Quantitative subanalysis of optical coherence tomography images in neovascular age-related macular degeneration may lead to the detection of novel retinal parameters that are capable of serving as surrogate endpoints in clinical trials of this disorder.
Abstract
Purpose.
To investigate the effect of changes in retinal morphology on contrast sensitivity and reading ability in patients with neovascular age-related macular degeneration (AMD) in the Avastin (bevacizumab; Genentech, South San Francisco, CA) for choroidal neovascularization (ABC) Trial.
Methods.
Contrast sensitivity obtained with Pelli-Robson charts, reading ability assessed with Minnesota Reading charts, and Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity (VA) obtained by protocol refraction, were recorded. Raw Stratus optical coherence tomography (OCT; Carl Zeiss Meditec, Inc., Dublin, CA) images were analyzed with the publicly available software OCTOR, which allows precise delineation of any retinal compartment of interest. Thickness and volume were calculated for neurosensory retina, subretinal fluid (SRF), subretinal tissue, and pigment epithelium detachment, and the resulting measurements were correlated with each visual function parameter.
Results.
One hundred twenty-two patients with newly diagnosed neovascular AMD and enrolled in the ABC Trial, were evaluated. Increased subretinal tissue volume correlated with decreased contrast sensitivity (Pearson's correlation coefficient, r = −0.4944, P = 0.001). A modest correlation was detected between SRF volume and contrast sensitivity (r = −0.2562, P = 0.004). Increased retinal thickness at the foveal center also correlated with decreased visual function (ETDRS VA: r = −0.4530, P < 0.001).
Conclusions.
The strongest correlation detected between the functional parameters assessed and any of the OCT-derived morphologic parameters was that between decreased contrast sensitivity and increased subretinal tissue. In the future, assessment of contrast sensitivity and reading ability, in combination with quantitative subanalysis of retinal compartments, may lead to the identification of parameters relevant to functional improvement and ultimate prognosis in patients with newly diagnosed neovascular AMD (www.controlled-trials.com number, ISRCTN83325075).
In recent years, the treatment of neovascular age-related macular degeneration (AMD) has been revolutionized by the introduction of antiangiogenic therapies such as ranibizumab (Lucentis), and bevacizumab (Avastin; both from Genentech, Inc., South San Francisco, CA).1–3 Optical coherence tomography (OCT), an imaging modality that provides cross-sectional images of the neurosensory retina, plays a critical role in this treatment by allowing noninvasive monitoring of disease activity.4,5 Although it is presumed that anatomic improvements demonstrated by OCT will ultimately result in clinical benefits, many studies have failed to show a consistent correlation between reduction in OCT-derived retinal thickness and improvement in visual function.3,6 Such failures may be due, at least in part, to the limitations of the automated image-analysis software provided by current OCT systems.7,8 In addition, however, the structurally heterogeneous nature of the disorder and the reliance on high-contrast distance visual acuity as the primary measure of visual function in these studies may play a role.9,10
CNV, the hallmark of neovascular AMD, results in a wide range of alterations in retinal morphology, with intraretinal, subretinal, and RPE components.11,12 CNV lesions also vary widely, both in size, and in location relative to the fovea. Although high-contrast distance visual acuity is largely a function of foveal integrity, other visual parameters, such as reading ability and contrast sensitivity, may be more reflective of alterations in macular structure as a whole.10,13–15 Thus, evaluation of contrast sensitivity and reading ability may allow detection of stronger and more consistent correlations with OCT-derived retinal parameters. The detection of any such robust relationship is of interest for clinical trials, where the use of validated morphologic parameters as surrogate endpoints could lead to increased accuracy, reduced costs, and shortened duration.16–18 Furthermore, in clinical practice, measurement of reading ability and contrast sensitivity may also allow the clinician to better assess individual visual performance. Contrast sensitivity, for example, is closely linked with both orientation and mobility and, in patients with macular disease, may be markedly reduced despite near-normal distance visual acuity.19
To address these questions and others, we have developed a software tool (OCTOR) that allows the user to manually quantify any retinal structure of interest.20 Using this tool, we have identified novel OCT parameters that show modest correlations with Snellen visual acuity in neovascular AMD.6,9 In the current report, we evaluate the effect of changes in retinal morphology on reading ability and contrast sensitivity, using OCTOR, in a recently completed phase III/IV clinical trial: the Avastin (bevacizumab) for choroidal neovascularization (ABC) Trial.21
Materials and Methods
Data Collection
The ABC trial is a double-masked randomized controlled trial that commenced in August 2006, in which intravitreal bevacizumab injections were compared to standard therapy in the treatment of neovascular AMD.21 The patients enrolled in the ABC Trial were randomized to intravitreal bevacizumab or to the standard therapy available at the time of trial initiation (photodynamic therapy with verteporfin, intravitreal pegaptanib, or sham treatment). For inclusion in the study, the patients were required to have previously untreated subfoveal CNV secondary to AMD, with no evidence of significant ocular comorbidity. Full details of the clinical trial design, as well as the inclusion and exclusion criteria, have been reported.21 The ABC Trial was conducted according to the ICHGCP (International Conference on Harmonization for Good Clinical Practice in clinical research), as set out in the European Union Clinical Trials Directive (2001) and associated U.K. Regulations (2004), which adhere to the principles of the Declaration of Helsinki.
StratusOCT (Carl Zeiss Meditec, Inc., Dublin, CA) images, using the Radial Lines protocol of six high-resolution B-scans (512 A-scans per 6-mm B-scan) or the Fast Macular Thickness protocol of six lower-resolution B-scans (128 A-scans per 6-mm B-scan), were collected at baseline for each patient enrolled in the study. Data for each case were exported to disk by using the export feature available in the StratusOCT analysis software (ver. 4.0).
Each patient's best-refracted visual acuity was recorded at the time of enrollment by using Early Treatment Diabetic Retinopathy (ETDRS) visual acuity charts at a starting distance of 4 m. Contrast sensitivity was measured with Pelli-Robson charts, whereas reading ability was measured with Minnesota Reading (MNREAD) charts according to standard protocols.22 Color photos and fluorescein angiographic images were also obtained for each patient at enrollment.
Computer-Assisted Grading Software
The software used for OCT analysis (OCTOR) was written by Doheny Image Reading Center software engineers to facilitate viewing and manual grading. This software, which effectively operates as a painting program and calculator, imports data exported from the StratusOCT machine and allows the grader to use a computer mouse to draw various boundaries in the retinal cross-sectional images. OCTOR is publicly accessible at http://www.diesel.la and has been described and validated in previous reports.20,23
Analogous to the StratusOCT software, OCTOR provides a report showing the calculated thickness and volume values for the nine ETDRS macular subfields, as well as the mean and SD of the foveal center point thickness. OCTOR also provides both maximum and minimum thickness values for any given morphologic space.
Grading Procedure
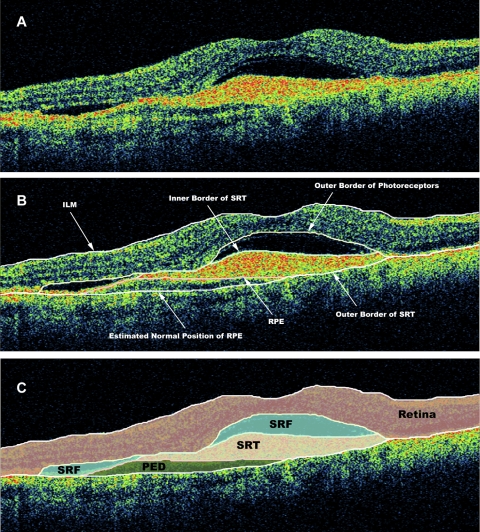
OCT scans were analyzed by certified OCT graders at the Doheny Image Reading Center (PAK, YO), who were masked to associated visual acuity information at the time of grading. Boundaries drawn in each of the six OCT B-scans included the internal limiting membrane, the outer border of the photoreceptors, the borders of subretinal fluid (SRF) and subretinal tissue (if present), the inner surface of the retinal pigment epithelium (RPE), and the estimated normal position of the RPE layer (in cases of RPE elevation). All boundaries were drawn in accordance with the standard OCT grading protocol of the Doheny Image Reading Center.23 All OCT scans included in the study met reading center criteria for sufficient image quality, including the absence of significant artifactitious variations in signal intensity or generalized reductions in signal strength. No minimum value for signal strength was set, as manual grading with OCTOR often allows quantitative information to be accurately derived from images with low signal strength (image sets are only excluded when the grader cannot accurately delineate the inner and outer retinal boundaries). After completion of grading, OCTOR was used to calculate output parameters for the various morphologic spaces: retina, SRF, subretinal tissue, and pigment epithelium detachment (PED; Fig. 1).
Figure 1.
OCT B-scan demonstrating (A) subretinal tissue, SRF, and PED. (B) The clinically relevant boundaries—the internal limiting membrane (ILM), the outer border of the photoreceptors, the RPE, the inner border of the subretinal tissue, the outer border of the subretinal tissue (SRT), and the estimated normal location of the RPE layer—were graded by OCTOR (computer-assisted manual grading) software. (C) The software then computed the volumes of the spaces (retina, SRT, SRF, and PED) defined by these boundaries.
Statistical Methods
The mean and SD of the foveal center point thickness, as well as the total volume (subfields 1–9), were calculated for each space in each case. Volume was measured in cubic millimeters, and thickness was measured in micrometers.
Univariate and multivariate regression was used to test for associations between visual function parameters and OCT parameters. Stepwise regression was used for selection of independent parameters when the improvement χ2 P-value was < 0.15. Linearity was examined by testing for higher-order polynomial terms for each continuous variable in the final multivariate model. To reduce potential collinearity, we did not include highly correlated variables (r ≥ 0.90) in the same model. Statistical analysis and graph generation were performed with commercially available software (Intercooled Stata for Windows, ver. 9; Statacorp LP, College Station, TX).
Results
Baseline Characteristics
One hundred twenty-two patients, with newly diagnosed neovascular AMD and enrolled in the ABC Trial, were evaluated. We used the Radial Lines scan protocol to image 79 (65%) eyes on StratusOCT and the Fast Macular Thickness scan protocol on the remaining 43 (35%). Of the 122 patients included in our analysis, 76 (62%) were women and 46 (38%) were men. The mean age of the patients was 80 years (SD 7.1) and the median age was 81 years (range, 58–93). The mean visual acuity at the time of initial diagnosis was 52.15 letters (SD 11.62; range, 25–70). The mean contrast sensitivity at the time of initial diagnosis was 0.84 ± 0.33 log CS. The mean reading acuity at the time of initial diagnosis was 0.89 ± 0.30 log RAD (reading acuity determination). The neovascular lesions were categorized by fluorescein angiography as classic with no occult (8 eyes, 7%), predominantly classic (20 eyes, 16%), minimally classic (37 eyes, 30%), and occult with no classic (57 eyes, 47%).
Morphologic Outcomes of OCTOR Analysis
The mean thickness of the neurosensory retina at the foveal center point was 269 ± 91 μm (mean ± SD). Retinal thickness was greatest in lesions classified as classic with no occult (306 ± 105 μm) and least in minimally classic (258 ± 64 μm) lesions. SRF was present at baseline in 104 (85%) eyes and had a total volume of 0.55 ± 1.16 mm3. SRF volume was greatest in classic with no occult lesions (0.72 ± 1.15 mm3) and least in minimally classic lesions (0.39 ± 0.48 mm3). PED was present at baseline in 104 (85%) eyes and had a total volume of 0.78 ± 0.92 mm3. PED volume was greatest in occult (0.91 ± 0.99 mm3) and minimally classic (0.95 ± 0.99 mm3) lesions and least in classic with no occult (0.16 ± 0.38 mm3) lesions. Subretinal tissue was present at baseline in 109 (89%) eyes and had a total volume of 0.56 ± 0.61 mm3. Subretinal tissue volume was greatest in classic with no occult lesions (1.09 ± 0.90 mm3) and least in occult with no classic lesions (0.30 ± 0.44 mm3).
Contrast Sensitivity
The association between contrast sensitivity and each of the OCT parameters is summarized in Table 1.
Table 1.
Univariate Model of Contrast Sensitivity and OCT Predictive Factors
| Factor | Model R2 | Parameter Estimate | P |
|---|---|---|---|
| Foveal center point thickness (μm) | |||
| Neurosensory retina | 0.0308 | −0.0006444 | 0.053 |
| SRF | 0.0039 | 0.0003467 | 0.492 |
| Subretinal tissue | 0.0802 | −0.0010277 | 0.002 |
| PED | 0.0058 | −0.0002544 | 0.404 |
| Total volume (mm3) | |||
| Neurosensory retina | 0.0160 | −0.0438253 | 0.165 |
| SRF | 0.0657 | −0.0740246 | 0.004 |
| Subretinal tissue | 0.2445 | −0.2684146 | 0.001 |
| PED | 0.0058 | −0.0276908 | 0.404 |
Bold data indicate significant results.
Neurosensory Retina.
No statistically significant association was detected between contrast sensitivity and either total volume of the neurosensory retina (Pearson correlation coefficient [r] = −0.1265, P = 0.165) or thickness of the neurosensory retina at the foveal center (r = −0.1755, P = 0.053).
Subretinal Fluid.
Decreased contrast sensitivity correlated with an increased total volume of SRF (r = −0.2562, P = 0.004), but not with the thickness of SRF at the foveal center (r = 0.0628, P = 0.492).
Subretinal Tissue.
Decreased contrast sensitivity demonstrated a statistically significant correlation with an increased total volume of subretinal tissue (r = −0.4944, P < 0.001) and, to a lesser extent, with increased thickness of subretinal tissue at the foveal center (r = −0.2831, P = 0.002).
Pigment Epithelium Detachment.
No statistically significant association was detected between contrast sensitivity and either the total volume of PED (r = −0.0762, P = 0.241) or the thickness of PED at the foveal center (r = −0.1189, P = 0.192).
Reading Ability
The association between reading ability and each of the OCT parameters is summarized in Table 2.
Table 2.
Univariate Model of Reading Ability and OCT Predictive Factors
| Factor | Model R2 | Parameter Estimate | P |
|---|---|---|---|
| Foveal center point thickness (μm) | |||
| Neurosensory retina | 0.0956 | 0.0010175 | 0.001 |
| SRF | 0.0013 | 0.0001752 | 0.699 |
| Subretinal tissue | 0.0922 | 0.0009879 | 0.001 |
| PED | 0.0028 | −0.0001015 | 0.563 |
| Total volume (mm3) | |||
| Neurosensory retina | 0.0485 | 0.0684034 | 0.015 |
| SRF | 0.0212 | 0.0377354 | 0.109 |
| Subretinal tissue | 0.1865 | 0.2101417 | <0.001 |
| PED | 0.0008 | −0.0094513 | 0.751 |
Bold data indicate significant results.
Neurosensory Retina.
Decreased reading ability correlated with increased thickness of the neurosensory retina at the foveal center (r = 0.3091, P = 0.001) and with the total volume of the neurosensory retina (r = 0.2202, P = 0.015).
Subretinal Fluid.
No statistically significant association was detected between reading ability and either total volume of SRF (r = 0.1457, P = 0.109) or thickness of SRF at the foveal center (r = 0.0354, P = 0.699).
Subretinal Tissue.
Decreased reading ability demonstrated a statistically significant correlation with an increased total volume of subretinal tissue (r = 0.4318, P < 0.001) and, to a lesser extent, with increased thickness of subretinal tissue at the foveal center (r = 0.3036, P = 0.001).
Pigment Epithelium Detachment.
No statistically significant association was detected between reading ability and either the total volume of PED (r = −0.029, P = 0.751) or the thickness of PED at the foveal center (r = −0.052, P = 0.563).
Visual Acuity
The association between ETDRS visual acuity and each of the OCT parameters was also evaluated and is summarized in Table 3.
Table 3.
Univariate Model of ETDRS Visual Acuity and OCT Predictive Factors
| Factor | Model R2 | Parameter Estimate | P |
|---|---|---|---|
| Foveal center point thickness (μm) | |||
| Neurosensory retina | 0.2053 | −0.0579249 | <0.001 |
| SRF | 0.0234 | 0.0294218 | 0.092 |
| Subretinal tissue | 0.0300 | −0.0218945 | 0.056 |
| PED | 0.0011 | 0.0024761 | 0.716 |
| Total volume (mm3) | |||
| Neurosensory retina | 0.0900 | −3.620042 | 0.001 |
| SRF | 0.0194 | −1.399761 | 0.126 |
| Subretinal tissue | 0.0661 | −4.858363 | 0.001 |
| PED | 0.0114 | 1.351158 | 0.241 |
Bold data indicate significant results.
Multivariate OCT Models of Visual Function
Multiple regression analysis, with a visual function parameter as the dependent variable in each case, yielded the models summarized in Table 4. These models had a coefficient of determination (R2) of 24.45%, 22.74%, and 22.17% for contrast sensitivity, reading ability, and ETDRS visual acuity, respectively.
Table 4.
Multivariate Models of Visual Function and OCT Predictive Factors*
| Factor | Model R2 | Improvement χ2P* |
|---|---|---|
| Model 1 (ETDRS visual acuity) | 0.2217 | |
| Thickness of neurosensory retina at FCP (μm) | <0.0001 | |
| Total volume of subretinal tissue (mm3) | 0.116 | |
| Model 2 (contrast sensitivity) | 0.2445 | |
| Total volume of subretinal tissue (mm3) | <0.0001 | |
| No other significant variables | ||
| Model 3 (reading ability) | 0.2274 | |
| Thickness of neurosensory retina at foveal central subfield (μm) | 0.0130 | |
| Total volume of subretinal tissue (mm3) | <0.0001 |
FCP, foveal center point.
Stepwise regression was used to select the variables that were independently associated with visual acuity.
Discussion
In this cross-sectional study of patients enrolled in a recently completed phase III/IV clinical trial, we identified several novel OCT-derived parameters that demonstrated moderate, but statistically significant, correlations with contrast sensitivity and reading ability in patients with newly diagnosed neovascular AMD.
In neovascular AMD, the abnormal blood vessels that constitute the choroidal neovascular membrane may pass directly into the subretinal space after their initial penetration of Bruch's membrane (type 2 CNV on histology).24 In these initial growth phases, the CNV membrane is often highly vascular and appears on OCT as an amorphous lesion of medium to high reflectivity above the RPE. As the CNV lesion becomes less active over time, the vascular component typically regresses, whereas the fibrous component increases, resulting in disciform scar formation that appears on OCT as a well demarcated highly hyperreflective lesion.25 In the present study, an increased volume of subretinal tissue correlated significantly with a decrease in all three parameters of visual function in particular, 24% of the variation in contrast sensitivity at baseline could be explained by an increased total volume of subretinal tissue (r = −0.4944, P = 0.001). Since the positioning of the fibrovascular tissue in the subretinal space may disrupt communication between the photoreceptors and the RPE, a deleterious effect on contrast sensitivity is not surprising. In addition, disciform scar formation may be associated with loss of the overlying photoreceptor layer and irreversible reduction in visual function, often seen as disruption of the inner segment–outer segment junction (IS/OS) on newer spectral domain OCT systems.26,27 Manual segmentation of spectral domain OCT images may allow quantification of IS/OS disruption and measurement of photoreceptor outer segment thickness, as well as allowing more precise delineation of fibrovascular tissue in the subretinal space. (On StratusOCT, it may be difficult to differentiate the fibrovascular tissue from coexisting hemorrhage, lipid, or thick fibrin, all of which may be present in the subretinal space and appear hyperreflective.)
In other cases of neovascular AMD, growth of CNV in the sub-RPE space (type 1 CNV on histology) produces an elevated lesion that is often visible on clinical examination and is termed a fibrovascular PED.24,28 Growth of the lesion in the sub-RPE space is often accompanied by leakage of serous fluid or by frank hemorrhage, leading to the formation of serous or hemorrhagic PEDs.29 In the ABC Trial, increased PED volume was not associated with decreased contrast sensitivity or reading ability, a finding consistent with those in previous angiographic studies.30 This lack of correlation is perhaps not surprising, as the presence of the PED beneath the RPE may not always result in disruption of photoreceptor-RPE interactions. Furthermore, any changes in contrast sensitivity or reading ability may be dependent on the structural characteristics of the PED. Such an analysis was not performed in the present study, in part due to the relative inability of StratusOCT to visualize the areas underneath the highly reflective RPE. Recently, the use of spectral domain OCT with multiple B-scan averaging has allowed improved visualization of the sub-RPE space.31 Using this method, fibrovascular PEDs can often be seen to consist of solid layers of hyperreflective material, separated by hyporeflective clefts, whereas areas of solid material—the apparent fibrovascular proliferation—can be seen to adhere to the outer surface of the RPE in serous PEDs. In the future, quantitative subanalysis of such images may facilitate the detection of significant associations with visual function in this disorder.
As the choroidal neovascular membrane grows, either in the subretinal or sub-RPE spaces, it is often accompanied by profuse leakage from its immature blood vessels. Consequently, pockets of fluid commonly accumulate between the neurosensory retina and the RPE, and these areas may be seen on OCT as hyporeflective spaces.23 In the current report, a weak, but statistically significant, correlation was detected between an increased total volume of SRF and a decrease in contrast sensitivity (r = −0.2562, P = 0.004), a relationship not seen for either reading ability or ETDRS visual acuity. When fluid exudation is serous, SRF pockets are seen on OCT as homogenous hyporeflective spaces; when the exudate contains fibrin or red blood cells, the area of SRF may be sparsely hyperreflective. Profuse fibrinous exudation in neovascular AMD, seen after PDT or in particularly active classic CNV lesions, may result in the formation of distinct SRF compartments separated by fibrinous membranes.32 The increased sensitivity of spectral domain OCT allows enhanced visualization of the subretinal space, and assessment of the optical density of SRF compartments may facilitate detection of more robust correlations with visual function parameters.33
In neovascular AMD, disruption of the external limiting membrane (ELM)–photoreceptor complex in the outer retina, by the active CNV lesion, results in the accumulation of fluid in the neurosensory retina.34 Initially, this fluid collection manifests as diffuse thickening of the outer nuclear layer. However, with more severe fluid exudation, cystoid spaces, seen on OCT as round or oval hyporeflective areas, may form.35,36 Thus, thickening of the neurosensory retina is a common feature in patients with neovascular AMD.37 In the present study, subjects enrolled in the ABC Trial showed a moderate correlation at baseline between increased retinal thickness at the foveal center and decreased visual function. Approximately 20% of the variation in ETDRS visual acuity in these patients could be explained by thickening of the neurosensory retina (r = −0.4530, P < 0.001). Failure to detect a stronger correlation may be related to the heterogeneous structural characteristics of this disorder. Retinal thickening is less commonly seen in lesions where CNV growth is contained entirely beneath the RPE9 or in lesions more advanced at initial presentation and thus associated with substantial photoreceptor degeneration.5 Furthermore, in some patients, geographic atrophy, and thus reduced retinal thickness, may predate the occurrence of CNV.38
Our study has several strengths: (1) the utilization of manual segmentation, performed at a dedicated OCT image reading center, to quantify any morphologic space of interest in an objective, reproducible, manner; (2) the correlation of OCT findings with multiple visual function parameters obtained with trained personnel in the context of a multicenter clinical trial (in particular, the use of ETDRS visual acuity obtained after protocol refraction; (3) the replication, using ETDRS visual acuity, of OCT-VA correlations previously determined with quantitative subanalysis in the context of a single-center, retrospective series in which best-corrected Snellen visual acuities were used.9
Our study also had several limitations. Accurate data were not available regarding disease duration, a potentially key determinant of visual acuity at presentation in patients with neovascular AMD. In addition, the correlations detected in the present study are cross-sectional in nature. Further analysis is necessary to determine the way in which changes in visual acuity correspond to changes in OCT parameters over time. Finally, the study was performed with StratusOCT, which is the most commonly used OCT device worldwide but is based on older, time domain technology and thus is limited by slower scanning speeds. Future clinical trials are likely to adopt the next generation of OCT technology, spectral domain OCT, for the analysis of anatomic parameters.39 The high speed of spectral domain OCT allows dense raster scanning of the macula, reducing the need for interpolation algorithms and thus increasing the accuracy of any quantitative information. The rapid scanning of spectral domain OCT also allows multiple B-scan averaging, which reduces coherent speckle noise. The resulting improvement in image quality may allow more detailed delineation of the complex morphologic changes that occur in neovascular AMD, in particular detailed assessments of the photoreceptor inner and outer segments and their interaction with the RPE.31 Unfortunately, however, manual segmentation of individual OCT B-scans from a dense spectral domain OCT dataset is unlikely to be feasible in large a number of patients.37 As a first step, it is important to determine the optimal OCT B-scan density for the provision of both quantitative and qualitative information in the management of patients with neovascular AMD. With such knowledge, manual segmentation of spectral domain OCT datasets may allow exploratory analysis of novel OCT parameters, improvement in automated segmentation algorithms may then allow such parameters to be quantified in large clinical trials. In the interim, until large spectral domain OCT datasets in the context of clinical trials become available, analysis of existing StratusOCT datasets may help guide the selection of candidate parameters for quantification in future studies.
In summary, the strongest correlation detected between the functional parameters assessed and any of the OCT-derived morphologic parameters was that between decreased contrast sensitivity and increased total volume of subretinal tissue. A combination of increased thickness of the neurosensory retina at the foveal center and increased subretinal tissue volume was also associated with decreased reading ability. In the future, assessment of contrast sensitivity and reading ability is likely to acquire renewed importance for the clinician in the management of neovascular AMD, either in the assessment of visual performance in patients who maintain adequate levels of distance visual acuity, or in the evaluation of the relative efficacy of new therapies. Furthermore, assessment of contrast sensitivity and reading ability, in combination with quantitative subanalysis of retinal compartments using spectral domain OCT, may lead to the identification of parameters relevant for functional improvement and ultimate prognosis in patients with newly diagnosed neovascular AMD.
Footnotes
Supported in part by National Institutes of Health Grant EY03040 and National Eye Institute Grant R01 EY014375, and by research support from Carl Zeiss Meditec and Optovue, Inc. to the Doheny Image Reading Center.
Disclosure: P.A. Keane, None; P.J. Patel, None; Y. Ouyang, None; F.K. Chen, None; F. Ikeji, None; A.C. Walsh, None; A. Tufail, None; S.R. Sadd, None
References
- 1. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444 [DOI] [PubMed] [Google Scholar]
- 2. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431 [DOI] [PubMed] [Google Scholar]
- 3. Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26:383–390 [DOI] [PubMed] [Google Scholar]
- 4. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583 [DOI] [PubMed] [Google Scholar]
- 5. Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148:43–58.e1 [DOI] [PubMed] [Google Scholar]
- 6. Keane PA, Chang KT, Liakopoulos S, Jivrajka RV, Walsh AC, Sadda SR. Effect of ranibizumab retreatment frequency on neurosensory retinal volume in neovascular AMD. Retina. 2009;29:592–600 [DOI] [PubMed] [Google Scholar]
- 7. Keane PA, Mand PS, Liakopoulos S, Walsh AC, Sadda SR. Accuracy of retinal thickness measurements obtained with cirrus optical coherence tomography. Br J Ophthalmol. 2009;93:1461–1467 [DOI] [PubMed] [Google Scholar]
- 8. Keane PA, Liakopoulos S, Jivrajka RV, et al. Evaluation of optical coherence tomography retinal thickness parameters for use in clinical trials for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:3378–3385 [DOI] [PubMed] [Google Scholar]
- 9. Keane PA, Liakopoulos S, Chang KT, et al. Relationship between optical coherence tomography retinal parameters and visual acuity in neovascular age-related macular degeneration. Ophthalmology. 2008;115:2206–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moutray T, Alarbi M, Mahon G, Stevenson M, Chakravarthy U. Relationships between clinical measures of visual function, fluorescein angiographic and optical coherence tomography features in patients with subfoveal choroidal neovascularisation. Br J Ophthalmol. 2008;92:361–364 [DOI] [PubMed] [Google Scholar]
- 11. Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617 [DOI] [PubMed] [Google Scholar]
- 12. Keane PA, Liakopoulos S, Ongchin SC, et al. Quantitative subanalysis of optical coherence tomography after treatment with ranibizumab for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:3115–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hogg RE, Chakravarthy U. Visual function and dysfunction in early and late age-related maculopathy. Prog Retin Eye Res. 2006;25:249–276 [DOI] [PubMed] [Google Scholar]
- 14. Ergun E, Maár N, Radner W, Barbazetto I, Schmidt-Erfurth U, Stur M. Scotoma size and reading speed in patients with subfoveal occult choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2003;110:65–69 [DOI] [PubMed] [Google Scholar]
- 15. Patel PJ, Chen FK, Rubin GS, Tufail A. Intersession repeatability of contrast sensitivity scores in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:2621–2625 [DOI] [PubMed] [Google Scholar]
- 16. Csaky KG, Richman EA, Ferris FL. Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008;49:479–489 [DOI] [PubMed] [Google Scholar]
- 17. Duivenvoorden R, de Groot E, Stroes ES, Kastelein JJ. Surrogate markers in clinical trials: challenges and opportunities. Atherosclerosis. 2009;206:8–16 [DOI] [PubMed] [Google Scholar]
- 18. Lloyd R, Harris J, Wadhwa S, Chambers W. Food and Drug Administration approval process for ophthalmic drugs in the US. Curr Opin Ophthalmol. 2008;19:190–194 [DOI] [PubMed] [Google Scholar]
- 19. Hazel CA, Petre KL, Armstrong RA, Benson MT, Frost NA. Visual function and subjective quality of life compared in subjects with acquired macular disease. Invest Ophthalmol Vis Sci. 2000;41:1309–1315 [PubMed] [Google Scholar]
- 20. Sadda SR, Joeres S, Wu Z, et al. Error correction and quantitative subanalysis of optical coherence tomography data using computer-assisted grading. Invest Ophthalmol Vis Sci. 2007;48:839–848 [DOI] [PubMed] [Google Scholar]
- 21. Patel PJ, Bunce C, Tufail A, ABC Trial Investigators OB A randomised, double-masked phase III/IV study of the efficacy and safety of Avastin(R) (Bevacizumab) intravitreal injections compared to standard therapy in subjects with choroidal neovascularisation secondary to age-related macular degeneration: clinical trial design. Trials. 2008;9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mansfield J, Ahn S, Legge G, Leubeker A. A new reading acuity chart for normal and low vision. In: Ophthalmic Visual Optics: Non-Invasive Assessment of the Visual System. OSA Technical Digest. Vol 3 Washington, DC: Optical Society of America; 1993:232–235 [Google Scholar]
- 23. Joeres S, Tsong JW, Updike PG, et al. Reproducibility of quantitative optical coherence tomography subanalysis in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:4300–4307 [DOI] [PubMed] [Google Scholar]
- 24. Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137:496–503 [DOI] [PubMed] [Google Scholar]
- 25. Keane PA, Liakopoulos S, Chang KT, et al. Comparison of the optical coherence tomographic features of choroidal neovascular membranes in pathological myopia versus age-related macular degeneration, using quantitative subanalysis. Br J Ophthalmol. 2008;92:1081–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hayashi H, Yamashiro K, Tsujikawa A, Ota M, Otani A, Yoshimura N. Association between foveal photoreceptor integrity and visual outcome in neovascular age-related macular degeneration. Am J Ophthalmol. 2009;148:83–89.e1 [DOI] [PubMed] [Google Scholar]
- 27. Sayanagi K, Sharma S, Kaiser PK. Photoreceptor status after anti-vascular endothelial growth factor therapy in exudative age-related macular degeneration. Br J Ophthalmol. 2009;93:622–626 [DOI] [PubMed] [Google Scholar]
- 28. Macular Photocoagulation Study Group Subfoveal neovascular lesions in age-related macular degeneration: guidelines for evaluation and treatment in the macular photocoagulation study. Arch Ophthalmol. 1991;109:1242–1257 [PubMed] [Google Scholar]
- 29. Coscas F, Coscas G, Souied E, Tick S, Soubrane G. Optical coherence tomography identification of occult choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2007;144:592–599 [DOI] [PubMed] [Google Scholar]
- 30. Pauleikhoff D, Löffert D, Spital G, et al. Pigment epithelial detachment in the elderly: clinical differentiation, natural course and pathogenetic implications. Graefes Arch Clin Exp Ophthalmol. 2002;240:533–538 [DOI] [PubMed] [Google Scholar]
- 31. Spaide RF. Enhanced depth imaging optical coherence tomography of retinal pigment epithelial detachment in age-related macular degeneration. Am J Ophthalmol. 2009;147:644–652 [DOI] [PubMed] [Google Scholar]
- 32. Keane PA, Aghaian E, Ouyang Y, Chong LP, Sadda SR. Acute visual decrease post-photodynamic therapy with verteporfin: spectral domain optical coherence tomographic features. Ophthalmic Surg Lasers Imaging. In press [DOI] [PubMed] [Google Scholar]
- 33. Ahlers C, Golbaz I, Einwallner E, et al. Identification of optical density ratios in subretinal fluid as a clinically relevant biomarker in exudative macular disease. Invest Ophthalmol Vis Sci. 2009;50:3417–3424 [DOI] [PubMed] [Google Scholar]
- 34. Gass JDM. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. Vol 1 4th ed St Louis: Mosby; 1997:49–70 [Google Scholar]
- 35. Kashani AH, Keane PA, Dustin L, Walsh AC, Sadda SR. Quantitative subanalysis of cystoid spaces and outer nuclear layer using optical coherence tomography in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:3366–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ting TD, Oh M, Cox TA, Meyer CH, Toth CA. Decreased visual acuity associated with cystoid macular edema in neovascular age-related macular degeneration. Arch Ophthalmol. 2002;120:731–737 [DOI] [PubMed] [Google Scholar]
- 37. Ahlers C, Golbaz I, Stock G, et al. Time course of morphologic effects on different retinal compartments after ranibizumab therapy in age-related macular degeneration. Ophthalmology. 2008;115:e39–e46 [DOI] [PubMed] [Google Scholar]
- 38. Sunness JS. Choroidal neovascularisation and atrophy. Br J Ophthalmol. 2006;90:398–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keane PA, Bhatti RA, Brubaker JW, Liakopoulos S, Sadda SR, Walsh AC. Comparison of clinically relevant findings from high-speed Fourier-domain and conventional time-domain optical coherence tomography. Am J Ophthalmol. 2009;148:242–248.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]