The ARVO 2009 Summer Eye Research Conference (SERC 2009) on Ophthalmic Drug Delivery Systems was held July 31 and August 1, 2009, at the National Institutes of Health (NIH) in Bethesda, Maryland. The conference provided an opportunity to gather a diverse group of more than 200 experts from both academic ophthalmology and the ophthalmic pharmaceutical industry, including laboratory researchers and clinicians, to discuss recent advances in delivery systems that convey ocular drugs to the posterior segment and how these systems might be successfully used in commercial products.
The two-day meeting comprised the following nine sessions: (1) eye anatomy and ocular barriers to drug transport, (2) the vitreous humor in drug delivery, (3) intravitreal drug delivery, (4) transscleral drug delivery for retinal diseases, (5) preclinical benchmarks for retinal drug therapy, (6) animal models for evaluating drug delivery systems, (7) topical therapy for retinal diseases, (8) clinical trials, and (9) new data from abstracts.
This meeting was co-sponsored by the Association in Research in Vision and Ophthalmology (ARVO) and the NIH to expand and continue ongoing collaborative interchange and synergic endeavors that were addressed at an earlier conference, held May 4 and 5, 2007, and sponsored by the Pfizer Ophthalmics Research Institute Conference.1 SERC 2009 was organized by Cheryl L. Rowe-Rendleman, PhD (Omar Consulting Group, LLC), Michael R. Robinson, MD (Allergan Inc.), and Henry F. Edelhauser, PhD (Emory University). Edelhauser and Paul A. Sieving, MD, PhD, started off the meeting with introductory comments.
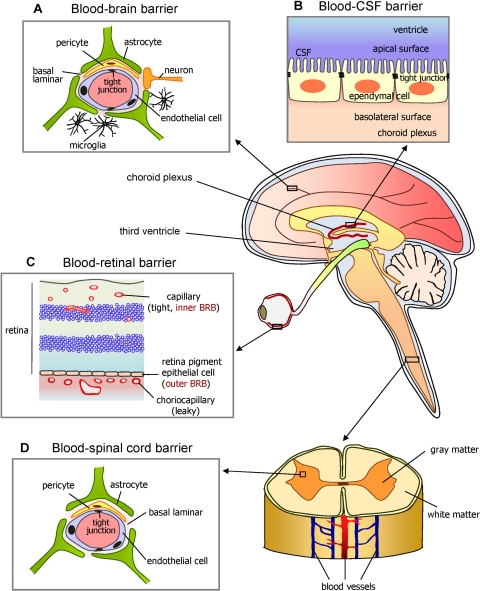
Edelhauser began by briefly discussing the history of basic science research involving ophthalmic drug delivery. He touched on the difficulty in finding a flawless technique to deliver drugs targeting diseases that directly affect the retina and vitreous humor, owing to the anatomic barriers and physiologic clearance mechanisms of the blood–neural barriers (BNBs). The BNBs comprise the blood–retinal barrier (BRB) posteriorly and the blood–aqueous barrier (BAB) anteriorly (Fig. 1). He also mentioned the importance of the transcellular and paracellular transport pathways across or between epithelial or endothelial BRBs (Fig. 2). Edelhauser described the purpose of SERC 2009, designed as a forum in which researchers and clinicians could have an open dialog about their work, during and between meeting sessions, all in the service of advancing the field of ophthalmology.
Figure 1.
Diagram of the brain and spinal cord illustrating how the eye's inner and outer blood-retinal barriers (BRBs) fit into the overall scheme of blood-neural barriers (BNBs). Barriers between the blood and neural tissues are collectively referred to as BNBs and include the blood-brain barrier (A), the blood-CSF barrier (B), the BRB (C), and the blood-spinal cord barrier (D). Other similar specialized barriers in the body include the blood-labyrinth barrier in the inner ear, the blood-nerve barrier in the peripheral nervous system, and the blood-testis barrier. Their function is to act as semipermeable paracellular passive diffusion barriers or gates to large and hydrophilic solutes. Small, lipophilic essential nutrients and toxic metabolites are delivered or removed, respectively, through passive or active site-specific transcellular carrier–mediated influx or efflux transporters. Considering that several retinal disorders are accompanied by dysfunction or breakdown of this BRB and their associated cell-cell signaling mechanisms, elucidating the nature of the BRB is important for understanding normal health and disease. The static morphologic structure responsible for all these organ-specific barriers is the tight junction (TJ). Counterintuitively, curative drug therapy to these protected sites requires that drugs circumvent these naturally protective barriers. Reprinted with permission from Choi YK, Kim KW. Blood-neural barrier: its diversity and coordinated cell-to-cell communication. BMB Rep. 2008;41:345–352.
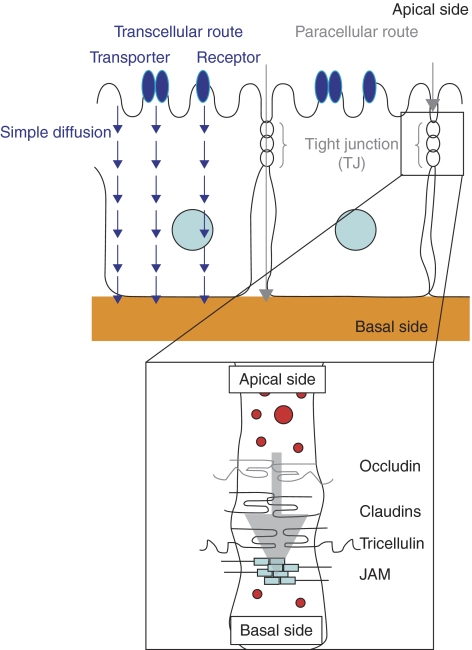
Figure 2.
Diagram of the transcellular and paracellular transport pathways across or between epithelial or endothelial BRBs of the posterior segment. Tight junctions (TJs) are the most apical component of both the epithelial or endothelial intercellular junctional complexes. TJs are crucial for the formation and maintenance of epithelial and endothelial paracellular barriers since they semipermeably regulate intercellular passive diffusion of large or hydrophilic ions and solutes. Moreover, a complex network of TJ proteins have been identified, with the assembly and dynamic maintenance of various claudin proteins being the most crucial in regard to dictating the selectivity of this paracellular barrier. Using the transcellular route, drugs are delivered by simple passive diffusion to bind with cell surface membrane–bound transporters (cell surface receptors, pumps, channels, and transporters) where they can directly cross the cell via passive diffusion again or through active transport mechanisms. Various influx and efflux transporters have been found for small lipophilic peptides, organic anions, and cations. Transporter-mediated drug delivery is tissue-specific and has low toxicity since transmembrane concentration gradients are not required for it work. The transcellular pathway is not suitable for high-throughput production of drug candidates though, unlike the paracellular pathway, which is suitable for high-throughput production since drug modification is not needed and one method can be applied for various drugs. Knowing the rate-limiting tissue barrier based on the physiochemical properties of a drug helps when optimizing the absorption of passively penetrated drugs. Reprinted with permission from Matsuhisa K, Kondoh M, Takahashi A, Yagi K. Tight junction modulator and drug delivery. Expert Opin Drug Deliv. 2009;6:509–515.
Sieving, director of the National Eye Institute (NEI), outlined the major ophthalmic blinding diseases in the United States and their pathophysiologies, pointing out their economic costs to society. In conclusion, he noted that, many times, the most crucial step in successfully translating promising drugs from the bench to the bedside is identifying the best drug delivery route.
Given the emphasis on translational science and the nonsynchronized arrangement of lectures and posters, this summary of SERC 2009 presents the major topics of the meeting by theme, not necessarily in the order of their original presentation. Each lecture has been included in the material for the pertinent area of study (see meeting agenda in the Supplementary Material, http://www.iovs.org/cgi/content/full/51/11/5403/DC1).
The basic science part of this article focuses on the anatomic barriers to the five major modes of ocular drug delivery: intraocular, periocular, hybrid, topical, and systemic. The second half is a review of the clinical and regulatory components of translational science.
Treatments and Unmet Needs
Gerald J. Chader, PhD, provided an overview of the steps that scientists in industry and academia are taking to finding new ophthalmic treatments and what he believes to be the basic keys to success in translational research. He declared that correctly assessing the major unmet needs in the treatment of ophthalmic diseases is the first step in moving from the bench to the bedside. Subsequently, a series of steps are needed to develop and clinically implement a safe and effective new treatment, be it a drug, device, or procedure.
Chader then summarized the current state-of-the-art technology that is being used to treat some major posterior segment diseases, noting that diseases sharing a locus may have different unmet needs.
Combined Anterior and Posterior Segment Diseases
Endophthalmitis, uveitis, and glaucoma are common combined anterior and posterior segment diseases, but each presents a different unmet need. For endophthalmitis, the unmet need is prevention. When prevention fails, treatment is most effectively performed with prompt intravitreal and subconjunctival antibiotics, with or without vitrectomy and systemic antibiotics. For uveitis, good short-term control of the disease can be obtained with topical, periocular, or systemic anti-inflammatory or immunosuppressive drugs (e.g., corticosteroids). The major unmet need involves the treatment of chronic or recurrent uveitic disease, because long-term treatment with these agents commonly results in toxicity and complications due to cumulative dose-related side effects, such as cataract and glaucoma. Finally, for glaucoma, although fair to adequate control of intraocular pressure can be obtained with topical drugs and various anterior segment procedures and surgeries, a treatment for optic nerve or ganglion cell neurodegeneration is still largely unavailable.
Posterior Segment Diseases
Concerning strictly posterior segment diseases, the two major blinding diseases in the United States are diabetic retinopathy (DR) and age-related macular degeneration (AMD). DR is the main cause of irreversible blindness in adults aged 20 to 65 years, with incidence rates of 56% for nonproliferative DR and 29% for proliferative DR. Macular edema (ME) occurs in approximately 10% of diabetics. AMD is the main cause of irreversible blindness in adults older than 65 years. The prevalence of all forms of AMD in the 65- to 74-year age group is 20%, and it is closer to 35% in older age groups.2
Degenerative Diseases
The two other classes of visually significant retinal diseases discussed at SERC 2009 were degenerative and tumorigenic diseases: retinitis pigmentosa, Leber's congenital amaurosis, and the chorioretinal tumors, which include retinoblastoma, choroidal melanoma, and intraocular lymphoma.
In some patients with DR, the sequelae of retinopathic disease may be partially preventable or slowed down with proper diet, weight management, and intensive blood glucose control with systemic medications, such as insulin or oral hypoglycemic agents. Despite efforts at prevention, DR remains a chronic disease with a high prevalence rate among people with diabetes. When DR progresses to the point of becoming a vision-threatening complication, laser photocoagulation and vitreous surgery are only modestly effective in recovering or maintaining visual potential. The recent advances in the development of new therapeutic agents, such as intravitreal drugs (e.g., steroids or anti-vascular endothelial growth factor), also have been helpful, but more effective agents are still greatly needed.
For AMD, although oral antioxidants in the form used in the Age-Related Eye Disease Study can modestly reduce the progression rate at some stages of dry AMD, it was emphasized that no truly effective treatment is currently available for patients with advanced disease. In contrast, for wet AMD, intravitreally delivered drugs that target vascular endothelial growth factor, such as ranibizumab (Lucentis; Genentech, South San Francisco, CA) and bevacizumab (Avastin; Genentech), can be effective in stabilizing vision in the short term, although their effectiveness in the long term is still unclear. Monthly injections of anti-VEGF therapies to maximize visual potential are a significant treatment burden on the patient. As a result, the need for better treatments for AMD remains.
Chronic retinal degenerative diseases such as retinitis pigmentosa continue to lack effective treatment, as current options are either minimally effective (vitamin A) or are still under investigation in animal models (ribozyme techniques). However, treatment of a specific type of retinal degeneration, Leber's congenital amaurosis, recently has taken a large leap forward. It is the first successful human gene therapy in which a specific disease-causing mutation is replaced in the retina via an adenovirus-associated delivery system.
Tumors
Hans E. Grossniklaus, MD, and A. Linn Murphree, MD, discussed the current treatments for tumors and the related unmet needs. We are still in need of new treatment options for chorioretinal tumors, such as choroidal melanoma, since such conventional treatments as enucleation and/or local plaque radiation brachytherapy do not significantly improve long-term survival due to indolent micrometastatic disease, most commonly involving the liver. Although current local treatments are good, there is room for improvement. Similarly, intraocular lymphoma treatment must receive more consideration, as the prognosis is poor with current conventional treatments, primarily owing to the disease's origins in the central nervous system.
Although retinoblastoma has some fairly effective local and systemic treatment options, such as cryotherapy, chemoreduction and, sometimes, periocular chemotherapy, enucleation is still frequently used in patients with advanced disease. Intra-arterial chemotherapeutic drug delivery, however, is an exciting new treatment option that looks promising for the treatment of late-stage retinoblastoma, for which enucleation was formerly the only viable option. However, the long-term morbidity of this disease still makes prevention through genetic screening or possibly gene therapy the preferred goal.
Identifying and Overcoming Barriers to New Treatments
Chader then discussed the barriers to and challenges in developing and implementing safe and effective new treatment options in the clinic. He identified five key barriers: (1) developing an effective product, (2) identifying and implementing the best method of delivery, (3) using the appropriate animal model for testing the drug's safety and efficacy, (4) identifying an adequate patient sample and developing a well-considered treatment design or plan for a clinical trial to attain a satisfactory endpoint, and (5) locating a company to finance the product and guide it into the commercial market.
Chader then touched on factors that are of paramount importance in having a successful product or drug. First, the drug must be safe for the patient over the short and long term. Second, the drug must have adequate bioavailability to reach the targeted tissues in therapeutic concentrations levels—that is, the delivered therapeutic agent must reach the back of the eye in sufficient concentrations, do so in a timely manner, and remain there long enough to maintain the necessary therapeutic effect. Third, the timing of the product's launch on the commercial market is important, because the first product on the market, even if only mildly effective, typically remains quite successful until a better product emerges. Fourth, the earlier in the disease's evolution that the intervention can be introduced, the higher is its potential for overall effectiveness in preventing or slowing the disease process. Finally, if two drugs are roughly equal in their safety and efficacy, the drug that is easier to deliver, is tolerated better, and enables greater patient adherence to the regimen is likely to dominate the market. Chader mentioned that without a successful and convenient drug delivery system, drugs that are very effective at the bench or in preclinical testing often fail clinically.
Market Potential
Chader stated that the main driving force for translational research is the commercial ophthalmic market, in which U.S. consumers spent $10 billion in 2007 and are predicted to spend $14 billion in 2009.3 The ultimate marker of success is whether the treatment makes it to the clinic and thereby becomes available to treat illness.
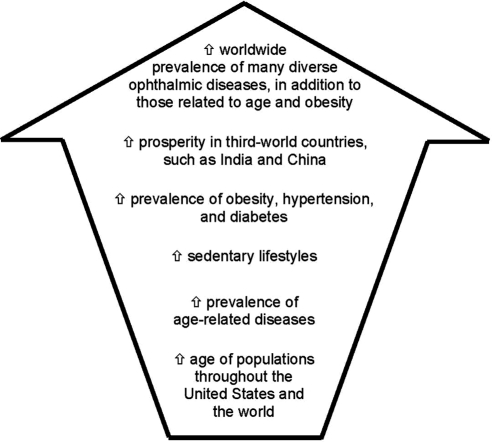
Chader emphasized the growth potential in this largely untapped market: 55% of all debilitating ocular diseases are posterior segment diseases, yet only 5% of ophthalmic pharmaceutical sales in 2007 were for treating posterior segment diseases.3 Moreover, the predictions of the future prevalence rates of posterior segment diseases issued in epidemiologic studies should also be considered: increasing aging of populations throughout the United States and the world, paired with other factors, including an increase in the prevalence of age-related diseases and sedentary lifestyles, will ultimately lead to a far greater worldwide prevalence of many diverse ophthalmic diseases, in addition to those related to only aging and obesity (Fig. 3).3 Chader also raised a very important social consideration: after cancer, Americans fear blindness most.4
Figure 3.
Factors identified in epidemiologic studies as predictors of future prevalence rates of posterior segment diseases.
He concluded by mentioning a financial consideration that should interest both the U.S. federal government and the ophthalmic health care industry: Blindness and irreversible sight impairment cost an estimated $50 billion each year in the United States and, thus, improving on the treatments available today could substantially reduce the financial burden on society.3 Chader hastened to emphasize that improvements in drug discovery and delivery will not help all blind people.
Understanding Eye Diseases' Pathogeneses and Pathophysiologies
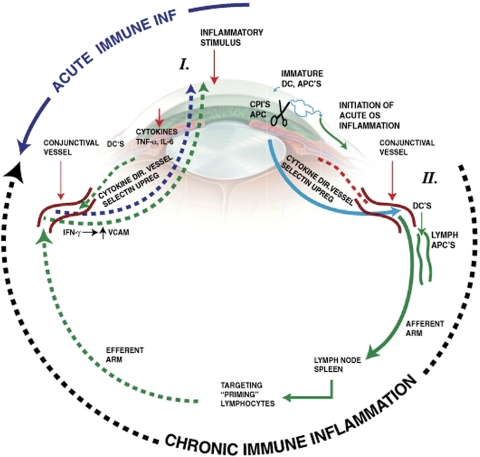
In subsequent talks, Grossniklaus illustrated that an important step in helping to develop successful treatments for ophthalmic diseases is first to understand the pathogenesis or pathophysiology of these major ophthalmic diseases. These aspects are oftentimes best understood through evaluation of the clinicopathologic correlations of their disease-causing mechanisms. Although disease origins are beyond the scope of this article, the current understanding of the causative processes involved are inflammation and immunology (observed in dry and wet AMD), neovascularization (DR and wet AMD), aging and oxidative damage (dry and wet AMD), and genetic mutations (e.g., retinal degenerations, retinoblastoma, choroidal melanoma, and intraocular lymphoma) (Fig. 4).
Figure 4.
Diagram of acute and chronic ocular immune inflammation. Acute inflammation is initiated by associated tissue damage and by a naïve cellular immune response through immature antigen presenting cells in the eye. This results in the subsequent recruitment of acute-phase inflammation cells from the conjunctival and episcleral blood vessels. In contrast, chronic immune inflammation involves procurement and processing of antigens by mature antigen presenting cells in the eye that migrate to the local afferent arm of the lymph drainage system in the conjunctiva, where antigen-APCs drain into regional lymph nodes and the spleen. This then results in a primed T-cell response that migrates out of the lymph system back into the eye via the blood vessels in the eye, including conjunctival and episcleral blood vessels. The cells adhere to vascular endothelium and enter the tissue through diapedesis. APCs indicate antigen presenting cells; CPIs, corneal proteases; DC, dendritic cells; TNF-α, tumor necrosis factor alpha; IL-6, interleukin 6; IFN-γ, interferon gamma. Reprinted with permission from McDermott AM, Perez V, Huang AJW, et al. Pathways of corneal and ocular surface inflammation: a perspective from the Cullen symposium. Ocul Surf. 2005;3:S131–S138. Artist: Elaine Kurie.
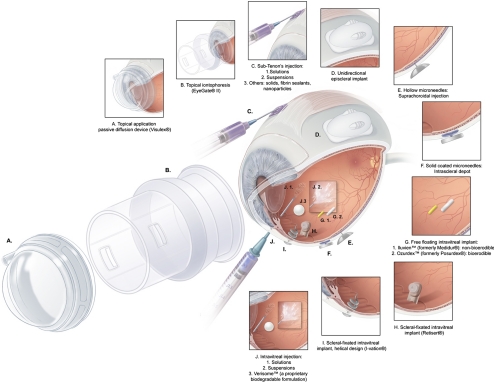
Since the main theme of SERC 2009 was centered on posterior segment drug delivery, many presenters described basic drug delivery concepts, including anatomic barriers and physiological clearance mechanisms. The treatment of retinal diseases is challenging, since the same anatomic, physiologic, and immune properties that effectively protect and nourish the healthy eye also hinder the efficient absorption of pharmaceutical drugs. Routes of drug administration that are currently used in the clinical setting to treat posterior segment diseases are the intraocular (i.e., intravitreal injection) and periocular (i.e., sub-Tenon's injection) routes (Fig. 5).5
Figure 5.
Diagram of common ocular drug delivery methods discussed at the ARVO 2009 Summer Eye Research Conference including various adjuvant implant devices. Adapted with permission from Lee SS, Robinson MR. Novel drug delivery systems for retinal diseases. Ophthalmic Res. 2009;41:124–135. © 2009 S. Karger AG, Basel.
Intraocular Drug Delivery
Intraocular drug delivery is the only mode that currently directly broaches the BRB and thereby attains the highest peak intravitreal or intraretinal drug concentrations. Therefore, compared with the other drug delivery routes, it achieves the highest intraocular bioavailability in posterior segment tissues (Table 1) such as the cone-containing macula or fovea.1,6,7 However, intraocular drug delivery is the most invasive, in that it involves penetrating the globe and thus is not free of injection-related complications. These complications may include raised IOP, floaters, vitreous hemorrhage, transient blurry vision, retinal hemorrhage, retinal tears, retinal detachment, endophthalmitis, and cataracts—with rates of endophthalmitis and retinal detachment per injection reaching 0.2% and 0.05%, respectively.8,9 In his poster presentation at SERC 2009, Daniel Roth, MD, suggested using superior rather than inferior injections, owing to the lower associated rates of endophthalmitis (0.01%, versus 0.2% for inferior injection; P = 0.011) (Roth DB, et al. IOVS 2009;50:ARVO E-Abstract 3566).
Table 1.
Comparison of Drug Delivery Modes for Retinal Diseases
| Drug Delivery Mode | Intravitreal Injection | Sub-Tenon's Injection | Suprachoroidal/Intrascleral Hollow Microneedle Injection | Topical Drops | Systemic Oral Pills |
|---|---|---|---|---|---|
| Pathway to target posterior segment | Direct | Transscleral | Transchoroidal | Transconjunctival/transscleral | Trans-RPE |
| Safety | |||||
| Risk | Highest injection risk | Minimal injection risk, mild systemic exposure | Minimal injection risk, minimal systemic exposure | Safest, but moderate systemic exposure | Minimal local exposure; highest systemic exposure |
| Adverse Events | Vitreous hemorrhage, retinal detachment, endophthalmitis | Subconjunctival hemorrhage | Subconjunctival hemorrhage, suprachoroidal hemorrhage | Conjunctival redness and irritation | Gastrointestinal upset |
| Efficacy | Most direct and effective; only mode that directly penetrates BRB | Much less bioavailable to the vitreous and retina due to anatomic barriers and several clearance mechanisms | 80-fold more bioavailable than sub-Tenon's injection; avoids subconjunctival/episclera clearance mechanism | Worst bioavailability and second worst duration of action; convenient and can be self-administered at home | Second worst bioavailability and worst duration of action; convenient and can be self-administered at home |
| Anatomic barrier(s) | Vitreous, retina | Subconjunctival/episclera, sclera, choroid, Bruch's membrane, RPE retina | Sclera, choroid, Bruch's membrane, RPE, retina | Conjunctival episclera, sclera, choroid, Bruch's membrane, RPE retina | Choroid, RPE, retina |
| Peak bioavailability | |||||
| Intravitreal | 100% | 0.01%–0.1% | 0.8%–70% | 0%–0.0004% | 0%–2% |
| Intra-aqueous humor | 3% | 0.008%–0.8% | 0.02%–2.1% | 0.0007%–5% | 1%–2% |
| Volume of distribution | Local | Local and systemic washout | Local | Local and systemic washout | Systemic and local |
| Clearance mechanism(s) | Outward vitreoretinal diffusion; aqueous humor flow | Subconjunctival/episclera; choriocapillaris | Choriocapillaris | Lacrimal tear flow, subconjunctival/episclera; choriocapillaris | Hepatic, choriocapillaris |
| Metabolism | RPE, ciliary body | Choroid, RPE, ciliary body | Choroid, RPE, ciliary body | Choroid, RPE, ciliary body | Hepatic, choroid, RPE, ciliary body |
| Duration of action | 21 Hours to 7 weeks | 6 Hours to 1 month | 18 Hours to 3 months | 30 Minutes to 4 hours | <30 Minutes |
Moreover, the presence of some moderate clearance mechanisms (posterior transretinal and anterior aqueous humor elimination pathways) cause the peak drug concentration levels achieved with intraocular administration to decline to nontherapeutic trough levels over time, unless the injections are given frequently and repeatedly, which imposes a significant treatment burden on the patient, a high clinical burden on ophthalmic health care providers, and a cumulative risk of adverse effects from each subsequent injection.1,6,7,10
The disadvantage caused by the short to medium duration of action of intravitreal drug solutions (Table 1) has been partially alleviated through product formulation (e.g., drug physiochemical changes, suspensions, free-floating or scleral-fixated intravitreal biodegradable implants, and biodegradable micro- or nanoparticles) or drug device development (e.g., free-floating or scleral-fixated, nondegradable intravitreal implants or nondegradable micro- or nanopaticles). Because implant devices are associated with risks and complications related to surgical placement and long-term drug exposure, they may be best suited for the treatment of chronic conditions that require extended drug therapy.
Overall, when compared with other modalities, intravitreal injection, and implantation may be the best in terms of efficacy and the worst in terms of safety (Table 1). Currently, despite its shortcomings, intravitreal administration is the preferred drug delivery route to treat diseases of the posterior segment.1,6–8
Static Anatomic Permeability Barriers.
Several tissues in the eye are generally regarded as static and have been considered in the past as simple diffusive barriers. To understand the pharmacokinetics of intravitreal drug delivery better, it was repeatedly emphasized in several SERC 2009 lectures, including that of Clive G. Wilson, PhD, that it is important to understand the molecular structure of the normal human vitreous gel and that normal vitreous gel undergoes age-related degenerative liquefaction. How age-related changes affect the distribution and clearance of drugs after intravitreal administration is an area of active research. The breakdown of the spacing elements of the heterotypic collagen fibrils and the loss of surface type IX collagen molecules results in the aggregation of fibrils in some parts of the vitreous and the loss of collagen fibrils in other parts, which is then converted into liquid vitreous.11
At infancy, 100% of a human's vitreous is in the gel phase and 0% is in the liquid phase, whereas in senescence, 49% is in the gel phase and 51% is in the liquid phase.11 The age-related liquefaction process does not occur uniformly within the vitreous cavity; pockets of liquid vitreous (where intravitreal drugs can collect) form in the central vitreous, where they enlarge and coalesce.11,12 Eventually, the vitreous liquefaction combined with age-related weakening of postbasal vitreoretinal adhesions predisposes elderly individuals to posterior vitreous detachment (PVD), in which the cortical vitreous gel splits away from the inner limiting lamina of the retina, usually extending as far anteriorly as the posterior border of the vitreous base.
PVDs can be localized, partial, or complete. Typically during initiation of a PVD, the residual vitreous body collapses, and the liquefied vitreous leaks through a hole in the posterior cortical vitreous, which then helps dissect the residual posterior cortical vitreous from the retina.12 During the evolution of a PVD, separation of the posterior cortical vitreous from the peripheral retina at the vitreous base does not occur, because of the strong perpendicular adhesion of vitreal collagen fibrils within the superficial retina.11,12
PVD occurs spontaneously, with a lifetime occurrence rate of approximately 25%.11,12 Normal vitreous humor has a discontinuity only in the cortical vitreous at the optic nerve and is thinned over the macula, but with PVD, the cortical vitreous often develops a second discontinuity called a premacular hole, which may help drugs delivered intravitreally to preferentially target the macula.
During sessions 1 and 2 of the SERC 2009, it was shown that immediately after intravitreal injection, drugs initially concentrate near the injection site or in the cisternae described by Worst over the short-term (8 hours to 2 days), forming vitreous concentration gradients (Laude A, et al., manuscript submitted) before distributing throughout the entire vitreous cavity and then reaching steady state equilibrium levels (Lee SS, et al. IOVS 2009;50:ARVO E-Abstract 5950).13 If fluorescein is delivered as a microsphere preparation, the particles delineate the injection bleb and the diffusion front moves ahead of the retained depot.
W. L. Fowlks14 showed that posterior meridional pararetinal flow occurred in addition to diffusion along the outer 2 mm of the vitreous after the injection of India ink into the vitreous of anesthetized rabbits. The duration of this temporary concentration gradient is not affected significantly by head or eye movement, but it is significantly decreased with age-related vitreous syneresis, since drug diffusivity and convection flow increases, resulting in increased drug distribution and even drug clearance (Lee SS, et al. IOVS 2009;50:ARVO E-Abstract 5950). However, Uday B. Kompella, PhD, showed that with intravitreal fluocinolone acetonide sustained-release devices the location of implant placement in the vitreous (anterior placement behind lens versus posterior placement near the retina) influences drug levels in various tissues. Specifically, when the implant was placed in the posterior region, drug levels were higher in the posterior retina, choroid-retinal pigment epithelium (RPE), and sclera and lower in the anterior retina, choroid-RPE, and sclera.
Thus, posterior placement of the implant is likely to support release of the drug to target tissues in case of retinal disorders. It would be beneficial if such posterior placement also reduced drug levels in lens and trabecular meshwork, tissues associated with side effects of corticosteroids. Although the posterior implant reduced drug levels in the lens, no such advantage was found with respect to corticosteroid levels in the trabecular meshwork, possibly due to the high affinity of the drug for this tissue. These trends, although apparent at the end of 2 weeks, were more prominent at the end of 4 weeks. These observations are helpful in controlling implant location and, hence, drug targeting.
Kompella thinks that after intravitreal placement of an implant, drug levels are not likely to achieve a uniform level throughout the vitreous. However, it is probable that a slow-release system achieves zones of steady state concentration, with the contours dictated by the clearance mechanisms of a given therapeutic agent. The reason for such zones or contours of steady state concentration is not necessarily diffusion limitation, but rather the presence of continuous dynamic clearance mechanisms in the posterior segment.
Kay D. Rittenhouse, PhD, added that despite these delivery platform–induced concentration gradients, the physiochemical properties of the drug are ultimately responsible for efficacy and bioavailability. Once the drug is released into a dissolving solution, the drug's intrinsic properties then dictate the overall efficacy and safety profile. She further elaborated on seeming inconsistencies in rank order of drugs of a similar class or structure. Data obtained from different preclinical models used to establish potency profiles for glucocorticoids, for example, have resulted in different outcomes. These outcomes are most likely due to the complex relationship between drug aqueous solubility and lower potency (enhancing ready drug access to tissues and rapid clearance from the tissues), versus high-potency drugs with low aqueous solubility and potentially low sustained concentrations within the eye.
In comparison to the hypocellular vitreous humor, the retina is primarily a cellular central nervous system tissue with 15- to 20-nm wide intercellular spaces that do not contain tight junctions (TJs).15 Thus, both small hydrophilic and lipophilic drugs can easily permeate the retina. Drugs with a cationic charge were found to be the most resistant to permeating the retina, followed by drugs with a large molecular size.16 This suggests that vitrectomy does not solve all the drug penetration problems, because the drugs also must enter or cross the retina.
Dynamic Physiologic Clearance Mechanisms.
There are two main mechanisms of drug clearance for intravitreally administered drugs in the eye: the anterior elimination pathway via counterdirectional bulk aqueous flow and the posterior elimination pathway via vitreoretinochoroidal bulk flow due to hydrostatic and osmotic pressure gradients in the inner, middle, and outer coats of the posterior segment.7 An additional mechanism to consider is the transcellular carrier–mediated transporters found on the RPE. Influx transporters enhance the penetration of drugs and efflux transporters inhibit retention of drugs across the outer (o)BRB.17
The duration of action of an intravitreally administered drug may in part depend on the retention of the injected drug at the site of administration. The longer the intravitreal half-life, the greater the anticipated duration of therapeutic response (i.e., a longer half-life makes less frequent doses feasible). In general, animal experiments have shown that the half-lives of drugs that are eliminated through both the retina and the aqueous humor, such as small lipophilic drugs, tend to be shorter than the half-lives of drugs eliminated primarily via the anterior route, such as large hydrophilic drugs. This shorter half-life may reflect the longer distance and smaller surface area for drug elimination found anteriorly and, secondarily, the transcellular carrier–mediated transport mechanisms found posteriorly. Limited information has been obtained about the biotransformation or metabolism of exogenously administered small molecules within the vitreous and retina. Thus, their impact on clearance mechanisms is generally unknown.
Intravitreal drug elimination also depends on the molecular weight (MW) of the drug, with larger MW (>70,000) drugs displaying longer half-lives. Quantitative structure–pharmacokinetic relationship approaches attempt to derive relationships between the physiochemical properties of drugs, such as the MW, lipophilicity, ionic charge, and solubility, and their pharmacokinetic properties, such as the volume of distribution, bioavailability, and duration of action. The quantitative structure–pharmacokinetic relationship between a drug's physicochemical properties and elimination in the vitreous was recently shown by Kompella et al.,10 who used a logarithmic regression model depending on multiple physiochemical properties, including MW, lipophilicity, and dose number (dose/solubility) of the drug.
One poster presenter, S. Kevin Li, PhD, showed that he could noninvasively monitor the intravitreal clearance of drugs in vivo by using fluorine magnetic resonance imaging (19F MRI). According to Li, although 19F MRI is a laboratory-based technique and is not currently feasible for use in a clinical setting because of its sensitivity and technical difficulties, noninvasive monitoring of the clearance of fluorine-containing drugs using 19F MR spectroscopy could advance our understanding of intravitreal clearance mechanisms and eventually allow clinicians to maximize their dosage regimens.
Dynamic Physiologic Metabolism.
The retina is believed to have the highest metabolic rate per unit weight of any tissue in the body.18,19 Thus, metabolism of essential nutrients such as glucose, amino acids, and vitamins extensively takes place in the inner coat (e.g., retina and vitreous humor) of the eye.19 Drug-metabolizing enzymes are also present in many ocular tissues, but the ciliary body and the RPE are the most active sites of xenobiotic metabolism in the eye, especially due to cytochrome P-450 and lysosomal enzymes.7 However, their influence is so small in comparison to anatomic permeation barriers and physiologic clearance mechanisms that they will not be discussed any further, except in the systemic drug delivery section, where systemic hepatic metabolism is much more pronounced than the local metabolism of the eye.
Adjuvants.
As previously mentioned, one means to overcoming the short to medium duration of action of intravitreal drug solutions is the use of one of several available sustained drug release systems. These systems may act through formulation modification to decrease the solubility of the drug via a suspension or to enhance the residence time in vitreous humor via a biodegradable implant, or through the use of a sustained-release delivery device. A review of the currently available or upcoming intravitreal devices was presented by Baruch Kuppermann, MD, PhD (Table 2).20
Table 2.
Currently Available or Upcoming Intravitreal Drug Delivery Devices
| Implant | Drug | Type | Duration of Action | Clinical Trial Progress | Disease |
|---|---|---|---|---|---|
| Vitrasert | Ganciclovir | Scleral-fixated non-biodegradable reservoir | 6 months | FDA approved March 4, 1996 | CMV retinitis |
| Retisert | Fluocinolone | Scleral-fixated non-biodegradable reservoir | 3 years | FDA approved April 11, 2005 | Uveitis |
| I-vation | Triamcinolone | Minimally invasive scleral screw helical implant | 18–36 months | Phase 1 | DME |
| Iluvien/formerly Medidur | Fluocinolone | Free-floating non-biodegradable implant | 3 years | Phase 3 | DME |
| Ozurdex/formerly Posurdex | Dexamethasone | Free-floating biodegradable implant | 6 months | FDA approved June 17, 2009 Phase 3 | ME associated with BVO and CVO, DME, uveitis DME, uveitis |
| Verisome drug platform | Triamcinolone | Free-floating non–polymer-based biodegradable platform | 1 year | Phase 2 | ME associated with BVO |
BVO, branch vein occlusion; CMV, cytomegalovirus retinitis; DME, diabetic macular edema; ME, macular edema; CVO, central vein occlusion. All names and products are the properties of the respective companies, Vitrasert and Retisert (Bausch & Lomb, Rochester, NY), I-Vation (Surmodics, Eden Prarie, MN), Iluvien (pSivida, Watertown, MA), Ozurdex (Allergan, Irvine, CA), and Versiome (Icon Bioscience, Sunnyvale, CA).
The Retisert fluocinolone acetonide implant (Bausch & Lomb, Rochester, NY) was the first device of its kind to provide sustained delivery of a drug, for the treatment of chronic noninfectious uveitis affecting the posterior segment of the eye for up to 3 years. A predecessor implant, Vitrasert (Bausch & Lomb) contains ganciclovir and is indicated for the treatment of cytomegalovirus (CMV) retinitis. The Vitrasert is larger than the Retisert, and implantation therefore requires a larger scleral incision. The evolution of intravitreal devices is toward smaller sizes, longer drug release duration, and less-invasive procedures, such as being injectable and office-based rather than requiring sclerotomies in the operating room.
Kuppermann emphasized that since multiple corticosteroid delivery devices are relatively effective, the final determinant of success may be a combination of durability of the effect and safety issues, such as rates of cataract formation and steroid-induced glaucoma. For example, Retisert has been shown to have a rate of cataract formation of nearly 100% just 2 to 3 years after placement, and roughly a 40% rate of severe steroid-induced glaucoma that requires surgical intervention. By comparison, the Ozurdex (Allergan, Inc., Irvine, CA) dexamethasone implant approved by the U.S. Food and Drug Administration (FDA) for ME due to retinal vein occlusion lasts 3 to 6 months, but has a much lower rate of cataract formation and steroid-induced glaucoma. Study results that became available in December 2009 from a pivotal 2-year trial of the Iluvien (pSivida, Watertown, MA) fluocinolone acetonide implant in 956 patients with diabetic macular edema indicated that a system that delivers lowered rather than higher dosages of the steroid over 24 months produced the best balance between safety and efficacy.
Another highlight of SERC 2009 were two talks given on the recent development of an implantable intravitreal device that uses genetically modified ARPE-19 RPE cells (transfected with plasmid gene insertion techniques) to release a therapeutic growth factor protein, ciliary neurotrophic factor (CNTF), with zero-order kinetics.21,22 Weng Tao, MD, PhD, explained that to avoid immune rejection, the cells are encapsulated within a semipermeable, hollow-fiber membrane, intravitreal implant device so that the CNTF protein is released to ocular tissues but the modified RPE cells are immunologically isolated from the patient. CNTF is a neurotrophic growth factor with profound antiapoptotic effects that prolong the lives of dying photoreceptor cells. Phase 2 trials showed that this delivery system was safe, and a dose-dependent biological effect on the retina was observed. Potential visual benefit was also shown in patients with geographic atrophy.23 The duration of action of this encapsulated cell therapy device was found to be up to 2 years. Next-phase clinical trials are under way to continue to evaluate encapsulated cell technology (ECT)-CNTF's effectiveness for the treatment of dry AMD and retinitis pigmentosa.
Periocular Drug Delivery Routes
Periocular drug delivery using the transscleral absorption pathway is one of the safest means of achieving consistent therapeutic drug concentrations in the inner coat of the posterior segment (Table 1).5,6,24 Periocular drug delivery systems, however, require drugs to pass through several layers of ocular tissue (episclera, sclera, choroid, Bruch's membrane, and RPE) to reach the retina or vitreous humor.7,25,26 As a result, steep drug concentration gradients develop, with the highest concentration developing at the sclera, the lowest concentration in the vitreous, and the second lowest in the retina. In addition, the clearance mechanisms in the subconjunctival space near the limbus appear to be robust, which can affect the amount and retention time of depot drug that is available for absorption transsclerally, especially when given via subconjunctival injection (Fig. 5).7,26–28 This clearance mechanism is perhaps less important for drug delivery by sub-Tenon's injection.25
Although the static anatomic barriers to various periocular drug delivery techniques have been examined in detail, particularly the sclera, the literature on the contributions of the dynamic clearance mechanisms and the metabolic impediments to various periocular drug delivery techniques unfortunately is still lacking.7 Current knowledge shows that the combined effects of several static anatomic barriers and dynamic clearance mechanisms generally make periocular drug delivery one of the least effective ways of attaining high peak therapeutic intraocular drug concentrations in the retina or vitreous humor (Table 1).5,6 Another reason for this inefficiency is that the posterior transretinal elimination pathway from the vitreous humor occurs in the outward direction. Thus, inward-directed periocular drug delivery goes against the natural bulk clearance flow in the posterior segment of the eye.
Taken together, these factors result in low intraocular bioavailability of drug delivered by the various periocular drug techniques, compared with that of intravitreal injection (Table 1). However, the periocular route does seem to achieve better bioavailability in the outer and middle coats of the eye. The presence of several clearance mechanisms, as alluded to earlier, which are not found with intravitreal injection, also cause periocular drug delivery to have a shorter duration of action than intravitreal injection (Table 1).7,25,26 This route has been made a more viable competitor with intravitreal injection by partially alleviating the latter disadvantage through formulation modifications and drug device development.
Overall, when the safety and efficacy of this drug delivery route is compared with that of the others for the treatment of disease in the inner coat of the posterior segment (Table 1), periocular injection is one of the best for safety, and it is in the middle to low end for efficacy, after intrinsic drug properties are factored in, such as potency and exposure–response relationships. It appears that it is ideally suited as a treatment option for mild to moderate acute posterior segment disease or for preventative drug therapy. For serious acute posterior segment diseases or chronic or recurrent diseases, periocular injection is best used as a supplement to other delivery routes. In contrast, for diseases of the middle (e.g., uveal tract) and outer coats (e.g., sclera and cornea) of the eye, this route of drug delivery is currently one of the most preferred, largely because of the higher drug exposure to the target tissues.
With a few modifications, this route has the greatest potential to surpass intravitreal injection as the preferred treatment option for disease of the inner coat of the eye, since it deposits drug locally immediately adjacent to the targeted tissue without being overly invasive. A formulation or device with zero- or first-order sustained-release kinetics may be the most promising research area that could supplant intravitreal injection as the preferred drug delivery route and relieve clinicians of the treatment burden of cumulative injection-related complications.
Static Anatomic Permeability Barriers.
The static anatomic barriers of sub-Tenon drug injection are the sclera, choroid, and RPE.25,29 The sclera is a soft connective tissue consisting of 68% water, with the remainder composed of collagen, proteoglycans, fibrocyte constituents, elastin, blood vessel constituents, and other substances.30 It therefore acts more like a sponge and a possible depot reservoir site than as a true barrier. The thickness of the sclera varies in different parts of the globe from 0.3 to 1.0 mm, typically being thinnest under the rectus muscle insertion sites or at the equator and being thickest posteriorly near the optic nerve.31 It is also perforated by numerous arteries, veins, and nerves, which again lowers the possibility of any significant barrier effects.
For large or hydrophilic drugs, the RPE (oBRB) has been found to be 10- to 100-times less permeable than the sclera and 14 to 16 times less permeable than the choroid–Bruch's membrane complex, whereas for small lipophilic drugs, the RPE, choroid–Bruch's membrane complex, and sclera are relatively equal barriers.32 Molecules that can passively diffuse across the RPE, whether by transcellular or paracellular pathways, show similar permeability values in the outward (retina-to-choroid) and inward (choroid-to-retina) directions, whereas molecules that are actively transported across the RPE via transcellular carrier–mediated transporters show differences in permeability between the two directions.16,33
The transscleral clearance mechanisms (discussed later) may also have an inhibitory role on transscleral drug permeation if the drug depot amount (volume and concentration) and scleral surface area of exposure is less than the saturation rate of the clearance mechanisms, particularly the subconjunctival–episcleral lymphatic and blood vessel clearance mechanisms.7 In addition, small, lipophilic, and/or cationic drugs may bind to melanin (acidic and polyanionic polymeric pigmented compounds) in the RPE or choroid, thereby reducing their permeation.34,35 Eumelanin is found in the pigmented melanocytes of the uvea and in melanosomes of RPE cells, both of which modify the pharmacokinetics and duration of action of these drugs at the cellular level, since less peak free drug is available, but more is released in a slower, sustained manner.34 The affinity for melanin correlates positively with the lipophilicity, alkalinity, or cationic charge of the drug being applied.36
Overall, the rate-limiting factors for posterior segment drug delivery using the periocular drug delivery route depends on the individual physiochemical properties of the drug itself—predominantly the molecular radius (≤8 nm molecular radii drugs) and the lipophilicity of the drug, and, secondarily, the shape, MW, protein and melanin binding properties, and ionic charge of the drug.25,30,35
Dynamic Physiologic Clearance Mechanisms.
When the clearance mechanisms of sub-Tenon's injection are compared to intravitreal injection, substances delivered by the sub-Tenon's route are dynamically eliminated from ocular tissues through subconjunctival–episcleral blood and lymph vessel flow (∼5%–80% drug removal rate) and choriocapillaris blood flow (∼2%–20% drug removal rate).5,24,25 The subconjunctival–episcleral clearance mechanism is composed of four separate vascular plexi: superficial subconjunctival plexus (found below the epithelium), deep subconjunctival plexus (found within Tenon's fascia but just above the episclera), superficial episcleral plexus (found in the most superficial episclera), and deep episcleral plexi (found in the deepest episclera). All four plexi are most prominent along a 4-mm zone anterior to the rectus muscle insertion sites, whereas they are markedly less vascular posteriorly. These vascular plexi contain nonfenestrated endothelia with 20-nm intercellular spaces and no TJs.26,30 Whereas the episclera and sclera are devoid of lymphatic plexi, the conjunctiva has two prominent subconjunctival lymphatic plexi, the superficial and deep subconjunctival, which run in tandem with the two vascular ones.26
Sustained-release drug depots with release rates that exceed the innate saturable clearance rates will deliver drugs transsclerally into the retina and vitreous.37 Thus, estimating this cutoff point is important when developing sustained-release drug formulations or devices for placement in the sub-Tenon's space.27,28 The excellent subconjunctival–episcleral clearance mechanism best explains why the first-order kinetics of devices that permit the sustained release of drugs rapidly becomes smaller over time as the drug release rate from the depot eventually falls below the saturation point required to overcome the clearance mechanisms. Note that clearance rates vary according to the specific physiochemical properties of each specific drug being injected or implanted. Uptake by the rapid blood flow of the choroid is another mechanism by which drugs can be eliminated, but it has not been studied as well as the subconjunctival–episcleral clearance mechanism.
Adjuvants.
A major adjuvant used to overcome the short to medium duration of action of periocular drug solutions is the development of several sustained drug release systems, whether through formulation modifications or through various sustained release drug delivery devices. Many of these innovative options have been discussed and published previously, and include liposomes, microspheres, nanoparticles, and biodegradable fibrin sealants.5
Some new ideas on this topic discussed at SERC 2009 were included in a review by Thierry Nivaggoli, PhD, on how drug formulation of the immunomodulatory drug sirolimus (rapamycin; an inhibitor of the mammalian target of rapamycin) could significantly enhance its duration of action when given by subconjunctival injection. By altering the formulation of this drug from an aqueous solution to a nonaqueous suspension (lowering its MW and solubility, and increasing its lipophilicity) and by increasing its depot concentration, Nivaggoli showed they could increase the duration of action from 2 to 6 months after a single subconjunctival injection.
Another innovative strategy discussed was the conversion of episcleral depot drug solutions to a solid form, as with a tissue tablet. Ashkan Khalili, MD, summarized his work with colleagues at the Moorfields Eye Hospital on developing a tissue tablet of bevacizumab that, when placed in the subconjunctival space, could increase the duration of action of the antibody to vascular endothelial growth factor (VEGF) from 2 hours to 6 days (Khalili A, et al. IOVS 2009;50:ARVO E-Abstract 5992).
Another possibility was presented in the poster session by Richard Eiferman, MD, who reported that he could extend the duration of action of dexamethasone from 2 weeks to nearly 6 months by placing an impermeable backing on a collagen wafer and inserting it in the sub-Tenon's space.38 This creates unidirectional transscleral diffusion into the posterior segment.
One of the highlights of the meeting was a presentation on a new innovative sealed drug depot reservoir by Murphree, from the Vision Center at Childrens Hospital in Los Angeles.39 The episcleral drug reservoir is an impermeable silicone exoplant, which prevents washout by the subconjunctival–episcleral clearance mechanism and enhances net unidirectional transscleral drug delivery to retina and vitreous.
According to Murphree, experiments using sodium fluorescein showed that containment in the episcleral drug reservoir increased intravitreal bioavailability by 30- to 40-fold and the duration of action by 21-fold compared to standard sub-Tenon's injection.40 He also mentioned that effective transscleral delivery of chemotherapeutic agents, such as carboplatin and topotecan, has been demonstrated and that biopeptides as large as bevacizumab (160 kDa) can be successfully delivered using this system.
Murphree and the company developing the system, 3T Ophthalmics, have received NCI Rapid Access to Intervention Development (RAID) funding for their Investigational New Drugs (IND) application. This funding will cover the clinical supplies necessary to begin phase 1 and 2 clinical trials with sustained-release formulations of both carboplatin and topotecan for salvage therapy of eyes with retinoblastoma. These studies should begin in the spring or summer of 2010. He also mentioned that this device could be used for drugs in both liquid and solid form and that combination therapies could be developed with either two separate devices or one device with two cavities.
Hybrid Drug Delivery
To avoid some of the shortcomings in safety and bioavailability with intravitreal or periocular injection, minimally invasive hollow and solid microneedles (<1 mm diameter) have been developed to deliver drugs into the cornea, sclera, or suprachoroidal space.41 Solid, drug-coated microneedles are used for intracorneal and intrascleral drug delivery, improved bioavailability, and duration of action. Hollow microneedles are used for intrascleral and suprachoroidal delivery of a sustained-release drug depot in a tissue layer, with clearance mechanisms that are minimal or less than those in the subconjunctival or sub-Tenon's space. Microneedles allow for better retinochoroidal targeting than periocular drug delivery, because it is closer to the target tissue.42,43
A hollow microcatheter cannulation drug delivery technique has also been developed for suprachoroidal drug delivery. It is more directly invasive than the hollow microneedle approach, but microcatheter delivery may be promising for sustained drug delivery if it can be shown that continuous infusion of drugs into the suprachoroidal space can be tolerated better than a one-time injection with a microneedle.44
Overall, despite being relatively new and having few long-term data, the hybrid delivery route is arguably the second best for safety (tied with periocular and just behind topical) and second best for efficacy (the best being intraocular). This ranking has not been definitively proven in clinical trials. Another drawback that has not yet been calculated is the greater expense associated with this technology.
Static Anatomic Permeability Barriers.
The static anatomic barriers to suprachoroidal injection are the porous tissues of the choroid, interfibrillar spaces in Bruch's membrane, TJs in the RPE, and the intercellular spaces of the retina. With intrascleral injection, one must also account for the barrier posed by the sclera (50-nm wide interfibrillar spaces). In summary, the permeation barriers are quite similar to those encountered with sub-Tenon's injection, except that the contribution of the episclera and all or part of the sclera can be subtracted. An added benefit, as far as injection volumes are concerned is that the suprachoroidal space can accept volumes up to 0.5 mL, whereas intrascleral injections are limited to 10 to 35 μL.45
Dynamic Physiologic Clearance Mechanisms.
The main clearance mechanism to both suprachoroidal and intrascleral injection is the choriocapillaris blood flow. However, with subconjunctival injection, it was determined that this clearance mechanism is minor (25% of the clearance rate) when compared with the subconjunctival–episcleral clearance mechanism.7 The circumvention of the subconjunctival–episcleral clearance mechanism may be the main reason that, in animal studies, microneedle hybrid drug delivery techniques have produced up to an 80-fold greater intraocular bioavailability than have periocular drug delivery techniques and a 3-fold longer duration of action.
Adjuvants.
As this drug delivery route has only one major shortcoming—short to medium duration of action—the main adjuvant is a sustained drug release system. Several options are available: a suprachoroidal microcannulation technique; a change in drug formulations to maximize drug depot retention; an increase of drug binding in the delivered tissues, perhaps to melanin in melanocytes of the choroid or RPE or to proteoglycans and collagen in the sclera; and intrascleral implants or devices.
An innovative idea discussed by Francine Behar-Cohen, MD, PhD, at SERC 2009 was using gene therapy to convert the normal ciliary muscle into an intraocular bioreactor that secretes the desired therapeutic protein.46–48 In studies conducted in a rat model of uveitis, she used a microneedle-enabled gene delivery technique to transfect the ciliary muscle with plasmids encoding three different variants of the p55 tumor necrosis factor-α soluble receptor. She found that the transfected ciliary muscle self-produced and release intraocular monomeric tumor necrosis factor-α–soluble receptors for up to 8 months. She also showed preclinical data that suggest that these receptors successfully induced local immunomodulation of experimental intraocular inflammation.
Topical Drug Delivery
Topical instillation of ophthalmic drops is the most common method used to administer pharmaceutical agents for ocular disease; 90% of ophthalmic drug formulations are for topical drop use. Methods of topical drug delivery include eye drops, ointments, suspensions, contact lenses, and some drug delivery devices. The topical route of administration is preferred for many classes of drugs when treating diseases of the anterior segment because of ease of access and patient compliance and because drops can easily achieve therapeutic concentration levels in anterior segment tissues. The anatomy and physiology of the eye seem to pose almost insurmountable barriers for topical drug delivery to the posterior segment tissues, but topically administered drugs have been shown to reach the back of the eye.2
The barriers to productive topical absorption of drugs into the anterior chamber are well documented.2 Further posterior movement via transcorneal absorption is thought to be prevented by the iridolenticular diaphragm and the anterior-directed aqueous humor bulk flow. In classic studies, David Maurice, PhD,49 calculated that topical drugs could reach the vitreous cavity, most likely by either the conjunctival-scleral-choroidal-RPE-retinal route or possibly the conjunctival-orbital-optic nerve head pathway, with an intravitreal bioavailability ranging from 0.0001% to 0.0004%.
Despite the extremely poor intravitreal and intraretinal bioavailability, the advantages of topical drug delivery for retinal diseases are quite appealing because it minimizes the chance of systemic side effects and is minimally invasive. Overall, if bioavailability can be improved, topical approaches to retinal disease can be convenient, self-administered, and can lower the overall treatment burden for chronic diseases.
Overall, if the specific physiochemical properties of a topical drug solution allow it to reach the retina or vitreous at therapeutic concentrations, a topical drug delivery route can be considered the best for safety and convenience. Currently, because of work in various eye models, it is thought that topical drug delivery for retinal diseases may be tied for last along with systemic delivery when it comes to efficacy. Before the topical approach can be considered a viable competitor with intravitreal injection, most conventional topical drug solutions must undergo modification, such as changes in formulation or modification for use in adjuvant drug delivery devices that will increase conjunctival penetration for better intravitreal and intraretinal bioavailability and, secondarily, greater residence time in the conjunctival cul-de-sac for longer duration of action.50
Peter A. Campochiaro, MD, summarized several preclinical experiments in mice and clinical trials in which topically administered drugs, such as nepafenac, TG100801, mecamylamine, and pazopanib were used.51–53 These studies showed that it is possible to achieve therapeutic effects in the vitreous and retina after topical delivery, in some cases by the transconjunctival–transscleral pathway. Despite these occasional successes, he mentioned that there have been many failures. Therefore, it is difficult to predict which drugs can achieve adequate therapeutic levels in the inner coat of the posterior segment after topical drug delivery and whether penetration can be enhanced by structural modifications or a particular formulation. Thus, experimental testing in animal models is critical. As the list of drugs that achieve therapeutic levels in the retina and choroid after topical administration increases, it may be possible to identify structural characteristics that promote ocular penetration and to specifically design drugs and/or prodrugs accordingly.
Static Anatomic Permeability Barriers.
The static anatomic barriers to topical drops are the corneal epithelium, conjunctival epithelium, sclera, choroid, Bruch's membrane, RPE, and the retina.30,54 The conjunctival epithelium is 55 times more permeable than the corneal epithelium and allows the passive diffusion of 12-fold larger solutes, primarily because of the combined affect of the “leaky” goblet cell subpopulation of surface cells and the larger surface area for drug contact (conjunctival surface area is 17 times greater than that of the cornea).
Because the permeability across the RPE is 10 times higher than that across the conjunctival epithelium, the conjunctiva is the rate-limiting anatomic barrier to passive paracellular penetration into the posterior segment of large and/or hydrophilic drugs.2 In contrast, for small, lipophilic drugs, the combined effects of the RPE and conjunctival epithelium are the rate-limiting anatomic barriers to passive or active transcellular penetration, primarily due to the xenobiotic efflux carrier-mediated transporter (pumps).
In summary, the permeation barriers to topical drug delivery are most similar to those of subconjunctival injection, except that the effects of the conjunctival epithelium, which decreases drug permeation approximately fivefold, must be added to the effects of the other noted barriers.2,54
Dynamic Physiologic Clearance Mechanisms.
Topical drops encounter more local clearance mechanisms before reaching the inner coat of the posterior segment than do drugs delivered by any other route. At tear turnover rates of 0.5 to 2.2 μL/min and removal at 50% to 100%, the lacrimal gland–derived tear clearance mechanism plays a major role in drug clearance from the ocular surface. Topical drop solutions typically remain on the ocular surface for an average of only 5 minutes before being washed away by the lacrimal gland-derived tears into the nasolacrimal duct. Drugs that manage to penetrate tissues may be cleared by the subconjunctival–episcleral blood and lymph vessel flow and the choriocapillaris blood flow. The cumulative effect of all the clearance mechanisms clearly explains the short duration of action of topical drug delivery and also partially explains the extremely poor intraretinal and intravitreal bioavailability of most commercially available topical drug solutions.
Adjuvants.
As this drug delivery route has two major shortcomings, extremely poor bioavailability to the inner coat of the posterior segment and a short duration of action, the main adjuvants are penetration enhancers and sustained-release drug delivery systems.
Drug development studies indicate that paracellular penetration by topical drugs can be improved by several mechanisms including: (1) opening TJs by using preservatives in topical medications or by iatrogenic epithelial scraping, (2) increasing drug lipophilicity through the use of prodrugs or other analogues, such as surfactants, and (3) binding the drugs to dendrimers that use carrier-mediated influx transporters.
One promising option presented by Ashim Mitra, PhD, at SERC 2009 involves encapsulating drugs in a biodegradable carrier with a hydrophilic exterior and a lipophilic interior. Mitra described a novel mixed micellar-based formulation of LX-214 (Lux Biosciences, Jersey City, NJ)—vitamin E TPGS and octoxynol-40 polymer—that allowed hydrophobic immunosuppressant and anti-inflammatory steroid drugs to reach the retina in therapeutic concentrations by permeation through the transconjunctival–transscleral pathway. In pharmacokinetic studies, topical application of a voclosporin preparation reached peak intraretinal and intravitreal bioavailability measurements of 0.2% and 0.001%, respectively. According to Mitra, the mixed micelle coating, with a hydrophilic exterior and a hydrophobic interior, helps the drugs evade the clearance mechanisms of the eye. An interesting finding was that the vitreous humor drug levels for both voclosporin and dexamethasone were below detectable limits, suggesting that the micelles release the drug in the choroid, just behind the RPE. Mitra noted that the safety and efficacy of this approach will be evaluated in phase 2 and 3 clinical trials soon.55
Mark Kester, PhD, discussed two experimental topical drug delivery alternatives: nanoliposomes and calcium phosphate nanoparticles, also known as nanojackets.56–61 He described proof-of-concept studies that explored the potential of subconjunctival or topical delivery of minocycline or doxycycline in encapsulated nanoliposomes (<80 nm in diameter). The drugs could be used in DR for their matrix metalloproteinase and other anti-inflammatory effects. In addition, he discussed indocyanine green–encapsulated nanojackets (<30 nm in diameter) for near infrared imaging of posterior segments of the eye. He mentioned that nanoparticles can also be pegylated on the exterior surface to evade the immune system and even to selectively deliver their contents to specific targets.
Two more highlights of the meeting were presentations on iontophoresis given by Jean-Marie Parel, PhD, and Mike Patane, PhD. Iontophoresis is an old technology that has recently been modified into a new innovative drug delivery platform. Recent clinical trials have demonstrated that iontophoresis is sufficiently safe and capable of delivering steroids to ocular tissue to treat uveitis. Development in iontophoresis may bring these devices to the U.S. commercial market by 2011.
Parel discussed the history of iontophoresis in medicine and followed by summarizing his experiences since the 1970s using the coulomb-controlled iontophoresis unit for transcorneal and transscleral drug delivery (Parel JM, et al., manuscript in preparation).46 Iontophoresis is a noninvasive method of propelling charged active compounds (e.g., low MW drugs, high MW biological proteins, and gene therapeutics) into either the anterior or posterior segment.62 It is performed by applying a small electrical current that has the same charge as the drug to create repulsive electromotive forces. He presented several examples of diseases and conditions that had been treated with ionized drugs that were driven into ocular tissues by coulomb-controlled iontophoresis to overcome poor ocular penetration properties. These included fungal keratitis, uveitis, cytomegalovirus retinitis, retinoblastoma, and proliferative vitreoretinopathy. He also showed how the sclera and cornea act as a drug reservoir or sponge, providing slow, sustained release of drugs to the retina, choroid, or cornea. He also noted that, after conventional iontophoresis, the drug concentrations in the vitreous humor, aqueous humor, and lens were almost nil.
An iontophoretic device has been evaluated in phase 2 clinical trials for the treatment of dry eye.63 Patane followed Parel with a discussion on EyeGate II (Eye Gate Pharma, Waltham, MA). He summarized results of the phase 1 safety and tolerability studies and described how procedures with the device could be routinely performed in-office in as few as 1 to 4 minutes. Preclinical and clinical studies to date have shown that iontophoresis can increase intraocular bioavailability over 100-fold more than topical drops and is able to increase the duration of action of drugs by more than threefold. Compared with past iontophoresis device models, the Eyegate II design spreads the electrical charge over a scleral surface area of 1 cm2, which is larger than that of the conventional devices described by Parel.
Previously published sustained-release formulation modifications and drug release devices such as gels, suspensions, ointments, biomucoadhesives, hydrogel contact lenses, corneal collagen shields, and conjunctival cul-de-sac insert devices, will not be reviewed. A novel device presented by Joseph Ciolino, MD, in the poster session is a prototype drug-eluting contact lens made of a polymer-drug film encapsulated within a poly-2-hydroxyethyl methacrylate (pHEMA) hydrogel. The release rate of the drug could be modified by changing the characteristics of the film's MW or its polymer-to-drug ratio. The prototype lenses that were made incorporating ciprofloxacin produced drug release with 0-order kinetics up to 4 weeks in duration.64
Kongara Papangkorn, MD, presented a novel topical drug delivery device composed of a plastic dome that is applied to and sealed on the bulbar conjunctiva. The dome decreases lacrimal gland–derived tear clearance or washout and, when combined with formulation adjuvants, such as oxymetazoline or calcium ions, resulted in intraocular drug concentrations from passive diffusion that were comparable to those of iontophoresis and markedly higher than standard topical drop application (Higuchi JW, et al. IOVS 2009;50:ARVO E-Abstract 5996; Papangkorn K, et al. 2009;50:ARVO E-Abstract 5318)65 For example, experiments with rabbits that were treated with dexamethasone sodium phosphate resulted in an intraretinal bioavailability of 4% and an intravitreal bioavailability of 1%. Based on the results of these experiments, Papangkorn reasoned that three major factors influence passive topical drug diffusion to the posterior segment tissues: (1) the contact surface area of the drug, (2) the contact time of the drug on the eye surface, and (3) the concentration of the drug in the applicator. By using their unique topical drug delivery device combined with oxymetazoline or Ca2+, they hope to deliver drugs noninvasively into the inner coat of the posterior segment with a brief treatment period of 5 to 20 minutes.
Systemic Drug Delivery Routes
Systemic penetration of many drugs, particularly large or hydrophilic ones, into the posterior segment of the eye is restricted by the BRB. For example, fluorescein transverses the choriocapillaris and penetrates the choroid in a matter of minutes as the choroidal tissue rapidly equilibrates with the blood. Further movement from the choroid into internal ocular structures such as the retina and vitreous humor, especially of large and/or hydrophilic drugs, is restricted by the RPE (oBRB) and TJs of the retinal vasculature (iBRB). Small lipophilic drugs, however, can penetrate the oBRB and iBRB, achieving appreciable concentrations in the retina and vitreous humor after systemic administration.
The dilution effect of the systemic blood volume (large volume of distribution) creates the need to use larger doses of drugs when they are administered systemically rather than in a local injection, to yield a sufficient concentration gradient in the choroid and retina. This need for a larger dose is true even for small, lipophilic drugs.2 Also, because of the rapid blood flow rate in the choroid and retinal vasculature, the duration of action of the drug is usually too brief to result in meaningful therapeutic effects.
Overcoming all these shortcomings necessitates large, continuous systemic drug infusions. These are often associated with significant systemic side effects or toxicities, some quite serious. Since passive and active transcellular transport mechanisms are present in the RPE and because small, lipophilic drugs seem to have little trouble in crossing the BRB, some drugs with specific physiochemical properties, with or without carrier molecules, may take advantage of these pathways to bypass the selective passive paracellular diffusion barrier posed by the BRB; however, the problem of their extremely short duration of action remains unresolved.
Overall, the efficacy of systemic drug delivery to the posterior segment of the eye is extremely low—tied for last with topical drops—and its safety rating is poor due to the potential risk of systemic side effects and toxicities.
Static Anatomic Permeability Barriers.
The primary static anatomic barriers to oral medications are the choriocapillaris, Bruch's membrane, the RPE, and the retina, as well as the well-known problems in absorption from the gastrointestinal tract of oral drugs.30 Because systemic blood flow from the ophthalmic artery also supplies the optic nerve via the short posterior ciliary arteries, some leakage may occur into the vitreous cavity or retina due to defects in the blood–optic nerve barrier at the level of the prelaminar optic nerve head.66,67
In summary, the permeation barriers are most similar to those seen with suprachoroidal hybrid delivery techniques, except that systemic volume effects and rapid ocular blood flow must be accounted for, in addition to the effects of oral drug delivery on the gastrointestinal tract.
Dynamic Physiologic Volume of Distribution, Clearance, and Metabolic Mechanisms.
There are several major physiologic barriers to oral delivery of a drug to the eye. One is the volume of distribution of the drug—that is, the amount of drug in the body divided by the concentration in the blood. Thus, the oral route has a substantial diluting effect on the applied original drug concentration. In addition, oral medications have high systemic clearance rates due to first-pass clearance and metabolism in the liver and short exposure times in the choroid and retinal vasculature due to the rapid flow rate in the systemic bloodstream and choroid.
The blood flow in the human choroid is the highest in the entire human body per gram of tissue (higher than in the cortex of the kidney). More than 70% of all the blood in the eye is found in the choroid nearest the retina, probably to accommodate the mitochondrial-rich photoreceptors of the retina, which have the highest metabolic rate and demands for oxygen and nutrients per gram of tissue of all cells in the human body. In the entire retinal layer, oxygen consumption is 1.5 times greater than that of the kidney, 3 times greater than that of the cerebral cortex, and 6 times greater than that of the cardiac muscle.19,68–70 Because the impinging light is converted largely into heat, the retina must have a very effective cooling system, again provided by the rapid choroidal blood flow that serves to conduct away the heat produced by absorption of light in the retina and adjacent ocular tissues. Finally, once all these systemic factors are accounted for, there are several dynamic local elimination pathways, such as the choriocapillaris or retina clearance mechanism and the transcellular xenobiotic efflux transporters of the RPE.
In summary, the combination of the systemic volume of distribution, the fast systemic and choroidal blood flow rates, hepatic clearance mechanisms, and smaller local clearance mechanisms result in this delivery route's brief duration of action. The net effect is low to medium bioavailability to the retina and vitreous, depending on whether the drug's physiochemical properties allow it to cross the BRB easily.
Adjuvants.
Most systemic drugs are formulated with excipients that help overcome their tendency toward a brief duration of action and poor to medium bioavailability to the inner coat of the posterior segment. One novel scheme to enhance the bioavailability of systemic drugs to the retina are penetration enhancers that help reversibly open up the BRB or improve transcellular penetration. Because TJ proteins are not very antigenic, it is difficult to develop antibodies against their extracellular domain, a fact that has severely hampered the development of TJ modulators.
At SERC 2009, Matthew Campbell, BSc, discussed a new experimental treatment option along these lines that employs small interfering (si)RNA technology to block claudin-5 and reversibly open rabbit inner-BRB TJs.71 Experiments in his laboratory have shown that intravenously administered claudin-5 siRNA transiently opened the inner BRB TJs to small MW (<750) tracers for 1 to 2 days. He mentioned that there are several other ways to deliver molecules to suppress claudin-5, and their recent work used short hairpin RNA delivered by adenoassociated virus.
Another novel approach to treat retinal disease by systemic drug delivery was discussed by V. Michael Holers, MD, and Bärbel Rohrer, PhD. Holers reviewed the role of the complement pathway in the pathogenesis of ARMD and described how dysregulation of the alternate complement pathway, especially in the C3 amplification loop, may be a reasonable target for treating AMD and inflammatory retinal diseases by administering the intravenous fusion protein complement receptor 2 and factor H (CR2-fH), to recognize and inhibit complement-activation products.72–74 Complement receptor 2 recognizes C3d, a tissue-bound activation product of complement-mediated inflammation (e.g., drusen), whereas the fH component of the fusion molecule is the most potent inhibitor of the alternative complement pathway.
Rohrer discussed animal experiments that show that oxidative stress sensitizes RPE cells to complement-mediated attack by decreasing regulatory cell surface membrane-bound complement inhibitors to the alternative pathway. This sensitization was further compounded by the fact that oxidative stress also alters RPE cells in such a way that soluble fH in the serum is less functionally protective.75 Complement-mediated attack of the RPE then results in sublytic activation of the membrane attack complex resulting in vascular endothelial growth factor VEGF release and breakdown of the oBRB.75 In humans, this cascade of events can result in either dry geographic atrophy or wet AMD. Rohrer concluded with evidence from several preclinical studies showing that systemic CR2-fH therapy protects the retina using experimental mouse models of retinal degeneration and choroidal neovascularization.
Making the Transition from Bench to Bedside
Currently, the medical treatment of vitreoretinal disease is limited because it is difficult to deliver pharmacologically active levels of drugs to these tissues for extended periods of time. Injection of drugs in the sub-Tenon's space avoids the risks of intravitreal injections, but many drugs cannot be effectively delivered to the back of the eye. Intravitreal injections can successfully deliver drugs to the retina, but the need for multiple injections increases both risk and treatment burden. Because of the short to medium duration of actions with either drug delivery route, there is an unmet need for safe, effective, controlled, sustained-release ocular delivery systems.
Several approaches are being studied to improve drug delivery to the back of the eye. Microneedles show great promise in balancing safety and efficacy, and this technology should be explored and more fully developed. Also, although neither the topical nor the systemic route to the posterior segment is an efficient pathway for delivering many drugs to the vitreous and retina, recent evidence suggests that these routes should not be completely abandoned, especially since systemic and topical formulations allow self-administered treatments at home and thereby lessen the clinical burden that ophthalmologists face when treating chronic age-related diseases of the eye.
To translate some of the experimental approaches described at SERC 2009 to effective research and development efforts a teamwork-based multidisciplinary approach is necessary, combining expertise from several areas, including biomaterials, bioengineering, pharmacology, toxicology, and clinical ophthalmology. In many cases, this teamwork approach has already been used, and the next step to disseminating this expertise in science into medical therapy involves know-how in clinical trial design to help identify appropriate human disease targets, acceptable clinical endpoints or biomarkers, and test populations. Collaborations between scientists in academia, physicians in the clinic, and professionals in industry are essential for bridging the gap from the bench to the commercial market. SERC 2009 also explored the practical steps in translational medicine as discoveries at the laboratory bench are translated to the bedside. The remainder of this summary will present themes of translational research, not necessarily in the order of their presentation at the conference.
Use of Patents to Protect Research
It is important that translational researchers understand how patents are used to protect inventions and intellectual property from being used, made, or sold by others for up to 20 years.
Phillip Reilly, JD, PhD, discussed the relevant policies in the United States regarding the regulation of utility patents for drug delivery. He explained that inventors must demonstrate that the subjects of their patents are useful, novel, and nonobvious. The criteria for utility can be subjective in some cases, but those for novelty and nonobviousness have induced heightened regulation of drug delivery patents. In these cases, patent offices rely on the recorded dates of seminal inventions, the order of claims presented, and prior art or relevant research in the same field as the patent application to satisfy criteria for nonobviousness and novelty.
Adequate knowledge of the scope of an invention, gained through literature searches or practical experience, is important in the defense of intellectual property. The claims are the most important part of a drug patent; these define the scope of an invention and defend it against encroachment by others by delineating and excluding areas of applicability. Reilly cautioned that loss of protection for an adequately described invention can occur if scientists and clinicians share their results with others or make them public before adequate measures are taken to file patents.
Additional impediments to patent security include lack of, or inadequate, laboratory notebook records and failure to understand the scope of prior art. Although patents grant protection for an idea, they do not automatically grant the right to practice an invention. With drugs and drug delivery systems, this right is granted by regulatory agencies such as the FDA through the process of clinical trials.
Effective Conduct of Clinical Trials
Emily Chew, MD, focused on the steps involved in progressing from early pilot studies (phase 1 and 2) to larger phase 3 trials. She emphasized the importance of the early pilot studies in providing useful data and in vetting drug candidates for larger phase 2 and 3 trials. Her examples included early-phase studies performed with ocular von Hippel-Lindau disease and AMD.
Curtis Meinert, PhD, discussed some common pitfalls in clinical trial design and described how a multidisciplinary team can successfully design and conduct a clinical trial. Meinert identified three common design pitfalls: (1) the absence of randomization in the study, (2) using too small a sample size, and (3) lack of sufficient follow-up time in clinical studies. Other design pitfalls included using historical controls as the comparison group, using unapproved surrogate outcome measures to reduce sample size requirements, and failing to provide sample size calculations.
Meinert concluded by summarizing some obstacles to successful clinical trials that occur at different levels of planning, such as issues regarding organization (e.g., no governance structure, no coordinating center, no monitoring body, and nonfunctional clinics), operations (e.g., basing the decision to follow-up on treatment status, open treatment assignment schemes, allowing the baseline period to extend beyond the moment of randomization, randomization without checking eligibility, and no performance monitoring), data analysis (e.g., analysis by treatment administered, overemphasis of P values, dredged results, and changing the outcome measure post hoc), publications (e.g., lack of publication, use of ghostwriters, use of nonindexed journals, and premature presentations of data). In the process of translational medicine, clinical trials expertise is needed to avoid the pitfalls specific to ocular drug delivery systems.
Karl Csaky, MD, PhD, discussed the appropriate endpoints for clinical trials in DR, dry AMD, branch retinal vein occlusion, and wet AMD. Extensive papers authored by various study groups for each of these have been published and will not be discussed here.76–78 Csaky emphasized that many drug formulations are not optimized for ophthalmic drug delivery, and need to be adapted for use in an existing or developing drug delivery system. He concluded that after the thorough evaluation of clinical trial data, the process continues with the refinement of drug delivery methods, which may involve input from the FDA as well as involvement from the Centers for Devices and Radiologic Health.
Future Directions for Basic and Translational Research
Throughout SERC 2009, participants discussed several areas in which they think basic science research and clinical studies in ophthalmic drug delivery should be focused for the purpose of fostering successful translational research. Six were identified:
A better understanding must be developed of the nature and effect of dynamic physiologic processes of the eye, such as clearance mechanisms and metabolism of drugs in specific tissue layers. It was noted that the challenge of isolating the dynamic barriers during data acquisition currently prevents the direct comparison of one dynamic barrier to another. It was repeatedly emphasized that an understanding of the importance of each dynamic barrier is key to improving the design of current drug delivery systems. In addition, it was stressed during the conference that live animal model studies or noninvasive studies on live human subjects are needed, as ex vivo work may not fully address relevant live dynamic physiologic processes.
Each static anatomic barrier encountered for each specific drug delivery technique must be studied, so that all individual layers of tissue or ocular fluid cavities are more fully understood. For example, with periocular injection techniques, the permeability properties of the choroid, RPE, retina, and vitreous should be individually assessed and compared to existing data from studies on the sclera and Bruch's membrane.
Drug–protein or drug–pigment binding must be better characterized, to guide attempts to modify natural sustained-release mechanisms for drug delivery to the posterior segment.
More research is needed in formulation modifications that alter physicochemical properties of both new and old drugs in ways that facilitate delivery through known paracellular and transcellular pathways. Although the paracellular and transcellular passive diffusion pathways offer an advantage, in that one drug delivery method may be generalized to various drugs, the active transcellular carrier–mediated transporters across the BRB should be further studied as they permit site-specific targeting without the need to create transmembrane concentration gradients and aid in tissue-specific targeting.
Ophthalmic drug delivery via nanotechnology-based products (<1 μm in diameter) must be further explored, as it fulfills three crucial criteria of ophthalmic drug delivery by enhancing drug permeation, controlled drug release, and selective drug targeting.
Innovation and development of ophthalmic gene delivery deserve further study, given the extensive potential of this technology.
Conclusions and an Action Plan for Advancing Translational Research in Ophthalmic Drug Delivery
Effective treatment of posterior segment ophthalmic diseases is a formidable challenge for scientists and clinicians in the ophthalmic pharmaceutical field. The challenges include the anatomic and physiologic barriers that can impede pharmacologically active levels of drug from reaching the targeted tissues inside the eye, immune reactions and clearance mechanisms to certain drugs and drug delivery materials, and the often irreversible nature of vision loss.
Although it was not one of the goals of this conference, an action plan to sustain future innovations and success in the clinic is needed to marshal the growing interest in this topic, as demonstrated by the attendance of scientists and clinicians from the pharmaceutical engineering, medical, and academic sectors. To maintain these discussions and see the successful application of more drug delivery systems in ophthalmology, we propose:
Improved cooperation between basic and applied science teams at academic institutions, where many novel drug delivery systems are discovered.
Cooperation between pharmaceutical companies and basic and applied science investigators, to facilitate the transfer of technology, which will enable the development and commercialization of novel products.
Uniform techniques for obtaining ocular tissue samples for comparative pharmacokinetic studies. Samples of posterior ocular tissues—including neuroretina (macula and peripheral retina), retinal pigment epithelium, choroid, and sclera—should be obtained in a consistent fashion, to improve data comparison.
Clear guidance, including early and frequent communication with regulatory agencies, to help sponsors select the appropriate path to regulatory approval of novel ocular drug delivery products.
Meetings and societies that accommodate discourse among basic, applied, and clinical researchers to help promote future collaborative activities in device and drug development and ophthalmic translational research.
Incentives for individuals and institutions that use a team science approach to train scientists and physicians to use translational strategies for problem-solving in ophthalmology.
Journals that, by encouraging and accepting translational research papers in such areas as preclinical testing results, development of analytical methods, strategies that enable development of investigational drugs, and critical analysis of trials that do not meet their endpoints, provide researchers with an outlet to publish translational work.
The application of technological advances in vision science is progressing at a rapid rate. Advances in nanotechnology, gene therapy, and biomaterials, for example, hold promise for providing new solutions to the challenge of ocular drug delivery. To facilitate collaboration among scientists and companies, many universities have established technology transfer offices that provide the researcher with legal advice and the tools to deal with patent protection and contract writing. Scientists who have a better understanding of mechanisms that facilitate collaborative research will play key roles in the effective development of new drug delivery methods.
The ultimate goal is to design drugs and delivery devices that can more effectively target disease. Greater knowledge of drug pharmacokinetics in both normal and diseased ocular tissue would greatly hasten further developments in the field.
Supplementary Material
Acknowledgments
The authors thank the Association for Research in Vision and Ophthalmology and the National Eye Institute for their support of SERC 2009, Rhonda Williams at ARVO for her dedicated and tireless management of the project, and Thea Gray for editing the manuscript.
Footnotes
Supported by NIH Core Grant 5P30EY006360-23 (HFE, HEG, TWO), NIH Grant SR24 EY017045 (HFE, HEG, UBK), and NIH Grant R24 EY017404 (GSH); the Alcon Research Institute (GSH); and an unrestricted grant from Research to Prevent Blindness (TWO).
Disclosure: H.F. Edelhauser, Alcon (C); C.L. Rowe-Rendleman, Omar Consulting (E), Taligen Therapeutics (C); M.R. Robinson, Allergan (E); D.G. Dawson, None; G.J. Chader, None; H.E. Grossniklaus, None; K.D. Rittenhouse, Pfizer (E); C.G. Wilson, None; D.A. Weber, MacuSight (E); B.D. Kuppermann, Allergan (F, C), Alimera (F), Genentech (F, C), NeoVista (F), Novagali (F), Optherion (F), Renegeneron (F), Thrombogenics (F); Braun (C), CoMentis (C), Fovea (C), Glaukos (C), Novartis (C), Ophthotech (C), Pfizer (C), ScyFix (C), Surmodics (C), TargeGen (C), VitreoRetinal Technologies (C); K.G. Csaky, Ophthotech (I), Potentia (I); Allergan (C), Genentech (C), GlaxoSmithKline (C), Heidelberg Engineering (C), Novartis (C); T.W. Olsen, None; U.B. Kompella, Allergan (F, C); V.M. Holers, Taligen Therapeutics (I, E, P); G.S. Hageman, Optherion (I); B.C. Gilger, None; P.A. Campochiaro, Alcon (F), Alimera (F), CoMentis (F), TargeGen (F), Genentech (F, C), GlaxoSmithKline (C), Regeneron (C); S.M. Whitcup, Allergan (E); W.T. Wong, None
References
- 1. Edelhauser HF, Boatright JH, Nickerson JM. and the Third ARVO/Pfizer Research Institute Working Group Drug delivery to posterior intraocular tissues: third annual ARVO/Pfizer ophthalmics research institute conference. Invest Ophthalmol Vis Sci. 2008;49:4712–4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005;57:2010–2032 [DOI] [PubMed] [Google Scholar]
- 3. Business Insights: The Ophthalmic pharmaceutical market outlook to 2014: competitive landscape, pipeline analysis, growth opportunities and market forecasts. http://www.Pharmalicensing.com Accessed January 2, 2010
- 4. Yuker HE. Perceptions of severely and multiply diasabled persons. J Multiple Handicapped Person. 1988;1:5–15 [Google Scholar]
- 5. Geroski DH, Edelhauser HF. Drug delivery for posterior segment diseases. Invest Ophthalmol Vis Sci. 2000;41:961–964 [PubMed] [Google Scholar]
- 6. Ghate D, Edelahauser HF. Ocular drug delivery. Expert Opin Drug Deliv. 2006;3:275–287 [DOI] [PubMed] [Google Scholar]
- 7. Kim SH, Lutz RJ, Wang NS, Robinson MR. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007;39:244–254 [DOI] [PubMed] [Google Scholar]
- 8. Frederici TJ. Intravitreal injections: AAO's Focal Points. Clinical Modules for Ophthalmologists. 2009;27(8, module 2):1–12 [Google Scholar]
- 9. Jager RD, Aiello LP, Patel SC, Cunningham ET. Risks of intravitreous injection: a comprehensive review. Retina. 2004;24:676–698 [DOI] [PubMed] [Google Scholar]
- 10. Durairaj C, Shah JC, Senapati S, Kompella UB. Prediction of vitreal half-life based on drug physicochemical properties: quantitative structure–pharmacokinetic relationships (QSPKR). Pharm Res. 2009;26:1236–1260 [DOI] [PubMed] [Google Scholar]
- 11. Le Goff MM, Bishop PN. Adult vitreous structure and postnatal changes. Eye. 2008;22:1214–1222 [DOI] [PubMed] [Google Scholar]
- 12. Bishop PN. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog Retin Eye Res. 2000;19:323–344 [DOI] [PubMed] [Google Scholar]
- 13. Worst JG, Los LI. Comparative anatomy of the vitreous body in rhesus monkeys and man. Doc Ophthalmol. 1999;82:169–178 [DOI] [PubMed] [Google Scholar]
- 14. Fowlks WL. Meridional flow from the corona ciliaris through the pararetinal zone of the rabbit vitreous. Invest Ophthalmol Vis Sci. 1963;2:63–71 [PubMed] [Google Scholar]
- 15. Smelser GK, Ishikawa T, Pei YF. Electron microscopic studies of intra-retinal spaces: diffusion of particulate materials. In: Rohen JW. ed. Eye Structure, II. Stuttgart, Germany: Schattauer-Verlag; 1965 [Google Scholar]
- 16. Törnquist P, Alm A, Bill A. Permeability of ocular vessels and transport across the blood-retinal-barrier. Eye. 1990;4:303–309 [DOI] [PubMed] [Google Scholar]
- 17. Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv Drug Deliv Rev. 2006;58:1136–1163 [DOI] [PubMed] [Google Scholar]
- 18. Gaudana R, Jwala J, Boddu SHS, Mitra AK. Recent perspectives in ocular drug delivery. Pharm Res. 2009;26:1197–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Winkler BS. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981;77:667–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee SS, Robinson MR. Novel drug delivery systems for retinal diseases. Ophthalmic Res. 2009;41:124–135 [DOI] [PubMed] [Google Scholar]
- 21. MacDonald IM, Sauvé Y, Sieving PA. Preventing blindness in retinal disease: ciliary neurotrophic factor intraocular implants. Can J Ophthalmol. 2007;42:399–402 [PubMed] [Google Scholar]
- 22. Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci. 2006;103:3896–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. US National Institutes of Health ClinicalTrials.org. NCT00447954 CgI. A Study of an Encapsulated Cell Technology (ECT) Implant for Patients With Atrophic Macular Degeneration. Phase II study. 2009. http://clinicaltrials.gov/ct2/show/NCT00447954 Accessed December 24, 2009
- 24. Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert Opin Drug Deliv. 2004;1:99–114 [DOI] [PubMed] [Google Scholar]
- 25. Ghate D, Brooks W, McCarey BE, Edelhauser HF. Pharmacokinetics of intraocular drug delivery by periocular injections using ocular fluorophotometry. Invest Ophthalmol Vis Sci. 2007;48:2230–2237 [DOI] [PubMed] [Google Scholar]
- 26. Robinson MR, Lee S, Kim H, et al. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp Eye Res. 2006;82:479–487 [DOI] [PubMed] [Google Scholar]
- 27. Amrite AC, Edelhauser HF, Singh SR, Kompella UB. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis. 2008;14:150–160 [PMC free article] [PubMed] [Google Scholar]
- 28. Amrite AC, Kompella UB. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J Pharm Pharmacol. 2005;57:1555–1563 [DOI] [PubMed] [Google Scholar]
- 29. Vaezy S, Clark JI. Quantitative analysis of the microstructure of the human cornea and sclera using 2-D Fourier methods. J Microscopy. 1994;175:93–99 [DOI] [PubMed] [Google Scholar]
- 30. Dawson DG, Ubels JL, Edelhauser HF. Cornea and sclera. In: Kaufman P, Ulm A. eds. Adler's Physiology of the Eye. 11th ed New York: Elsevier; In press [Google Scholar]
- 31. Olsen TW, Aabert SY, Geroski DH, Edelahauser HF. Human sclera: thickness and surface area. Am J Ophthalmol. 1998;125:237–241 [DOI] [PubMed] [Google Scholar]
- 32. Pitkänen L, Ranta VP, Moilanen H, Urtti A. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest Ophthalmol Vis Sci. 2005;46:641–646 [DOI] [PubMed] [Google Scholar]
- 33. Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979;23:279–296 [DOI] [PubMed] [Google Scholar]
- 34. Pitkänen L, Ranta VP, Moilanen H, Urtti A. Binding of betaxolol, metoprolol and oligonucleotides to synthetic and bovine ocular melanin, and prediction of drug binding to melanin in human choroid-retinal pigment epithelium. Pharm Res. 2007;24:2063–2070 [DOI] [PubMed] [Google Scholar]
- 35. Cheruvu NP, Kompella UB. Bovine and porcine transscleral solute transport: influence of lipophilicity and the choroid-Bruch's layer. Invest Ophthalmol Vis Sci. 2006;47:4513–4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pitkänen L. Retinal Pigment Epithelium as a Barrier in Drug Permeation and as a Target of Non-Viral Gene Delivery. Kuopia, Finland: University of Kuopio and Kuopio University Hospital; 2007. PhD Thesis [Google Scholar]
- 37. Amrite AC, Ayalasomayajula SP, Cheruvu NP, Kompella UB. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci. 2006;47:1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DeVore DP, Eiferman RA, Keates EU. inventors. Collagenesis, Inc, assignee. Compound delivery using rapidly dissolving collagen film. U.S. patent 6,197,934 March 6, 2001
- 39. Carvalho RAP, Murphree AL, Schmitt EE. inventors. Implantable and sealable system for unidirectional delivery of therapeutic agents to tissues. U.S. patent 7,195,774 March 27, 2007
- 40. Pontes de Carvalho RA, Krausse ML, Murphree AL, Schmitt EE, Campochiaro PA, Maumenee IH. Delivery from episcleral exoplants. Invest Ophthalmol Vis Sci. 2006;47:4532–4539 [DOI] [PubMed] [Google Scholar]
- 41. McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles for gene and drug delivery. Annu Rev Biomed Eng. 2000;2:289–313 [DOI] [PubMed] [Google Scholar]
- 42. Jiang J, Gill HS, Ghate D, et al. Coated microneedles for drug delivery to the eye. Invest Ophthalmol Vis Sci. 2007;48:4038–4043 [DOI] [PubMed] [Google Scholar]
- 43. Jiang J, Moore JS, Edelhauser HF, Prausnitz MR. Instrsclera drug delivery to the eye using hollow microneedles. Pharm Res. 2009;26:395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olsen TW, Feng X, Wabner K, et al. Cannulation of the suprachoroidal space: a novel drug delivery methodology to the posterior segment. Am J Ophthalmol. 2006;142:777–787 [DOI] [PubMed] [Google Scholar]
- 45. Jiang J, Moore JS, Edelhauser HF, Prausnitz MR. Intrascleral drug delivery to the eye using hollow microneedles. Pharm Res. 2009;26:395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bejjani RA, Andrieu C, Bloquel C, et al. Electrically assisted ocular gene therapy. Surv Ophthalmol. 2007;52:196–208 [DOI] [PubMed] [Google Scholar]
- 47. Bloquel C, Bejjani R, Bigey P, et al. Plasmid electrotransfer of eye ciliary muscle: principles and therapeutic efficacy using hTNF-alpha soluble receptor in uveitis. FASEB J. 2006;20:389–391 [DOI] [PubMed] [Google Scholar]
- 48. Touchard E, Bloquel C, Bigey P, et al. Effects of ciliary muscle plasmid electrotransfer of TNF-alpha soluble receptor variants in experimental uveitis. Gene Ther. 2009;16:862–873 [DOI] [PubMed] [Google Scholar]
- 49. Maurice DM. Drug delivery to the posterior segment from drops. Surv Ophthalmol. 2002;47:S41–S52 [DOI] [PubMed] [Google Scholar]
- 50. Loftsson T, Sigurdsson HH, Konradsdottir F, et al. Topical drug delivery to the posterior segment of the eye: anomatomical and physiological considerations. Pharmazie. 2008;63:171–179 [PubMed] [Google Scholar]
- 51. Takahashi K, Saishin Y, Saishin Y, et al. Topical nepafenac inhibits ocular neovascularization. Invest Ophthalmol Vis Sci. 2009;44:409–415 [DOI] [PubMed] [Google Scholar]
- 52. Doukas J, Mahesh S, Umeda N, et al. Topical administration of a multi-targeted kinase inhibitor suppresses choroidal neovascularization and retinal edema. J Cell Physiol. 2008;216:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kiuchi K, Matsuoka M, Wu JC, et al. Mecamylamine suppresses basal and nicotine-stimulated choroidal neovascularization. Invest Ophthalmol Vis Sci. 2008;49:1705–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang AJW, Tseng SCG, Kenyon KR. Paracellular permeability of corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1989;30:684–689 [PubMed] [Google Scholar]
- 55. Lux Biosciences ClinicalTrials.org. NCT00851734. A Dose-Escalation Study to Assess the Safety and Tolerability of LX214 Ophthalmic Solution in Healthy Volunteers Followed by an Open-Label Evaluation of LX214 in Patients With Keratoconjunctivitis Sicca (KCS). http://clinicaltrials.gov/ct2/show/NCT00851734 Accessed January 2, 2010
- 56. Altinoğlu EI, Russin TJ, Kaiser JM, et al. Near-infrared emitting fluorophore-doped calcium phosphate nanoparticles for in vivo imaging of human breast cancer. ACS Nano. 2008;28:2075–2084 [DOI] [PubMed] [Google Scholar]
- 57. Heakal Y, Kester M. Nanoliposomal short-chain ceramide inhibits agonist-dependent translocation of neurotensin receptor 1 to structured membrane microdomains in breast cancer cells. Mol Cancer Res. 2009;7:724–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kester M, Heakal Y, Fox T, et al. Calcium phosphate nanocomposite particles for in vitro imaging and encapsulated chemotherapeutic drug delivery to cancer cells. Nano Lett. 2008;8:4116–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kline CL, Shanmugavelandy SS, Kester M, Irby RB. Delivery of PAR-4 plasmid in vivo via nanoliposomes sensitizes colon tumor cells subcutaneously implanted into nude mice to 5-FU. Cancer Biol Ther. 2009;8:1831–1837 [DOI] [PubMed] [Google Scholar]
- 60. Morgan TT, Muddana HS, Altinoglu EI, et al. Encapsulation of organic molecules in calcium phosphate nanocomposite particles for intracellular imaging and drug delivery. Nano Lett. 2008;8:4108–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sun Y, Fox T, Adhikary G, et al. Inhibition of corneal inflammation by liposomal delivery of short-chain, C-6 ceramide. J Leukoc Biol. 2008;83:1512–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Elijarrat-Binstock E, Domb AJ. A non-invasive ocular drug delivery. J Control Release. 2006;110:479–489 [DOI] [PubMed] [Google Scholar]
- 63. EyeGate Pharma Clintrials.org. NCT00765804. Safety and Efficacy Study of Iontophoresis and Dexamethasone Phosphate to Treat Dry Eye. Phase II study. http://clinicaltrials.gov/ct2/show/NCT00765804 Accessed January 2, 2010
- 64. Ciolino JB, Hoare TR, Iwata NG, et al. A drug-eluting contact lens. Invest Ophthalmol Vis Sci. 2009;50:3346–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Miller DJ, Li SK, Tuitupou AL, et al. Passive and oxymetazoline-enhanced delivery with a lens device: pharmacokinetics and efficacy studies with rabbits. J Ocul Pharmacol Ther. 2008;24:385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tso MOM, Shih CY, McLean IW. Is there a blood-brain barrier at the optic nerve head? Arch Ophthalmol. 1975;93:815–825 [DOI] [PubMed] [Google Scholar]
- 67. Hofman P, Hoyng P, vanderWerf F, et al. Lack of blood–brain barrier properties in microvessels of the prelaminar optic nerve head. Invest Ophthalmol Vis Sci. 2001;42:895–901 [PubMed] [Google Scholar]
- 68. Birol G, Wang S, Budzynski E, et al. Oxygen distribution and consumption in the macaque retina. Am J Physiol Heart Circ Physiol. 2007;283:H1696–H1704 [DOI] [PubMed] [Google Scholar]
- 69. Hayreh SS. Posterior ciliary artery circulation in health and disease: The Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2004;45:749–757 [DOI] [PubMed] [Google Scholar]
- 70. Williamson TH, Harris A. Ocular blood flow measurement. Br J Ophthalmol. 1994;78:939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Campbell M, Nguyen AT, Kiang AS, et al. An experimental platform for systemic drug delivery to the retina. Proc Natl Acad Sci. 2009;106:17817–17822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rohrer B, Long Q, Coughlin B, et al. A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:3056–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol Rev. 2008;223:300–316 [DOI] [PubMed] [Google Scholar]
- 74. Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176:1305–1310 [DOI] [PubMed] [Google Scholar]
- 75. Thurman JM, Renner B, Kunchithapautham K, et al. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem. 2009;284:16939–16947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Csaky KG, Richman EA, Ferris FL., 3rd Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008;49:479–489 [DOI] [PubMed] [Google Scholar]
- 77. Sunness JS, Applegate CA, Bressler NM, Hawkins BS. Designing clinical trials for age-related geographic atrophy of the macula: enrollment data from the geographic atrophy natural history study. Retina. 2007;27(2):204–210 [DOI] [PubMed] [Google Scholar]
- 78. Scott IU, VanVeldhuisen PC, Oden NL, Ip MS, Blodi BA. and SCORE Study Investigator Group SCORE Study report 1: baseline associations between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophthalmology. 2009;116(3):504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.