasone, 2mM l-glutamine, 1% (vol/vol) MEM nonessential amino acids, 100 U penicillin G sodium, and 100 μg streptomycin sulfate. Cells were cultured for three additional days to allow for the formation of canalicular networks between cells. Culture medium was replaced daily.
Accumulation of [14C]CDCA and [3H]TCA in WT and TR− rat SCH.
On day 4 of culture, hepatocytes were rinsed three times (20 s per each rinse) with 2 ml per well of warm standard HBSS with Ca2+ (three wells) or HBSS without Ca2+ (three wells). Following the washes, 2 ml of warm HBSS with or without Ca2+ were added, and cells were incubated at 37°C for 10 min. Incubation of SCH with Ca2+-containing HBSS maintains the tight junctions between cells, and the bile canalicular structures formed between cells remain intact. Incubation of cells in Ca2+-free HBSS disrupts the tight junctions, allowing the contents of the canaliculi to be washed away. After incubation, the HBSS was double aspirated from each well and 1.5 ml of dosing solution consisting of HBSS with Ca2+, substrate (1μM unlabeled CDCA plus 0.2μM [14C]CDCA or 1μM TCA plus trace [3H]TCA), or inhibitor (specified concentrations of TRO or MK571 or vehicle [0.1% (vol/vol) DMSO]) were added. Cells were incubated at 37°C for 10 min. Following incubation, the dosing solution was aspirated from the cells, and uptake was stopped by rinsing cells three times for 20 s each with 2 ml ice-cold HBSS with Ca2+ per wash. After washing, the HBSS was aspirated and 1 ml of lysis buffer (0.5% [vol/vol] Triton X-100 in PBS) was added to each well, and plates were shaken on a rotating plate shaker for 20 min. Aliquots (500 μl) of sample and 100 μl aliquots of dosing solution were collected for quantification of radioactivity by liquid scintillation counting; 500 μl aliquots were reserved for protein quantification using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Accumulation of [14C]CDCA and [3H]TCA in BioCoat plates without cells was subtracted to correct for nonspecific binding to the collagen substratum.
Temperature-dependent accumulation of [14C]CDCA and [3H]TCA in WT rat SCH.
Accumulation in WT rat SCH of [14C]CDCA and [3H]TCA in the presence of vehicle was measured at 37°C using the above method. Accumulation at 4°C was carried out using the same method, except that all reagents were kept at 4°C, and all incubations were performed on ice.
Measurement of taurine- and glycine-conjugated CDCA species in WT rat SCH.
In order to determine the extent of CDCA metabolism to taurine or glycine conjugates (TCDCA or GCDCA, respectively) during the 10-min incubation in the accumulation studies, WT rat SCH were treated using the above method for measuring accumulation of CDCA at 37°C, except that the dosing solution contained either vehicle (0.1% DMSO) or 1μM unlabeled CDCA only. Following the final washes, wells were aspirated and plates were stored at −70°C until analysis by liquid chromatography-tandem mass spectrometry (LC-MS/MS) for TCDCA and GCDCA, as well as for the rodent-specific BAs tauromuricholic acid (TMCA) and glycomuricholic acid (GMCA), which are metabolites of CDCA. CDCA, TCDCA, and GCDCA were measured using standard curves prepared with stable isotope equivalents; TMCA and GMCA were estimated from standard curves for TCA and glycocholic acid (GCA). Ten microliters of d4-TCDCA, d4-GCDCA, d8-TCA, and d4-GCA solutions in methanol were added to previously frozen untreated rat SCH plates to yield a final concentration of 0.5–100 pmol per well (0.5–200 pmol per well for TCA) as standards. Lysis solution (750 μl; 70:30 [vol/vol] methanol:water containing 19 pmol per well d4 TCA as an internal standard) was added to each well of study plates and to the plates containing standards. Plates were shaken on a rotating plate shaker at a speed of 500 rpm for 15 min. The total contact time of the lysis solution with cells, prior to filtration, was ∼20 to 30 min. The cell lysates were transferred to a Whatman 96-well Unifilter Plate (Whatman, Florham Park, NJ) with 25 μm melt blown polypropylene over 0.45 μm polypropylene membrane. Lysate was filtered into a Greiner 96-well Deepwell Plate by centrifugation at 2000 × g for 5 min. Filtrate was evaporated to dryness under nitrogen gas, reconstituted in 200 μl of sample diluent (60:40 [vol/vol] methanol:10mM ammonium acetate [native pH]), and mixed for 15 min on the plate shaker at 500 rpm. The reconstituted samples were transferred to a Whatman 96-well Unifilter Plate with 0.45 μm PVDF (polyvinylidene fluoride) membrane and collected into a Costar 3956 96-well plate (Corning, Corning, NY) by centrifugation at 2000 × g for 5 min. The 96-well plate was sealed with a silicone capmat prior to LC-MS/MS analysis. Liquid handling during these procedures was accomplished using a Hamilton Microlab STAR liquid handling workstation and Tomtec Quadra 96 320 96-well simultaneous pipetting workstation.
LC-MS/MS analysis.
Chromatographic separation of a 10 μl sample injection volume was accomplished using a Shimadzu binary high-performance liquid chromatography system (Columbia, MD) incorporating LC-10ADvp pumps, a CTO-10Avp oven, a Shimadzu HTc 96-well autosampler, and a Thermo Scientific Hypersil GOLD C18 column (100 × 1.0 mm, 3 μm) with matching guard and precolumn filter. The mobile phase was initially 70% [60% 0.5mM ammonium acetate (native pH):40% methanol]:30% [20% 0.5mM ammonium acetate (native pH):80% methanol]. From 2–15 min, the gradient was ramped to 100% [20% 0.5mM ammonium acetate (native pH):80% methanol] and then stepped back to initial conditions [60% 0.5mM ammonium acetate (native pH):40% methanol] over 1 min. The flow rate through the column was 50 μl/min, and the column was maintained at 35°C. The autosampler was maintained at 4°C and rinsed with 1500 μl of 50:50 (vol/vol) methanol:water after aspiration. Methanol (100%; 10 μl/min) was added as a postcolumn solvent to the MS. A Thermo Electron TSQ Quantum Discovery MAX (Thermo Fisher Scientific) with an Ion Max ESI source in negative ion electrospray ionization mode was used for tandem mass spectrometry. The scan type was selected reaction monitoring. The transitions monitored at unit resolution are listed in Table 1.
TABLE 1.
Transitions Monitored at Unit Resolution for LC-MS/MS Analysis of Parent CDCA and Taurine- and Glycine-Conjugated CDCA Metabolites in Cell Lysates from WT Rat SCH Following a 10-min Incubation with 1μM CDCA
| Analyte | Molecular weight | Salt | Retention time (min) | Precursor m/z | Product m/z | Calibration curve range |
| TCA | 515.7 | None | 5.7 | 514 | 124 | n/a |
| d4-TCA | 519.7 | None | 5.7 | 514 | 124 | Internal standard |
| d8-TCA | 545.73 | Na+ | 5.7 | 522 | 128 | 0.5–200 pmol per well |
| GCA | 465.62 | None | 5.8 | 464 | 74 | n/a |
| d4-GCA | 469.65 | None | 5.8 | 468 | 74 | 0.5–100 pmol per well |
| TCDCA | 499.7 | None | 8.1 | 498 | 80 | n/a |
| d4-TCDCA | 503.73 | None | 8.1 | 502 | 80 | 0.5–100 pmol per well |
| GCDCA | 449.62 | None | 8.1 | 448 | 74 | n/a |
| d4-GCDCA | 453.65 | None | 8.1 | 452 | 74 | 0.5–100 pmol per well |
| CDCA | 392.57 | None | 10.2 | 5–1000 pmol per well |
Initial uptake of [14C]CDCA and [3H]TCA in WT rat suspended hepatocytes.
The initial uptake of substrate (0.5μM unlabeled CDCA plus 0.5μM [14C]CDCA, 25 nCi/ml, or 1μM TCA plus trace [3H]TCA, 60 nCi/ml) in WT rat suspended hepatocytes was measured in the presence of vehicle, 10μM TRO, or 50μM MK571 using standard methods (Leslie et al., 2007) with modifications. Uptake was performed in Na+-containing buffer to measure total uptake (Na+-dependent and Na+-independent) and in Na+-free choline-containing buffer to measure Na+-independent uptake. Na+-dependent uptake was calculated as the difference in uptake between the two conditions. The viability of freshly isolated hepatocytes was >90% as measured by trypan blue exclusion. Briefly, cells were washed 2× in ice-cold buffer containing sodium chloride or in buffer in which choline chloride was substituted for sodium chloride (137mM NaCl or choline chloride, 0.8mM MgSO4, 10mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 1.2mM CaSO4, 0.86mM K2HPO4, 0.14mM KH2PO4, and 5mM glucose, pH 7.4). Cells were resuspended at 1.0 × 106 cells/ml in the same buffer and kept on ice for immediate use. Aliquots of cells (4 ml) in bottom-inverted Erlenmeyer flasks were preincubated at 37°C in a shaking water bath for 5 min. Vehicle (0.3% DMSO), 10μM TRO, or 50μM MK571 was added 15 s prior to the addition of [14C]CDCA or [3H]TCA. At 15, 30, and 45 s, 200 μl samples of the cell suspension were removed, placed in a 0.4 ml polyethylene tube over a top layer of silicone oil:mineral oil (82:18 [vol/vol], 100 μl) and a bottom layer of 3M KOH (50 μl), and immediately centrifuged. Radioactivity in the cell pellet and in the supernatant was measured by liquid scintillation counting. Adherent fluid volume was determined by incubating cells with [14C]inulin (60 nCi/ml) using the method of Baur et al. (1975). Uptake was normalized to protein concentrations in the incubation mixtures as measured at the end of each experiment using the BCA assay (Pierce Biotechnology, Inc., Rockford, IL).
Data analysis.
The biliary excretion index (BEI), which represents the percentage of accumulated substrate that is excreted into bile canaliculi, was calculated using B-CLEAR technology (Qualyst, Inc., Durham, NC) from the following equation: BEI = [(Accumulationstandard buffer−AccumulationCalcium-free buffer)/Accumulation standard buffer] × 100% (Liu et al., 1999). Statistical analysis (one-way ANOVA and Dunnett’s multiple comparison test or two-way ANOVA with Bonferroni’s multiple comparison test) was performed using GraphPad Prism 5.03. In all cases, a p value <0.05 was considered statistically significant.
RESULTS
Accumulation of [14C]CDCA Species in WT and TR− Rat SCH
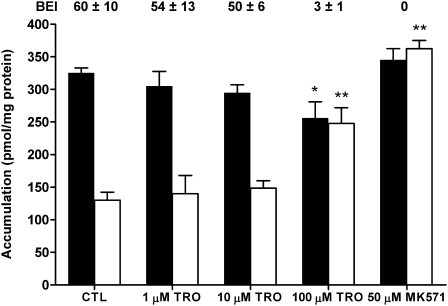
Accumulation of [14C]CDCA species in cells + bile and cells was compared in WT and TR− rat SCH, respectively, following a 10-min coincubation with 1.2μM [14C]CDCA and vehicle control (CTL), increasing concentrations of TRO (1–100μM) or 50μM MK571. In WT rat SCH, 1 and 10μM TRO had no significant effect on accumulation of [14C]CDCA species in cells + bile or cells compared with CTL, but 100μM TRO significantly decreased cell + bile accumulation, increased cellular accumulation nearly twofold compared with CTL, and markedly inhibited the biliary excretion of [14C]CDCA species; the BEI was reduced from ∼60 to 3% (Fig. 1). MK571 completely inhibited the biliary excretion and significantly increased cellular accumulation of [14C]CDCA species 2.8-fold over CTL.
FIG. 1.
Accumulation of [14C]CDCA species in cells + bile (black bars) or cells (white bars) in WT rat SCH following a 10-min incubation with 1μM [14C]CDCA or vehicle control (0.1% DMSO; CTL), 1, 10, or 100μM TRO, or 50μM MK571. The BEI was calculated as described in “Materials and Methods” section. Data represent the mean ± SE of triplicate determinations in at least n = 3 livers; *p < 0.05 versus CTL cells + bile; **p < 0.05 versus CTL cells.
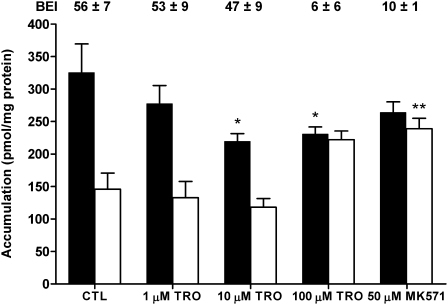
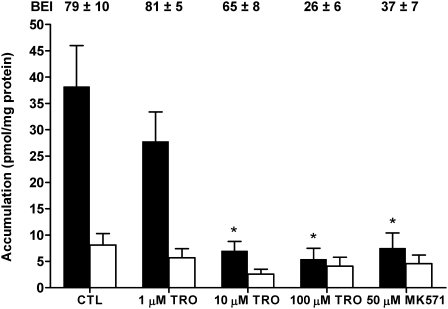
Accumulation of [14C]CDCA species and [3H]TCA also was measured in TR− rat SCH to determine whether loss of Mrp2 altered the biliary excretion of [14C]CDCA species. Accumulation of [14C]CDCA species in CTL TR− cells + bile and cells (Fig. 2) was similar to WT CTL values (Fig. 1). TRO (10 and 100μM) significantly decreased cells + bile accumulation of [14C]CDCA species. Cellular accumulation of [14C]CDCA species was notably increased over CTL in the presence of 100μMTRO and 50μM MK571, and BEI values decreased from ∼56 in CTL to ∼6% and ∼10%, respectively, consistent with inhibition of the biliary excretion of [14C]CDCA species. For comparison, TCA accumulation also was measured in TR− SCH (Fig. 3). [3H]TCA accumulation in CTL cells + bile was ∼8.5-fold lower than the accumulation of [14C]CDCA species in cells + bile of TR− rat SCH, similar to differences in [14C]CDCA accumulation (Fig. 1) and [3H]TCA accumulation published previously (Marion et al., 2007) in WT rat SCH. In contrast to [14C]CDCA species, both 10 and 100μM TRO, as well as 50μM MK571, significantly decreased cells + bile accumulation of [3H]TCA; although there was a trend toward decreased cellular accumulation of TCA in TR− rat SCH, the differences were not statistically significant.
FIG. 2.
Accumulation of [14C]CDCA species in cells + bile (black bars) or cells (white bars) in TR− rat SCH following a 10-min incubation with 1.2μM [14C]CDCA or vehicle control (0.1% DMSO; CTL), 1, 10, or 100μM TRO, or 50μM MK571. The BEI was calculated as described in “Materials and Methods” section. Data represent the mean ± SE of triplicate determinations in at least n = 3 livers; *p < 0.05 versus CTL cells + bile; **p < 0.05 versus CTL cells.
FIG. 3.
Accumulation of [3H]TCA in cells + bile (black bars) or cells (white bars) in TR− rat SCH following a 10-min incubation with 1μM [3H]TCA or vehicle control (0.1% DMSO; CTL), 1, 10, or 100μM TRO, or 50μM MK571. The BEI was calculated as described in “Materials and Methods” section. Data represent the mean ± SE of triplicate determinations in at least n = 3 livers; *p < 0.05 versus CTL cells + bile; **p < 0.05 versus CTL cells.
The BEI of [14C]CDCA species was similar between control WT and TR− rat SCH, and TRO decreased the BEI of [14C]CDCA species in a concentration-dependent manner. Although MK571 ablated the biliary excretion of [14C]CDCA in WT cells, the effect in TR− rat SCH was not as pronounced. BEI values for [3H]TCA in TR− rat SCH also were decreased by TRO and MK571, but the decreases in BEI observed with 100μM TRO and MK571 for [3H]TCA were less than the decreases in BEI for [14C]CDCA species at the same concentrations.
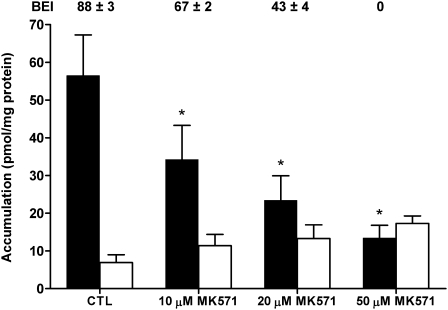
MK571-Mediated Inhibition of [3H]TCA Accumulation Is Concentration Dependent
MK571 significantly inhibited [3H]TCA accumulation in cells + bile in WT rat SCH in a concentration-dependent manner (Fig. 4), and there was a clear trend toward increased cellular accumulation of [3H]TCA with increasing MK571 concentration. The BEI of [3H]TCA also was decreased in a concentration-dependent manner; 50μM MK571 completely ablated biliary excretion of [3H]TCA in WT rat SCH.
FIG. 4.
Accumulation of [3H]TCA in cells + bile (black bars) or cells (white bars) in WT rat SCH following a 10-min incubation with 1μM [3H]TCA and vehicle control (0.1% DMSO; CTL) or 10, 20, or 50μM MK571. The BEI was calculated as described in the “Materials and Methods” section. Data represent the mean ± SE of triplicate determinations in n = 3 livers; *p < 0.05 versus CTL.
Accumulation of [14C]CDCA Species and [3H]TCA Is Temperature Dependent
The accumulation of [14C]CDCA species and [3H]TCA was measured at 4°C in WT rat SCH in order to rule out passive uptake. As expected, the uptake of [14C]CDCA species and [3H]TCA into cells + bile and cells was almost entirely ablated at 4°C, and biliary excretion was negligible, consistent with temperature-dependent active transport processes for both BAs (data not shown).
Unconjugated CDCA Is Metabolized in WT Rat SCH
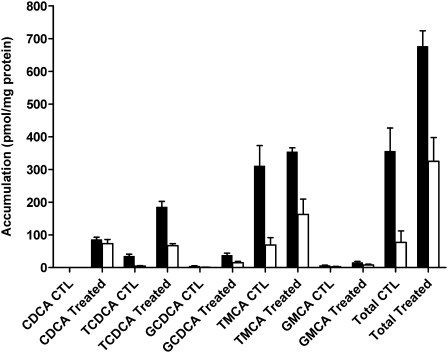
In order to determine the extent of CDCA metabolism during the 10-min incubation period in the accumulation studies, BAs were measured by LC-MS/MS in rat SCH incubated with vehicle (0.1% DMSO; CTL) or 1μM unlabeled CDCA; results are shown in Figure 5. Analysis revealed that endogenous unconjugated CDCA was below the limit of quantification in both cells + bile and cells in CTL SCH. In CTL cells, modest amounts of endogenous TCDCA (6% of total) and small amounts of GCDCA and GMCA (1 and 3% of total, respectively) were measured, whereas TMCA was the most abundant BA measured (90% of total) (Table 2). TMCA was excreted extensively into bile (86% of total), whereas TCDCA was excreted into bile to a lesser extent (11% of total); GCDCA and GMCA accounted for 1 and 2%, respectively, of the BAs in bile (Table 2).
FIG. 5.
Parent CDCA and formed CDCA species (taurine- and glycine-conjugated CDCA), TMCA, and GMCA in cells + bile (solid bars) and cells (white bars) of WT rat SCH following a 10-min incubation with vehicle (0.1% DMSO; CTL) or 1μM unlabeled CDCA. Data represent the mean ± SD of triplicate determinations in n = 1 liver.
TABLE 2.
Accumulation (% Total) of CDCA, TCDCA, GCDCA, TMCA, and GMCA in Cells and Bile of Rat SCH Following a 10-Min Incubation with Vehicle (0.1% DMSO; CTL) or with 1μM Exogenous CDCA (Treated)
| % of total | CTL |
Treated |
||
| Cells | Bile | Cells | Bile | |
| CDCA | 0 | 0 | 22 | 4 |
| TCDCA | 6 | 11 | 21 | 34 |
| GCDCA | 1 | 1 | 5 | 6 |
| TMCA | 90 | 86 | 50 | 54 |
| GMCA | 3 | 2 | 2 | 2 |
Following a 10-min incubation of WT rat SCH with exogenously administered CDCA, unconjugated CDCA accumulated in cells; cellular TCDCA increased ∼15-fold, GCDCA increased ∼14-fold, and GMCA increased approximately threefold compared with CTL values (Fig. 5). Biliary excretion of CDCA and GMCA in the bile of cells exposed to exogenous CDCA was negligible, whereas TCDCA in bile increased approximately fourfold. Interestingly, although cells + bile accumulation of TMCA did not change between CTL and treated cells, cellular accumulation increased approximately twofold and accumulation in bile decreased 21%. Overall, following the 10-min incubation, the accumulation in cells + bile of exogenously administered unlabeled CDCA including its conjugates (the difference in total BAs before and after exogenous CDCA exposure) was about 322 pmol/mg protein, which is consistent with the accumulation of [14C]CDCA species in cells + bile in WT CTL SCH (∼325 pmol/mg protein, Fig. 1). This indicates that the CDCA species detected by LC-MS/MS represent the majority, if not all, of the parent and metabolites. As summarized in Table 2, following exogenous exposure to CDCA, the amount of TCDCA, GCDCA, and unconjugated CDCA increased as a percentage of the total BAs measured in cells and bile, whereas the percentage of TMCA within the cells and bile decreased as a percentage of the total; only modest changes in GMCA were noted.
Initial Uptake of [14C]CDCA Is Primarily Na+-Independent, Whereas [3H]TCA Uptake Is Primarily Na+-Dependent
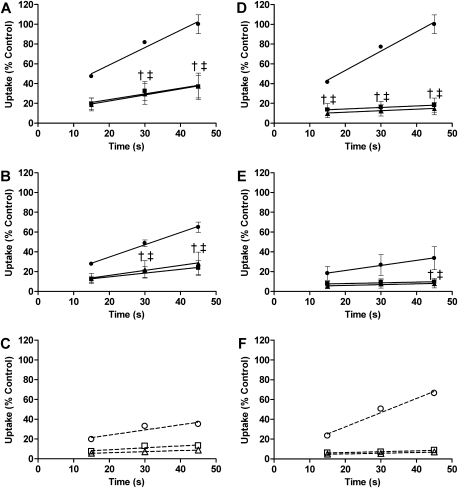
Initial uptake of [14C]CDCA and [3H]TCA was measured in WT rat suspended hepatocytes at 15, 30, and 45 s (Fig. 6). Pilot studies indicated that uptake of both BAs was linear through 90 s (data not shown). TRO and MK571 significantly inhibited total [14C]CDCA uptake to a similar extent when compared with CTL (Fig. 6A). Na+-independent uptake of [14C]CDCA at 45 s in CTL cells (Fig. 6B) was 65 ± 5% of total uptake of [14C]CDCA at 45 s in CTL cells in the presence of Na+ (set at 100%; Fig. 6A) and was double the Na+-independent uptake of [3H]TCA at 45 s in CTL cells (Fig. 6E). Both TRO and MK571 significantly inhibited Na+-independent [14C]CDCA uptake (Fig. 6B); Na+-dependent uptake (Fig. 6C) also was inhibited. For comparison, initial uptake of [3H]TCA was measured under the same conditions as [14C]CDCA. TRO and MK571 significantly inhibited total [3H]TCA uptake to a similar extent at all time points (Fig. 6D). Na+-independent uptake of [3H]TCA at 45 s in CTL cells (Fig. 6E) was 34 ± 12% of total [3H]TCA uptake at 45 s in CTL cells (set at 100%; Fig. 6D), consistent with previous reports demonstrating that TCA uptake in rat hepatocytes is mediated primarily by a Na+-dependent process (Kemp et al., 2005; Van Dyke et al., 1982). Both TRO and MK571 significantly inhibited Na+-independent [3H]TCA uptake at 45 s (Fig. 6E) and also inhibited Na+-dependent [3H]TCA uptake (Fig. 6F).
FIG. 6.
Accumulation of [14C]CDCA (A, B, and C) or [3H]TCA (D, E, and F) in suspended rat hepatocytes in the presence of 1μM [14C]CDCA or 1μM [3H]TCA and vehicle (•,○ 0.3% DMSO; CTL), 10μM TRO (▪,□), or 50μM MK571 (▴,▵). Lines represent the linear regression of the data using GraphPad Prism 5.03. Total accumulation (Na+-dependent and independent) was measured in Na+-containing buffer (A and D). Na+-independent uptake was measured in choline-containing buffer (B and E). Na+-dependent uptake was calculated by subtracting Na+-independent accumulation from total accumulation (C and F; open symbols and dashed lines). Data represent the mean ± SE of triplicate determinations in n = 3 experiments; †p < 0.05 versus CTL for TRO, and ‡p < 0.05 versus CTL for MK571.
DISCUSSION
Inhibition of BA transport, which may cause toxic intracellular accumulation of BAs, is one hypothesized mechanism of TRO toxicity. Although a number of in vivo and in vitro studies have reported that TRO inhibits transport of the prototypic BA TCA, the present study demonstrates that TRO differentially affects the disposition of BAs, specifically CDCA and TCA, in primary rat hepatocytes following acute exposure. The accumulation of [14C]CDCA in cells + bile in WT rat SCH was approximately sixfold higher than accumulation of [3H]TCA in cells + bile [historically ∼40 to 70 pmol/mg protein (Lee et al., 2010; McRae et al., 2006; Wolf et al., 2010)]. This is consistent with previously published data indicating that the rate of uptake of CDCA in suspended rat hepatocytes was ∼10-fold higher than of TCA (Iga and Klaassen, 1982). In contrast to reports using [3H]TCA as a model substrate (Ansede et al., 2010; Kemp et al., 2005; Marion et al., 2007), exposure to 1 or 10μM TRO did not significantly decrease accumulation of [14C]CDCA species in cells + bile or in cells. In fact, treatment with 100μM TRO significantly increased intracellular accumulation and completely ablated the biliary excretion of [14C]CDCA species. These results are significant because they are the first to demonstrate that TRO causes intracellular accumulation of a BA species in hepatocytes in vitro without uncoupling uptake from efflux, as discussed below.
MK571 also inhibited biliary excretion and caused significant cellular accumulation of [14C]CDCA species. Hepatic MRP3/Mrp3 and MRP4/Mrp4 are upregulated under cholestatic conditions in both rat (Denk et al., 2004; Donner and Keppler, 2001) and human (Gradhand et al., 2008; Scheffer et al., 2002) liver, and are postulated compensatory routes for basolateral BA efflux. MK571 was expected to increase intracellular [14C]CDCA accumulation by inhibiting basolateral efflux via Mrps; complete ablation of biliary excretion was not anticipated because Bsep is responsible for transporting both conjugated and unconjugated BAs into bile, and MK571 has not been reported to inhibit Bsep. In contrast to Bsep, which transports monovalent BAs, Mrp2 transports sulfate- and glucuronide-conjugated (divalent) BAs (Konig et al., 1999). Impaired biliary excretion of [14C]CDCA by MK571 suggested that unconjugated [14C]CDCA may be metabolized completely to an Mrp2 substrate (i.e., sulfate or glucuronide conjugate) during the 10-min incubation, or that MK571 inhibited Bsep-mediated biliary excretion of [14C]CDCA species in rat SCH.
Mrp2-deficient TR− rat hepatocytes were utilized to elucidate a potential role for Mrp2 in the transport of [14C]CDCA species, and to compare the effect of MK571 on transport of [14C]CDCA species versus [3H]TCA. Accumulation of [14C]CDCA species in cells + bile and cells, and the BEI, were similar in vehicle-treated TR− compared with WT rat SCH, indicating that the lack of Mrp2 did not affect the disposition of [14C]CDCA species in rat SCH. Furthermore, MK571 and TRO had similar effects on intracellular accumulation of [14C]CDCA species in WT and TR− rat SCH. These data suggest that CDCA and/or CDCA metabolites are Bsep substrates and that MK571 inhibited Bsep-mediated biliary excretion.
In rats, ∼95 to 98% of BAs are conjugated to taurine, and the remainder to glycine, whereas in humans, 75% of BAs are conjugated to glycine and 25% to taurine (Alvaro et al., 1986); conjugation increases aqueous solubility, facilitating biliary excretion (Kullak-Ublick et al., 2004). LC-MS/MS analysis revealed that when CDCA was administered exogenously to SCH, unconjugated CDCA, TCDCA, and GCDCA increased within the cells during the 10-min incubation. Of the total BAs in bile following exposure to CDCA, 88% of total biliary BAs were conjugated to taurine, 8% were conjugated to glycine, and 4% remained as unconjugated CDCA (Table 2). The present results are consistent with data from Hoffman et al. (1975), showing that when unconjugated CDCA was injected into rats, >90% was excreted into the bile as the taurine conjugate, <5% as unconjugated CDCA, and a small amount was excreted as the glycine conjugate (Hoffman et al., 1975). Both TRO and MK571 increased cellular accumulation of [14C]CDCA species in WT and TR− rat SCH; these results raise the possibility that in humans, TRO may cause intracellular accumulation of CDCA/CDCA species. GCDCA, in particular, is cytotoxic and has been shown to induce apoptosis in primary rat hepatocytes (Kaplowitz and DeLeve, 2003).
Several studies have shown that TRO inhibits uptake and biliary excretion of TCA in rat (Ansede et al., 2010; Kemp et al., 2005; Marion et al., 2007) and human (Marion et al., 2007) suspended hepatocytes and SCH, leading to unchanged or decreased intracellular accumulation. Consistent with these reports, in the present studies, 10 and 100μM TRO significantly decreased accumulation of [3H]TCA in cells + bile of TR− rat SCH, but had no significant effect on cellular accumulation. In contrast, Jemnitz et al. (2010) used the method of Lengyel et al. (2008) to assess the effects of TRO on basolateral and canalicular efflux of TCA in SCH. In these studies, [3H]TCA was preloaded into rat and human SCH by incubating them with [3H]TCA in the absence of inhibitor; then, [3H]TCA was washed off, and cells were incubated with various inhibitors or vehicle in standard HBSS or in Ca2+- and Mg2+-free HBSS buffer. At the end of the 10-min incubation, effluxed [3H]TCA was measured in the buffer and in cells + bile or in cells. Using this method, 100μM TRO decreased biliary excretion and increased intracellular accumulation of [3H]TCA in both rat and human SCH without affecting basolateral efflux of [3H]TCA into the medium. These results established that TRO can cause the intracellular accumulation of TCA, which had not been shown using other methods in SCH. By preloading hepatocytes with TCA, any inhibitory effects of TRO on BA uptake were bypassed. However, this paradigm, which uncouples the uptake and efflux of BAs, does not realistically represent the in vivo situation. The intracellular accumulation of a substrate in hepatocytes is dependent upon both uptake and efflux processes. BAs are highly conserved through enterohepatic cycling; basolateral reuptake of BAs from the blood greatly exceeds BA synthesis, and intracellular BAs are excreted rapidly into the bile canaliculi. This vectorial transport of BAs from blood to bile represents the normal circulation of BAs. In previous studies using TCA to examine the disposition of BAs following TRO exposure, it appears that TRO-mediated inhibition of [3H]TCA uptake precluded any effects on intracellular [3H]TCA accumulation (Ansede et al., 2010; Kemp et al., 2005; Marion et al., 2007).
Suspended rat hepatocytes were utilized to examine the effects of TRO on the uptake of CDCA versus TCA. In previous studies, TRO inhibited TCA uptake in suspended rat (Kemp et al., 2005) and human (Marion et al., 2007) hepatocytes and in basolateral membrane vesicles (Snow and Moseley, 2006). Based on results in SCH, TRO and MK571 were expected to have little, if any, effect on initial uptake of CDCA in suspended rat hepatocytes. The uptake of CDCA reportedly occurs partially by a nonsaturable Na+-independent mechanism, hypothesized to be passive diffusion, and partially by a saturable process (Bartholomew and Billing, 1983; Iga and Klaassen, 1982; Van Dyke et al., 1982). In cultured rat hepatocytes, uptake of CDCA was significantly, but not completely, reduced by the removal of Na+ and also by ouabain, a Na+-K+-ATPase inhibitor (Van Dyke et al., 1982), suggesting a Na+-dependent component of uptake (i.e., Ntcp). Results of the present studies confirmed that the majority of [3H]TCA uptake was Na+-dependent, whereas [14C]CDCA uptake was primarily Na+-independent. Surprisingly, both TRO and MK571 decreased Na+-dependent and Na+-independent initial uptake of [14C]CDCA. MK571 reportedly inhibits OATP1B3 (Letschert et al. 2005) and OATP2B1 (Letschert et al., 2006), which would explain inhibition of the Na+-independent component of CDCA uptake. MK571 also inhibited Na+-dependent TCA uptake into hepatocytes. To our knowledge, there are no prior reports of MK571-mediated inhibition of NTCP/Ntcp.
Decreased initial uptake of [14C]CDCA in suspended rat hepatocytes is compatible with results in SCH. Given that total intracellular accumulation of BAs is dependent on uptake processes as well as canalicular and basolateral efflux processes, then total accumulation of [14C]CDCA species in cells in the presence of 10μM TRO may be the result of decreased uptake coupled with decreased efflux, resulting in no change in net accumulation relative to CTL. However, the increased intracellular [14C]CDCA accumulation with 100μM TRO may indicate that higher concentrations of TRO inhibit biliary excretion of [14C]CDCA to a greater extent than uptake. Thus, TRO may have different effects on uptake and efflux depending on the concentration at the site of transport.
In conclusion, TRO differentially affected the uptake and accumulation of CDCA species compared with TCA in rat SCH, causing an intracellular increase in CDCA species but not TCA. This supports the hypothesis that impaired BA transport is a potential mechanism of TRO hepatotoxicity. In addition to the known inhibitory effect of MK571 on MRPs, our results suggest that MK571 also inhibited Ntcp and Bsep. Overall, these results demonstrate that inhibitors of BA transport proteins may have differential effects on the disposition of individual BAs and suggest that use of a single BA substrate for transport studies (i.e., TCA) may yield an incomplete picture of a compound’s effects on overall BA disposition. Therefore, multiple BA species should be evaluated as more cytotoxic BAs may accumulate in hepatocytes, potentially contributing to toxicity.
FUNDING
National Institutes of Health, National Institute of General Medical Sciences (GM41935 to K.L.R.B); National Institutes of Health, National Institute of Environmental Health Sciences (T32-ES007126 to T.L.M.).
References
- Alvaro D, Cantafora A, Attili AF, Ginanni Corradini S, De Luca C, Minervini G, Di Biase A, Angelico M. Relationships between bile salts hydrophilicity and phospholipid composition in bile of various animal species. Comp. Biochem. Physiol. B. 1986;83:551–554. doi: 10.1016/0305-0491(86)90295-6. [DOI] [PubMed] [Google Scholar]
- Ansede JH, Smith WR, Perry CH, St Claire RL, III, Brouwer KR. An in vitro assay to assess transporter-based cholestatic hepatotoxicity using sandwich-cultured rat hepatocytes. Drug Metab. Dispos. 2010;38:276–280. doi: 10.1124/dmd.109.028407. [DOI] [PubMed] [Google Scholar]
- Bartholomew TC, Billing BH. The effect of 3-sulphation and taurine conjugation on the uptake of chenodeoxycholic acid by rat hepatocytes. Biochim. Biophys. Acta. 1983;754:101–109. doi: 10.1016/0005-2760(83)90086-3. [DOI] [PubMed] [Google Scholar]
- Baur H, Kasperek S, Pfaff E. Criteria of viability of isolated liver cells. Hoppe Seylers Z. Physiol. Chem. 1975;356:827–838. doi: 10.1515/bchm2.1975.356.s1.827. [DOI] [PubMed] [Google Scholar]
- Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 2002;123:1649–1658. doi: 10.1053/gast.2002.36591. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Calderon PB, Taper HS, Roberfroid MB. Comparative hepatotoxicity of cholic acid, deoxycholic acid and lithocholic acid in the rat: in vivo and in vitro studies. Toxicol. Lett. 1992;61:291–304. doi: 10.1016/0378-4274(92)90156-e. [DOI] [PubMed] [Google Scholar]
- Denk GU, Soroka CJ, Takeyama Y, Chen WS, Schuetz JD, Boyer JL. Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J. Hepatol. 2004;40:585–591. doi: 10.1016/j.jhep.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Donner MG, Keppler D. Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology. 2001;34:351–359. doi: 10.1053/jhep.2001.26213. [DOI] [PubMed] [Google Scholar]
- Funk C, Pantze M, Jehle L, Ponelle C, Scheuermann G, Lazendic M, Gasser R. Troglitazone-induced intrahepatic cholestasis by an interference with the hepatobiliary export of bile acids in male and female rats. Correlation with the gender difference in troglitazone sulfate formation and the inhibition of the canalicular bile salt export pump (Bsep) by troglitazone and troglitazone sulfate. Toxicology. 2001a;167:83–98. doi: 10.1016/s0300-483x(01)00460-7. [DOI] [PubMed] [Google Scholar]
- Funk C, Ponelle C, Scheuermann G, Pantze M. Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: in vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol. Pharmacol. 2001b;59:627–635. [PubMed] [Google Scholar]
- Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J. Biol. Chem. 1998;273:10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- Gradhand U, Lang T, Schaeffeler E, Glaeser H, Tegude H, Klein K, Fritz P, Jedlitschky G, Kroemer HK, Bachmakov I, et al. Variability in human hepatic MRP4 expression: influence of cholestasis and genotype. Pharmacogenomics J. 2008;8:42–52. doi: 10.1038/sj.tpj.6500451. [DOI] [PubMed] [Google Scholar]
- Greim H, Czygan P, Schaffner F, Popper H. Determination of bile acids in needle biopsies of human liver. Biochem. Med. 1973;8:280–286. doi: 10.1016/0006-2944(73)90032-x. [DOI] [PubMed] [Google Scholar]
- Hoffman NE, Iser JH, Smallwood RA. Hepatic bile acid transport: effect of conjugation and position of hydroxyl groups. Am. J. Physiol. 1975;229:298–302. doi: 10.1152/ajplegacy.1975.229.2.298. [DOI] [PubMed] [Google Scholar]
- Iga T, Klaassen CD. Uptake of bile acids by isolated rat hepatocytes. Biochem. Pharmacol. 1982;31:211–216. doi: 10.1016/0006-2952(82)90213-1. [DOI] [PubMed] [Google Scholar]
- Jemnitz K, Veres Z, Vereczkey L. Contribution of high basolateral bile salt efflux to the lack of hepatotoxicity in rat in response to drugs inducing cholestasis in human. Toxicol. Sci. 2010;115:80–88. doi: 10.1093/toxsci/kfq044. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N, DeLeve LD. Drug-Induced Liver Disease. New York, NY: Marcel Dekker; 2003. [Google Scholar]
- Kemp DC, Zamek-Gliszczynski MJ, Brouwer KLR. Xenobiotics inhibit hepatic uptake and biliary excretion of taurocholate in rat hepatocytes. Toxicol. Sci. 2005;83:207–214. doi: 10.1093/toxsci/kfi020. [DOI] [PubMed] [Google Scholar]
- Kis E, Ioja E, Nagy T, Szente L, Heredi-Szabo K, Krajcsi P. Effect of membrane cholesterol on BSEP/Bsep activity: species specificity studies for substrates and inhibitors. Drug Metab. Dispos. 2009;37:1878–1886. doi: 10.1124/dmd.108.024778. [DOI] [PubMed] [Google Scholar]
- Konig J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim. Biophys. Acta. 1999;1461:377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Semin. Liver Dis. 2000;20:273–292. doi: 10.1055/s-2000-9426. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–342. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- LeCluyse EL, Bullock PL, Parkinson A, Hochman JH. Cultured rat hepatocytes. In: Borchardt RT, Smith P, Wilson G, editors. Models for Assessing Drug Absorption and Metabolism. New York: Plenum Press; 1996. pp. 121–159. [Google Scholar]
- Lee JK, Paine MF, Brouwer KLR. Sulindac and its metabolites inhibit multiple transport proteins in rat and human hepatocytes. J. Pharmacol. Exp. Ther. 2010;334:410–418. doi: 10.1124/jpet.110.165852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel G, Veres Z, Tugyi R, Vereczkey L, Molnar T, Glavinas H, Krajcsi P, Jemnitz K. Modulation of sinusoidal and canalicular elimination of bilirubin-glucuronides by rifampicin and other cholestatic drugs in a sandwich culture of rat hepatocytes. Hepatol. Res. 2008;38:300–309. doi: 10.1111/j.1872-034X.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Watkins PB, Kim RB, Brouwer KLR. Differential inhibition of rat and human Na+-dependent taurocholate co-transporting polypeptide (NTCP/SLC10A1) by bosentan: a mechanism for species differences in hepatotoxicity. J. Pharmacol. Exp. Ther. 2007;321:1170–1178. doi: 10.1124/jpet.106.119073. [DOI] [PubMed] [Google Scholar]
- Letschert K, Faulstich H, Keller D, Keppler D. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol. Sci. 2006;91:140–149. doi: 10.1093/toxsci/kfj141. [DOI] [PubMed] [Google Scholar]
- Letschert K, Komatsu M, Hummel-Eisenbeiss J, Keppler D. Vectorial transport of the peptide CCK-8 by double-transfected MDCKII cells stably expressing the organic anion transporter OATP1B3 (OATP8) and the export pump ABCC2. J. Pharmacol. Exp. Ther. 2005;313:549–556. doi: 10.1124/jpet.104.081224. [DOI] [PubMed] [Google Scholar]
- Liu X, LeCluyse EL, Brouwer KR, Lightfoot RM, Lee JI, Brouwer KLR. Use of Ca2+ modulation to evaluate biliary excretion in sandwich-cultured rat hepatocytes. J. Pharmacol. Exp. Ther. 1999;289:1592–1599. [PubMed] [Google Scholar]
- Maglova LM, Jackson AM, Meng XJ, Carruth MW, Schteingart CD, Ton-Nu HT, Hofmann AF, Weinman SA. Transport characteristics of three fluorescent conjugated bile acid analogs in isolated rat hepatocytes and couplets. Hepatology. 1995;22:637–647. [PubMed] [Google Scholar]
- Marion TL, Leslie EM, Brouwer KLR. Use of sandwich-cultured hepatocytes to evaluate impaired bile acid transport as a mechanism of drug-induced hepatotoxicity. Mol. Pharm. 2007;4:911–918. doi: 10.1021/mp0700357. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y. Metabolic and non-metabolic factors determining troglitazone hepatotoxicity: a review. Drug Metab. Pharmacokinet. 2006;21:347–356. doi: 10.2133/dmpk.21.347. [DOI] [PubMed] [Google Scholar]
- McRae M, Rezk NL, Bridges AS, Corbett AH, Tien HC, Brouwer KLR, Kashuba AD. Plasma bile acid concentrations in patients with human immunodeficiency virus infection receiving protease inhibitor therapy: possible implications for hepatotoxicity. Pharmacotherapy. 2010;30:17–24. doi: 10.1592/phco.30.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae MP, Lowe CM, Tian X, Bourdet DL, Ho RH, Leake BF, Kim RB, Brouwer KLR, Kashuba AD. Ritonavir, saquinavir, and efavirenz, but not nevirapine, inhibit bile acid transport in human and rat hepatocytes. J. Pharmacol. Exp. Ther. 2006;318:1068–1075. doi: 10.1124/jpet.106.102657. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Nakayama F, Koga A. Effect of chenodeoxycholic and ursodeoxycholic acids on isolated adult human hepatocytes. Dig. Dis. Sci. 1984;29:1123–1130. doi: 10.1007/BF01317087. [DOI] [PubMed] [Google Scholar]
- Noe J, Hagenbuch B, Meier PJ, St-Pierre MV. Characterization of the mouse bile salt export pump overexpressed in the baculovirus system. Hepatology. 2001;33:1223–1231. doi: 10.1053/jhep.2001.24171. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, Meier PJ, Stieger B. Genetic determinants of drug-induced cholestasis and intrahepatic cholestasis of pregnancy. Semin. Liver Dis. 2010;30:147–159. doi: 10.1055/s-0030-1253224. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, Stieger B, Meier Y, Kullak-Ublick GA, Meier PJ. Enterohepatic transport of bile salts and genetics of cholestasis. J. Hepatol. 2005;43:342–357. doi: 10.1016/j.jhep.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Scheffer GL, Kool M, de Haas M, de Vree JM, Pijnenborg AC, Bosman DK, Elferink RP, van der Valk P, Borst P, Scheper RJ. Tissue distribution and induction of human multidrug resistant protein 3. Lab. Invest. 2002;82:193–201. doi: 10.1038/labinvest.3780411. [DOI] [PubMed] [Google Scholar]
- Snow KL, Moseley RH. Effect of thiazolidinediones on bile acid transport in rat liver. Life Sci. 2006;80:732–740. doi: 10.1016/j.lfs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Soroka CJ, Ballatori N, Boyer JL. Organic solute transporter, OSTalpha-OSTbeta: its role in bile acid transport and cholestasis. Semin. Liver Dis. 2010;30:178–185. doi: 10.1055/s-0030-1253226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliacozzi D, Mozzi AF, Casetta B, Bertucci P, Bernardini S, Di Ilio C, Urbani A, Federici G. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin. Chem. Lab. Med. 2003;41:1633–1641. doi: 10.1515/CCLM.2003.247. [DOI] [PubMed] [Google Scholar]
- Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol. Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- Van Dyke RW, Stephens JE, Scharschmidt BF. Bile acid transport in cultured rat hepatocytes. Am. J. Physiol. 1982;243:G484–G492. doi: 10.1152/ajpgi.1982.243.6.G484. [DOI] [PubMed] [Google Scholar]
- Wolf KK, Vora S, Webster LO, Generaux GT, Polli JW, Brouwer KLR. Use of cassette dosing in sandwich-cultured rat and human hepatocytes to identify drugs that inhibit bile acid transport. Toxicol. In Vitro. 2010;24:297–309. doi: 10.1016/j.tiv.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Okada M, Akitaya S, Ohara H, Mikkaichi T, Ishikawa H, Sato M, Matsuura M, Saga T, Unno M, et al. Transport of fluorescent chenodeoxycholic acid via the human organic anion transporters OATP1B1 and OATP1B3. J. Lipid Res. 2006;47:1196–1202. doi: 10.1194/jlr.M500532-JLR200. [DOI] [PubMed] [Google Scholar]