In this prospective study, factors were examined that are independently associated with the increased risk of corneal infiltrative events caused by continuous silicone hydrogel lens wear. The findings will be helpful for practitioners as they select and monitor patients for continuous wear of silicone hydrogel lenses and for manufacturers as they design future generations of similar lenses.
Abstract
Purpose.
This study determined which microbiologic, clinical, demographic, and behavioral factors are associated with corneal infiltrative events (CIEs) during continuous wear of silicone hydrogel (SH) contact lenses.
Methods.
Subjects (n = 205) were fitted with lotrafilcon A lenses for continuous wear and observed for 1 year. The main exposures of interest were corneal staining and bacterial lens contamination. Kaplan-Meier (KM) plots were used to estimate the cumulative unadjusted probability of remaining CIE free, and Cox proportional hazards regression was used to model the hazard of having a CIE, as a function of key predictor variables.
Results.
The KM-unadjusted cumulative probability of remaining CIE free was 73.3%. Approximately 53% of subjects had repeated episodes of corneal staining (mild or greater), and 11.3% had repeated episodes of moderate or greater corneal staining. Corneal staining was not associated with the development of a CIE. The frequency of substantial bacterial bioburden on worn lenses at the time of a CIE was 64.7%, compared with only 12.2% during uncomplicated wear. The presence of substantial lens bacterial bioburden was associated with the development of a CIE (adjusted hazards ratio [HR], 8.66; 95% confidence interval [CI], 2.88–26.01). Smoking was also associated with a CIE (adjusted HR, 4.13; 95% CI, 1.27–13.45).
Conclusions.
Corneal staining is common during continuous wear of SH lenses, but it is not associated with the development of a CIE. Smoking and substantial lens bacterial bioburden pose prominent risks of a CIE. In this study, more than 70% of the total risk of CIE in those with substantial lens bioburden is attributable to this exposure. (ClinicalTrials.gov number, NCT00727402).
Silicone hydrogel (SH) contact lens materials have significantly improved the cornea's physiological response to contact lens wear compared with their low-oxygen-permeable (Dk) hydrogel counterparts.1–4 However, since their introduction into the global market, SH lenses have not reduced the risk of microbial keratitis compared with low-Dk hydrogels.5–7 In addition, compared with low-Dk hydrogel lenses, SH lenses are associated with a twofold increase in the incidence of corneal infiltrative events (CIEs).8,9
CIEs are a result of a stimulus that causes directed infiltration of leukocytes into the cornea.10 Overnight contact lens use9,11–14 and bacterial adhesion to lenses15–20 significantly increase the risk of CIEs. During uneventful extended wear of low-Dk lenses, approximately twice as many lenses are sterile compared with lenses worn during a CIE, and although pathogenic bacteria are occasionally cultured from lenses during uneventful extended wear, their presence is substantially and significantly higher during a CIE.17 An association between microbial contaminants on SH lenses and development of CIEs has not been reported.
With regard to the available data surrounding microbial contamination of contact lenses and CIEs,15,18–20 there is evidence that CIEs are acute inflammatory reactions to the presence of bacterial toxins, enzymes, and byproducts on lens surfaces.21,22 However, there are wide gaps in this knowledge. For example, no microorganisms are found on approximately 22% of lenses of patients experiencing a CIE,17 and often patients can exhibit heavy colonization of pathogenic bacteria on their lenses, yet remain event free.15,17,23
A break in the corneal epithelium may be a predisposing factor that initiates a CIE in the presence of microorganisms. There is a debate about whether corneal surface disruption, as manifested by superficial corneal fluorescein uptake (corneal staining), is a sufficient breach in the epithelial barrier to predispose oa patient to a CIE.24,25 Carnt et al.26 found a threefold increased risk of asymptomatic CIEs in subjects exhibiting solution-induced staining during soft lens daily wear. During extended wear, corneal staining posed a sevenfold greater risk for the subsequent development of a CIE.27 However, in neither of these studies was there a control for the effect of lens contamination.
Other risk factors for the development of CIE during continuous wear of SH lenses include younger age28–30 (especially when combined with smoking),31 being male,14,29 blepharitis and bulbar redness (Carnt N, et al. IOVS 2002;43:ARVO E-Abstract 3111), limbal redness,27 high refractive error,30 failure to achieve the intended wearing schedule,30 and a history of CIEs.31 In none of these studies was there a control for the effect of microbial lens contamination.
In this prospective study, we examined factors that are independently associated with increased risk of CIEs from continuous SH lens wear. The association between microbial contamination of SH lenses and the development of CIEs was of interest, as the incidence of CIEs is doubled during the continuous wearing of SH lenses compared with the wearing of low-Dk hydrogels,8,9 and microbial contamination has been a known risk factor for these types of inflammatory events during low-Dk lens wear.15–20 We used multivariate modeling to quantify and control the impact of lens microbial contamination on the risk for a CIE while other risk factors, such as corneal staining, were explored. The information reported herein will be helpful for practitioners as they select and monitor patients for continuous wear of SH lenses and for manufacturers as they design future generations of similar lenses.
Methods
The Longitudinal Analysis of Silcone Hydrogel Contact Lens (LASH) Study Cohort and Design
The LASH Study was a 12-month prospective cohort study of subjects fitted with lotrafilcon A lenses (Night and Day Lens; Ciba Vision, Duluth, GA) for up to 29 consecutive nights of continuous wear, with monthly disposal. The study was approved by the University Hospitals Case Medical Center Institutional Review Board, all subjects signed a written informed consent before participation, and the study adhered to the tenets of the Declaration of Helsinki. Baseline data for the study subjects are shown in Table 1.
Table 1.
Characteristics of 205 Subjects Enrolled in the LASH Study from October 2006 to February 2008
| Parameter | Distribution n (%) |
|---|---|
| Sex | |
| Female | 157 (76.6) |
| Age (mean 32.8 y, range 15–62) | |
| Under 21 | 26 (12.7) |
| Between 21 and 50 | 163 (79.5) |
| 50+ | 16 (7.8) |
| Previous soft lens wear experience | |
| Current or recent (within 12 months) users | 152 (74.1) |
| Neophytes | |
| Never wore | 21 (10.3) |
| Discontinued more than 12 months ago | 32 (15.6) |
| Race | |
| Caucasian | 115 (56.1) |
| African-American | 49 (23.9) |
| Asian | 31 (15.1) |
| Other | 10 (4.9) |
| Education (highest level achieved, of 203 reported) | |
| High school | 17 (8.4) |
| Some college | 53 (26.1) |
| College degree, 4-year | 61 (30.1) |
| Graduate work | 72 (35.5) |
| Smoking Status (of 201 reported) | |
| Current | 21 (10.5) |
| Never | 170 (84.6) |
| Former | 10 (5.0) |
| History of previous adverse event (of 204 reporting) | |
| Yes | 90 (44.1) |
| No | 93 (45.6) |
| Not applicable | 21 (12.3) |
| Contact lens power | |
| Greater than ±5.00 D | 63 (30.9) |
Neophyte subjects were defined as subjects who had not worn contact lenses for the preceding 12 months or longer. Any neophyte subject first entered a 2-week daily wear phase with a hydrogen peroxide–based disinfection system (Clear Care; Ciba Vision). All daily wear–adapted neophytes and all other lens-wearing subjects began continual overnight use and returned for visits after 1 week and 1, 4, 8, and 12 months. The subjects also returned for unscheduled visits during acute symptomatic events as needed. They documented scheduled and unscheduled lens removals in diaries that were used to calculate lens age at each visit. Although 29 nights of CW was attempted by each subject on a monthly basis, if premature lens removal occurred, the subjects cleaned and disinfected their lenses with the disinfection system before reinsertion. At every visit, including the unscheduled ones, each eye was assessed for the presence of a CIE according to definitions adopted from the standards listed in the “Institute for Eye Research/L.V. Prasad Eye Institute (IER/LVPEI) Guide To Corneal Infiltrative Conditions.”32 In addition, a clinical severity matrix, modified from that originally described by Aasuri et al.,33 and Morgan et al.34 was used to generate a cumulative CIE score per eye for each subject visit (Table 2). A score >2 indicated the presence of an active CIE.
Table 2.
Scoring of Clinical Features in the Clinical Severity Matrix
| Parameter | 0 | 1 | 2 | 3 | Point Total |
|---|---|---|---|---|---|
| Symptoms | None | Mild | Moderate | Severe | |
| Lid swelling | Absent | Present | |||
| Conjunctival redness | Absent | Localized | Generalized | ||
| Infiltrate shape | Round | Irregular | |||
| Infiltrate size, mm (largest) | ≤1.0 | 1.0–2.0 | ≥2.0 | ||
| Number of infiltrates | 1–4 | 5–10 | >10 | ||
| Fluorescein staining | Absent | Present | |||
| Surrounding cornea | Clear | Slight haze | Severe haze | ||
| Endothelial debris | Absent | Present | |||
| Hypopyon | Absent | Present | |||
| Effect of lens discontinuation | Resolving | No change | Slight worsening | Significant worsening | |
| Total |
Collection and Classification of Covariates
Demographic and social covariates of interest included age, race, refractive error, history of adverse events, level of education, smoking status, and health status between visits. Health status was modeled as a binary time-dependent covariate and noted as positive if a subject reported experiencing a cold, flu, or other viral infection between visits. Ocular covariates were drawn from the affected eye at any visit (including unscheduled visits) preceding a CIE, or any visit for event-free subjects, unless otherwise noted. Efron Grading Scales35,36 were used for grading blepharitis, meibomian gland dysfunction, corneal neovascularization, epithelial microcysts, and corneal edema. The IER grading scales37 were used for grading upper tarsal plate redness and roughness, limbal redness, bulbar redness, and conjunctival and corneal staining. Aqueous tear production was assessed with an unstimulated, anesthetized Schirmer test.
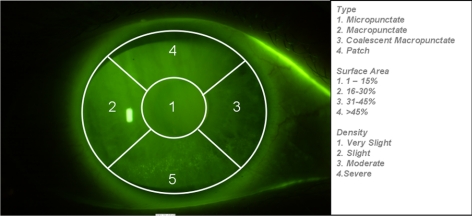
Corneal staining was assessed after addition of sodium fluorescein (BioGlo ophthalmic strips; Hub Pharmaceuticals, Rancho Cucamoga, CA) with a filter (Wratten 15; Eastman Kodak, Rochester, NY) in combination with a cobalt blue filter over the light source, and recorded by masked readers examining digitally acquired photographs. The digital photographs were acquired with a slit lamp (Photo slit lamp; Carl Zeiss Meditec, Dublin, CA) and a modified version the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study's photographic slit lamp protocol for corneal staining.38 Type, surface area, and density of corneal staining were recorded in each of five corneal zones according to modified versions of the IER staining criteria, as listed in Figure 1. Each image series was graded by two trained readers and adjudicated by a third reader. Staining variables of interest were those that represented chronicity or higher severity and were modeled as binary fixed covariates. The presence of at least mild or moderate stain was determined using the criteria as listed in Table 3. Chronicity was classified as positive if the subjects experienced two or more episodes of staining across visits.
Figure 1.
Example of grading of photographic corneal staining. Each of the five corneal zones was graded for staining type, surface area, and density. In this example, zone 5 was graded by the masked readers as a macropunctate stain, with a 31% to 45% surface area, and moderate density. On this visit, this eye was classified with overall moderate stain. This subject ended the study event free.
Table 3.
Corneal Staining Criteria and Characteristics of 1047 Subject Visits in the LASH Cohort
| At Least Mild Stain |
At Least Moderate Stain |
|||||
|---|---|---|---|---|---|---|
| Zones (n) | Surface Area | Density | Zones (n) | Surface Area | Density | |
| Micropunctate | ||||||
| >1 | ≥1 | ≥1 | 5 | ≥1 | ≥1 | |
| ≥1 | >2 | ≥1 | >1 | 4 | ≥1 | |
| ≥1 | ≥1 | >1 | >1 | 1 | 4 | |
| >2 | >2 | ≥1 | ||||
| >1 | >2 | >2 | ||||
| Frequency of subject visits | 41.7% | 8.0% | ||||
| Macropunctate | ||||||
| ≥1 | ≥1 | ≥1 | 5 | ≥1 | ≥1 | |
| >1 | ≥1 | 4 | ||||
| ≥1 | 4 | ≥1 | ||||
| >1 | >2 | ≥1 | ||||
| Frequency of subject visits | 11.5% | 1.9% | ||||
| Coalescent Macropunctate | ||||||
| ≥1 | ≥1 | ≥1 | >1 | ≥1 | ≥1 | |
| Frequency of subject visits | 8.5% | 1.4% | ||||
| Patch | ||||||
| ≥1 | ≥1 | ≥1 | ≥1 | ≥1 | ≥1 | |
| Frequency of subject visits | 1.5% | |||||
| Overall Stain | ||||||
| Any of the above criteria | Any of the above criteria | |||||
| Frequency of subject visits | 51.2% | 17.7% | ||||
* Through 4 months (768 subject visits), 55.6% of visits had at lest mild staining and 19.8% had at least moderate staining documented.
Aerobic cultures were performed from samples taken from lid margins and conjunctival surfaces at baseline, and after 1 week and 4 months of wear. Lenses were aseptically removed and aerobically cultured after 1 week and 4 months of wear. Lids, conjunctivae, and contact lenses were also cultured during any adverse event. The methods and results of lid and conjunctival cultures have been published.39 The methods for culturing contact lenses used an adaptation of an agar overlay technique that has been used routinely in extended-wear clinical trials.16–18,20,40,41 The culture technique and definition of bacterial bioburden used in this study have also been published.39 Briefly, the bacterial bioburden was stratified into three categories: (1) the presence of any bacterial species, regardless of whether it was considered a pathogenic or commensal microbiota; (2) the presence of pathogenic species only; and (3) “substantial bioburden” if either high levels of commensal ocular biota or organisms of low pathogenicity were present, or if any level of pathogenic organisms was detected.42–44 The second column in Table 4 lists the magnitude of the bacterial load necessary for classification of a substantial bioburden, stratified by species. Lens bioburden was modeled as both a time-dependent and a fixed covariate.
Table 4.
Bacteria Isolated from Contact Lenses Stratified by Presence of CIE and Levels Considered to be Significant
| Organism | Magnitude of CFU/lens Required for Definition of Substantial Bioburden | No CIE |
Active CIE |
||||
|---|---|---|---|---|---|---|---|
| Frequency of Isolation*n (%) | Range CFU Per Lens | Frequency Substantial Bioburden n (%) | Frequency of Isolation†n (%) | Range CFU Per Lens | Frequency Substantial Bioburden n (%) | ||
| CNS‡ | >10 | 226 (32.0) | 1–200 | 65 (9.2) | 13 (45.8) | 1–112 | 6 (20.7) |
| Serratia marcescens | >0 | 5 (0.7) | 6–1000 | 5 (0.7) | 4 (13.8) | 10–1000 | 4 (13.8) |
| Corynebacterium‡ | >100 | 4 (0.6) | 100–1000 | 2 (0.3) | 5 (17.2) | 100–1000 | 4 (13.8) |
| Pseudomonas aeruginosa | >0 | 2 (0.3) | 20–100 | 2 (0.3) | 3 (10.3) | 30–2000 | 3 (10.3) |
| Viridans group streptococcus | >0 | 8 (1.1) | 1–100 | 8 (1.1) | 3 (10.3) | 1–2000 | 3 (10.3) |
| Stenotrophomonas maltophilia | >0 | 2 (0.3) | 2000 | 2 (0.3) | 1 (3.5) | 500 | 1 (3.5) |
| Staphylococcus aureus | >0 | 18 (2.5) | 1–60 | 18 (2.5) | 1 (3.5) | 20 | 1 (3.5) |
| Klebsiella pneumoniae | >0 | 0 | NA | 0 | 1 (3.5) | 15 | 1 (3.5) |
| Lactobacillus sp | >0 | 1 (0.1) | 200 | 1 (0.1) | 0 | NA | 0 |
| Haemophilus parainfluenzae | >0 | 1 (0.1) | 1 | 1 (0.1) | 0 | NA | 0 |
| Bacillus‡ | >10 | 3 (0.4) | 2–100 | 2 (0.3) | 0 | NA | 0 |
| Enterobacter asburiae | >0 | 2 (0.3) | 100 | 2 (0.3) | 0 | NA | 0 |
| Enterobacter cloacae | >0 | 1 (0.3) | 3–100 | 1 (0.3) | 0 | NA | 0 |
| Proteus mirabilis | >0 | 1 (0.3) | 200 | 1 (0.3) | 0 | NA | 0 |
| Klebsiella oxytoca | >0 | 1 (0.1) | 7 | 1 (0.3) | 0 | NA | 0 |
| Pseudomonas fluorescens | >0 | 1 (0.1) | 33 | 1 (0.3) | 0 | NA | 0 |
| Escherichia coli | >0 | 1 (0.1) | 1000 | 1 (0.3) | 0 | NA | 0 |
| Candida parapsilosis | >10 | 2 (0.3) | 2–10 | 0 | 0 | NA | 0 |
n = 706 lenses cultured.
n = 29 lenses cultured.
Normal microbiota or organism of low ocular pathogenicity.
Statistical Methods
The sample size was based on the null hypothesis that the proportion of subjects experiencing a CIE is the same in those with corneal staining and those without (type I error α = 0.05, with 80% power). With the normal (χ2) approximation of the binomial test, estimated prevalence of CIEs of 0.2 in the exposed and of 0.01 in the unexposed, as extracted from previous studies,27 a combined total of 98 subjects was needed. Up to five more factors were hypothesized to play a role in the final model. Thus at least 20 observations for each additional variable were necessary.45 Therefore, 100 additional subjects were needed, increasing the desired sample size to 198. Last, to account for loss to follow-up, the sample size was increased by 5%.
Time-to-event analyses were conducted. Only the first occurrence of any CIE, regardless of score in either eye, was counted. If a subject had a bilateral first event, the eye with the more severe CIE was used. Data from visits preceding the CIE were drawn from the eye that had the event, with one exception: Lens microbiology assessments were drawn from the visits preceding the event as well as at the time of the event. For subjects who remained event free throughout the study, the covariates from the left eye were used in the analysis. Events were assumed to occur at the time of the visit in which they were first detected.
The cumulative unadjusted probability of remaining CIE free was estimated by the Kaplan-Meier (KM) method. Univariate analyses of exploratory variables were performed by using KM plots stratified by presence or absence of demographic, clinical, and microbiologic covariates. The log-rank test was used to test for differences between the KM curves when stratified by these variables. The univariate Cox proportional hazards regression was used to determine initial assessments of risk of each covariate. Graphic analyses (log–log plots) were used to test for the assumption of proportionality of hazards for each covariate. Biologically plausible covariates (age and sex) as well as the variables found to be significant at P < 0.10 during univariate analyses were entered in a multivariate Cox proportional hazards model (SAS, ver. 9.1.3; SAS Institute, Inc., Cary, NC).
Analyses were first performed on data obtained through the 12-month visit. Subsequent analyses were limited to the first 4 months of follow-up because routine lens cultures were not performed past this time point. Only subjects presenting for CIE or other problem-oriented visits provided cultures between 4 and 12 months. The study was originally designed in this manner because resident lens and ocular surface microbiota have not been shown to change with repeating sampling.41,46 Limiting the analysis to the first 4 months was performed to limit potential information bias that could occur by failure to capture microbiology data in the problem-free subjects past the 4-month visit.
Results
Subjects and Incidence
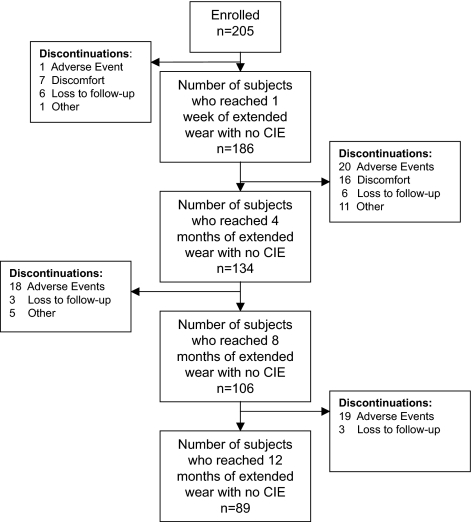
From October 2006 to February 2008, 205 subjects were enrolled and completed 1166 visits for 134.3 person-years of follow-up. This analysis was limited to the 1047 person-visits that occurred before the event of interest. Overall, 89 (43.4%) subjects completed the 12-month follow-up event-free. Fifty-eight (28.3%) subjects discontinued because of adverse events, 23 (11.2%) experienced discomfort precluding successful extended wear, 18 (8.8%) were lost to follow-up, and 17 (8.3%) discontinued for other reasons (pregnancy, relocation, excessive time commitment). Figure 2 outlines the discontinuations by length of time in the study. Those prematurely censored were more likely to be neophytes than were those subjects remaining under observation (P = 0.04).
Figure 2.
Flow chart of subject retention and discontinuation in the LASH Study.
The mean age of the lenses at time of collection was 26.3 days (range, 1–52 days), and the median age was 29 days. The majority (81.8%) of lenses were worn between 21 and 30 days, 3.9% were worn >30 days, and 14.3% were worn <21 days at the time of collection.
During the 12-month follow-up, there were 44 incident CIEs in 38 subjects (6 were bilateral events). There were 11 contact lens acute red eye (CLARE) responses, 10 contact lens peripheral ulcers (CLPUs), 17 events of infiltrative keratitis (IK), 5 events of asymptomatic infiltrative keratitis (AIK), and 1 asymptomatic infiltrate (AI). By severity, there were 10 eyes with a score of 4; 5 with a score of 5; 4 with a score of 6; 11 with a score of 7; and 5 with a score of 8. Eight CLPUs were diagnosed by an incident corneal scar and thus had a score of 0. There were no events of microbial keratitis. The KM unadjusted cumulative incidence after 12 months for remaining event free was 73.3% (95% CI, 65.0–79.9); thus, the cumulative incidence for development of a CIE was 26.7% (95% CI, 20.1–35.0). The KM unadjusted cumulative incidence after 4 months for development of a CIE was 16.7%.
Corneal Staining
Table 3 shows the corneal staining distribution across the 1047 subject visits. Across the 205 subjects, more than half (53.3%) had repeated episodes of mild (or more severe) corneal staining, more than one third (38.1%) had at least one episode of moderate staining or greater, and more than one tenth (11.3%) had repeated episodes of moderate corneal staining or higher. There were no univariate associations between any staining variables (stratified by type, overall severity, and chronicity) and the development of a CIE (log rank test P > 0.2). Figures 3A and 3B display representative KM curves stratified by presence or absence of at least one episode of moderate corneal stain or greater and presence or absence of repeated episodes of mild corneal stain or greater, respectively. Neither graph displays any evidence of a corneal staining effect throughout the entire study period.
Figure 3.
Unadjusted cumulative probability of remaining CIE free stratified by (A) the presence or absence of at least one episode of moderate corneal staining or greater and (B) the presence or absence of repeated episodes of mild corneal staining or greater.
Lens Bioburden
Table 4 displays the bacteria isolated from contact lenses stratified by the presence of a CIE and the frequency of substantial bioburden by species. In total, 17 bacterial and one yeast group or species were isolated. The most frequently isolated species was coagulase negative staphylococci (CNS) in both the unaffected and CIE groups, although the frequency of substantial bioburden with CNS in the CIE group was more than double that in the non-CIE group. Other species that displayed large differences in isolation frequency between the two groups included Serratia marcescens, Corynebacterium species, Pseudomonas aeruginosa, and viridans group streptococci, all present in greater than 10% of lenses cultured at the time of a CIE, compared with approximately 1% or less when no CIE occurred.
Table 5 presents the percentage of subjects with culture-positive lenses stratified by the presence of infiltrate and then by visit during the entire 12-month follow-up. The frequency of the presence of bacterial bioburden was always greater during a CIE, regardless of type of bioburden (substantial, pathogenic, or any). Although the frequency of bacterial bioburden was greater during symptomatic CIEs compared with asymptomatic CIEs, the differences were not statistically significant, probably because of the small samples in these categories. The frequency of substantial bacterial bioburden on worn lenses at the time of a CIE was 64.7%, compared with only 12.2% to 15.9% during uncomplicated wear.
Table 5.
Subjects with Culture Positive Lenses Stratified by Visit and Presence of Infiltrate
| No Infiltrative Event |
During Infiltrative Event |
P | ||||
|---|---|---|---|---|---|---|
| 1-wk Visit (n = 170) | 4-mo Visit (n = 123) | Any Event (n = 34) | Asymptomatic Events (n = 15) | Symptomatic Events (n = 19) | ||
| Substantial bacterial bioburden | 27 (15.9) | 15 (12.2) | 22 (64.7) | 8 (53.3) | 14 (73.7) | 0.1365* |
| Any bacterial species | 69 (40.6) | 40 (32.5) | 27 (79.4) | 10 (66.8) | 17 (89.5) | 0.0955* |
| Pathogenic species | 10 (5.9) | 6 (4.9) | 11 (32.4) | 3 (20.0) | 8 (42.1) | 0.1202* |
Data are the number of subjects (percentage of total group).
P comparing symptomatic to asymptomatic events.
Table 6 presents the univariate hazard for CIE by type of bacterial bioburden after 4 and 12 months of follow-up. The hazard for CIE was greater at 4 months than during the entire 12 months of follow-up for substantial and pathogenic bioburden. The presence of substantial lens bioburden (as opposed to only pathogenic or any species) resulted in the greatest hazard for a CIE at both time points and thus was used in subsequent multivariate analyses. Figure 4 displays the KM plot for development of a CIE stratified by substantial lens bioburden. The percent attributable risk in those subjects with substantial bioburden was 72.3%.
Table 6.
Univariate Hazard for CIE by Type of Bacterial Bioburden
| 4-mo Data | 12-mo Data | |
|---|---|---|
| Substantial bacterial bioburden | 7.47 (2.62–21.36) | 4.41 (2.21–8.79) |
| Pathogenic species | 4.35 (1.60–11.82) | 3.29 (1.67–6.49) |
| Any level or bacterial species | 2.74 (0.89–8.41) | 3.65 (1.52–8.76) |
Data are the Cox proportional hazards ratio (95% CI).
Figure 4.
The unadjusted cumulative probability of remaining CIE free, stratified by the presence or absence of substantial bioburden on study lenses.
Other Covariates
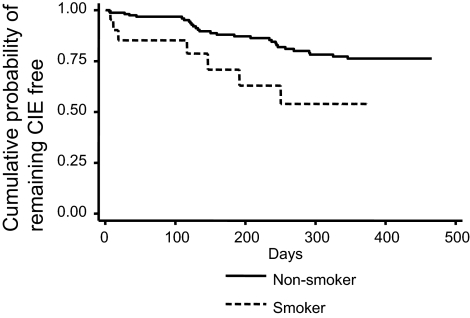
Of the other baseline demographic factors and clinical covariates measured during biomicroscopic evaluations, only two variables were significant at P < 0.10 for entry into the multivariate model: current smokers (HR, 2.48; 95% CI, 1.09–5.66) and conjunctival staining noted as grade 2 or higher (HR, 0.43, 95% CI; 0.21–0.87). Figure 5 displays the KM plot for development of CIE stratified by smoking status. Smokers had a relatively consistent increased cumulative incidence of a CIE compared with nonsmokers.
Figure 5.
The unadjusted cumulative probability of remaining CIE free, stratified by the presence or absence of smoking.
Time-Dependent Analyses
Lens bioburden, in addition to being a fixed variable, was assessed as a time-dependent covariate by quantifying the number of days a subject was exposed to a contaminated lens. In this analysis, subject intervals were used in the KM and Cox proportional hazards models and the numbers of days a subject was exposed to a contaminated lens was assumed to be the same as the known age of the lens at the time of culture. The unadjusted hazards for a CIE if a subject harbored substantial lens bioburden were 13.62 (95% CI, 4.39–42.23) and 5.87 (95% CI, 2.86–12.08), over 4 and 12 months of follow-up, respectively.
Almost half (46.4%) of the subjects experienced a cold, flu, or other viral infection at some time point during the study. If a subject experienced such an illness between visits over 12 months of follow-up, the unadjusted hazard for a CIE was 2.11 (95% CI, 1.02–4.36).
Multivariate Analyses
Table 7 presents the multivariate analyses through 4 and 12 months of follow-up using the fixed covariates found to be significant during univariate analyses as well as the biologically plausible covariates of age and sex. Through 4 months of follow-up, the adjusted hazards for a CIE were significantly greater for subjects harboring substantial lens bioburden and for smokers compared with their referents. Through 12 months of follow-up, the same trend was noted, but the hazards ratio was lower for lens bioburden than in the 4-month data.
Table 7.
Multivariate Analysis for Development of CIE
| Variable | 4-mo Data |
12-mo Data |
||||
|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | |
| Substantial lens bioburden | ||||||
| No | Referent | |||||
| Yes | 0.0001 | 8.66 | 2.88–26.01 | <0.0001 | 4.76 | 2.28–9.94 |
| Conjunctival stain | ||||||
| No | Referent | |||||
| Yes (>grade 2) | 0.6812 | 0.76 | 0.21–2.77 | 0.1838 | 0.60 | 0.28–1.27 |
| Smoking | ||||||
| Not currently | Referent | |||||
| Currently | 0.0184 | 4.13 | 1.27–13.45 | 0.0018 | 4.08 | 1.69–9.86 |
| Sex | ||||||
| Female | Referent | |||||
| Male | 0.8303 | 1.14 | 0.35–3.75 | 0.9009 | 0.96 | 0.43–2.12 |
| Age | ||||||
| >21 y | Referent | |||||
| ≤21 y | 0.8269 | 0.84 | 0.18–3.86 | 0.6495 | 1.23 | 0.51–2.98 |
In multivariate models with lens bioburden and health status used as time-dependent covariates, the hazards ratio for a CIE was 15.32 (95% CI, 4.86–48.30) and 4.67 (95% CI, 1.42–15.26) for substantial lens bioburden and smoking, respectively, through 4 months of follow-up. Through 12 months of follow-up, the hazards ratio for a CIE was 8.07 (95% CI, 3.75–17.38) and 5.46 (95% CI, 2.10–14.20) for substantial lens bioburden and smoking, respectively. Experiencing a cold, flu, or other viral infection was no longer significant (P > 0.191).
Discussion
In the LASH Study, the unadjusted cumulative incidence for a CIE with continuous wear of lotrafilcon A SH lenses was approximately 26.7% after 1 year. Contact lens–related CIEs are the end result of the normal defense mechanisms of the ocular surface, as it encounters foreign substances. Although CIEs are known to occur in healthy noncontact lens wearers,47,48 it is well documented that contact lens use significantly increases the risk and severity.17,48–54 On the high end, Sankaridurg et al.55 reported the incidence of CIEs as 36% in a group of subjects wearing low-Dk disposable lenses for extended wear.
The incidence of CIEs reported herein is higher than that previously reported for this lens type and mode of wear,27,30,56 as a result of differences in outcome definition and frequency of follow-up. For example, Chalmers et al.30 reported an incidence of 2.6% which represented symptomatic lotrafilcon A-wearing subjects in a phase IV postmarket surveillance study. Their study captured only the events that resulted in a red or uncomfortable eye that needed clinician intervention. We (LSF and SD) reported a cumulative incidence of 5.7% after 1 year, and 10.3% after 3 years in a separate longitudinal study of 317 lotrafilcon A lens wearers observed every 6 months across 19 clinical sites.27 Biomicroscopy signs were graded according to the 0- to 4-point pictorial Efron Grading Scale. Subtle AIs or incident CLPU scars may not have been noted. In the LASH Study, all CIEs were recorded, including AIK, AIs, and CLPU events detected as incident scars. In addition, the shorter intervals between visits in the LASH Study provided more opportunity to capture transient asymptomatic events. Last, subjects were frequently reminded to present promptly for any episode of red eye or discomfort, regardless of severity and perceived speed of resolution. These factors enabled us to capture more incident CIE events.
Mild corneal staining was common in this study; however, the staining evident at study visits was not associated with the occurrence of a CIE. Sodium fluorescein stains areas of loss of epithelial integrity; it does not penetrate the intact corneal tissue or stain vital tissue.57 In this regard, there was sufficient rationale to consider subtle epithelial trauma (as captured by the corneal staining response) as a plausible risk factor for CIEs. At least one infiltrative event, CLPU, has already been associated with minor ocular surface trauma58,59 and Gram-positive bacterial contamination of lens surfaces (Willcox M, et al. IOVS 1995;36:ARVO Abstract 734).16,18,60 Because first-generation SH lenses are stiffer than most hydrogel lenses and they are associated with other mechanical complications such as subepithelial arcuate lesions,61 it is feasible that mild mechanical epithelial trauma with SH materials is related to CIE production. The lack of an association between corneal staining and CIEs in this study does not rule out that an epithelial breach may be a predisposing factor in CIE development. It simply reflects that superficial corneal staining, as captured during uneventful clinical visits, even if present in a repeated fashion, is not associated with an eventual infiltrative response. In fact, our findings are supported by Fleiszig,62 who showed in animal models that the superficial epithelium can be severely abraded in the presence of virulent strains of Pseudomonas species with no increased susceptibility to infection.62
The lack of an association between corneal staining and CIEs found in the LASH Study contradicts findings from previous work by us (LSF, SD),27 which can be explained by differences in study design. In the previous study,27 a simple grading scheme for corneal staining was used and filters (Wratten; Eastman Kodak) were not required. As a consequence of that methodology, the incidence of staining in that cohort was only approximately 16%. Thus, in our previous study, we very likely underreported corneal staining, resulting in exposure misclassification.
As expected, the presence of substantial bacterial bioburden on worn contact lenses was significantly associated with the development of a CIE. In fact, other than smoking, it is the only statistically significant risk factor for CIEs in this study. This study is the first in which the association was quantified between overall bacterial bioburden on SH lenses and CIEs. The best estimate is at least an eightfold increased hazard for a CIE if substantial bioburden is present on SH lens surfaces; this estimate is taken from the multivariate analysis using 4-month data with lens bioburden modeled as a fixed covariate. The 12-month dataset is limited in relation to the exposure classification of lens bioburden. That is, only CIE subjects and disease control subjects were sampled past the 4-month visit; the CIE-free subjects retained the classification of lens bioburden, as was determined in the first 4 months of the study. These disease controls were CIE-free subjects who presented for other problems and non–CIE-related adverse events. The incidence of positive cultures in this group was greater than in the group with no events. Either these other adverse events were also associated with bacterial bioburden, or the discomfort secondary to the problem prompted lens manipulation and contamination. Selectively culturing lenses during problem-oriented visits resulted in a lower hazard for CIEs using the 12-month dataset compared to the 4-month dataset. Therefore, although the 4-month dataset assessed fewer CIEs, it is probably more accurate than the 12-month dataset because of potential exposure misclassification. Future studies can avoid this bias if culture of all remaining CIE-free subjects at the end of the follow-up period is included in the study design.
The time-dependent analyses resulted in significantly greater hazards of CIEs for substantial lens bioburden at both follow-up times. The time-dependent approach quantified the number of days a subject was exposed (or not) by using the age of the lens and microbiology status at time of culture. There are limitations to this approach, because it was assumed that the lens bioburden status assessed at time of culture had remained constant since the lens was inserted. In fact, there is no method of predicting when contamination (or lack of) occurs during each 30-day wear cycle. A presumed sterile lens from the package can become contaminated in a sporadic and unpredictable manner rather than in a steady accumulation over time,63 and a contaminated lens (from hand contact) can become sterile after 5 hours of lens wear.64 Despite these limitations, the time-dependent approach is desirable for covariates that change over time. The fact that the hazard for a CIE is significantly greater when bacterial bioburden is modeled as a time-dependent covariate suggests that lens bioburden lies in the causal CIE pathway and that its effect is more acute than not. In other words, the time-dependent approach is better able to capture the fact that the risk of a CIE is substantially diminished each time a new, sterile lens replaces a contaminated lens at the beginning of each 30-day wear cycle.
Of the 38 subjects with incident CIEs, aseptic removal techniques may not have been practiced in 12 of them, because lens removal was initiated as would be expected during a symptomatic event. Although the subjects presumably removed the lenses with washed hands and placed them in unpreserved saline within unused cases, there is still potential for hand contamination with normal skin microbiota. However, the effect of hand contamination on the results is dampened, as the saline used to transport the lenses most likely washed off some of the loosely bound organisms. In addition, of the 12 self-removed lenses, only four harbored CNS (normal skin flora); the remainder harbored high levels of pathogenic bacteria or were sterile.
The finding of an association between bacterial bioburden and CIEs is not novel15,16,19,20,59,65–67; however, this study is the first in which the association was quantified in SH lens wearers. Furthermore, certain SH lenses bind more microorganisms than do low-Dk lenses,68–70 which may explain why there is a higher rate of CIEs observed during SH lens wear.8,9 Normal ocular biota include CNS, Corynebacterium, Micrococcus, Bacillus, and Propionibacterium species.40,43,71–75 In the LASH study, the five most commonly isolated organisms associated with a CIE were Serratia marcescens, Pseudomonas aeruginosa, viridans group streptococci, Corynebacterium species, and CNS. Although some of these organisms are considered normal microbiota, our results suggest that they can initiate an immune response when found in high numbers on contact lenses worn in a static continuous-wear environment. In addition, some CNS such as certain strains of Staphylococcus epidermidis are pathogenic, such as invasive or biofilm-forming S. epidermidis strains.76,77 In this study, we were not able to distinguish which specific CNS species or strains were associated with a CIE, because isolates were not routinely speciated.
Although the predominant risk factor for development of CIE was bacterial contamination of lenses, approximately 20% of lenses worn during a CIE were sterile. There are several explanations for this finding. First, by design, only viable aerobic bacteria or yeast were cultured from the study lenses, because anaerobes have rarely been associated with contact lens–related adverse events. However, the Gram-positive anaerobic bacilli of the Propionibacterium genus are normal inhabitants of the eye,40,43 and have been cultured off worn contact lenses.40,63 Therefore, sterile lens cultures may simply reflect the absence of viable aerobic organisms. Furthermore, viable organisms are not necessary to initiate an immune response. Pearlman has shown that microbial breakdown products, such a lipopolysaccharide found on Gram-negative bacteria, can activate Toll-like receptors in the corneal epithelium and stimulate an inflammatory response in the absence of live organisms.21
Smoking has frequently been reported to be a risk factor for CIEs14,30,31 and microbial keratitis.34,78–80 In the present study, we also found that smoking was associated with a CIE. The mechanisms of this relationship are probably multifactorial and may be partly causal and partly confounding. The adverse effects of smoking may stem from toxins,81 increased pathogens in the subject's resident microbiota,82,83 changes in mucous membranes,84 or there may be a confounding effect with other unmeasured risk-taking behavior, as there is evidence of clustering of risky health behavior in the primary care setting.85,86
In summary, CIEs related to continuous wear of SH lenses are not associated with asymptomatic corneal staining captured during uneventful examinations at times preceding the event. The presence of substantial bacterial bioburden on lenses and smoking are prominent risk factors for development of a CIE during CW. The hazard for a CIE increases more than eightfold in the presence of substantial lens bioburden, and 72.3% of the total risk of CIE in those with substantial lens bioburden is attributable to this exposure. Based on this information, novel antimicrobial lens surfaces should lower this risk of CIE, as has been shown in animal models.87
Acknowledgments
The authors thank Saralee Bajaksouzian, MS, Mark Harrod, and Stephanie Burke for assistance with data collection and Desmond Fonn, MOptom, for data interpretation and manuscript review.
Footnotes
Supported primarily by National Eye Institute Grant NEI K23 EY015270-01 (LS-F); CIBA Vision; National Eye Institute Grant P30 EY11373; American Optometric Foundation, Prevent Blindness Ohio; Alcon Laboratories; Ohio Lions Eye Research Foundation; and Research to Prevent Blindness.
Disclosure: L. Szczotka-Flynn, Ciba Vision (F), Alcon Laboratories (F, R), Menicon (R), InSpire (R), Vistikon (R); J.H. Lass, None; A. Sethi, None; S. Debanne, None; B.A. Benetz, None; M. Albright, None; B. Gillespie, None; J. Kuo, None; M.R. Jacobs, None; A. Rimm, None
References
- 1. Keay L, Sweeney DF, Jalbert I, Skotnitsky C, Holden BA. Microcyst response to high Dk/t silicone hydrogel contact lenses. Optom Vis Sci. 2000;77:582–585 [DOI] [PubMed] [Google Scholar]
- 2. Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology. 2001;108:1279–1288 [DOI] [PubMed] [Google Scholar]
- 3. Papas EB, Vajdic CM, Austen R, Holden BA. High-oxygen-transmissibility soft contact lenses do not induce limbal hyperaemia. Curr Eye Res. 1997;16:942–948 [DOI] [PubMed] [Google Scholar]
- 4. Steffen RB, Schnider CM. The impact of silicone hydrogel materials on overnight corneal swelling. Eye Contact Lens. 2007;33:115–120 [DOI] [PubMed] [Google Scholar]
- 5. Dart JK, Radford CF, Minassian D, Verma S, Stapleton F. Risk factors for microbial keratitis with contemporary contact lenses: a case–control study. Ophthalmology. 2008;115:1647–1654, e1–3 [DOI] [PubMed] [Google Scholar]
- 6. Schein OD, McNally JJ, Katz J, et al. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Ophthalmology. 2005;112:2172–2179 [DOI] [PubMed] [Google Scholar]
- 7. Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115:1655–1662 [DOI] [PubMed] [Google Scholar]
- 8. Szczotka-Flynn L, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low Dk hydrogel extended contact lens wear: a meta-analysis. Optom Vis Sci. 2007;84:247–256 [DOI] [PubMed] [Google Scholar]
- 9. Radford CF, Minassian D, Dart JK, Stapleton F, Verma S. Risk factors for nonulcerative contact lens complications in an ophthalmic accident and emergency department: a case-control study. Ophthalmology. 2009;116:385–392 [DOI] [PubMed] [Google Scholar]
- 10. Josephson JE, Caffery BE. Infiltrative keratitis in hydrogel lens wearers. Int Contact Lens Clin. 1979;6:223–241 [Google Scholar]
- 11. Cutter GR, Chalmers RL, Roseman M. The clinical presentation, prevalence, and risk factors of focal corneal infiltrates in soft contact lens wearers. CLAO J. 1996;22:30–37 [PubMed] [Google Scholar]
- 12. Levy B, McNamara N, Corzine J, Abbott RL. Prospective trial of daily and extended wear disposable contact lenses. Cornea. 1997;16:274–276 [PubMed] [Google Scholar]
- 13. Efron N, Morgan PB, Hill EA, Raynor MK, Tullo AB. Incidence and morbidity of hospital-presenting corneal infiltrative events associated with contact lens wear. Clin Exp Optom. 2005;88:232–239 [DOI] [PubMed] [Google Scholar]
- 14. Morgan PB, Efron N, Brennan NA, Hill EA, Raynor MK, Tullo AB. Risk factors for the development of corneal infiltrative events associated with contact lens wear. Invest Ophthalmol Vis Sci. 2005;46:3136–3143 [DOI] [PubMed] [Google Scholar]
- 15. Holden BA, La Hood D, Grant T, et al. Gram-negative bacteria can induce contact lens related acute red eye (CLARE) responses. CLAO J. 1996;22:47–52 [PubMed] [Google Scholar]
- 16. Jalbert I, Willcox MD, Sweeney DF. Isolation of Staphylococcus aureus from a contact lens at the time of a contact lens-induced peripheral ulcer: case report. Cornea. 2000;19:116–120 [DOI] [PubMed] [Google Scholar]
- 17. Sankaridurg PR, Sharma S, Willcox M, et al. Bacterial colonization of disposable soft contact lenses is greater during corneal infiltrative events than during asymptomatic extended lens wear. J Clin Microbiol. 2000;38:4420–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sankaridurg PR, Sharma S, Willcox M, et al. Colonization of hydrogel lenses with Streptococcus pneumoniae: risk of development of corneal infiltrates. Cornea. 1999;18:289–295 [DOI] [PubMed] [Google Scholar]
- 19. Sankaridurg PR, Vuppala N, Sreedharan A, Vadlamudi J, Rao GN. Gram negative bacteria and contact lens induced acute red eye. Indian J Ophthalmol. 1996;44:29–32 [PubMed] [Google Scholar]
- 20. Sankaridurg PR, Willcox MD, Sharma S, et al. Haemophilus influenzae adherent to contact lenses associated with production of acute ocular inflammation. J Clin Microbiol. 1996;34:2426–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pearlman E, Johnson A, Adhikary G, et al. Toll-like receptors at the ocular surface. Ocul Surf. 2008;6:108–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sweeney DF, Naduvilath TJ. Are inflammatory events a marker for an increased risk of microbial keratitis? Eye Contact Lens. 2007;33:383–387; discussion 399–400 [DOI] [PubMed] [Google Scholar]
- 23. Szczotka-Flynn L, Pearlman E., Ghannoum M. Microbial contamination of contact lenses, lens care solutions and their accessories; a literature review. Eye Contact Lens. 2010;36:116–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ward KW. Superficial punctate fluorescein staining of the ocular surface. Optom Vis Sci. 2008;85:8–16 [DOI] [PubMed] [Google Scholar]
- 25. Willcox M. FDA Ophthalmic Devices Panel Meeting. 2008. Available at: http://www.fda.gov/ohrms/dockets/ac/08/slides/2008-4363oph1–08-willcox.pdf Accessed September 27, 2009
- 26. Carnt N, Jalbert I, Stretton S, Naduvilath T, Papas E. Solution toxicity in soft contact lens daily wear is associated with corneal inflammation. Optom Vis Sci. 2007;84:309–315 [DOI] [PubMed] [Google Scholar]
- 27. Szczotka-Flynn L, Debanne SM, Cheruvu VK, et al. Predictive factors for corneal infiltrates with continuous wear of silicone hydrogel contact lenses. Arch Ophthalmol. 2007;125:488–492 [DOI] [PubMed] [Google Scholar]
- 28. Brennan NA, Mullen B. Increased susceptibility of younger people to adverse reactions with silicone-hydrogel contact lend continuous wear. Optom Vis Sci. 2001;78:229 [Google Scholar]
- 29. du Toit R, John T, Sweeney D, Kalliris A, Tahhan N, Papas E. Association of age, gender and ethnicity with the incidence of adverse responses with extended wear of silicone hydrogel lenses. Optom Vis Sci. 2002;79(suppl)9 [Google Scholar]
- 30. Chalmers RL, McNally JJ, Schein OD, et al. Risk factors for corneal infiltrates with continuous wear of contact lenses. Optom Vis Sci. 2007;84:573–579 [DOI] [PubMed] [Google Scholar]
- 31. McNally JJ, Chalmers RL, McKenney CD, Robirds S. Risk factors for corneal infiltrative events with 30-night continuous wear of silicone hydrogel lenses. Eye Contact Lens. 2003;29:S153–S156; discussion S66, S92–S94 [DOI] [PubMed] [Google Scholar]
- 32. Institute for Eye Research Institute for Eye Research (IER)/LVPEI Guide to Corneal Infiltrative Conditions. Available at: http://www.siliconehydrogels.org/resources/index.asp Accessed September 25, 2009
- 33. Aasuri MK, Venkata N, Kumar VM. Differential diagnosis of microbial keratitis and contact lens-induced peripheral ulcer. Eye Contact Lens. 2003;29:S60–S62;discussion S83–S84, S192–S194 [DOI] [PubMed] [Google Scholar]
- 34. Morgan PB, Efron N, Hill EA, Raynor MK, Whiting MA, Tullo AB. Incidence of keratitis of varying severity among contact lens wearers. Br J Ophthalmol. 2005;89:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Efron N. Grading scales for contact lens complications. Appendix A. Contact Lens Complications. 2nd ed 2004:239–243 [Google Scholar]
- 36. Efron NMP, Katsara SS. Validation of grading scales for contact lens complications. Ophthalmic Physiol Opt. 2001;21:17–29 [PubMed] [Google Scholar]
- 37. Institute for Eye Research Institute for Eye Research Grading Scales. Available at: http://www.siliconehydrogels.org/resources/index.asp Accessed September 15, 2009
- 38. Barr JT, Schechtman KB, Fink BA, Pierce GE, Pensyl CD, Zadnik K, Gordon MO. CLEK Study Group Corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: baseline prevalence and repeatability of detection. Cornea. 1999;18:34–46 [PubMed] [Google Scholar]
- 39. Szczotka-Flynn LB, Bajaksouzian S, Jacobs M, Rimm A. Risk factors for contact lens bacterial contamination during continuous wear. Optom Vis Sci. 2009;28(8):918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Willcox MD, Power KN, Stapleton F, Leitch C, Harmis N, Sweeney DF. Potential sources of bacteria that are isolated from contact lenses during wear. Optom Vis Sci. 1997;74:1030–1038 [DOI] [PubMed] [Google Scholar]
- 41. Willcox MD, Harmis NY, Holden BA. Bacterial populations on high-Dk silicone hydrogel contact lenses: effect of length of wear in asymptomatic patients. Clin Exp Optom. 2002;85:172–175 [DOI] [PubMed] [Google Scholar]
- 42. Baleriola-Lucas C, Fukuda M, Willcox MD, Sweeney DF, Holden BA. Fibronectin concentration in tears of contact lens wearers. Exp Eye Res. 1997;64:37–43 [DOI] [PubMed] [Google Scholar]
- 43. Stapleton F, Willcox MD, Fleming CM, Hickson S, Sweeney DF, Holden BA. Changes to the ocular biota with time in extended- and daily-wear disposable contact lens use. Infect Immun. 1995;63:4501–4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramachandran L, Sharma S, Sankaridurg PR, et al. Examination of the conjunctival microbiota after 8 hours of eye closure. CLAO J. 1995;21:195–199 [PubMed] [Google Scholar]
- 45. Liu GLK. Sample size calculations for studies with correlated observations. Biometrics. 1997;53:937–947 [PubMed] [Google Scholar]
- 46. Gopinathan U, Stapleton F, Sharma S, et al. Microbial contamination of hydrogel contact lenses. J Appl Microbiol. 1997;82:653–658 [DOI] [PubMed] [Google Scholar]
- 47. Hickson S, Papas E. Prevalence of idiopathic corneal anomalies in a non contact lens-wearing population. Optom Vis Sci. 1997;74:293–297 [DOI] [PubMed] [Google Scholar]
- 48. Sankaridurg PR, Sweeney DF, Holden BA, et al. Comparison of adverse events with daily disposable hydrogels and spectacle wear: results from a 12-month prospective clinical trial. Ophthalmology. 2003;110:2327–2334 [DOI] [PubMed] [Google Scholar]
- 49. Serdahl CL, Mannis MJ, Shapiro DR, Zadnik K, Lightman JM, Pinilla C. Infiltrative keratitis associated with disposable soft contact lenses: case reports. Arch Ophthalmol. 1989;107:322–323 [DOI] [PubMed] [Google Scholar]
- 50. Skotnitsky C, Jalbert I, O'Hare N, Sweeney DF, Holden BA. Case reports of three atypical infiltrative keratitis events with high DK soft contact lens wear. Cornea. 2002;21:318–324 [DOI] [PubMed] [Google Scholar]
- 51. Snyder C. Infiltrative keratitis with contact lens wear: a review. J Am Optom Assoc. 1995;66:160–177 [PubMed] [Google Scholar]
- 52. Stapleton F, Dart J, Minassian D. Nonulcerative complications of contact lens wear. Relative risks for different lens types. Arch Ophthalmol. 1992;110:1601–1606 [DOI] [PubMed] [Google Scholar]
- 53. Stapleton F, Dart JK, Minassian D. Risk factors with contact lens related suppurative keratitis. CLAO J. 1993;19:204–210 [PubMed] [Google Scholar]
- 54. Stapleton F, Keay L, Jalbert I, Cole N. The epidemiology of contact lens related infiltrates. Optom Vis Sci. 2007;84:257–272 [DOI] [PubMed] [Google Scholar]
- 55. Sankaridurg PR, Sweeney DF, Sharma S, et al. Adverse events with extended wear of disposable hydrogels: results for the first 13 months of lens wear. Ophthalmology. 1999;106:1671–1680 [DOI] [PubMed] [Google Scholar]
- 56. Ciba Vision Summary of safety and effectiveness data for 30 day wear of Lotrafilcon A. 2001. Available at: http://www.fda.gov/cdrh/pdf/p000030b.pdf Accessed September 15, 2009
- 57. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–650 [DOI] [PubMed] [Google Scholar]
- 58. Holden BA, Sankaridurg PR, Jalbert I. Adverse events and infections: which ones and how many? In: Sweeney DF. ed. Silicone Hydrogels: The Rebirth of Continuous Wear Contact Lenses. 2000:181 [Google Scholar]
- 59. Wu PZ, Thakur A, Stapleton F, Willcox MD. Staphylococcus aureus causes acute inflammatory episodes in the cornea during contact lens wear. Clin Exp Ophthalmol. 2000;28:194–196 [DOI] [PubMed] [Google Scholar]
- 60. Szczotka L. Ocular microbiota associated with contact lens induced peripheral ulcer. Optom Vis Sci. 2000;76:165 [Google Scholar]
- 61. Jalbert I, Sweeney DF, Holden BA. Epithelial split associated with wear of a silicone hydrogel contact lens. CLAO J. 2001;27:231–233 [PubMed] [Google Scholar]
- 62. Fleiszig SM. The Glenn A. Fry award lecture 2005: the pathogenesis of contact lens-related keratitis. Optom Vis Sci. 2006;83:866–873 [DOI] [PubMed] [Google Scholar]
- 63. Sweeney DF, Stapleton F, Leitch C, Taylor J, Holden BA, Willcox MD. Microbial colonization of soft contact lenses over time. Optom Vis Sci. 2001;78:100–105 [DOI] [PubMed] [Google Scholar]
- 64. Mowrey-McKee MF, Sampson HJ, Proskin HM. Microbial contamination of hydrophilic contact lenses. Part II: Quantitation of microbes after patient handling and after aseptic removal from the eye. CLAO J. 1992;18:240–244 [PubMed] [Google Scholar]
- 65. Corrigan KM, Harmis NY, Willcox MD. Association of acinetobacter species with contact lens-induced adverse responses. Cornea. 2001;20:463–466 [DOI] [PubMed] [Google Scholar]
- 66. Keay L, Harmis N, Corrigan K, Sweeney D, Willcox M. Infiltrative keratitis associated with extended wear of hydrogel lenses and Abiotrophia defectiva. Cornea. 2000;19:864–869 [DOI] [PubMed] [Google Scholar]
- 67. Willcox MD, Hume EB. Differences in the pathogenesis of bacteria isolated from contact-lens-induced infiltrative conditions. Aust N Z J Ophthalmol. 1999;27:231–233 [DOI] [PubMed] [Google Scholar]
- 68. Henriques M, Sousa C, Lira M, et al. Adhesion of Pseudomonas aeruginosa and Staphylococcus epidermidis to silicone-hydrogel contact lenses. Optom Vis Sci. 2005;82:446–450 [DOI] [PubMed] [Google Scholar]
- 69. Kodjikian L, Casoli-Bergeron E, Malet F, et al. Bacterial adhesion to conventional hydrogel and new silicone-hydrogel contact lens materials. Graefes Arch Clin Exp Ophthalmol. 2008;246:267–273 [DOI] [PubMed] [Google Scholar]
- 70. Willcox MD, Harmis N, Cowell, Williams T, Holden BA. Bacterial interactions with contact lenses; effects of lens material, lens wear and microbial physiology. Biomaterials. 2001;22:3235–3247 [DOI] [PubMed] [Google Scholar]
- 71. Elander TR, Goldberg MA, Salinger CL, Tan JR, Levy B, Abbott RL. Microbial changes in the ocular environment with contact lens wear. CLAO J. 1992;18:53–55 [PubMed] [Google Scholar]
- 72. Fleiszig SM, Efron N. Microbial flora in eyes of current and former contact lens wearers. J Clin Microbiol. 1992;30:1156–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hovding G. The conjunctival and contact lens bacterial flora during lens wear. Acta Ophthalmol (Copenh). 1981;59:387–401 [DOI] [PubMed] [Google Scholar]
- 74. Iskeleli G, Bahar H, Eroglu E, Torun MM, Ozkan S. Microbial changes in conjunctival flora with 30-day continuous-wear silicone hydrogel contact lenses. Eye Contact Lens. 2005;31:124–126 [DOI] [PubMed] [Google Scholar]
- 75. Larkin DF, Leeming JP. Quantitative alterations of the commensal eye bacteria in contact lens wear. Eye. 1991;5:70–74 [DOI] [PubMed] [Google Scholar]
- 76. Li H, Xu L, Wang J, et al. Conversion of Staphylococcus epidermidis strains from commensal to invasive by expression of the ica locus encoding production of biofilm exopolysaccharide. Infect Immun. 2005;73:3188–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vallas V, Stapleton F, Willcox MD. Bacterial invasion of corneal epithelial cells. Aust N Z J Ophthalmol. 1999;27:228–230 [DOI] [PubMed] [Google Scholar]
- 78. Schein OD, Glynn RJ, Poggio EC, Seddon JM, Kenyon KR. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses: a case-control study. Microbial Keratitis Study Group. N Engl J Med. 1989;321:773–778 [DOI] [PubMed] [Google Scholar]
- 79. Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115:1655–1662 [DOI] [PubMed] [Google Scholar]
- 80. Lam DS, Houang E, Fan DS, Lyon D, Seal D, Wong E. Incidence and risk factors for microbial keratitis in Hong Kong: comparison with Europe and North America. Eye. 2002;16:608–618 [DOI] [PubMed] [Google Scholar]
- 81. Didilescu AC, Hanganu SC, Galie N, et al. The role of smoking in changing essential parameters in body homeostasis (in Romanian). Pneumologia. 2009;58:89–94 [PubMed] [Google Scholar]
- 82. Brook I. Effects of exposure to smoking on the microbial flora of children and their parents. Int J Pediatr Otorhinolaryngol. 2010;74:447–450 [DOI] [PubMed] [Google Scholar]
- 83. Brook I, Gober AE. Recovery of potential pathogens and interfering bacteria in the nasopharynx of smokers and nonsmokers. Chest. 2005;127:2072–2075 [DOI] [PubMed] [Google Scholar]
- 84. Samet JM. Adverse effects of smoke exposure on the upper airway. Tob Control. 2004;13(suppl 1):i57–i60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pronk NP, Peek CJ, Goldstein MG. Addressing multiple behavioral risk factors in primary care: a synthesis of current knowledge and stakeholder dialogue sessions. Am J Prev Med. 2004;27:4–17 [DOI] [PubMed] [Google Scholar]
- 86. Shankar A, McMunn A, Steptoe A. Health-related behaviors in older adults relationships with socioeconomic status. Am J Prev Med. 2010;38:39–46 [DOI] [PubMed] [Google Scholar]
- 87. Cole N, Hume E, Vijay AK, Sankaridurg P, Kumar N, Willcox M. In vivo performance of melimine as an antimicrobial coating for contact lenses in models of CLARE and CLPU. Invest Ophthalmol Vis Sci. 2009;51:390–395 [DOI] [PubMed] [Google Scholar]