Abstract
Mechanistic studies underlying dopaminergic neuron death may identify new drug targets for the treatment of Parkinson disease (PD). Epidemiological studies have linked pesticide exposure to increased risk for sporadic PD. Here, we investigated the role of c-Jun N-terminal kinase 3 (JNK3), a neural-specific JNK isoform, in dopaminergic neuron death induced by the pesticides rotenone and paraquat. The role of JNK3 was evaluated using RNA silencing and gene deletion to block JNK3 signaling. Using an antibody that recognizes all isoforms of activated JNKs, we found that paraquat and rotenone stimulate JNK phosphorylation in primary cultured dopaminergic neurons. In cultured neurons transfected with Jnk3-specific siRNA and in neurons from Jnk3−/− mice, JNK phosphorylation was nearly abolished, suggesting that JNK3 is the main JNK isoform activated in dopaminergic neurons by these pesticides. Paraquat- and rotenone-induced death of dopaminergic neurons was also significantly reduced by Jnk3 siRNA or Jnk3 gene deletion and deletion of the Jnk3 gene completely attenuated paraquat-induced dopaminergic neuron death and motor-deficits in vivo. Our data identify JNK3 as a common and critical mediator of dopaminergic neuron death induced by paraquat and rotenone, suggesting that it is a potential drug target for PD treatment.
Keywords: Dopaminergic neuron, JNK3, Paraquat, Parkinson disease, Reactive oxygen species, Rotenone
INTRODUCTION
Parkinson disease (PD) is the second most common aging-related neurodegenerative disorder, characterized by selective and progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the midbrain (1). Although several gene mutations have been linked to familial PD, the etiology of sporadic PD, which accounts for 90% to 95% of all PD cases, is largely undefined. The prevailing hypothesis is that increased risk for sporadic PD is most likely associated with multiple factors including exposure to environmental toxicants and a complex interaction between genetic factors and environmental exposure to neurotoxicants. At the time of PD diagnosis, approximately 80% of dopaminergic neuron terminals in the striatum and 60% of dopaminergic neurons in the SNpc are already lost in PD brains (2). Current therapy focuses on symptomatic relief and there is a great need to identify neuroprotective therapies that will slow disease progression. The elucidation of cell death mechanisms common to several models of PD may identify new drug targets for PD treatment.
Epidemiological studies have suggested a potential link between increased risk for PD and occupational exposure to pesticides, including paraquat (3). Alarmingly, a recent study based on exposure data from the Central Valley of California suggests that residential exposure to paraquat and maneb, 2 commonly used pesticides in that region, increases risk for PD (4). Paraquat and rotenone are pesticides used worldwide (5) that selectively kill dopaminergic neurons in primary cultures (6, 7). Chronic administration of paraquat or rotenone to rodents in vivo induces many key features of PD, including motor deficits, loss of dopaminergic neurons and the presence of α-synuclein-containing inclusion bodies (8–16). Thus, treatment of rodents or cultured cells with paraquat or rotenone provides a useful model to study mechanisms of dopaminergic neuron death associated with PD.
JNKs are mitogen-activated protein (MAP) kinases that are preferentially activated by cell stress including environmental stress and toxic chemical insults (17, 18). Notably, JNKs are activated by oxidative stress, a key mechanism underlying dopaminergic neuron death associated with PD pathogenesis (2, 19–22). Increasing in vitro and in vivo evidence suggests a role for JNK in the pathogenesis of dopaminergic neuron death in PD models (23). For example, (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) (MPTP) activates JNK and the JNK kinase MKK4 in the nigrostriatal system (24). Gene transfer of the JNK interacting protein-1, which interferes with JNK signaling, protects dopaminergic neurons in the MPTP model of PD (25). CEP-1347, a pharmacological inhibitor of mixed lineage kinases that are upstream kinase kinases of JNK, attenuates MPTP-mediated nigrostriatal dopaminergic neuron loss and MPTP-induced JNK activation (26). In addition, dopaminergic neuron death induced by rotenone, paraquat and 6-hydroxydopamine (6-OHDA) all require JNK activation (7, 27–30). There are 3 Jnk genes: Jnk1, Jnk2, and Jnk3 (31). The goal of this study was to determine whether JNK3, the only neural specific JNK isozyme, is critical for dopaminergic neuron death induced by paraquat or rotenone.
MATERIALS AND METHODS
Animals
Generation and characterization of the Jnk3−/− mice was described (32). JNK3 heterozygotes (Jnk3+/−) were bred to generate littermates of Jnk3+/+, Jnk3+/−, and Jnk3−/− embryos for culture or adult mice for in vivo paraquat administration.
Primary mesencephalic neuron cultures and drug treatments
Primary cultured dopaminergic neurons were prepared from mesencephalon of E14 C57/BL6 mouse embryos (Charles Rivers, Wilmington, MA), or Jnk3+/+, Jnk3+/− and Jnk3−/− individual embryos, as described (33). For single embryo cultures, PCR genotyping of the embryos was performed after the culture and the results were matched to each embryo at the end of the experiment. Therefore, all experiments were performed blinded regarding the status of Jnk3 genotype. Cells were plated (3–5 × 104 cells in 100 μl) on 9-mm-diameter Aclar embedding film (Electron Microscopy Sciences, Fort Washington, PA) that had been pre-coated with 100 μg/ml poly-D-lysine and 4 μg/ml laminin (BD Bioscience, Bedford, MA). The cultures were maintained at 37°C in a humidified 7% CO2 atmosphere. After overnight incubation, fresh culture medium was added. Thereafter, half of the medium was changed every 48 hours.
Rotenone (Sigma, St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO) as 10-mM stock solution and paraquat (Sigma) was dissolved in water as 400 mM stock. Drugs were diluted in N2 medium (Invitrogen, Carlsbad, CA) right before the drug treatments. When cell cultures were treated with rotenone, the final concentration of DMSO did not exceed 0.0001%. All drug treatments were performed in defined serum-free N2 medium. Half of the medium was replaced with N2 medium on the day before drug treatment and then again at the time of drug treatment. Cultures treated with vehicle were used as controls.
Immunoblot analysis
After treatments, protein lysates were prepared from cells and analyzed by SDS-PAGE gel electrophoresis and western blotting, as described (6). Anti-active caspase-3 and anti-phospho-JNK antibodies (p-Thr183 and p-Tyr185) were purchased from Cell Signaling Technology (Beverly, MA). Anti-β-actin antibody was from Sigma.
siRNA
siRNA against Jnk1, 2, or 3 and scrambled control, non-silencing siRNA were described (34) and purchased from Qiagen (Valencia, CA). Jnk1 siRNA sequence is 5’ GAAGCUCAGCCGGCCAUUUdTdT 3’; Jnk2 siRNA 5’ GCCUUGCGCCACCCGUAUAdTdT 3’; Jnk3 siRNA 5’ GCCAGGGACUUGUUGUCAAdTdT 3’; Scrambled siRNA 5’ UUCUCCGAACGUGUCACGUdTdT 3’. E14 Sprague–Dawley rat mesencephalic primary neurons were plated on 24-well or 48-well plates at 80% density and transfected with siRNA using TransMessenger Transfection Reagent (Qiagen) according to the manufacturer's protocol. The final siRNA concentration was 2.5 µg/ml. An enhanced GFP expression vector was co-transfected to identify transfected cells (4:1 for siRNA:enhanced GFP).
Immunocytochemistry and quantification of neurons and JNK phosphorylation
Neuron cultures were fixed with 4% paraformaldehyde /4% sucrose for 30 minutes at room temperature (RT) and blocked for 1 hour in blocking buffer (PBS containing 5% BSA, 5% normal goat serum, and 0.1% Triton X-100). Cells were then incubated with primary antibodies in blocking buffer at 4°C overnight. Primary antibodies included mouse monoclonal antibody against tyrosine hydroxylase (TH; 1:500; Sigma), rabbit polyclonal antibody against TH (1:50,000; Pel-Freez, Rogers, AR), and rabbit polyclonal antibody against phospho-JNK (1:100; Cell Signaling). After 3 washes with PBS, cells were incubated at RT for 1 hour with appropriate secondary antibodies: Alexa Fluor 488, 568 or 660 goat anti-rabbit IgG and Alexa Fluor 488, 568 or 660 goat anti-mouse IgG (1:200; Molecular Probes, Eugene, OR). Cells incubated as above but without primary antibodies were used as negative controls for staining specificity (data not shown).
Cells immunostained for TH and having neurites twice the length of the soma were scored as TH+ cells under a fluorescence microscope (Leica, Heidelberg, Germany). All TH+ cells on a 9-mm-diameter Aclar embedding film were scored. The total number of TH+ cells in vehicle control treated cultures of each genotype or siRNA-transfected group was defined as 100% survival. The number of TH+ cells in rotenone- or paraquat-treated cultures was divided by that in the respective vehicle control cultures and presented as percent survival.
To quantify the extent of JNK phosphorylation in TH+ neurons, cells were double-stained with anti-p-JNK and anti-TH antibody. Images of 8 random fields from each well were captured using a fluorescent microscope equipped with a digital camera (Axiovert 200M, Zeiss, Thornwood, NY). The staining intensity of p-JNK in the transfected TH+ neuron population was quantified using NIH Image J program (http://rsb.info.nih.gov/ij/). All transfected TH+ neurons in captured images were scored and the average p-JNK level per TH+ neuron was calculated. At least 50 TH+ cells were used to quantify p-JNK per data point. The p-JNK staining units were arbitrary and the relative intensity was normalized to vehicle control-treated group. All cell counting was performed in a blinded manner.
Drug administration in vivo
Male and female 10-week-old Jnk3+/+ (n = 11) and Jnk3−/− (n = 13) littermates were group-housed (4 to 5 per cage) in a room maintained under constant temperature (72–74°F) and humidity and a 12-hour light/dark cycle (light on at 06:00 a.m.) with ad libitum access to food and water. Mice were habituated to the vivarium for at least 1 week before commencement of experiments. Mice were injected i.p. with either saline (vehicle) (n = 5 for Jnk3+/+ and n = 6 for Jnk3−/−), or 10 mg/kg paraquat dichloride hydrate (Sigma) dissolved in saline (n = 6 for Jnk3+/+ and n = 7 for Jnk3−/−), 2X/week for 6 weeks for a total of 12 doses, a procedure modified from previous reports (13, 14, 35). Body weights were determined before each injection to ensure that there was no marked weight loss. Animals were cared for and treated in accord with National Institutes of Health and the University of Washington Animal Care Committee guidelines.
Behavioral tests and data analysis
At 24 hours after the last paraquat injection, mice were subjected to open field test and then rotarod test. All behavioral tests were carried out by the researcher without knowledge of the genotype of the animals. Data from both male and female mice were pooled and analyzed together because no statistically significant difference was observed between males and females.
For the open field test, mice were tested in a rectangular open field arena (48 × 48 cm, 30 cm high) made of white hard plastic board (36, 37). Locomotor activity was recorded with a video camera placed on the ceiling above the arena and connected to an automated video tracking system (Polytrack system, San Diego Instruments, San Diego, CA). Locomotor activity was measured and analyzed as total distance traveled (in cm) during the first 30 minutes.
At 3 days after the open-field test, mice were tested on a rotarod apparatus as described (36, 38). Briefly, mice were placed on the stationary cylinder of the rotarod apparatus (San Diego Instruments). Before the test, mice were habituated on the apparatus for at least 3 consecutive trials in which the rod was kept at constant speed (first trial at 0 rpm and the rest at 4 rpm). Once the animals were able to stay on the rod rotating at 4 rpm for at least 60 seconds, they were subjected to the rotarod test. Mice were placed individually on the rod rotating at an accelerating speed from 4 to 29 rpm in 300 seconds. The time before animals fell off the rod was recorded with a maximum cut-off of 300 seconds. Mice were tested for 8 consecutive trials with at least 5-minute intervals. The data from the last 4 trials were averaged as the latency to fall.
Immunohistochemistry and stereological quantification of neurons
After motor behavior tests, animals were intracardially perfused with heparinized saline followed by 4% paraformaldehyde. Brains were harvested and post-fixed in 4% paraformaldehyde overnight, and then cryoprotected in PBS with 30% sucrose for over 2 days. Fixed brains were cut into 40-μm sections and collected in cryoprotectant. Sections were stored at −20°C before staining.
Sections were washed with PBS, blocked for nonspecific binding, and incubated with antibody against TH (1:1000; Sigma) in PBS containing 0.1% Triton X-100, 5% BSA and 5% goat serum. Sections were washed and incubated with avidin-biotin solution using the Vectastain Elite kit (Vector Laboratories, Burlingame, CA) for 1 hour at RT. Sections were treated with 0.3% H2O2 for 10 minutes to quench endogenous peroxidase activity, then developed in 3–3'-diaminobenzidine tetrachloride (DAB) for 2 to 5 minutes. Sections were rinsed several times in tap water and coverslip-mounted.
TH+ neurons were counted stereologically and in a blinded manner, as described (39). Briefly, analysis was carried out with a Zeiss Axiovert 200M microscope equipped with motorized stage in 3 axes. The boundaries of SNpc were outlined using the set of anatomical landmarks according to the mouse brain atlas (40). The number of TH+ neurons was counted at high power (x60) at randomly chosen fields of the SNpc, and the number of total TH+ neurons on each slide was calculated following optical dissector rules using dedicated software (Stereology Module of Slidebook, Olympus). We began cell counting at the first slide of the SNpc section when TH+ neurons were visible, and then on every fourth slide throughout the entire SNpc. The estimate of total number of TH+ neurons in each brain was calculated using the optical dissector method (39). Brains of 4 representative animals from each genotype/treatment group were analyzed for the stereology cell counting and data presented as percent of TH+ neurons. The total number of TH+ cells in vehicle control-treated mice of each genotype was defined as 100% survival and the number of TH+ cells in paraquat-treated cultures was divided by that in the respective vehicle control mice and presented as percent survival. The remaining brains were analyzed by non-stereological TH cell counting which yielded similar results (data not shown).
Statistical analysis
Data were from at least 3 independent experiments, each with at least duplicate or triplicate determinations. Statistical analysis of data was performed using 2-way ANOVA and post-hoc Student t test.
RESULTS
Paraquat and rotenone induce JNK phosphorylation and cell death in primary cultured dopaminergic neurons
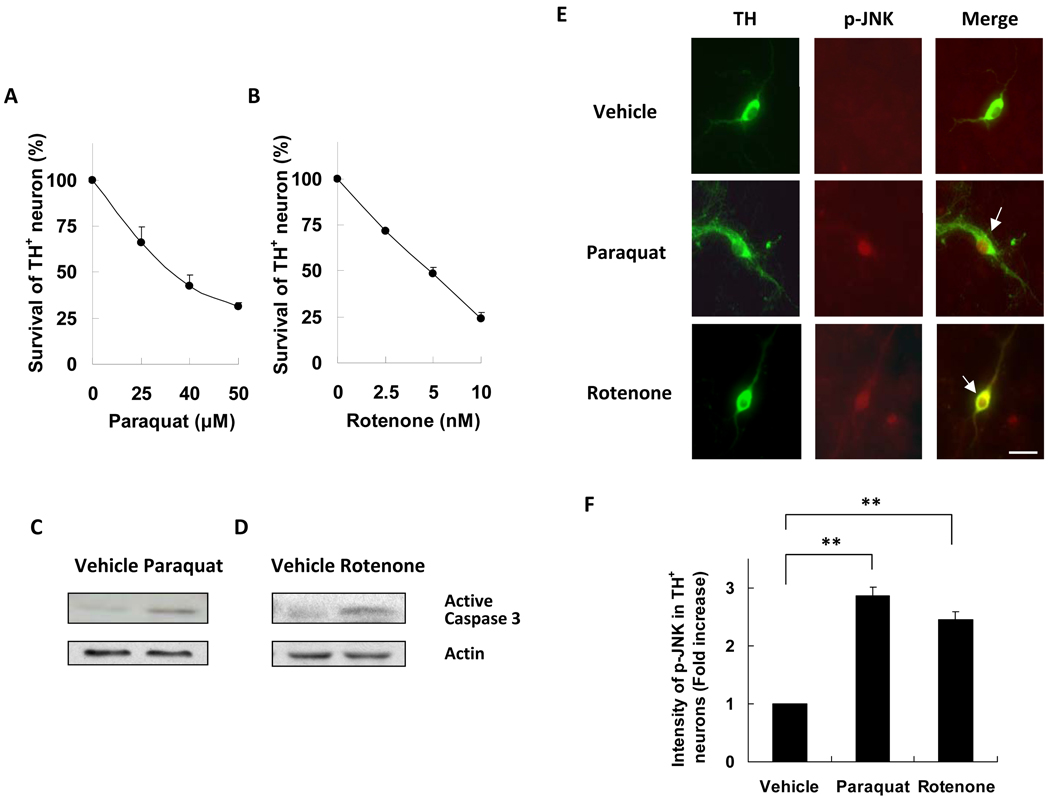
Paraquat and rotenone dose response curves for death of TH+ neurons were determined using primary neurons cultured from E14 mouse mesencephalon after 6 days in culture. Both paraquat and rotenone induced dopaminergic neuron death in a dose-dependent manner (Fig. 1A, B). Furthermore, caspase-3 was activated by both paraquat and rotenone (Fig. 1C, D), implicating apoptosis. To evaluate the contribution of JNK to cell death we monitored JNK activity using an antibody that recognizes all isoforms of phosphorylated and activated JNK (p-JNK). Treatment with either paraquat or rotenone stimulated JNK phosphorylation in primary dopaminergic neurons (Fig. 1E, F). JNK phosphorylation after paraquat treatment is consistent with data in a previous study (29).
Figure 1.
Treatment of paraquat and rotenone induces JNK phosphorylation and death of dopaminergic neurons. Primary ventral mesencephalic cultures were prepared from E14 mice and treated with paraquat, rotenone, or vehicle control. (A) Dose response of paraquat (24 hours)-induced dopaminergic neuron death. (B) Dose response of rotenone (24 hours)-induced dopaminergic neuron death. (C, D) Caspase 3 activation induced by paraquat (40 µM, 12 hours) or rotenone (5 nM, 12 hours). Actin was used as a loading control. (E) Paraquat (40 µM, 8 hours) and rotenone (5 nM, 8 hours) induce JNK phosphorylation in dopaminergic neurons. Images are representative photomicrographs of cells immunostained for tyrosine hydroxylase (TH) and for phosphorylated JNK (p-JNK). Arrows point to dopaminergic neurons with JNK phosphorylation, indicative of JNK activation. Scale bar = 20 µm. (F) Quantification of data in panel E for JNK phosphorylation in TH+ dopaminergic neuron; **p < 0.01.
siRNA silencing of Jnk3 attenuates paraquat- and rotenone-induced JNK phosphorylation and dopaminergic neuron death
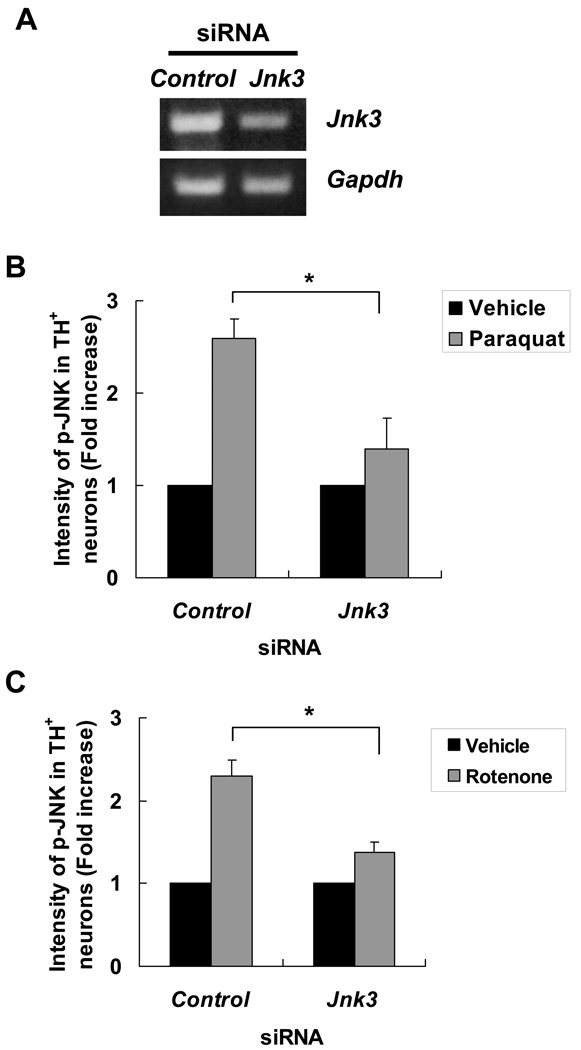
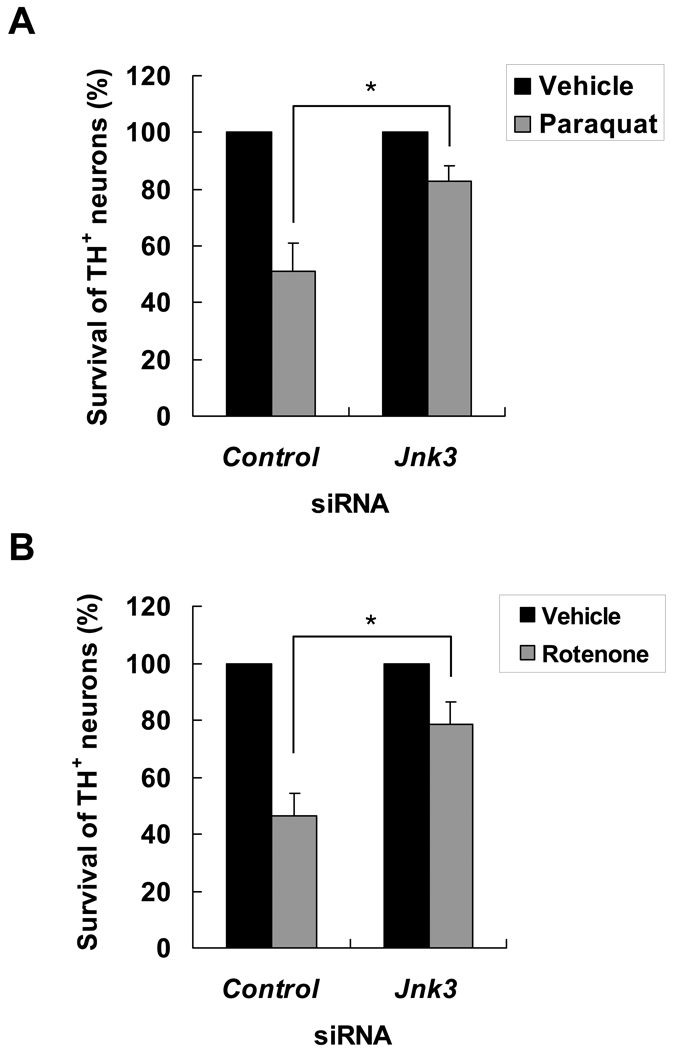
Semiquantitative RT-PCR confirmed that Jnk3 specific siRNA, but not the control siRNA, significantly reduced Jnk3 mRNA levels (Fig. 2A). Importantly, transfection of the Jnk3 siRNA significantly attenuated JNK phosphorylation after paraquat (Fig. 2B) or rotenone (Fig. 2C) treatment. However, JNK phosphorylation was not completely abolished by siRNA to JNK3. To assess the possibility that other JNK isoforms, JNK1 and 2, may also contribute, siRNA to JNK1 or JNK2 were utilized. The effectiveness and specificity of these siRNAs have been well documented (34). siRNA to JNK1 or JNK2 partially suppressed JNK phosphorylation, although the effect was much smaller than siRNA to JNK3 (Supplemental Fig. 1). These data suggest that JNK3 is the main JNK isozyme activated by paraquat and rotenone in dopaminergic neurons. Furthermore, transfection of the Jnk3 siRNA almost completely blocked paraquat- or rotenone-induced dopaminergic neuron death (Fig. 3.), suggesting that JNK3 activation plays a major role in these forms of cell death.
Figure 2.
JNK3 is the major JNK isoform in dopaminergic neurons activated by paraquat or rotenone. (A) Transfection of siRNA specific for Jnk3 reduces Jnk3 mRNA levels. E14 mesencephalic neurons were transfected with Jnk3 siRNA or control siRNA. Total mRNA was purified 24 hours later and analyzed by semiquantitative RT-PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. (B, C) Jnk3 siRNA reduced paraquat- (B) or rotenone (C)-induced JNK activation in dopaminergic neurons. At 24 hours after siRNA transfection, cultures were treated with 40 μM paraquat, 5 nM rotenone or their respective vehicle controls for another 8 hours. Cells were fixed and stained for tyrosine hydroxylase (TH) and p-JNK. The intensity of JNK phosphorylation in TH+ dopaminergic neurons was quantified. *p < 0.05.
Figure 3.
siRNA silencing of JNK3 protects dopaminergic neurons from paraquat and rotenone toxicity. (A, B) At 24 hours after transfection of siRNA, primary cultured E14 mesencephalic neurons were treated with 40 µM paraquat (A), 5 nM rotenone (B), or their respective vehicle control for another 24 hours. The cells were fixed and stained for TH. The number of TH+ neurons was normalized to vehicle control-treated groups. *p < 0.05.
Jnk3 gene deletion prevents paraquat- and rotenone-induced JNK phosphorylation and dopaminergic neuron death
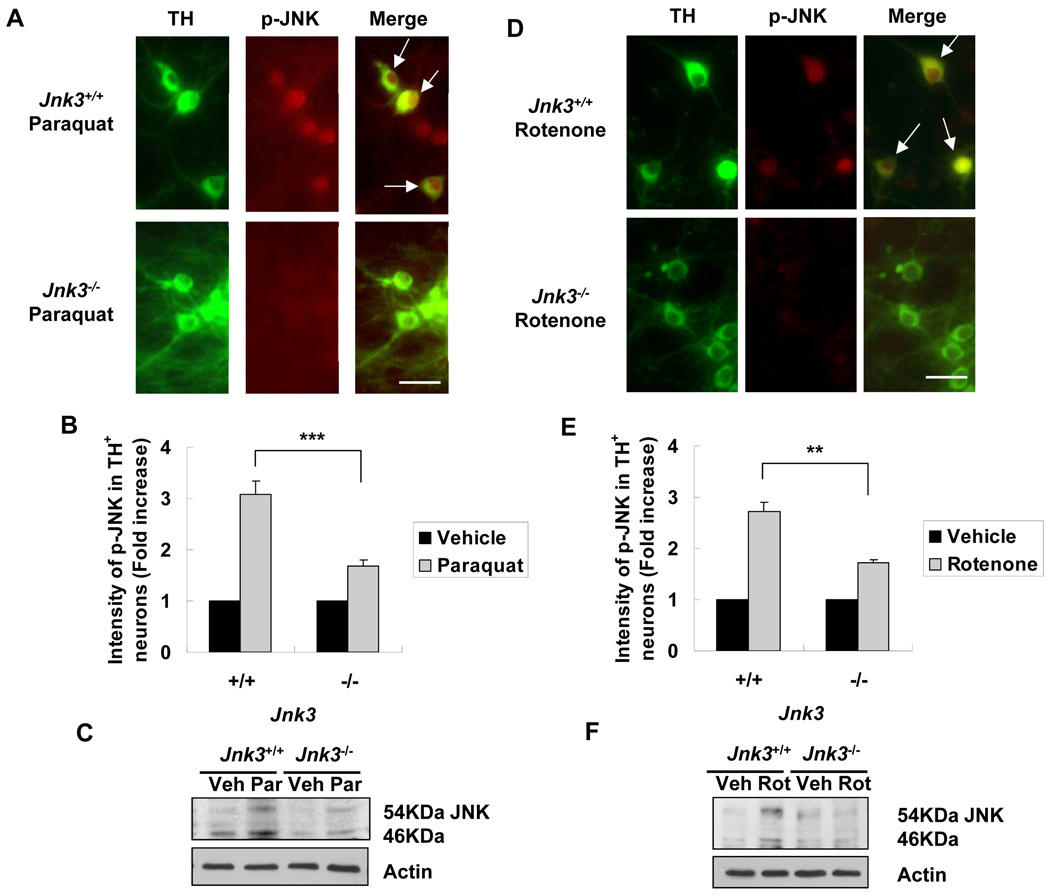
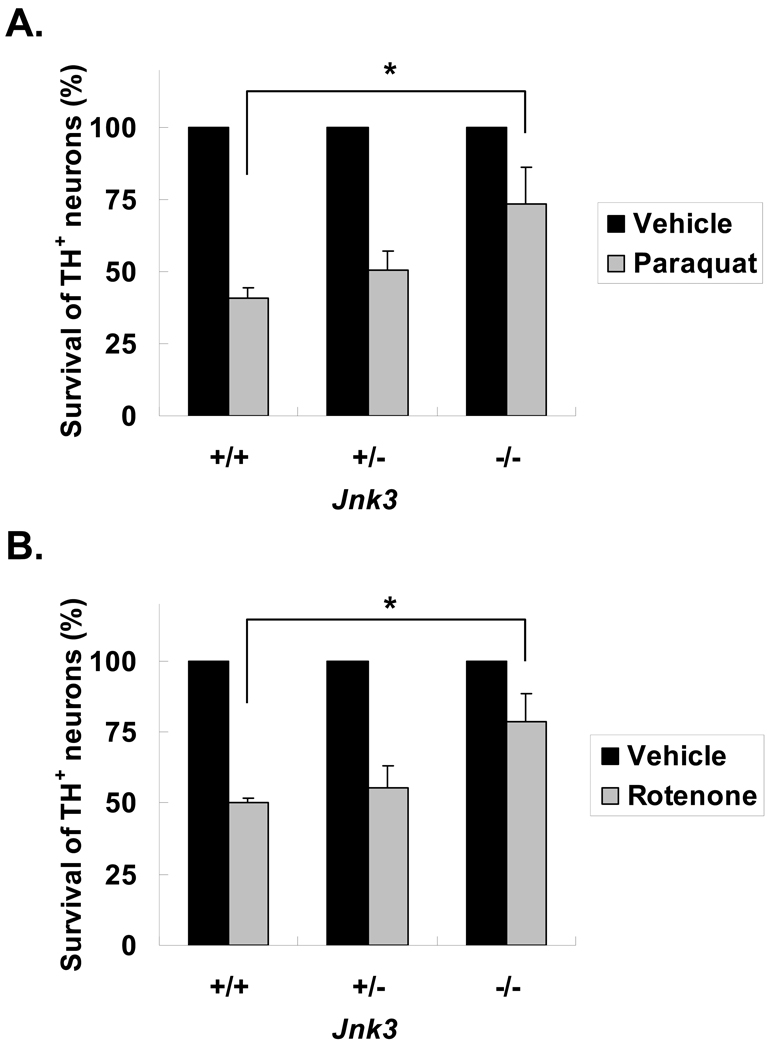
We used Jnk3 null mice to provide further genetic evidence supporting a role for JNK3 in dopaminergic neuron death. Jnk3+/− mice were bred to generate Jnk3−/− embryos so that Jnk3+/+ and Jnk3+/− littermates could be used as controls. Mesencephalic neurons were cultured from individual E14 embryos obtained from breeding Jnk3+/− mice. The genotype of each embryo was determined by PCR using residual tissue, and matched to each culture after cell counting at the completion of each experiment. Therefore, the experiments were performed blind without knowledge of the genotype of each culture. Consistent with the Jnk3 siRNA results, deletion of Jnk3 abrogated JNK phosphorylation in dopaminergic neurons (Fig. 4) and the death of these neurons caused by paraquat or rotenone (Fig. 5).
Figure 4.
Deletion of the Jnk3 gene diminishes JNK activation in culture in response to paraquat and rotenone. Jnk3 heterozygotes (Jnk3+/−) were mated and primary mesencephalic neurons were cultured separately from each E14 mouse fetus. At 6 days, cells were treated with 40 μM paraquat, 5 nM rotenone, or vehicle control for another 8 h. Cells were then fixed and immunostained for tyrosine hydroxylase (TH) and p-JNK. (A, D) Representative photomicrographs of cells immunostained for p-JNK and TH after treatment with paraquat (A) or rotenone (D). Arrows point to dopaminergic neurons with JNK phosphorylation. Scale bar = 20 µm. (B, E) Quantification of JNK phosphorylation in TH+ dopaminergic neurons. (C, F) After 8 hours of paraquat (C) or rotenone (F) treatment, cells were lysed and total protein was analyzed by immunoblotting for p-JNK. Actin was used as a loading control. Veh: vehicle; Par: paraquat; Rot: rotenone. **p < 0.01; ***p < 0.005.
Figure 5.
Tyrosine hydroxylase (TH)+ dopaminergic neurons cultured from Jnk3−/− mice are resistant to paraquat and rotenone toxicity. Cell cultures were prepared as in Figure 4, and treated on d 6 in vitro with 40 μM paraquat (A), 5 nM rotenone (B) or vehicle control. Numbers of surviving TH+ neurons were scored 24 hours later and normalized to the control group. *p < 0.05.
Dopaminergic neuron death induced by oxidative stress is also prevented by Jnk3 deletion
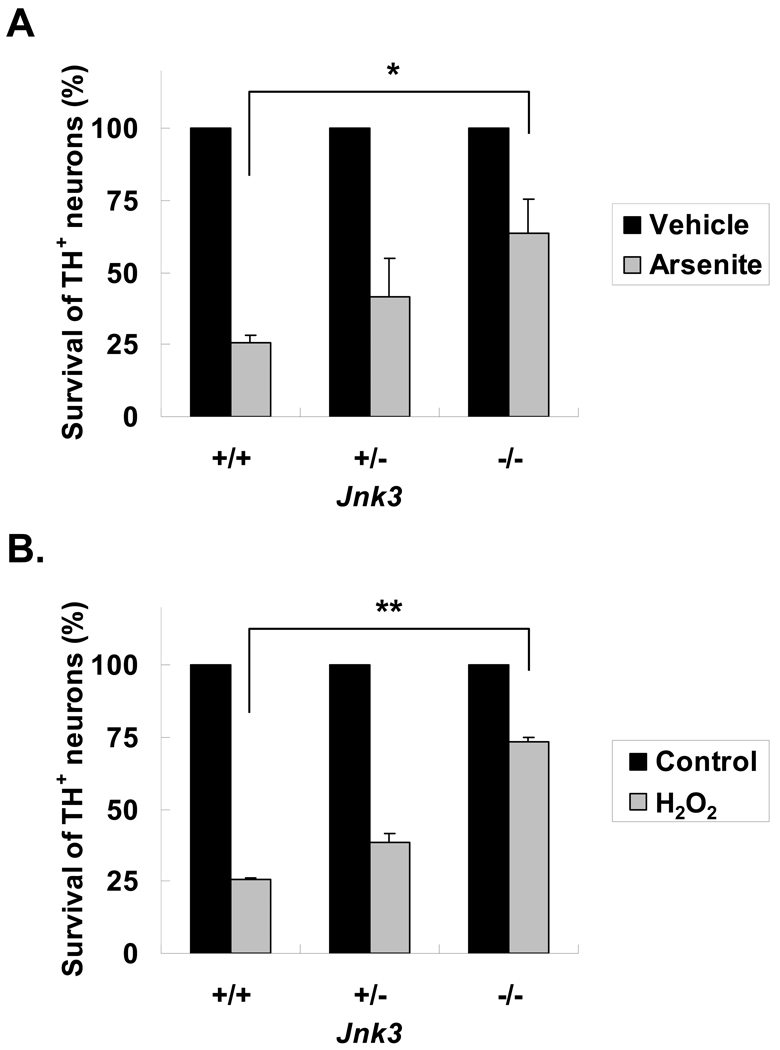
Oxidative stress has been implicated as a major underlying mechanism responsible for death of dopaminergic neurons associated with PD (41, 42); both rotenone and paraquat generate reactive oxygen species (ROS), which mediate their toxicity for dopaminergic neurons (data not shown and (43, 44)). Consequently, we also investigated whether JNK3 plays a general role in oxidative stress-induced dopaminergic neuron death using Jnk3 null mice. Sodium arsenite is known to induce cell death through JNK activation and oxidative stress in both neurons and non-neuronal cells (45–48) and H2O2 is often used as a direct and general tool to cause oxidative damage to cells. Primary cultured dopaminergic neurons prepared from Jnk3+/+ embryos were much more sensitive to sodium arsenite (Fig. 6A) and H2O2 toxicity (Fig. 6B) than those from Jnk3−/− embryos, suggesting that JNK3 contributes to the cell death of dopaminergic neurons caused by ROS.
Figure 6.
Jnk3 deletion prevents oxidative stress-induced dopaminergic neuron death. Cell cultures were prepared as in Figure 4, and treated with 10 μM Arsenite (A), 0.5 μM H2O2 (B), or vehicle control on day 6 in vitro. Numbers of surviving tyrosine hydroxylase (TH)+ neurons was scored 28 hours later and normalized to the vehicle control group. *p < 0.05; **p < 0.01.
Jnk3 deletion protects dopaminergic neurons against paraquat in vivo
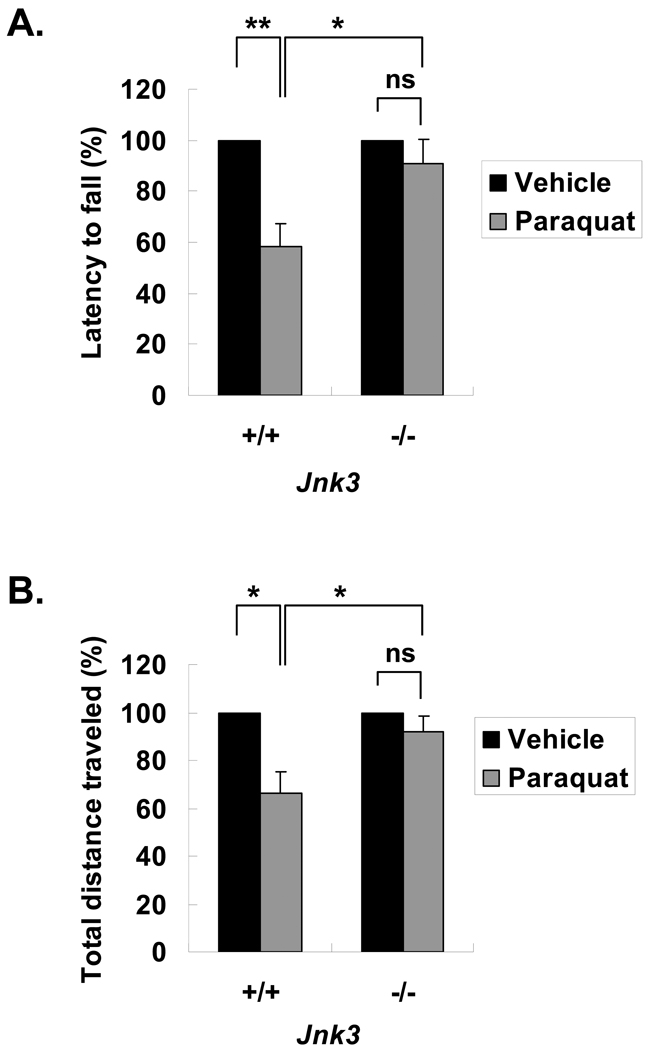
To evaluate the importance of JNK for dopaminergic neuron degeneration in vivo, mice were treated with paraquat. Motor function examined 1 day after the last injection. Paraquat treatment of Jnk3+/+ mice impaired performance on the rotarod and reduced the total distance traveled in the open field test (Fig. 7; Supplemental Table 1), indicating a deficit in motor function. In contrast, paraquat did not affect open field activity or rotarod performance of Jnk3−/− mice.
Figure 7.
Paraquat-induced motor deficits in mice are attenuated by Jnk3 deletion. Jnk3+/+ and Jnk3−/− littermates were treated with paraquat (10 mg/kg) or vehicle control saline 2X/week for 6 weeks, and assessed for motor functions. (A) Latency to fall in accelerated rotarod test. (B) Locomotor activity in open field test, quantified as total distance traveled. *p < 0.05; **p < 0.01; n.s. = not significant.
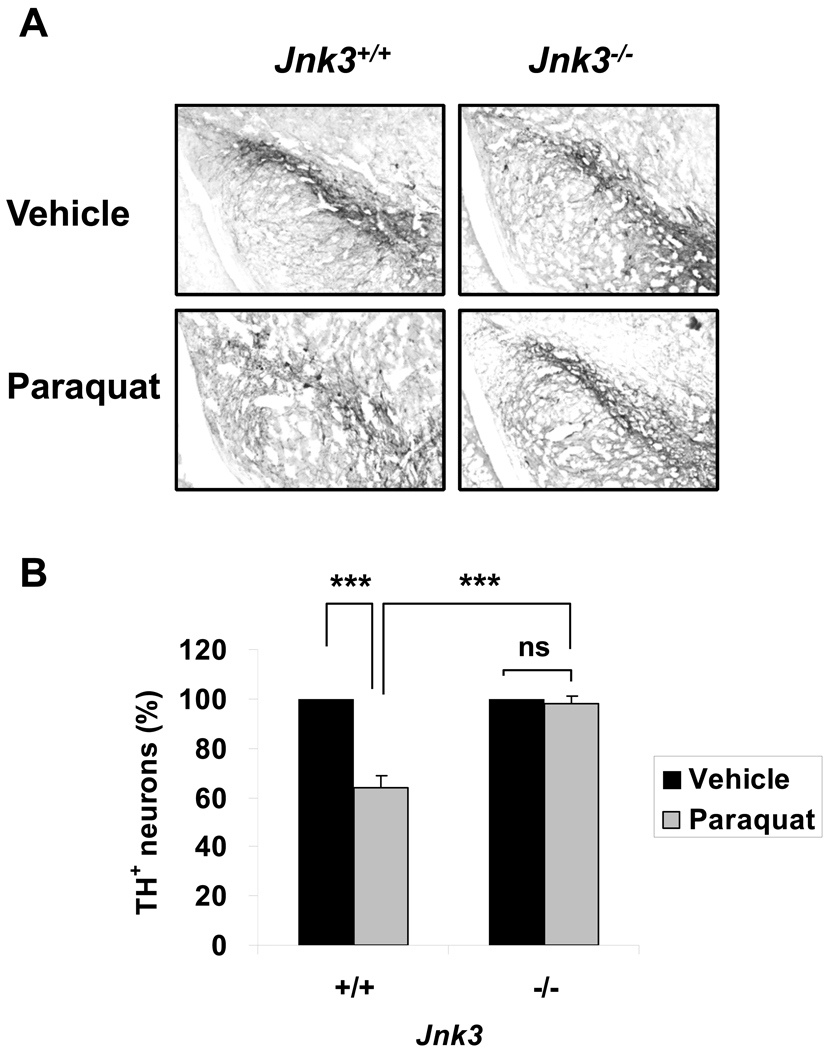
Paraquat reduced the number of TH+ dopaminergic neurons in the SNpc of Jnk3+/+ mice but not Jnk3−/− mice (Fig. 8). The number of dopamine transporter-positive neurons was similarly decreased in Jnk3+/+ mice but not in Jnk3−/− mice (percent survival of dopamine transporter-positive neurons in paraquat-treated Jnk3+/+ or Jnk3−/− mouse brains relative to the respective vehicle control-treated brains: 77.5% ± 2.0% and 99.2% ± 3.2%, respectively). These data demonstrate a critical role for JNK3 in the paraquat-model of PD in vivo in mice.
Figure 8.
Jnk3 deletion protects tyrosine hydroxylase (TH)+ dopaminergic neurons in the substantia nigra pars compacta (SNpc) from paraquat toxicity in vivo. Jnk3+/+ and Jnk3−/− littermates were treated with paraquat as in Figure 7. After motor function tests, mice were killed for TH immunostaining. (A) Representative photomicrographs of SNpc sections stained for TH. (B) Stereological quantification of the number of TH+ neurons in the SNpc, normalized to vehicle control-treated groups. ***p < 0.005; n.s. = not significant.
DISCUSSION
We tested the hypothesis that the neural specific JNK3 is a critical and common mediator of dopaminergic neuron death caused by paraquat and rotenone. Using RNA silencing and targeted gene deletion to block JNK3 expression, we found that JNK3 is the principal JNK isoform activated by rotenone and paraquat in primary dopaminergic neurons. Furthermore, blocking JNK3 expression protected primary dopaminergic neurons against paraquat and rotenone toxicity. Finally, Jnk3 null mice were protected from paraquat-induced motor deficits and dopaminergic neuron death in the SNpc. These data suggest a major role for JNK3 in dopaminergic neuron death in the pesticide-models for PD.
There was still a slight increase in JNK phosphorylation in paraquat- or rotenone-treated JNK3−/− cultures (Fig. 4) or in cultures transfected with siRNA to JNK3 (Fig. 2). Furthermore, siRNA to JNK1 or JNK2 partially suppressed JNK phosphorylation, although to a much lesser degree than siRNA to JNK3 did (Supplemental Fig. 1). Thus, while paraquat and rotenone induce JNK activation primarily through JNK3, the JNK1 and JNK2 isoforms may also contribute to this process to a much smaller degree.
JNK3 gene deletion or siRNA suppression of JNK3 expression also did not completely protect dopaminergic neurons in culture against paraquat and there was still a small loss of TH+ neurons after paraquat treatment (Figs. 3, 5). On the other hand, TH+ neurons in the brains of mice treated with paraquat were almost completely spared in JNK3 null mice (Fig. 8). This slight discrepancy may be the result of differences in experimental paradigms used for in vitro vs. in vivo studies. For example, 40 µM paraquat was used in the cell culture study, which killed approximately 60% of the TH+ neurons (in Fig. 5). This concentration is likely higher than that reached in the mouse brain after IP injection of paraquat in vivo, which killed about 38% of TH+ neurons. Paraquat at higher concentrations (e.g. 40 µM in culture) may kill TH+ neurons through JNK3-independent mechanisms.
The rotenone in vivo PD model was initially established in rats (8). Interestingly, Inden et al recently reported the rotenone mouse model after oral administration (16). It will be interesting in the future to determine whether JNK3 gene deletion protects dopaminergic neurons against rotenone in vivo in the mouse brain.
Oxidative damage has been implicated as a major convergence point whereby genetic, dietary, and environmental factors may cause dopaminergic neuron degeneration (41, 42). These include treatments with rotenone, paraquat, MPTP, and 6-OHDA (43, 44, 49, 50). Our data demonstrate that Jnk3 deletion also protects primary dopaminergic neurons against H2O2, a direct ROS, and against sodium arsenite, which induces cell death in multiple cell types through oxidative stress (45–48). Thus, JNK3 may be generally required for ROS-mediated dopaminergic neuron degeneration.
JNK activation has been reported in α-synuclein transgenic mouse brains and human PD brains (50, 51), suggesting a role for JNK activation in PD pathogenesis. The critical role for JNK in dopaminergic neuron death in the MPTP, 6-OHDA, rotenone and paraquat PD models suggests that pharmacological inhibitors of JNK signaling may be useful for PD treatment.
Disappointingly, a clinical trial using CEP1347 for PD treatment was terminated because it failed to offer significant improvement (52). However, among the 3 JNK isoforms, JNK1 and 2 are ubiquitously expressed in adult tissues and CNS neurons have extremely high basal JNK1/2 activities (46, 47, 53). JNK1 and JNK2 undoubtedly have important physiological functions in the adult, and side effects associated with inhibition of these enzymes would limit tolerable doses of JNK inhibitors. Consequently, general inhibition of all JNK isoforms, such as those achieved by CEP1347, may offer limited benefit to dopaminergic neurons at tolerable doses. By contrast, JNK3 is neural-specific (31, 54–56) and does not exhibit high basal activity in the brain (46, 47). Furthermore, JNK3 is expressed in striatum (55) and in SN neurons (57). Our data suggest that selective inhibition of the neural specific JNK3 isoform may be more specific for slowing PD progression. Furthermore, individual polymorphisms in Jnk3 gene may contribute to individual susceptibility to PD.
Deletion of the Jnk3 gene alone also protects many types of neurons against several other forms of injury, including ischemia/hypoxia, excitotoxicity, and axotomy of facial motor neurons or dorsal root ganglion neurons (32, 58, 59). Cortical neurons cultured from Jnk3−/− mice were also protected from β-amyloid toxicity (60). Thus, specific inhibition of JNK3 may provide a novel therapy for various forms of neurodegeneration including PD.
Supplementary Material
ACKNOWLEDGMENT
We thank Drs. Thomas Burbacher and Toby Cole for the use of their rodent behavior lab.
This work was supported by NIH grants ES012215 and ES013696 (ZX), by Post-doctoral Fellowship Program of KOSEF (WSC) and by NIH Environmental Pathology/Toxicology Training Grant (HK). This work was also facilitated by grant No. P30 HD02274 from the National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 2.Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 3.Brown TP, Rumsby PC, Capleton AC, et al. Pesticides and Parkinson's disease--is there a link? Environ Health Perspect. 2006;114:156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costello S, Cockburn M, Bronstein J, et al. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169:919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ecobichon DJ. Toxic effects of pesticides. In: Klaassen CD, editor. Casarett & Doull's Toxicology: The Basic Science of Poisons. New York, NY: The McGraw-Hill Companies, Inc.; 2001. pp. 763–810. [Google Scholar]
- 6.Hsuan SL, Klintworth HM, Xia Z. Basic fibroblast growth factor protects against rotenone-induced dopaminergic cell death through activation of extracellular signal-regulated kinases 1/2 and phosphatidylinositol-3 kinase pathways. J Neurosci. 2006;26:4481–4491. doi: 10.1523/JNEUROSCI.4922-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klintworth H, Newhouse K, Li T, et al. Activation of c-jun n-terminal protein kinase is a common mechanism underlying paraquat- and rotenone-induced dopaminergic cell apoptosis. Toxicol Sci. 2007;97:149–162. doi: 10.1093/toxsci/kfm029. [DOI] [PubMed] [Google Scholar]
- 8.Betarbet R, Sherer TB, MacKenzie G, et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 9.Sherer TB, Kim JH, Betarbet R, et al. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- 10.Manning-Bog AB, McCormack AL, Li J, et al. The herbicide paraquat causes up-regulation and aggregation of alpha -synuclein in mice. paraquat and alpha -synuclein. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- 11.McCormack AL, Di Monte DA. Effects of L-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. J Neurochem. 2003;85:82–86. doi: 10.1046/j.1471-4159.2003.01621.x. [DOI] [PubMed] [Google Scholar]
- 12.Manning-Bog AB, McCormack AL, Purisai MG, et al. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks AI, Chadwick CA, Gelbard HA, et al. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999;823:1–10. doi: 10.1016/s0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- 14.Thiruchelvam M, McCormack A, Richfield EK, et al. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson's disease phenotype. Eur J Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- 15.Thiruchelvam M, Richfield EK, Baggs RB, et al. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: Implications for Parkinson's disease. J Neurosci. 2000;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inden M, Kitamura Y, Takeuchi H, et al. Neurodegeneration of mouse nigrostriatal dopaminergic system induced by repeated oral administration of rotenone is prevented by 4-phenylbutyrate, a chemical chaperone. J Neurochem. 2007;101:1491–1504. doi: 10.1111/j.1471-4159.2006.04440.x. [DOI] [PubMed] [Google Scholar]
- 17.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 18.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 19.Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 20.Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link? Exp Neurol. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 22.Moore DJ, West AB, Dawson VL, et al. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 23.Peng J, Andersen JK. The role of c-Jun N-terminal kinase (JNK) in Parkinson's disease. IUBMB Life. 2003;55:267–271. doi: 10.1080/1521654031000121666. [DOI] [PubMed] [Google Scholar]
- 24.Saporito MS, Thomas BA, Scott RW. MPTP activates c-Jun NH(2)-terminal kinase (JNK) and its upstream regulatory kinase MKK4 in nigrostriatal neurons in vivo. J Neurochem. 2000;75:1200–1208. doi: 10.1046/j.1471-4159.2000.0751200.x. [DOI] [PubMed] [Google Scholar]
- 25.Xia XG, Harding T, Weller M, et al. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:10433–10438. doi: 10.1073/pnas.181182298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saporito MS, Brown EM, Miller MS, et al. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons In vivo. J Pharmacol Exp Ther. 1999;288:421–427. [PubMed] [Google Scholar]
- 27.Choi WS, Yoon SY, Oh TH, et al. Two distinct mechanisms are involved in 6-hydroxydopamine- and MPP+-induced dopaminergic neuronal cell death: role of caspases, ROS, and JNK. J Neurosci Res. 1999;57:86–94. doi: 10.1002/(SICI)1097-4547(19990701)57:1<86::AID-JNR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Newhouse K, Hsuan SL, Chang SH, et al. Rotenone-induced apoptosis is mediated by p38 and jnk map kinases in human dopaminergic SH-SY5Y cells. Toxicol Sci. 2004;79:137–146. doi: 10.1093/toxsci/kfh089. [DOI] [PubMed] [Google Scholar]
- 29.Peng J, Mao XO, Stevenson FF, et al. The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. J Biol Chem. 2004;279:32626–32632. doi: 10.1074/jbc.M404596200. [DOI] [PubMed] [Google Scholar]
- 30.Ries V, Silva RM, Oo TF, et al. JNK2 and JNK3 combined are essential for apoptosis in dopamine neurons of the substantia nigra, but are not required for axon degeneration. J Neurochem. 2008;107:1578–1588. doi: 10.1111/j.1471-4159.2008.05713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta S, Barrett T, Whitmarsh AJ, et al. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- 32.Yang DD, Kuan CY, Whitmarsh AJ, et al. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 33.Choi WS, Kruse SE, Palmiter RD, et al. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A. 2008;105:15136–15141. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Luo W, Reiser G. Proteinase-activated receptor-1 and -2 induce the release of chemokine GRO/CINC-1 from rat astrocytes via differential activation of JNK isoforms, evoking multiple protective pathways in brain. Biochem J. 2007;401:65–78. doi: 10.1042/BJ20060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves R, Thiruchelvam M, Baggs RB, et al. Interactions of paraquat and triadimefon: Behavioral and neurochemical effects. Neurotoxicology. 2003;24:839–850. doi: 10.1016/S0161-813X(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 36.Cole TB, Martyanova A, Palmiter RD. Removing zinc from synaptic vesicles does not impair spatial learning, memory, or sensorimotor functions in the mouse. Brain Res. 2001;891:253–265. doi: 10.1016/s0006-8993(00)03220-0. [DOI] [PubMed] [Google Scholar]
- 37.Fernagut PO, Chalon S, Diguet E, et al. Motor behaviour deficits and their histopathological and functional correlates in the nigrostriatal system of dopamine transporter knockout mice. Neuroscience. 2003;116:1123–1130. doi: 10.1016/s0306-4522(02)00778-9. [DOI] [PubMed] [Google Scholar]
- 38.Rozas G, Lopez-Martin E, Guerra MJ, et al. The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J Neurosci Methods. 1998;83:165–175. doi: 10.1016/s0165-0270(98)00078-8. [DOI] [PubMed] [Google Scholar]
- 39.Mandir AS, Przedborski S, Jackson-Lewis V, et al. Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci U S A. 1999;96:5774–5779. doi: 10.1073/pnas.96.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hof PR, Young WG, Bloom FE, et al., editors. Comparative cytoarchitectonic atlas of the c57bl/6 and 129/sv mouse brains with CD ROM. Netherlands: Elsevier; 2000. [Google Scholar]
- 41.Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease: Evidence supporting it. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- 42.Danielson SR, Andersen JK. Oxidative and nitrative protein modifications in Parkinson's disease. Free Radic Biol Med. 2008;44:1787–1794. doi: 10.1016/j.freeradbiomed.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherer TB, Betarbet R, Stout AK, et al. An in vitro model of Parkinson's disease: Linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCormack AL, Atienza JG, Johnston LC, et al. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J Neurochem. 2005;93:1030–1037. doi: 10.1111/j.1471-4159.2005.03088.x. [DOI] [PubMed] [Google Scholar]
- 45.Bernstam L, Nriagu J. Molecular aspects of arsenic stress. J Toxicol Environ Health, Part B Crit Rev. 2000;3:293–322. doi: 10.1080/109374000436355. [DOI] [PubMed] [Google Scholar]
- 46.Namgung U, Xia Z. Arsenite-induced apoptosis in cortical neurons is mediated by c-jun n-terminal protein kinase 3 and p38 mitogen-activated protein kinase. J Neurosci. 2000;20:6442–6451. doi: 10.1523/JNEUROSCI.20-17-06442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Namgung U, Xia Z. Arsenic induces apoptosis in rat cerebellar neurons via activation of JNK3 and p38 MAP kinases. Toxicol Appl Pharmacol. 2001;174:130–138. doi: 10.1006/taap.2001.9200. [DOI] [PubMed] [Google Scholar]
- 48.Cai B, Chang SH, Becker EB, et al. p38 MAP kinase mediates apoptosis through phosphorylation of BimEL at Ser-65. J Biol Chem. 2006;281:25215–25222. doi: 10.1074/jbc.M512627200. [DOI] [PubMed] [Google Scholar]
- 49.Pan J, Wang G, Yang HQ, et al. K252a prevents nigral dopaminergic cell death induced by 6-hydroxydopamine through inhibition of both mixed-lineage kinase 3/c-Jun NH2-terminal kinase 3 (JNK3) and apoptosis-inducing kinase 1/JNK3 signaling pathways. Mol Pharmacol. 2007;72:1607–1618. doi: 10.1124/mol.107.038463. [DOI] [PubMed] [Google Scholar]
- 50.Hunot S, Vila M, Teismann P, et al. JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:665–670. doi: 10.1073/pnas.0307453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frasier M, Walzer M, McCarthy L, et al. Tau phosphorylation increases in symptomatic mice overexpressing A30P alpha-synuclein. Exp Neurol. 2005;192:274–287. doi: 10.1016/j.expneurol.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 52.The Parkinson Study Group PRECEPT Investigators. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007;69:1480–1490. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- 53.Figueroa-Masot XA, Hetman M, Higgins MJ, et al. Taxol induces apoptosis in cortical neurons by a mechanism independent of Bcl-2 phosphorylation. J Neurosci. 2001;21:4657–4667. doi: 10.1523/JNEUROSCI.21-13-04657.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin JH, Mohit AA, Miller CA. Developmental expression in the mouse nervous system of the p49(3F12) SAP kinase. Mol Brain Res. 1996;35:47–57. doi: 10.1016/0169-328x(95)00181-q. [DOI] [PubMed] [Google Scholar]
- 55.Mohit AA, Martin JH, Miller CA. p493F12 kinase: a novel MAP kinase expressed in a subset of neurons in the human nervous system. Neuron. 1995;14:67–78. doi: 10.1016/0896-6273(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 56.Kumagae Y, Zhang Y, Kim OJ, et al. Human c-Jun N-terminal kinase expression and activation in the nervous system. Molecular Brain Research. 1999;67:10–17. doi: 10.1016/s0169-328x(99)00013-3. [DOI] [PubMed] [Google Scholar]
- 57.Lee JK, Park J, Lee YD, et al. Distinct localization of SAPK isoforms in neurons of adult mouse brain implies multiple signaling modes of SAPK pathway. Brain Res Mol Brain Res. 1999;70:116–124. doi: 10.1016/s0169-328x(99)00136-9. [DOI] [PubMed] [Google Scholar]
- 58.Kuan CY, Whitmarsh AJ, Yang DD, et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100:15184–15189. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keramaris E, Vanderluit JL, Bahadori M, et al. c-Jun N-terminal kinase 3 deficiency protects neurons from axotomy-induced death in vivo through mechanisms independent of c-Jun phosphorylation. J Biol Chem. 2005;280:1132–1141. doi: 10.1074/jbc.M410127200. [DOI] [PubMed] [Google Scholar]
- 60.Morishima Y, Gotoh Y, Zieg J, et al. {beta}-Amyloid induces neuronal apoptosis via a mechanism that involves the c-jun n-terminal kinase pathway and the induction of fas ligand. J Neurosci. 2001;21:7551–7560. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.