Abstract
Estradiol (E2) is the major endogenous estrogen, and its plasma concentration increases up to 100-fold during pregnancy in humans. Accumulating evidence suggests that an elevated level of E2 may influence hepatic drug metabolism, potentially being responsible for altered drug metabolism during pregnancy. We characterized effects of E2 on expression and activities of cytochrome P450 enzymes (CYPs) in an in vivo system using rats. To this end, female rats were treated with estradiol benzoate (EB) or known CYP inducers. Liver tissues were obtained after 5 days of treatment, and mRNA and protein expression levels as well as activities of major hepatic CYPs were determined by qRT-PCR, immunoblot, and microsomal assay. E2 increased CYP1A2 expression and activity to a smaller extent than β-naphthoflavone did. E2 also enhanced CYP2C expression (CYP2C6, CYP2C7, and CYP2C12) to levels comparable to those observed by phenobarbital. E2 upregulated CYP3A9 expression, while expression of CYP3A1 was downregulated. Expression of hepatic nuclear receptors (PXR and CAR) and the obligate redox partner of CYPs (POR) was downregulated in EB-treated rats, suggesting their potential involvement in regulation of CYP expression and activity by E2. In summary, in female rats E2 regulates expression of hepatic CYPs in a CYP isoform-specific manner although the directional changes are different from those clinically observed during human pregnancy. Further study is warranted to determine whether the changes in drug metabolism during human pregnancy are attributable to involvement of hormones other than E2.
Keywords: cytochrome P450, estradiol, nuclear receptors, p450 oxidoreductase, pregnancy, rat
1. Introduction
Medication use during pregnancy is prevalent; over 50% of pregnant women take at least one medication [1]. Pharmacokinetic profiles of drugs are different in these women as compared to those in nonpregnant women that typical drug doses established in males or nonpregnant women often result in treatment failure or toxicity [2]. Results from clinical studies suggest that altered hepatic metabolism is mainly responsible for the changes in drug disposition during pregnancy [3-5]. Pregnancy influences drug metabolism in a CYP isoform-dependent manner; the activities of CYP2A6, CYP3A4, CYP2C9, and CYP2D6 are increased by ∼50%, 100%, 20%, and 50%, respectively, whereas the activities of CYP2C19 and CYP1A2 are decreased by 50% and ∼40% in the 3rd trimester as compared to nonpregnant controls [3-5]. Activities of CYP1A2 and CYP2D6 have shown to change gradually during pregnancy, in a gestational period dependent manner [4-5]. Causative factors responsible for the changes in CYP activities during pregnancy remain unknown.
17β-Estradiol (E2) is the major endogenous estrogen. Its plasma concentration increases up to 100-fold during pregnancy in humans [6]. Accumulating evidence suggests that E2 may influence the rate and extent of drug metabolism, potentially being responsible for the CYP isoform-dependent changes in drug metabolism during pregnancy. For example, changes in hepatic drug elimination are similar for CYP1A2, CYP2A6, and CYP2C19 substrates in pregnant women and users of estrogen-based oral contraceptives [5]. Also, elimination of CYP2A6, CYP2B6, and CYP3A4 substrates is faster in females than in males whereas CYP1A2-mediated elimination is slower in females [7]. These directional changes in CYP activity in females are similar to those in pregnant women (as compared to the nonpregnant). Despite the evidence, the effects of E2 on major hepatic CYP expression and activities remain largely unknown.
Rats have been extensively used as an in vivo animal model for drug metabolism studies. The interspecies similarities and differences in CYP activities and the transcriptional regulators of CYP expression are well established. Between rats and humans, catalytic activities of CYP1A2 and CYP2E1 are well conserved while isoforms in the rest of major CYP families (i.e., CYP2B, CYP2C, CYP2D and CYP3A) show considerable differences in substrate specificity due to the distinct structures of the catalytic sites [8]. Interestingly however, mechanisms underlying transcriptional regulation of CYP expression appear well conserved between rats and humans regardless of CYP isoform. For example, in both humans and rats, expression of CYP1A, CYP2B/2C, and CYP3A is strongly enhanced upon activation of transcription regulators, aromatic hydrocarbon receptor (AhR), constitutive androstane receptor (CAR), and pregnane X receptor (PXR), respectively, while the CYP expression is downregulated by inflammatory mediators. This suggests that rats may serve as an animal model for studying regulation of CYP expression.
A number of researchers have examined the effects of E2 on hepatic CYP expression in rats [9-11]. However, these studies mainly focused on mechanisms underlying sex differences in CYP expression such that they involved surgical manipulation of animals (e.g., ovariectomy or hypophysectomy) and concentrated mostly on the CYP isoforms that show prominent sexual dimorphism such as CYP2C11 or CYP3A2. To date, there have not been comprehensive studies that address how E2 influences expression of major CYPs in intact female rats, reflecting the net effects of E2 on CYP expression.
The objective of this study was to determine whether E2 is potentially responsible for the altered CYP-mediated drug metabolism during pregnancy. To this end, we characterized the effects of E2 on the expression and activities of major hepatic CYPs in intact rats. In addition, the effects of E2 on the mRNA levels of major CYP transcriptional regulators and P450 oxidoreductase (POR) were examined to explore potential mechanisms underlying the regulation of CYP expression and activity by E2.
2. Materials and methods
2.1. Chemicals and reagents
Resorufin, 7-ethoxyresorufin, diclofenac, p-nitrophenol, p-nitrocatechol, 1′-hydroxymidazolam, phenobarbital (PB), dexamethasone (DX), β-naphthoflavone (BNF), estradiol benzoate (EB), potassium fluoride, sodium arsenate, NADP+, isocitric acid, magnesium chloride and isocitric acid dehydrogenase were obtained from Sigma (St. Louis, MO). Midazolam was purchased from Cerilliant (Round Rock, Texas). 4′-Hydroxydiclofenac was purchased from Axxora (San Diego, CA). Bufuralol and 1′-hydroxybufuralol were purchased from BD Biosciences (Franklin Lakes, NJ). Formic acid (ACS grade) and methanol (Optima grade) were purchased from Fisher Scientific (Pittsburgh, PA).
2.2. Drug treatment
Adult female Sprague-Dawley rats weighing 180-200 g (8-week old) were purchased from Harlan (Indianapolis, IN). After one week of acclimation, rats were administered with EB (1 mg/kg/day subcutaneous injection), BNF (40 mg/kg/day intraperitoneal injection), PB (60 mg/kg/day intraperitoneal injection), DX (60 mg/kg/day intraperitoneal injection), or vehicle (corn oil subcutaneous injection) [n = 4 each group except for control and DX groups (n = 3)]. On day 5, the livers were removed and weighed. Microsomes and total RNA were prepared from the liver tissues.
In parallel, two separate groups of rats (n = 3 per group) were treated with EB (1 mg/kg subcutaneous injection) for either 1 day or 5 days. After EB administration, 200 μl of blood was collected at various time points (pretreatment, 0.5, 1, 2, 4, 7, 11, and 24 hr post-injection). Plasma samples were obtained by immediate centrifugation of the blood and added with 50 μl of 2 M sodium arsenate and 10 μl of 50% potassium fluoride/ml plasma (plasma esterase inhibitors).
2.3. Pharmacokinetic analysis
Concentration of E2 in plasma was determined by an enzyme-linked immunosorbent assay (ELISA) kit following manufacturer's protocol (Cayman Chemical Company, Ann Arbor, MI). Maximum plasma concentration (Cmax) and time to reach Cmax after injection (Tmax) were determined by visual examination of the concentration vs. time profile. Area under the curve over dosing interval (AUC0-24 h) was estimated by using the linear trapezoidal rule. Average plasma concentration (Cave) over dosing interval (τ, 24 hr) was estimated by using the equation: Cave = AUCo-24 h/ τ.
2.4. Hepatic microsomal assays
Hepatic microsomes were prepared by differential ultracentrifugation of hepatic tissues as previously described [12]. Protein concentration of the prepared microsomes was determined by using BCA Protein Assay kits (Pierce, Rockford, IL), and CYP amount was measured by the method of Omura and Sato [13]. For microsomal reactions, an NADPH-generating system (1 mM NADP+, 5 mM isocitric acid, 0.2 U/ml isocitric acid dehydrogenase, and 5.0 mM MgCl2) was used. The reactions were initiated by adding NADP+ to drug-containing reaction media and terminated by adding three volumes of ice-cold acetonitrile. A control reaction was performed in the absence of NADP+. Preliminary experiments were conducted for each substrate compound to determine the microsomal protein concentration and the incubation time that lead to proportional increases in metabolite production. For each substrate compound, Vmax and Km were determined using Prism 5 software (GraphPad, La Jolla, CA). Statistical analysis was performed using Student's t-test.
2.5. Determination of metabolite concentrations
The microsomal samples were analyzed by LC/MS/MS (Agilent 1200 HPLC interfaced with Applied Biosystems Qtrap 3200) using an electrospray ion source. The mobile phase consisted of water (0.1% formic acid) and methanol. Separation was performed with a Zorbax Eclipse XDB-C8 column (4.6 × 50 mm, 3.5 μm) (Agilent Technologies, Santa Clara, CA) at a flow rate of 0.4 ml/min. MS detection of metabolites and internal standards was followed in a positive ion mode by examining multiple MRM pairs as described previously [14]. Concentration of resorufin, the metabolite of 7-ethoxyresorufin, was measured by a fluorescence plate reader (Synergy 4) (BioTek, Winooski, VT) at excitation and emission wavelengths of 530 and 582 nm, respectively.
2.6. RNA isolation and quantitative real time-PCR (qRT-PCR)
Total RNAs were isolated from liver tissues using Trizol® (Invitrogen, Carlsbad, CA). cDNA was prepared using High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) according to manufacturer's instructions. qRT-PCR was performed using StepOnePlus Real-Time PCR System (Applied Biosystems). TaqMan® Gene expression Primers (Applied Biosystems) were used for CYP1A2 (Rn00561082_m1), CYP2B1 (Rn01457880_m1), CYP2D2 (Rn00562419_m1), CYP2E1 (Rn00580624_m1), CYP3A1 (Rn01412959_g1), CYP3A9 (Rn00595977_m1), and β-actin (Rn0066789_m1). Expression levels of CYP2C6, CYP2C7, CYP2C12, pregnane X receptor (PXR), constitutive androstane receptor (CAR), aryl hydrocarbon receptor (AhR), and P450 oxidoreductase (POR) were determined by using SYBR® green expression master mix (Applied Biosystems). The following primers were designed by using Primer 3 software [15]: CYP2C6 (F: 5′-ATGGCAGCCCTGCCTCCTCT-3′, R: 5′-GGCATGCGGCTCCTGTCCTG-3′), CYP2C7 (F: 5′-TGCCTTTCTCAGCAGGAAAACGAGC-3′, R: 5′-ACAACTGCATGCGGGCCAGG-3′), CYP2C12 (F: 5′-TGATTGGGAGACACCGCAGCC-3′, R: 5′-AGGGCATGTGGATCCTGTCCAAC-3′), PXR (F: 5′-GGAGGGCAGGGGCTGACAGA-3′, R: 5′-GAAACACCGCAGGTAGCCGGA-3′), CAR (F: 5′-GGCGCCCACACTCGTCATGT-3′, R: 5′-GCCGGAGGCCTGAACTGCAC-3′), AhR (F: 5′-ATGAGCAGCGGCGCCAACAT-3′, R: 5′-ACTGTTTTCTGCACCGGCTTGC-3′), POR (F: 5′-AACCCGCCACGCACCAATGT -3′, R: 5′-ACAGCTCCTTGCCCTCGCCT -3′), and β-actin (F: 5′-AAGTCCCTCACCCTCCCAAAAG-3′, R: 5′-AAGCAATGCTGTCACCTTCCC-3′). The fold change in mRNA levels of CYP upon drug treatment was determined by normalizing the gene expression levels by those of β-actin (2-ΔΔCt method, [16]).
2.7. Western immunoblot analysis
Liver microsomes were resolved by SDS gel electrophoresis on an 8% polyacrylamide gel (8 μg microsomal protein/lane). Proteins were transferred to a nitrocellulose membrane (1.5 hr, 300 mA); loading of equal sample amounts was ensured by comparing signals after Ponceau S staining. The membrane was then blocked at room temperature for 1 hr in 5% (w/v) milk in Tris-buffered saline containing 0.05% (v/v) Tween 20 (TBST). Membranes were incubated for overnight at 4 °C in anti-CYP1A2 (1:1000 in 2% milk powder in TBST) (Chemicon International, Billerica, MA), anti-CYP3A1 (1:1500 in 2% milk in TBST) (Chemicon International), or anti-CYP2C (1:1000 in 2% milk in TBST) (Abcam, Cambridge, MA). Then, the membrane was incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG secondary antibody at room temperature for 1 hr (1:10,000 in 5% milk in TBST) (Abcam). Protein expression was detected by Supersignal ® West Pico Chemiluminescent substrate (Pierce, Rockford, IL) on Kodak films, and the signals were quantitated by using Adobe Photoshop.
3. Results
3.1. Effects of E2 on liver size, microsomal protein and CYP contents
To investigate the effects of E2 on CYP expression and activity, female rats were administered with vehicle, EB, or known inducers of CYP enzymes for 5 days. BNF, PB, and DX were used as prototypical inducers for CYP1A, CYP2C, and CYP3A, respectively [17]. EB treatment did not affect liver size or microsomal CYP contents (Table 1) as compared to the vehicle treatment. However, EB treatment increased concentration of total protein in the microsome by unknown mechanisms. BNF, DX, and PB increased the microsomal proteins and CYP contents as expected.
Table 1.
Liver weight, microsomal protein and total cytochrome P450 contents after drug treatment.
| Control | EB | BNF | PB | DX | |
|---|---|---|---|---|---|
| na | 3 | 4 | 4 | 4 | 3 |
| Liver (g) | 10.4 ± 1.8 | 9.2 ± 0.7 | 11.3 ± 0.5 | 10.3 ± 0.6 | 11.9 ± 1.9 |
| Microsomal protein (mg/ml) | 6.7 ± 0.8 | 9.6 ± 1.4* | 8.3 ± 2.4* | 9.3 ± 1.6* | 8.1 ± 1.5* |
| CYP (pmol/mg protein) | 0.32 ± 0.10 | 0.44 ± 0.04 | 0.78 ± 0.15* | 0.45 ± 0.13 | 0.63 ± 0.24* |
n is the number of animals used in each group.
p < 0.05 vs. control
3.2. Plasma concentration of E2 after EB injection
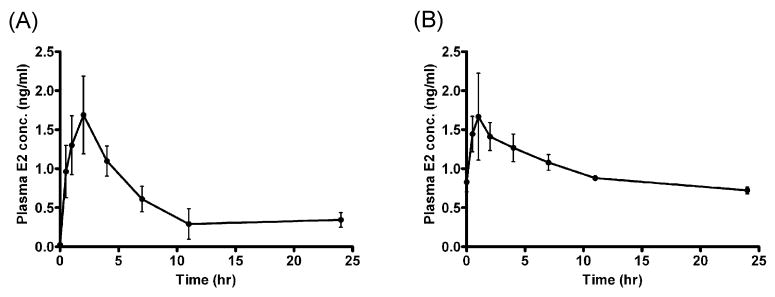
Plasma E2 concentration vs. time profiles obtained after a single or multiple doses (over 5 consecutive days) of EB are shown in Fig. 1. A single subcutaneous injection of EB led to Cmax of 1.68 ± 0.45 ng/ml and Tmax of 2 hr. E2 concentration at 24 hrs (0.34 ± 0.09 ng/ml) was higher than the pretreatment basal level (0.023 ± 0.001 ng/ml; Fig. 1 A). After multiple dosing, Cmax was 1.84 ± 0.46 ng/ml and Tmax of 2 hr. E2 concentration before the 5th dose was 0.83 ± 0.12 ng/ml, showing accumulation of E2 in the bodies. The average concentration of E2, estimated from AUC0-24 h, was also elevated from 0.56 ± 0.15 ng/ml on day 1 to 0.99 ± 0.04 ng/ml on day 5. These concentrations are comparable to the plasma E2 concentrations attainable during human pregnancy.
Fig. 1.
Plasma concentration of E2 vs. time profile after a single injection (A) and 5-day injections (B) of EB. Rats were administered with EB (1mg/kg subcutaneous injection), and blood samples were collected at various time points after EB administration. Concentration of E2 in plasma was determined by ELISA. The values are mean ± SEM (ng/ml).
3.3. Effects of E2 on mRNA expression of major CYPs
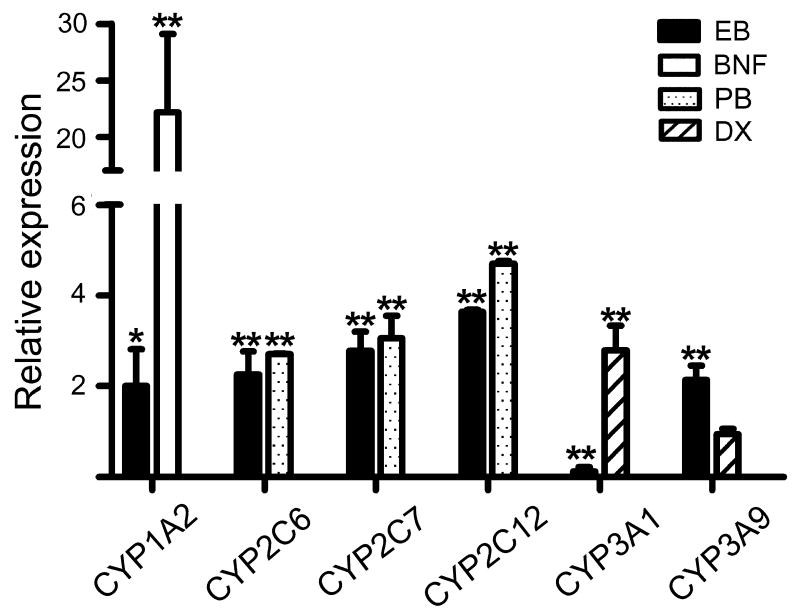
qRT-PCR was performed to investigate the effects of E2 on the mRNA expression of major hepatic CYPs in rat livers: CYP1A2, CYP2B1, CYP2C6, CYP2C7, CYP2C12, CYP2D2, CYP3A1, CYP3A9 and CYP2E1. The results showed that E2 differentially regulated the expression of individual CYP isoforms (Fig. 2). EB treatment increased expression of CYP1A2 by 2.0-fold (p = 0.049) as compared to vehicle treatment, whereas BNF increased CYP1A2 expression by over 20-fold. EB treatment led to upregulation of CYP2C isoforms by 2.2-, 2.7- and 4.0-fold for CYP2C6, CYP2C7, and CYP2C12, respectively. The induction in CYP2C expression by EB treatment was to a similar extent as the induction by PB (2.7-, 3.0- and 4.6-fold for CYP2C6, CYP2C7, and CYP2C12, respectively). EB treatment also increased CYP3A9 expression by 2.3-fold (p = 0.007) while downregulating CYP3A1 expression by 8.0-fold (p < 0.001). DX increased CYP3A1 expression (by 2.8-fold) but had no effect on CYP3A9 expression. EB treatment had insignificant effects on mRNA expression of CYP2B1, CYP2E1, and CYP2D2 (data not shown).
Fig. 2.
Effects of E2 on mRNA levels of rat CYPs. Rats were administered with EB (1 mg/kg/day), BNF (40 mg/kg/day), PB (60 mg/kg/day), DX (60 mg/kg/day), or vehicle (corn oil) for 5 days (n = 3-4/group). mRNA levels were determined by qRT-PCR. Data shown are relative CYP expression as compared to the control group (corn oil). *, p < 0.05; **, p < 0.01 vs. control.
3.4. Immunoblot analysis
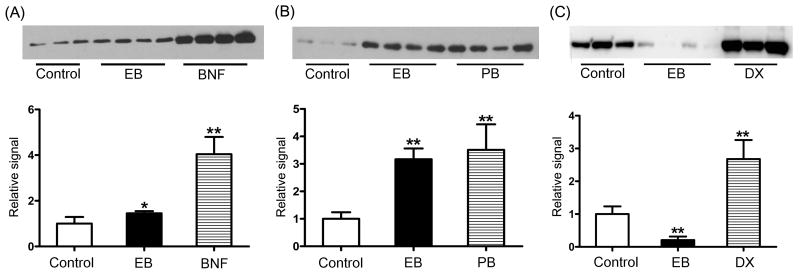
To determine whether E2-mediated mRNA changes in CYPs led to corresponding changes in the protein levels, immunoblot analysis was performed for CYP1A2, CYP3A1, and CYP2C. The results showed that EB treatment increased protein levels of CYP1A2 by 1.4-fold (p = 0.022), but to a much smaller extent than the increase observed with BNF (4.0-fold, p < 0.001) (Fig. 3A). EB treatment also increased CYP2C protein expression by 3.1-fold (p < 0.001), an increase comparable to that by PB (3.5-fold, p < 0.001) (Fig. 3B). On the other hand, EB treatment markedly decreased CYP3A1 expression (by 4.8-fold, p < 0.001) (Fig. 3C), consistent with the qRT-PCR results. The increased mRNA level of CYP3A9 by E2 (Fig. 2) was not confirmed by immunoblot analysis due to a lack of commercially available antibodies that specifically detect rat CYP3A9.
Fig. 3.
Effects of E2 on protein levels of CYP1A2 (A), CYP2C (B), and CYP3A1 (C). Western blot analysis was performed using hepatic microsomes prepared from rats administered with EB (1 mg/kg/day), BNF (40 mg/kg/day), PB (60 mg/kg/day), DX (60 mg/kg/day), or vehicle (corn oil) for 5 days. Eight micrograms of microsome from each treatment group were resolved on SDS-PAGE gel (8%). Results from quantitative analysis of the blots were shown at the bottom panel, expressed as relative signals in comparison with the control group (corn oil). *, p < 0.05; **, p < 0.01 vs. control.
3.5. Effects of E2 on CYP activities
To examine the effects of E2 on CYP activities, microsomal assays were performed using CYP-isoform specific probe substrates: 7-ethoxyresorufin (CYP1A2), diclofenac (CYP2C6/7), bufuralol (CYP2D2), p-nitrophenol (CYP2E1) and midazolam (CYP3A) [18]. EB treatment increased Vmax and intrinsic clearance (CLint) of 7-ethoxyresorufin O-dealkylation activity as compared to the vehicle treatment (by 1.5-fold and 1.7-fold, respectively) (Table 2); however, it did not affect Km, suggesting that intrinsic function of CYP1A2 was likely not influenced by EB treatment. The CLint and Vmax of p-nitrophenol hydroxylation were decreased by EB treatment by 70% and 50%, respectively. EB treatment had insignificant effects on diclofenac 4′-hydroxylation, bufuralol 1′-hydroxylation, and midazolam 1′-hydroxylation activities.
Table 2.
Kinetic parameters for metabolite formation from each probe substrate. Results are expressed as mean ± S.D. (n = 3-4/group)
| Km (μM) | Vmax (pmol/min/mg protein) | CLint (ml/min/mg protein) | |
|---|---|---|---|
| Ethoxyresorufin O-dealkylation (CYP1A2) | |||
| Control | 0.48 ± 0.06 | 53 ± 2 | 109 ± 13 |
| EB | 0.45 ± 0.05 | 80 ± 2* | 183 ± 37* |
| BNF | 1.35 ± 0.33* | 631 ± 4** | 463 ± 54** |
| Diclofenac 4′-hydroxylation (CYP2C6/7) | |||
| Control | 18.6 ± 5.5 | 432 ± 40 | 23.9 ± 5.2 |
| EB | 20.0 ± 4.0 | 434 ± 27 | 24.1 ± 8.6 |
| PB | 28.0 ± 0.6 | 902 ± 197* | 32.3 ± 7.7* |
| Midazolam 1′-hydroxylation (CYP3A) | |||
| Control | 8.0 ± 6.8 | 178 ± 42 | 16.1 ± 9.2 |
| EB | 1.7 ± 3.0 | 71 ± 10 | 7.1 ± 2.4 |
| DX | 8.6 ± 4.3 | 643 ± 72** | 85.2 ± 20.9* |
| p-Nitrophenol hydroxylation (CYP2E1) | |||
| Control | 4.8 ± 1.5 | 1308 ± 94 | 296 ± 139 |
| EB | 7.8 ± 1.9 | 649 ± 38* | 89 ± 24* |
| Bufuralol 1′-hydroxylation (CYP2D2) | |||
| Control | 5.6 ± 1.3 | 383 ± 30 | 97 ± 7 |
| EB | 4.0 ± 0.8 | 962 ± 23 | 120 ± 16 |
p < 0.05;
p < 0.01 vs. control
3.6. Effects of E2 on modulators of drug metabolism
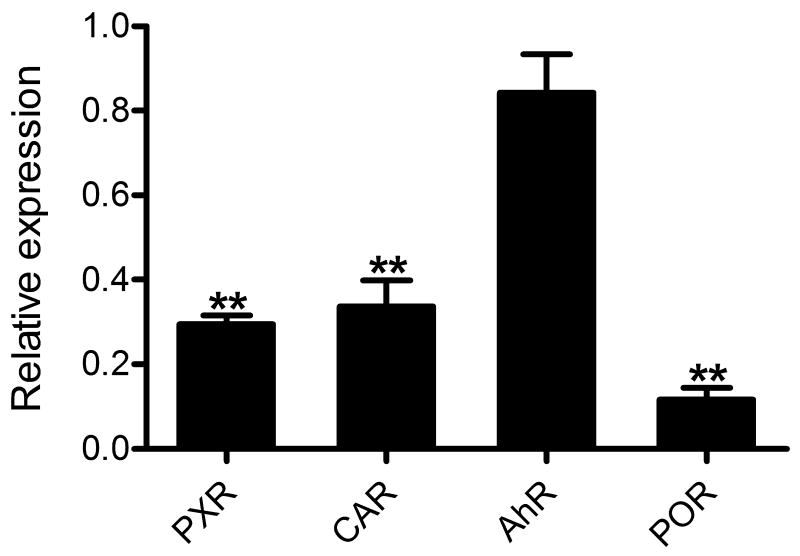
PXR, CAR, and AhR are transcriptional regulators that play key roles in modulating CYP expression [19]. On the other hand, POR is an obligate redox partner of CYP enzymes [20]. To explore potential mechanisms underlying E2-mediated regulation of CYP expression and activity, we examined whether EB treatment affects expression of PXR, CAR, AhR and POR. The results from qRT-PCR (Fig. 4) showed that EB treatment downregulated mRNA levels of PXR (3.4-fold, p < 0.001), CAR (2.9-fold, p < 0.001), and POR (8.6-fold, p < 0.001) as compared to the vehicle treatment, whereas EB treatment had an insignificant effect on AhR expression. These results suggest that in rats, E2 may influence CYP expression and activities by downregulating transcription factors and the redox partner.
Fig. 4.
Effects of E2 on mRNA levels of transcription factors and POR. Female rats were administered with EB (1 mg/kg/day) or vehicle (corn oil) for 5 days (n = 3-4/group). mRNA expression levels of PXR, CAR, AhR, and POR in the livers were determined by qRT-PCR. Data shown are relative CYP expression as compared to the control group (corn oil). *, p < 0.05; **, p < 0.01 vs. control.
4. Discussion
Pregnancy influences hepatic drug metabolism in a CYP isoform-dependent manner in humans. However, the responsible factors or the underlying mechanisms remain largely unknown. The objective of this study is to determine whether increasing plasma concentration of E2 during pregnancy is potentially responsible for the altered drug metabolism using rats as a model.
In the present study, to achieve the plasma E2 concentrations attainable during human pregnancy, we administered 1 mg/kg of EB by subcutaneous injection into female rats. This led to Cmax and Cave of 1.84 ± 0.46 ng/ml and 0.99 ± 0.04 ng/ml, respectively (Fig. 1). The Cave corresponds to the plasma E2 level in pregnant women during the first trimester [6], which is 40-fold higher than the baseline E2 concentrations in non-pregnant rats and women. Of note, high doses of estrogen are known to negatively influence hepatic functions in rats, e.g., increasing liver sizes and causing cholestasis [21], which may indirectly affect hepatic drug metabolism. At the dosage used in this study, EB treatment did not change liver weight or CYP concentration in hepatic microsomes in the rats (Table 1), suggesting an apparent lack of E2 effects on normal liver physiology.
Our results indicate that E2 modulates CYP expression in an isoform-specific manner: upregulation of CYP1A2, CYP2Cs, and CYP3A9 expression and downregulation of CYP3A1. The increased expression and activity of rat hepatic CYP1A2 by EB treatment are, in part, in agreement with a previous study where activity of rat intestinal CYP1A2 is enhanced by EB treatment [22]. The induction of CYP1A2 expression may be attributed to activation of an AhR-mediated regulatory mechanism which is involved in upregulation of hepatic and intestinal CYP1A expression [23]. Although it is currently unknown whether E2 is capable of activating AhR pathways in a direct manner (i.e., being an AhR ligand), results from previous studies have suggested that E2 may activate AhR by an indirect mechanism, potentiating the induction of CYP1A expression by other AhR ligands (e.g., endogenous AhR ligands) [24-25]. Our results show that EB treatment increased mRNA expression of another representative target gene of AhR, NADPH dehydrogenase quinine 1 (NQO1) [23] (by 2-fold; data not shown). These findings suggest that E2 upregulates CYP1A2 expression potentially by activating AhR-mediated regulatory pathways. On the other hand, the induction of CYP1A2 expression in rats by EB treatment did not correspond to the clinically reported reduction in metabolism of CYP1A2 substrates during human pregnancy. Considering the highly conserved regulatory mechanisms for CYP1A2 between humans and rats [8], our data suggest that pregnancy-specific factors other than E2 may be responsible for the decreased CYP1A2 activity during human pregnancy.
EB treatment significantly upregulated expression of CYP2C isoforms (CYP2C6, CYP2C7, and CYP2C12) (Fig. 2 and Fig. 3B), comparable to the induction observed from PB treatment. This result appears consistent with previous studies where administration of E2 enhanced expression of CYP2C7 and CYP2C12 in male rats [26] and increased mRNA expression and activity of CYP2C6 in rat hepatocytes [27-28]. Global upregulation of CYP2C shown in our study as well as others suggests that E2 may be responsible for the increased metabolism of CYP2C9 substrates in pregnant women [3-5]. Interestingly, despite significant induction in CYP2C expression by E2, diclofenac 4′-hydroxylation, a marker for CYP2C6 and CYP2C7 activities [29], did not increase in the EB-treated rats as compared to the vehicle-treated rats (Table 2). Downregulation of POR expression by E2 (Fig. 4) and subsequent decrease in CYP activity may provide a potential explanation. In both humans and rats, amount of POR in microsomes is the key determinant of CYP2C-mediated reaction rates such as warfarin 7-hydroxylation and 16α-steroid hydroxylation [30]. Whether pregnancy alters POR expression, and subsequently CYP2C9 activity in humans, remains unknown. Taken together, further study is warranted to determine the role of E2 in increased CYP2C9 activity during human pregnancy.
EB treatment had differential effects on expression of CYP3A1 and CYP3A9 (two major female hepatic CYP3A isoforms): marked downregulation of CYP3A1 and upregulation of CYP3A9 (Fig. 2). The opposing effects of E2 on CYP3A1 and CYP3A9 expression are potentially responsible for the minimal changes in CLint of midazolam 1′-hydroxylation (Table 2) in the EB-treated group as compared to the control because the reaction is mediated by both CYP3A1 and CYP3A9 enzymes [31]. The downregulation of CYP3A1 expression in the EB-treated rats may be attributed to the decreased expression of PXR (Fig. 4), a key transcription factor in modulating CYP3A1 expression [32]. Considering that CYP3A1 is the ortholog of human CYP3A4, our finding suggests that increased metabolism of CYP3A4 substrates in pregnant women [3-5] is mediated by yet to be characterized pregnancy factors other than E2. Such candidates include progesterone. Plasma concentration of progesterone rises about 100-fold during human pregnancy, and progesterone has shown to be a PXR activator in in vitro systems [35-36]. Potential involvement of progesterone in altered drug metabolism during human pregnancy is currently under investigation. On the other hand, CYP3A9 expression is not governed by PXR ([33] and Fig. 2). Interestingly, consistent with our data indicating increased CYP3A9 expression upon EB treatment, CYP3A9 expression has shown to be female-specific and enhanced by ethinylestradiol in rats [31, 34]. These directional changes in CYP3A9 expression are similar to those reported for CYP3A4 expression in pregnant women. The mechanism underlying estrogen-responsive expression of CYP3A9, although it currently remains unclear, may help us determine how E2 is potentially involved in the increased CYP3A4 expression during pregnancy.
Our results show that EB treatment has insignificant effects on expression and activity of CYP2D6, suggesting a lack of involvement of E2 in the increased metabolism of CYP2D6 substrates in pregnant women. EB treatment also shows minimal effects on CYP2E1 expression while decreasing Vmax and CLint of p-nitrophenol hydroxylation as compared to the vehicle-treated group. Although p-nitrophenol hydroxylation is mainly mediated by CYP2E1 in humans, in rats CYP3A1 also mediates the reaction with an efficiency ∼40% that of CYP2E1 [18]. Potentially, the decreased p-nitrophenol hydroxylation may reflect the reduced CYP3A1 expression in the EB-treated rats. Our immunoblot results also show that protein levels of CYP2E1 were not affected by EB treatment (data not shown).
Taken together, we have characterized the in vivo effects of E2 (at high E2 concentrations attainable during human pregnancy) on hepatic CYP expression and activities in rats. Our results show that E2 modulates CYP expression in an isoform-specific manner, leading to downregulation of CYP3A1 expression and upregulation of CYP1A2, CYP2C6, CYP2C7, CYP2C12, and CYP3A9. The directional changes mostly did not reflect those clinically reported during human pregnancy, suggesting that pregnancy-specific changes other than elevated E2 level are potentially responsible for the changes in major CYP activities during human pregnancy. Further studies appear warranted to identify such factors and to better understand the mechanisms underlying the discrepancies between our results and the clinically reported data. Information obtained from the current study should be of great value in better understanding the effects of estrogen on drug metabolism and guiding future approaches to investigating CYP regulation during pregnancy.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development [Grant HD055313 and fellowship K12HK055892].
Abbreviations
- AhR
aryl hydrocarbon receptor
- BNF
β-naphthoflavone
- CAR
constitutive androstane receptor
- CYP
cytochrome P450
- DX
dexamethasone
- E2
17β-estradiol
- EB
estradiol benzoate
- PB
phenobarbital
- POR
P450 oxidoreductase
- PXR
pregnane X receptor
Footnotes
Classification: Pharmacokinetics and Drug Metabolism
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Su-Young Choi, Email: schoi32@uic.edu.
Liam Fischer, Email: lfisch4@uic.edu.
Kyunghee Yang, Email: khyang1213@gmail.com.
Hyejin Chung, Email: hjc@uic.edu.
Hyunyoung Jeong, Email: yjeong@uic.edu.
References
- 1.Andrade SE, Gurwitz JH, Davis RL, Chan KA, Finkelstein JA, Fortman K, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004;191:398–407. doi: 10.1016/j.ajog.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 2.Little BB. Pharmacokinetics during pregnancy: evidence-based maternal dose formulation. Obstet Gynecol. 1999;93:858–68. doi: 10.1016/s0029-7844(98)00444-x. [DOI] [PubMed] [Google Scholar]
- 3.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 4.Anderson GD, Carr DB. Effect of pregnancy on the pharmacokinetics of antihypertensive drugs. Clin Pharmacokinet. 2009;48:159–68. doi: 10.2165/00003088-200948030-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hodge LS, Tracy TS. Alterations in drug disposition during pregnancy: implications for drug therapy. Expert Opin Drug Metab Toxicol. 2007;3:557–71. doi: 10.1517/17425225.3.4.557. [DOI] [PubMed] [Google Scholar]
- 6.Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112:1095–100. doi: 10.1016/0002-9378(72)90185-8. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GD. Gender differences in pharmacological response. Int Rev Neurobiol. 2008;83:1–10. doi: 10.1016/S0074-7742(08)00001-9. [DOI] [PubMed] [Google Scholar]
- 8.Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–94. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- 9.Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215–28. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waxman DJ, Dannan GA, Guengerich FP. Regulation of rat hepatic cytochrome P-450: age-dependent expression, hormonal imprinting, and xenobiotic inducibility of sex-specific isoenzymes. Biochemistry. 1985;24:4409–17. doi: 10.1021/bi00337a023. [DOI] [PubMed] [Google Scholar]
- 11.Dannan GA, Guengerich FP, Waxman DJ. Hormonal regulation of rat liver microsomal enzymes. Role of gonadal steroids in programming, maintenance, and suppression of delta 4-steroid 5 alpha-reductase, flavin-containing monooxygenase, and sex-specific cytochromes P-450. J Biol Chem. 1986;261:10728–35. [PubMed] [Google Scholar]
- 12.Jeong H, Chiou WL. Role of P-glycoprotein in the hepatic metabolism of tacrolimus. Xenobiotica. 2006;36:1–13. doi: 10.1080/00498250500485115. [DOI] [PubMed] [Google Scholar]
- 13.Omura T, Sato R. The Carbon Monoxide-Binding Pigment of Liver Microsomes. Ii. Solubilization, Purification, and Properties. J Biol Chem. 1964;239:2379–85. [PubMed] [Google Scholar]
- 14.Choi S, Sainz B, Jr, Corcoran P, UpRichard S, Jeong H. Characterization of increased drug metabolism activity in DMSO-treated Huh7 hepatoma cells. Xenobiotica. 2009 doi: 10.1080/00498250802613620. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 16.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 17.Martignoni M, de Kanter R, Grossi P, Mahnke A, Saturno G, Monshouwer M. An in vivo and in vitro comparison of CYP induction in rat liver and intestine using slices and quantitative RT-PCR. Chem Biol Interact. 2004;151:1–11. doi: 10.1016/j.cbi.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi K, Urashima K, Shimada N, Chiba K. Substrate specificity for rat cytochrome P450 (CYP) isoforms: screening with cDNA-expressed systems of the rat. Biochem Pharmacol. 2002;63:889–96. doi: 10.1016/s0006-2952(01)00843-7. [DOI] [PubMed] [Google Scholar]
- 19.Timsit YE, Negishi M. CAR and PXR: the xenobiotic-sensing receptors. Steroids. 2007;72:231–46. doi: 10.1016/j.steroids.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen AL, Kasper CB. Differential contributions of NADPH-cytochrome P450 oxidoreductase FAD binding site residues to flavin binding and catalysis. J Biol Chem. 2000;275:41087–91. doi: 10.1074/jbc.M008380200. [DOI] [PubMed] [Google Scholar]
- 21.Rahner C, Stieger B, Landmann L. Structure-function correlation of tight junctional impairment after intrahepatic and extrahepatic cholestasis in rat liver. Gastroenterology. 1996;110:1564–78. doi: 10.1053/gast.1996.v110.pm8613064. [DOI] [PubMed] [Google Scholar]
- 22.Shiverick KT, Delorme AM, Scammell JG, Fregly MJ. Effects of chronic treatment with thyroxine and estradiol on estrogen concentration in serum and on hepatic microsomal catechol estrogen formation in female rats. J Pharmacol Exp Ther. 1982;221:564–9. [PubMed] [Google Scholar]
- 23.Brauze D, Widerak M, Cwykiel J, Szyfter K, Baer-Dubowska W. The effect of aryl hydrocarbon receptor ligands on the expression of AhR, AhRR, ARNT, Hif1alpha, CYP1A1 and NQO1 genes in rat liver. Toxicol Lett. 2006;167:212–20. doi: 10.1016/j.toxlet.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar S, Jana NR, Yonemoto J, Tohyama C, Sone H. Estrogen enhances induction of cytochrome P-4501A1 by 2,3,7, 8-tetrachlorodibenzo-p-dioxin in liver of female Long-Evans rats. Int J Oncol. 2000;16:141–7. doi: 10.3892/ijo.16.1.141. [DOI] [PubMed] [Google Scholar]
- 25.Murray IA, Reen RK, Leathery N, Ramadoss P, Bonati L, Gonzalez FJ, et al. Evidence that ligand binding is a key determinant of Ah receptor-mediated transcriptional activity. Arch Biochem Biophys. 2005;442:59–71. doi: 10.1016/j.abb.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Bandiera S, Dworschak C. Effects of testosterone and estrogen on hepatic levels of cytochromes P450 2C7 and P450 2C11 in the rat. Arch Biochem Biophys. 1992;296:286–95. doi: 10.1016/0003-9861(92)90574-g. [DOI] [PubMed] [Google Scholar]
- 27.Endoh A, Natsume H, Igarashi Y. Dual regulation of 21-hydroxylase activity by sex steroid hormones in rat hepatocytes. J Steroid Biochem Mol Biol. 1995;54:163–5. doi: 10.1016/0960-0760(95)00129-n. [DOI] [PubMed] [Google Scholar]
- 28.Natsume H, Endoh A, Nakagawa Y, Igarashi Y. Regulation of steroid 21-hydroxylation by 17 beta-estradiol in rat liver: in vivo and in vitro study. Endocr J. 1993;40:197–206. doi: 10.1507/endocrj.40.197. [DOI] [PubMed] [Google Scholar]
- 29.Dickmann LJ, Tay S, Senn TD, Zhang H, Visone A, Unadkat JD, et al. Changes in maternal liver Cyp2c and Cyp2d expression and activity during rat pregnancy. Biochem Pharmacol. 2008;75:1677–87. doi: 10.1016/j.bcp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaminsky LS, Guengerich FP. Cytochrome P-450 isozyme/isozyme functional interactions and NADPH-cytochrome P-450 reductase concentrations as factors in microsomal metabolism of warfarin. Eur J Biochem. 1985;149:479–89. doi: 10.1111/j.1432-1033.1985.tb08950.x. [DOI] [PubMed] [Google Scholar]
- 31.Jager W, Correia MA, Bornheim LM, Mahnke A, Hanstein WG, Xue L, et al. Ethynylestradiol-mediated induction of hepatic CYP3A9 in female rats: implication for cyclosporine metabolism. Drug Metab Dispos. 1999;27:1505–11. [PubMed] [Google Scholar]
- 32.Hartley DP, Dai X, He YD, Carlini EJ, Wang B, Huskey SE, et al. Activators of the rat pregnane X receptor differentially modulate hepatic and intestinal gene expression. Mol Pharmacol. 2004;65:1159–71. doi: 10.1124/mol.65.5.1159. [DOI] [PubMed] [Google Scholar]
- 33.Mahnke A, Strotkamp D, Roos PH, Hanstein WG, Chabot GG, Nef P. Expression and inducibility of cytochrome P450 3A9 (CYP3A9) and other members of the CYP3A subfamily in rat liver. Arch Biochem Biophys. 1997;337:62–8. doi: 10.1006/abbi.1996.9752. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Strobel HW. Regulation of CYP3A9 gene expression by estrogen and catalytic studies using cytochrome P450 3A9 expressed in Escherichia coli. Arch Biochem Biophys. 1997;344:365–72. doi: 10.1006/abbi.1997.0230. [DOI] [PubMed] [Google Scholar]
- 35.Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, et al. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol. 2000;14:27–39. doi: 10.1210/mend.14.1.0409. [DOI] [PubMed] [Google Scholar]
- 36.Jeong H, Choi S, Song JW, Chen H, Fischer JH. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008;38:62–75. doi: 10.1080/00498250701744633. [DOI] [PMC free article] [PubMed] [Google Scholar]