A characteristic study of the lacrimal functional unit and wound healing in PKCα knockout mice was conducted to explore the PKCα function in the lacrimal functional unit.
Abstract
Purpose.
Protein kinase C (PKC) α plays a major role in the parasympathetic neural stimulation of lacrimal gland (LG) secretion. It also has been reported to have antiapoptotic properties and to promote cell survival. Therefore, the hypothesis for the present study was that PKCα knockout (−/−) mice have impaired ocular surface–lacrimal gland signaling, rendering them susceptible to desiccating stress and impaired corneal epithelial wound healing. In this study, the lacrimal function unit (LFU) and the stressed wound-healing response were examined in PKCα−/− mice.
Methods.
In PKCα+/+ control mice and PKCα−/− mice, tear production, osmolarity, and clearance rate were evaluated before and after experimental desiccating stress. Histology and immunofluorescent staining of PKC and epidermal growth factor were performed in tissues of the LFU. Cornified envelope (CE) precursor protein expression and cell proliferation were evaluated. The time course of healing and degree of neutrophil infiltration was evaluated after corneal epithelial wounding.
Results.
Compared with the PKCα+/+ mice, the PKCα−/− mice were noted to have significantly increased lacrimal gland weight, with enlarged, carbohydrate-rich, PAS-positive acinar cells; increased corneal epithelia permeability, with reduced CE expression; and larger conjunctival epithelial goblet cells. The PKCα−/− mice showed more rapid corneal epithelial healing, with less neutrophil infiltration and fewer proliferating cells than did the PKCα+/+ mice.
Conclusions.
The PKCα−/− mice showed lower tear production, which appeared to be caused by impaired secretion by the LG and conjunctival goblet cells. Despite their altered tear dynamics, the PKCα−/− mice demonstrated more rapid corneal epithelial wound healing, perhaps due to decreased neutrophil infiltration.
Protein kinase C (PKC) is an important intracellular signaling molecule that is present in mammalian cells as a family of at least 12 closely related isozymes with serine-threonine kinase activity. The isoforms in this family are divided into three groups: classic PKCα, -βI, -βII, and -γ, which are activated by Ca2+ and diacylglycerol (DAG); newly identified PKCδ, -ε, -μ, -η, and -θ, which are activated by DAG only; and Ca2+ and DAG-independent, atypical PKCι/λ and -ζ. PKC is involved in the intracellular transduction of a variety of signals related to proliferation, differentiation, migration, adhesion, transformation, and protection from apoptosis, that are triggered by guanine-nucleotide–binding, protein-coupled (G-protein) receptors; tyrosine kinase receptors; and other membrane-signaling molecules.1 The PKC isoforms are activated in specific processes in a tissue-specific manner.
Lacrimal acinar cells have been reported to contain five different PKC isoforms: α, β, μ, γ, ε, and ι/λ. With the exception of ι/λ, all are activated by DAG, and some also require elevation of cytosolic Ca2+.2 Both Ca2+ and PKCα activate exocytic fusion of preformed secretory vesicles with the apical plasma membrane, so that the content proteins are released into the forming lacrimal gland fluid.3,4 It has been documented that the PKC-α and -μ isoforms control exocytosis in the lacrimal gland.5,6 The lacrimal gland is innervated by parasympathetic and sympathetic nerves, with the former predominating. Cholinergic agonists activate phospholipase C (PLC), to generate inositol 1,4,5-trisphosphate (IP3) and DAG. IP3 releases intracellular Ca2+ from IP3-sensitive stores and stimulates protein secretion by activating Ca2+ calmodulin-dependent protein kinase and PKCα,5 whereas DAG stimulates protein secretion by activating PKCα. Both the Ca2+ and the PKC-dependent pathways are equally potent in stimulating protein secretion. Thus, in parasympathetic lacrimal secretion, PKCα accounts for more than 50% of cholinergic agonist–induced protein secretion.7 Acinar cells of the lacrimal gland secrete water, electrolytes, protein, and mucin into tear fluid, mostly in response to neural stimulation. The tears secreted by lacrimal glands contain supportive and protective factors for the ocular surface, including the cornea. In 1998, the ocular surface (cornea, conjunctiva, and meibomian glands), the lacrimal glands, and the neural network that connects them were described as the lacrimal functional unit (LFU),8 which controls secretion of the three major components of the tear film. The overall purpose of the LFU is to maintain the clarity of the cornea and quality of the image projected onto the retina.
Conjunctival goblet cell mucin secretion is under neural control, often stimulated by activation of afferent sensory nerves in the cornea. The efferent arc of this neural reflex is mediated by autonomic parasympathetic and sympathetic nerves that surround the goblet cells or by antidromic stimulation of the sensory nerves in the conjunctiva and collateral sensory nerves from the cornea.9 It has been documented that cholinergic muscarinic agonists, increased Ca2+, and PKC activation stimulate conjunctival goblet cell secretion.10,11 However, the contributory role of the various PKC isozymes present in the goblet cells remains to be determined.
PKCα is activated by a variety of stimuli, including ligands binding to G-protein receptors and to tyrosine kinase receptors, and also by physical stresses such as hypoxia and mechanical strain. Therefore, this isotype plays an important role in the control of major cellular functions, including proliferation, apoptosis, differentiation, and motility, depending on where and when it is activated and what substrates it acts on.
The proliferative effect of PKCα is mediated via activation of the extracellular signal–regulated kinase/mitogen-activated protein kinase (ERK/MAPK) cascade. PKCα is also involved in cell differentiation and cell migration and adhesion by interacting with the integrin β1, ezrin, radixin, and moesin (ERZ) proteins and the F-actin-binding proteins, which are involved in the maintenance of cell survivor and extension.12 Some studies have reported that the overexpression of PKCα in human skin keratinocytes has no effect on proliferation or differentiation.13 The contribution of PKCα to corneal epithelial cell proliferation and differentiation after wound healing has not been evaluated.
Programmed cell death occurs by apoptosis and cornification in the epithelia.14–16 PKC has been reported to increase expression of the cornified envelope precursor gene, SPRR1, which may promote death by cornification.17
Besides its proliferative and cornification functions, PKCα has been noted to inhibit inflammation by negatively regulating NFKB-induced cytokine expression—particularly, that of interleukin (IL)-1.18
We evaluated the morphology and secretory function of the LFU in PKCα−/− mice. The effects of PKCα knockout on tear production and tear concentrations of lacrimal growth factors, such as epidermal growth factor (EGF), that support the ocular surface were assessed after desiccating stress. Markers of proliferation, cornification, and apoptosis were compared in PKC−/− mice and PKC+/+ wild-type mice. Furthermore, the effects of PKCα deficiency on corneal epithelial wound healing were evaluated. Specifically, corneal epithelial morphology, proliferation, inflammation, and healing rate after experimental corneal epithelial debridement, were compared in the two mouse strains.
Materials and Methods
Animal and Genotype
PKCα gene–deficient mice were generated by homologous recombination and were provide as a gift from Michael Leitges (Laboratory for Lymphocyte Signaling, University of Cologne, Cologne, Germany).19 The PKC isoenzymes were adapted from the rat to the mouse. Targeted embryonic stem (ES) cells (PKCα−/−) were injected into NMRI albino mouse blastocysts to generate chimeras. Germ line–transmitting male chimeras were crossed to 129/SV females to give rise to F1 heterozygous offspring on a pure 129 background. Intercrosses of these mice were used to establish a homozygous, PKC α-deficient (−/−) mouse line. Male mice from this line were compared with age-matched male 129/SV PKCα control (+/+) mice.19 Coat color was used to detect germ line transmission in mice homozygous for PKCα deficiency. All studies were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and all animal research protocols were approved by the Baylor College of Medicine Center for Comparative Medicine.
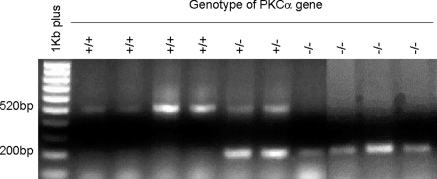
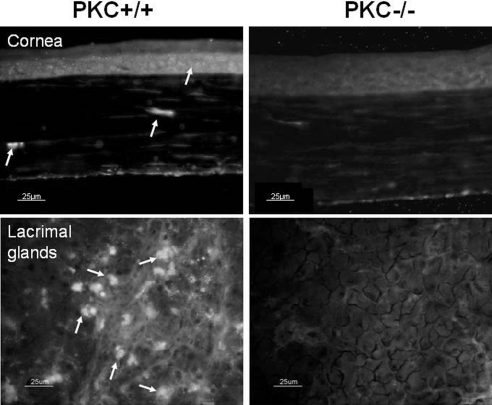
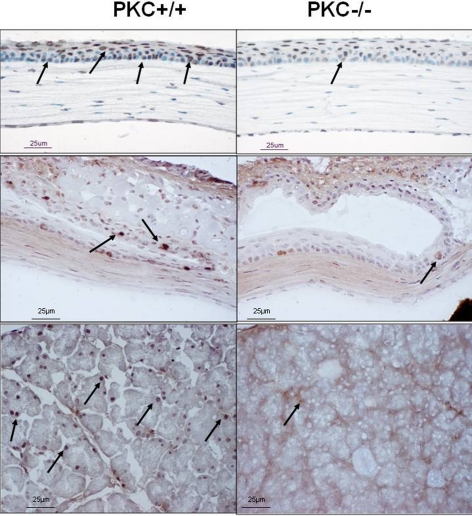
Twenty PKCα gene knockout mice (PKCα−/−) and 20 wild-type control mice (PKCα+/+) at age 2 months were used. Each set of 20 mice contained 10 female and 10 male mice. Genotypes of 1-month-old pups from homozygous parents were tested by using genomic DNA isolated from their tails (Genomic DNA Isolation kit; Sigma-Aldrich, St. Louis, MO). PCR amplification was performed on a thermal cycler (DNA Thermal Cycler 480; GeneAmp PCR kit; Applied Biosystems, Foster City, CA) with the Pkca/Neo gene primer pair, GGCGAACAGTTCGGCTGCGCGAGCCCC and GAGCCCTTGGGTTTCAAGTATAGA, yielding a 520-bp fragment from homozygous PKCα+/+ mice and a 200-bp fragment from homozygous PKCα−/− mice, with both bands detected in the heterozygotes (+/−) (Fig. 1). Further confirmation of PKC deficiency was detected by the absence of PKCα protein expression shown by immunofluorescent staining in the cornea and lacrimal glands of the PKCα−/− mice (Fig. 2).
Figure 1.
Genotype determination by PCR of mouse tail genomic DNA using PKCα-specific primer pairs. The PCR products were analyzed by 1.5% agarose gel electrophoresis. A 520-bp band was obtained from the wild-type mice (+/+), a 200-bp band from the PKCα knockout mice (−/−), and both bands from the heterozygotes (+/−).
Figure 2.
Immunofluorescent staining of PKC (arrow) in cornea (top) and lacrimal gland (bottom) from PKCα+/+ (left) and PKCα−/− mice (right).
Experimental Dry Eye Model
A modification of a previously reported mouse model of experimental dry eye (EDE) was used.20 Briefly, age and sex-matched mice were placed in a low-humidity (<35%) environment and exposed to an air draft for 18 hours per day. They received SC injections of 0.5 mg/0.2 mL scopolamine hydrobromide (Sigma-Aldrich, St. Louis, MO) in alternating hindquarters, four times per day (9 AM, Noon, 3 PM, and 6 PM) to inhibit tear secretion.
Aqueous Tear Production
Tear volumes were measured by using phenol red–impregnated cotton threads (Quick Zone; Oasis, Glendora, CA), held in the tear meniscus of the lateral canthus for 20 seconds. The measured uptake of tear fluid in millimeters was compared with a standard curve prepared from cotton threads of known uptake volumes of a stock basic solution (1500 mL of 0.9% saline and 5 mL of 5 N NaOH) over 20 seconds, with volumes in the range that would be expected in mouse tears.
Fluorescein Clearance Test
The fluorescein clearance test is a measure of total tear production, tear spread, and tear drainage.21 This test was performed by instilling 1 μL of 2% sodium fluorescein into the conjunctival sac. After 15 minutes, 1 μL of phosphate-buffered saline (PBS) was instilled, and the fluorescein-stained tear fluid was collected atraumatically from the lateral tear meniscus for 20 seconds under a surgical microscope with a 1-μL volume glass capillary tube (Drummond Scientific Co., Broomhall, PA). The tear sample collected from the capillary tube was eluted into a 1.5-mL tube containing 99 μL PBS and centrifuged for 5 minutes. The solution was transferred to a single well in a 96-well plate. The fluorescein concentration was measured with a fluorophotometer (CytoFluor II; Perseptive Biosystems, Framingham, MA), using 485-nm excitation and 530-nm emission filters.
Corneal Permeability to AlexaFluor Dextran
The corneal uptake of 10-kDa AlexaFluor dextran (AFD; Molecular Probes, Eugene, OR) was measured by using a modification of a previously reported technique.22 Briefly, 1 μL of 0.3% AFD was instilled onto the ocular surface 15 minutes before euthanatization. Excised corneas were rinsed four times with 200 μL balanced salt solution (BSS; Alcon Laboratory, Inc., Fort Worth, TX) and placed in 200 μL balanced salt solution. The solution containing the corneal tissue was protected from light and placed on an orbital shaker. The concentration of eluted AFD was measured with 485-nm excitation and 530-nm emission filters at 10, 20, and 60 minutes on a fluorophotometer (CytoFluor II; Perseptive Biosystems).
Tear Collection
PBS (1.5 μL) containing 0.1% bovine serum albumin (BSA) was instilled into the conjunctival sac. The tear fluid and buffer were collected with a 1-μL volume glass capillary tube (Drummond Scientific Co.) from the tear meniscus in the lateral canthus.
Collection of tear fluid to measure sodium concentration was performed before and after 3 days of desiccation stress. Briefly, 1 μL of distilled water was instilled into the conjunctival sac for 20 seconds. The diluted tear fluid was collected with a 0.5-μL glass capillary tube (Drummond Scientific Co.) from the tear meniscus in the lateral canthus and immediately added to 4.5 μL of distilled water. The samples were then capped to prevent evaporation and frozen for further analysis.
Tear Sodium and Osmolarity Measurements
Tear sodium and osmolarity measurements were performed by using a published method,20 with modifications. Briefly, 5 μL of a red mitochondrial probe (CoroNa Red, cat. no. C-24431; Molecular Probes, Eugene, OR) in DMSO was added to each sample. The samples were placed in individual wells of a round-bottomed, 96-well plate (cat. no. 163320; Nunc, Rochester, NY). A multiwell plate reader (CytoFluor II; Perkin Elmer, Wellesley, MA) was then used to measure fluorescence intensity. A standard curve was created with the fluorescence values from 500 mM of the mitochondrial dye in DMSO placed in a 10-μL sample with a known sodium concentration. The sodium concentration of the mouse tear sample was then calculated from the standard curve, by using a dilution factor based on the previously measured tear volume, to give the following formula: total tear sodium concentration (mM) = measured tear sodium concentration × (1 + tear volume)/tear volume. In four different mice of each strain, tear sodium was measured in both eyes.
The osmolarity of the mouse tear sample derived from sodium concentration was then calculated by comparing the sodium concentration in the tear sample with the osmolarity of a standard curve established from samples of known sodium concentrations, whose osmolarity was determined by using a vapor pressure osmometer (model 3300; Advanced Instruments Inc., Norwood, MA) at the clinical core laboratory of the Department of Pathology at Methodist Hospital (Houston, TX).
The serum osmolarity of the two mouse strains was determined by taking blood from the orbital venous sinus immediately after euthanatization. The serum was separated from the blood by centrifuging at 14,000 rpm (Microcentrifuge model 5417 C, 18,000g; Eppendorf, Fremont, CA) at room temperature for 10 minutes, and the osmolarity was measured (Table 1).
Table 1.
Comparison of Tear Sodium, Tear Osmolarity, and Serum Osmolarity in Two Strain Mice before and after 3 Days EDE Treatment
| Tear Sodium (mM) |
Tear Osmolarity (mOsm/L) |
Serum Osmolarity (mOsm/L) |
||||
|---|---|---|---|---|---|---|
| Baseline | EDE | Baseline | EDE | Baseline | EDE | |
| PKCα+/+ | 171 ± 60 | 264 ± 89* | 317 ± 112 | 491 ± 165* | 324 ± 3.9 | 330 ± 2.7 |
| PKCα−/− | 194 ± 42 | 303 ± 71* | 359 ± 77 | 561 ± 132* | 324 ± 5.4 | 331 ± 7.3 |
Data are expressed as the mean ± SD.
P < 0.05, Mann-Whitney test.
Lacrimal Gland Weight
After euthanatization, both exorbital lacrimal glands were removed from PKCα+/+ and PKCα−/− mice. The lacrimal glands' weight and volume and the mouse's body weight were measured. The lacrimal gland density (weight/volume) and the lacrimal gland/body weight ratio were compared between the two mouse strains.
Histology and Immunofluorescent Staining
After the mice were euthanatized, the eyeballs, together with the eyelids and conjunctiva, were excised; embedded in a mixture of 75% (vol/vol) OCT compound (Sakura Finetek USA., Inc., Torrance, CA) and 25% (vol/vol) aqueous mounting medium (Immu-Mount; Thermo-Shandon, Pittsburgh, PA); and fresh frozen in liquid nitrogen. Sections (10 μm thick) were cut and stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS), to detect the carbohydrate-rich cells, which stain for a high proportion of carbohydrate macromolecules (i.e., conjunctival goblet cells and granules in acinar cell). For immunofluorescent staining, sections were fixed with 100% methanol at 4°C for 10 minutes and blocked with 5% normal goat serum in PBS for 30 minutes. The primary antibody was applied for 1 hour at room temperature. These monoclonal antibodies and goat polyclonal antisera were reactive with mouse EGF (Monosan, Uden, Netherlands), PKCα (BD Transduction Laboratories, San Jose, CA), MUC-5AC (a gift from Marcia Jumblatt, University of Louisville, Louisville, KY) and SPRR2 (Apotech, Epalinges, Switzerland). After the sections were washed with PBS, the species-specific secondary antibody (AlexaFluor-488 conjugate; 1:100 dilution; Molecular Probes) was applied for 1 hour in a dark incubation chamber. After another wash with PBS, the sections were mounted with antifade medium (Gel-Mount; Fisher, Atlanta, GA) containing 1 μg/mL Hoechst 33342 dye, and a coverslip was applied. The sections were examined and photographed with an epifluorescence microscope (Eclipse 400; Nikon, Tokyo, Japan) and a digital camera (model DMX 1200; Nikon).
BrdU Incorporation
BrdU is a nucleoside that can be incorporated into DNA in place of thymidine to measure DNA synthesis and cell proliferation. Mice were injected with 100 μg BrdU/g body weight intraperitoneally for 1 to 2 hours before euthanatization. BrdU was detected by immunostaining frozen sections that were fixed in cold methanol at 4°C for 10 minutes, followed by incubation with 2 N HCl at 37°C for 1 hour, to denature the DNA, and neutralization in boric acid (pH 8.5) for 20 minutes. Anti-BrdU polyclonal antibody (1:100) was applied, followed by incubation with anti-rabbit secondary antibody (1:300) with 3,3′-diaminobenzidine (DAB) and hematoxylin counterstaining. The BrdU labeling index was assessed by point-counting positively stained cells through an inverted microscope (TE200; Nikon), equipped with a 40× objective lens. A total of 500 to 951 nuclei were counted in six to eight representative fields because this number was considered to be the minimum requirement to observe representative labeling.23 The labeling index was expressed as the number of positively labeled nuclei/total number of nuclei × 100%.
Corneal Epithelial Wound Healing
A central corneal wound was made as previously described.24,25 Age- and sex-matched PKCα+/+ and PKCα−/− mice were used in the experiments. Briefly, the central corneal epithelium was marked with a 3-mm trephine and then debrided with a golf club–shaped spatula (Accutome, Malvern, PA) under a dissecting microscope. After the corneal wound was created, the rate of closure was measured in digital images of fluorescein-stained corneas obtained every 6 hours.
Immunofluorescent Staining of Whole Mount Corneas
A protocol described in another publication24 was used for morphometric analysis of the corneal response to injury. Immunofluorescent staining using anti-Gr-1-FITC (PharMingen, San Diego, CA) to detect neutrophils and anti-tubulin (Sigma, St. Louis, MO) to detect cells undergoing mitosis was performed in excised corneas with the limbus retained. Nuclear morphology and cell division were assessed by staining with 1 μM 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO) in flat, whole mount corneas. The pattern of microscopic analysis included counting inflammatory cells (i.e., neutrophils) or healing (dividing epithelial cells) in nine microscopic fields (40× objective; field of view diameter, 0.53 mm) across the cornea from limbus to limbus. Digital images were captured and saved for analysis (DeltaVision; Applied Precision, Issaquah, WA). At least six corneas were examined in each group, and the average number of cells in each field was calculated and used for statistical analysis. The limbus was defined as the intervening zone between the cornea and sclera, and was considered the most peripheral field.
Statistical Analysis
Based on the normality of the data distribution, the t-test or Mann-Whitney test was used for statistical comparison of assay results between groups. P < 0.05 was regarded as statistically significant.
Results
Phenotype of PKCα−/− Mice
Aqueous Tear Production and Clearance.
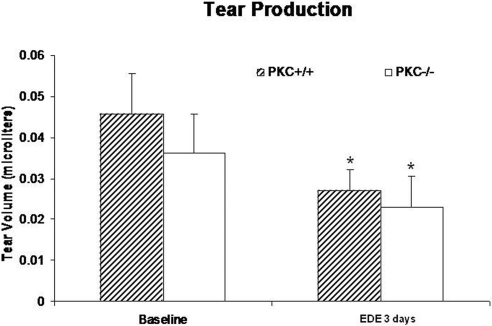
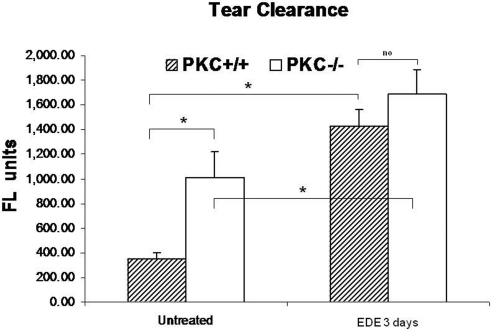
Aqueous tear production was reduced in the PKCα−/− mice (mean ± SD, n = 11) compared with that in age-matched the PKCα+/+ mice (mean ± SD, n = 16), with or without imposition of EDE for 3 days, but the difference between strains did not reach statistical significance in either situation (Fig. 3). However, there was a significant difference between EDE and baseline in each of the mouse strains (P < 0.05; Fig. 3). PKCα−/− mice had significantly delayed tear fluorescein clearance compared with age-matched PKC+/+ mice, without or with EDE (P < 0.05; Fig. 4).
Figure 3.
Tear production in PKCα+/+ and PKCα−/− mice without (baseline) and after EDE for 3 days. *Significant difference between EDE and baseline in each mouse strain (P < 0.05), Mann-Whitney test.
Figure 4.
Comparison of tear fluorescein clearance between PKCα+/+ and PKCα−/− mice before and after experimental dry eye. Data show mean and SD (error bars) fluorescence units. *P < 0.05; Mann-Whitney test.
Tear and Serum Osmolarity.
Tear sodium concentration in PKCα+/+ (n = 4) and PKCα−/− mice (n = 4), respectively, measured 171 ± 60 (mean ± SD) and 194 ± 42 mM at baseline and 264 ± 89 and 303 ± 71 mM after 3 days of EDE. The increase in sodium concentration after 3 days of EDE was significantly different from baseline in the PKCα+/+ and the PKCα−/− mice (P < 0.05); however, there was no difference between strains in either case.
Tear osmolarity, calculated from the sodium ion concentration, increased significantly after EDE in both strains, to an average of 491 ± 165 mOsm/L in the PKCα+/+ mice and 561 ± 132 mOsm/L in the PKCα−/− mice, compared with a baseline of 317 ± 112 osm/L (P < 0.05) and 359 ± 77 osm/L (P < 0.05), respectively.
The serum osmolarity of the PKCα+/+ mice (n = 4) was 324 ± 3.9 mOsm/L at baseline and 330 ± 2.7 mOsm/L after 3 days of EDE, whereas the serum osmolarity of the PKCα−/− mice (n = 4) was 324 ± 5.4 mOsm/L at baseline and 331 ± 7.3 mOsm/L after 3 days of EDE. There was no significant difference between baseline and EDE measurements in either strain.
Lacrimal Gland Weight.
The lacrimal glands of the PKCα−/− mice weighed more (0.069 ± 0.03 g, n = 4) than those of the age-matched PKCα+/+ mice (0.038 ± 0.018 g, n = 4, P < 0.05; Fig. 5). Similarly, lacrimal gland density (weight/volume) in the PKCα−/− mice (2.76 ± 0.6 mg/mm3) was higher than that in the age-matched PKCα+/+ mice (1.21 ± 0.071, P < 0.05), as was the ratio of lacrimal gland to body weight (2.13 ± 0.57 in the PKCα−/− mice, 1.21 ± 0.049 in the PKCα+/+ mice; P < 0.05).
Figure 5.
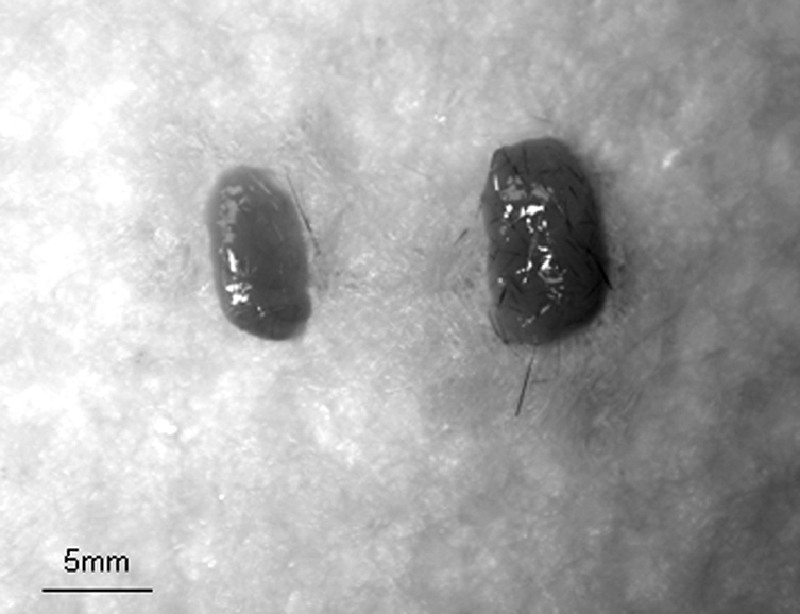
Lacrimal gland appearance in PKCα+/+ (left) and PKCα−/− (right) mice.
Corneal Epithelial Permeability to AFD.
Decreased corneal epithelial barrier function is a key feature of human dry eye. We assessed it by measuring corneal permeability to AFD. There was no difference in AFD permeability in the corneas of the PKC−/− and the age-matched PKCα+/+ mice as three males and two females at baseline. After EDE for 3 days, the corneas of the PKCα−/− mice were significantly more permeable to AFD (475 ± 25 U, n = 5) than were those of the age-matched PKCα+/+ mice (300 ± 25 U, n = 5, P < 0.05, by Mann-Whitney test). This finding suggests that PKCα−/− mice are more susceptible to corneal barrier disruption after experimental desiccating stress.
Morphology of the LFU.
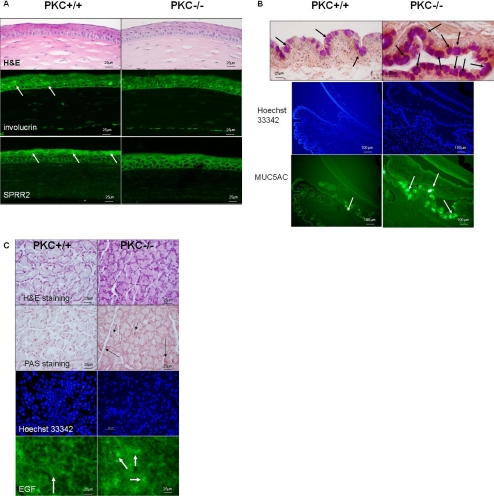
There was no difference in corneal epithelial morphology between the 2-month-old PKCα−/− mice and the age-matched wild-type mice (Fig. 6A). Immunofluorescent staining of mouse corneal frozen sections was performed to evaluate the expression by corneal epithelial cells of the cornification markers involucrin and SPRR2, which may have a role in corneal barrier functioning. The intensity of both involucrin and SPRR2 immunofluorescent staining in the corneal epithelia of nonstressed mice was lower in the PKCα−/− than in the PKCα+/+ mice (Fig. 6A).
Figure 6.
Histology and immunofluorescent (IF) staining of cornea, conjunctiva, and lacrimal gland of PKCα+/+ and PKCα−/− mice. (A) Corneal epithelia: H&E staining and IF for involucrin and SPRR2 in mice subjected to EDE for 3 days (arrows, IF+ staining). (B) Conjunctiva: PAS staining (top, arrows, secretory granules) and IF for MUC5AC (arrows, IF+ staining) with Hoechst 33342 as the counterstain. (C) Lacrimal glands: H&E and PAS staining (arrows, secretory granules) and IF for EGF (arrows, IF+ staining) with Hoechst 33342 as the counterstain.
In the conjunctiva, enlarged carbohydrate-rich goblet cells with stronger PAS staining were observed in the PKC−/− mice compared with those in the PKCα+/+ mice at baseline (before EDE). Correspondingly, greater MUC5AC staining was detected in the conjunctiva of the PKCα−/− than in the PKC+/+ mice (Fig. 6B).
Lacrimal gland histologic sections stained with H&E and PAS showed larger carbohydrate-rich acinar cells containing more PAS-positive granules in samples from the PKCα−/− mice. The number of lacrimal gland acinar cells in the PKCα−/− samples was similar to that in the wild-type, but they appeared to be filled with secretory granules that were not being discharged into the lumen. Furthermore, the lacrimal glands of the PKCα−/− mice had stronger staining for EGF, a growth factor produced by the acinar cells and secreted into the tears, than in the PKC+/+ mice, but the difference was without statistical significance (immunofluorescent staining intensity: PKC+/+ mice: 50.35 ± 17.62; PKCα−/− mice: 52.95 ± 18.17; P > 0.05; Fig. 6C).
BrdU Incorporation Index.
BrdU incorporation was evaluated as a marker of cell proliferation in tissues of the LFU. The PKCα+/+ mice (n = 4) had a greater percentage of BrdU-positive cells in the cornea, conjunctival epithelia, and lacrimal gland acinar cells (21.25% ± 5.78%, 8.49% ± 2.83%, and 11.11% ± 4.37%, respectively) than did the PKCα−/− mice (n = 4, 10.85% ± 3.92%, 2.83% ± 0.95%, and 3.39% ± 1.49%, respectively; all P < 0.05%). These findings indicate that PKCα is essential in initiating proliferation in the cells of the LFU (Fig. 7).
Figure 7.
BrdU incorporation (arrows) in corneal (top) and conjunctival (middle) epithelia and lacrimal gland (bottom) in PKCα+/+ and PKCα −/− mice (n = 4).
Wound Healing
Effect of PKCα Deficiency on Corneal Re-epithelialization after Wounding.
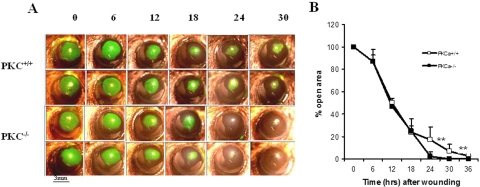
A standard model of corneal wound healing was established by removal of a 3-mm-diameter area of epithelium from the center of the cornea. The epithelial healing was monitored by fluorescein staining of the exposed stroma in the PKCα−/− and PKCα+/+ mice. At 6, 12, and 18 hours after wounding, the size of the wounded areas in PKCα+/+ and the PKCα−/− mice were similar. At 24 and 36 hours after creation of the epithelial defect, wound areas in the PKCα−/− mice were reduced to 2% and 0% of the original area, respectively. In contrast, wound areas in the PKCα+/+ mice were reduced to 17% and 7% (Fig. 8). Complete epithelialization was accelerated between 18 and 36 hours in the postwound PKCα−/− mice compared with that in the wild-type mice.
Figure 8.
Accelerated corneal epithelial wound healing in PKCα−/− mice. (A) Fluorescein-stained corneal images after corneal epithelial debridement in PKCα+/+ and PKCα−/− mice. (B) Percentage of the original wound area is plotted over time and was calculated from the area of fluorescein staining at each time point. Data are mean ± SD (n = 6, **P < 0.01).
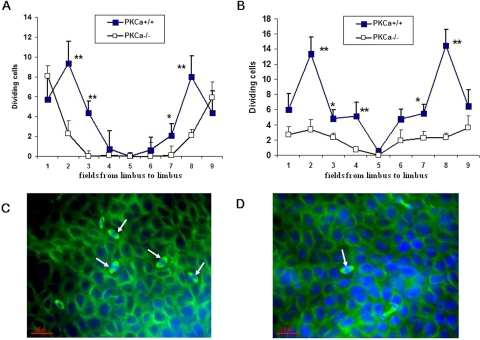
PKCα has been observed to have a regulatory role in cell proliferation that is crucial to corneal wound healing.26 Epithelial basal cell division was noted to be decreased in the PKCα−/− mice at 18 and 30 hours after epithelial debridement compared with that in the wild-type mice (Fig. 9).
Figure 9.
Effects of PKCα deficiency on dividing basal cells after corneal epithelial debridement. Dividing basal epithelial cells in each region of the cornea at 18 (A) and 30 (B) hours, respectively, after epithelial debridement. Representative photos of dividing cells (arrow) stained by tubulin (green) in PKCα+/+ (C) and PKCα−/− mice (D) at 18 hours after wounding. DAPI (blue) was used for counterstaining. *P < 0.05 and **P < 0.01.
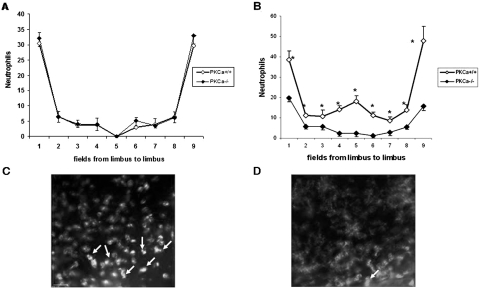
Effect of PKCα Deficiency on Neutrophil Accumulation in the Cornea.
Experimental neutropenia induced by depletion of neutrophils has been noted to accelerate corneal epithelial wound closure.27,28 PKCα activation in keratinocytes is a crucial event that orchestrates cutaneous neutrophil responses by increasing the concentration of two chemotactic factors: cytokine-induced neutrophil chemoattractant and macrophage inflammatory protein (MIP)-2 in murine plasma.29–31 To determine whether the accelerated corneal epithelial healing observed in the PKCα−/− mice is linked to reduced neutrophil migration, we analyzed neutrophil accumulation at 18 and 30 hours after epithelial debridement. Neutrophil migration into the avascular cornea stroma in the PKCα-deficient and wild-type mice was similar at 18 hours (Fig. 10A). In contrast to the response of the wild-type mice, the PKCα−/− mice had a markedly blunted accumulation of neutrophils in every field from limbus to limbus at 30 hours after wounding (P < 0.005, Fig. 10B). These results demonstrate that neutrophil recruitment that occurs during the inflammatory phase after corneal epithelial wounding is markedly reduced in the absence of PKCα.
Figure 10.
Neutrophil infiltration in PKCα-deficient mice after epithelial debridement. Neutrophil migration to corneal stroma in each region of the cornea at 18 (A) and 30 (B) hours, respectively, after epithelial debridement. *P < 0.005. Representative photos of neutrophils (arrow) stained by anti-Gr-1-FITC in PKCα+/+ (C) and PKCα−/− mice (D) at 30 hours after wounding.
Discussion
Effects of PKCα Deficiency on the Morphology and Function of the Lacrimal LFU
PKCα gene knockout was noted to affect the morphology and function of the LFU. These changes included reduced tear production, secretory paralysis of the lacrimal gland acinar and conjunctival goblet cells, increased permeability of corneal epithelia after experimental desiccating stress, and decreased cornification of the corneal epithelia.
Loss of PKCα appears to block the parasympathetic pathway of lacrimal gland acinar and conjunctival goblet cell secretion. This effect was signified by the enlarged cell size and stronger carbohydrate-rich cells stained in both tissues, and the failure of exocytosis, due to the lack of the PKCα gene in the exocytic process of preformed secretory vesicles with an apical plasma membrane. The PKCα−/− mice showed an elevated ocular surface response to experimental desiccating stress, with increased disruption of corneal epithelial barrier function. These changes may be directly due to the loss of the PKCα gene or may be secondary to the reduced secretion of the corneal supportive factors by the lacrimal gland and conjunctival goblet cells. Secretion of one such factor, EGF, was noted to be decreased.
Effect of PKCα Deficiency on Corneal Epithelial Wound Healing and Inflammation
Traumatic corneal epithelial abrasion is a very common condition encountered in clinical practice. To further characterize the healing response after corneal epithelial debridement, we used our standardized epithelial wound model to evaluate the healing rate, proliferation, and inflammatory cell infiltration of the corneal epithelium. PKCs are key intracellular signal transduction mediators that are involved in various cell functions throughout the body, including cell growth and differentiation. Their known functions suggest that they have a pivotal role in corneal wound healing. Studies have already established the presence of PKCα in normal rabbit corneal epithelium.32–34 Its expression increased during epithelial wound healing. An oligonucleotide-targeting rabbit PKCα was found to inhibit the wound-healing process in vitro by more than 50%.34 In contrast to the predictions made on the basis of these previously reported studies, we found that PKCα−/− mice show accelerated corneal epithelial wound healing and faster re-epithelialization compared with PKCα+/+ mice.
The mechanism of these conflicting results is not clear. One factor that influences healing rate is the local inflammatory response that may have a positive or negative impact on the healing rate, as debated in a recent review.27 The neutrophil is the predominant cell type in the acute phase of inflammation, soon followed by a second wave of monocyte infiltration. Although it is widely recognized that the infiltration of neutrophils into injured tissue protects wounds from invading pathogens, more recent studies suggest that neutrophils inhibit the wound repair process, including that in skin28 and corneal epithelium.25,29 The present study showed that neutrophil infiltration into the injured cornea was markedly decreased in the PKCα−/− mice and is consistent with the finding of a study linking neutrophil infiltration to PKCα activation in the epidermis.30,31,35 Therefore, deficient neutrophil infiltration is one of several possible explanations for the accelerated corneal epithelial re-epithelialization in the present study.
PKCα-modulated events in the cornea appear to have greater effects on epithelial healing than PKCα deficiency has on lacrimal gland function. A reduced corneal epithelial healing rate would be expected based on the reduced tear production that was observed in these mice. In summary, PKCα has essential functions in regulating tear secretion by the LFU and the ocular surface response to desiccating stress and corneal epithelial trauma. The details of the mechanism should be further investigated.
Footnotes
Supported by a grant from Fight for Sight; National Institutes of Health Grant EY011915 (SCP), National Eye Institute, Bethesda, MD; and an unrestricted grant from Research to Prevent Blindness.
Disclosure: Z. Chen, None; Z. Li, None; S. Basti, None; W.J. Farley, None; S.C. Pflugfelder, None
References
- 1. Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334:661–665 [DOI] [PubMed] [Google Scholar]
- 2. Zoukhri D, Hodges RR, Sergheraert C, Toker A, Dartt DA. Lacrimal gland PKC isoforms are differentially involved in agonist-induced protein secretion. Am J Physiol. 1997;272:C263–C269 [DOI] [PubMed] [Google Scholar]
- 3. Sundermeier T, Matthews G, Brink PR, Walcott B. Calcium dependence of exocytosis in lacrimal gland acinar cells. Am J Physiol Cell Physiol. 2002;282:C360–C365 [DOI] [PubMed] [Google Scholar]
- 4. Zoukhri D, Hodges RR, Willert S, Dartt DA. Immunolocalization of lacrimal gland PKC isoforms: effect of phorbol esters and cholinergic agonists on their cellular distribution. J Membr Biol. 1997;157:169–175 [DOI] [PubMed] [Google Scholar]
- 5. Dartt DA. Regulation of tear secretion. Adv Exp Med Biol. 1994;350:1–9 [DOI] [PubMed] [Google Scholar]
- 6. Zoukhri D, Hodges RR, Sergheraert C, Dartt DA. Protein kinase C isoforms differentially control lacrimal gland functions. Adv Exp Med Biol. 1998;438:181–186 [DOI] [PubMed] [Google Scholar]
- 7. Zoukhri D, Hodges RR, Dicker DM, Dartt DA. Role of protein kinase C in cholinergic stimulation of lacrimal gland protein secretion. FEBS Lett. 1994;351:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589 [DOI] [PubMed] [Google Scholar]
- 9. Dartt DA, Kessler TL, Chung EH, Zieske JD. Vasoactive intestinal peptide-stimulated glycoconjugate secretion from conjunctival goblet cells. Exp Eye Res. 1996;63:27–34 [DOI] [PubMed] [Google Scholar]
- 10. Rios JD, Zoukhri D, Rawe IM, Hodges RR, Zieske JD, Dartt DA. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Invest Ophthalmol Vis Sci. 1999;40:1102–1111 [PubMed] [Google Scholar]
- 11. Dartt DA, Rios JD, Kanno H, et al. Regulation of conjunctival goblet cell secretion by Ca(2+)and protein kinase C. Exp Eye Res. 2000;71:619–628 [DOI] [PubMed] [Google Scholar]
- 12. Nakashima S. Protein kinase C alpha (PKC alpha): regulation and biological function. J Biochem (Tokyo). 2002;132:669–675 [DOI] [PubMed] [Google Scholar]
- 13. Ohba M, Ishino K, Kashiwagi M, et al. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol Cell Biol. 1998;18:5199–5207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340 [DOI] [PubMed] [Google Scholar]
- 15. Matassa AA, Kalkofen RL, Carpenter L, Biden TJ, Reyland ME. Inhibition of PKCalpha induces a PKCdelta-dependent apoptotic program in salivary epithelial cells. Cell Death Differ. 2003;10:269–277 [DOI] [PubMed] [Google Scholar]
- 16. Ruvolo PP, Deng X, Carr BK, May WS. A functional role for mitochondrial protein kinase Calpha in Bcl2 phosphorylation and suppression of apoptosis. J Biol Chem. 1998;273:25436–25442 [DOI] [PubMed] [Google Scholar]
- 17. Deng J, Chen Y, Wu R. Induction of cell cornification and enhanced squamous-cell marker SPRR1 gene expression by phorbol ester are regulated by different signaling pathways in human conducting airway epithelial cells. Am J Respir Cell Mol Biol. 2000;22:597–603 [DOI] [PubMed] [Google Scholar]
- 18. Han Y, Meng T, Murray NR, Fields AP, Brasier AR. Interleukin-1-induced nuclear factor-kappaB-IkappaBalpha autoregulatory feedback loop in hepatocytes: a role for protein kinase calpha in post-transcriptional regulation of ikappabalpha resynthesis. J Biol Chem. 1999;274:939–947 [DOI] [PubMed] [Google Scholar]
- 19. Leitges M, Plomann M, Standaert ML, et al. Knockout of PKC alpha enhances insulin signaling through PI3K. Mol Endocrinol. 2002;16:847–858 [DOI] [PubMed] [Google Scholar]
- 20. Stewart P, Chen Z, Farley W, Olmos L, Pflugfelder SC. Effect of experimental dry eye on tear sodium concentration in the mouse. Eye Contact Lens. 2005;31:175–178 [DOI] [PubMed] [Google Scholar]
- 21. Afonso AA, Monroy D, Stern ME, Feuer WJ, Tseng SC, Pflugfelder SC. Correlation of tear fluorescein clearance and Schirmer test scores with ocular irritation symptoms. Ophthalmology. 1999;106:803–810 [DOI] [PubMed] [Google Scholar]
- 22. Dursun D, Wang M, Monroy D, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002;43:632–638 [PubMed] [Google Scholar]
- 23. Selvamurugan N, Kwok S, Alliston T, Reiss M, Partridge NC. Transforming growth factor-beta 1 regulation of collagenase-3 expression in osteoblastic cells by cross-talk between the Smad and MAPK signaling pathways and their components, Smad2 and Runx2. J Biol Chem. 2004;279:19327–19334 [DOI] [PubMed] [Google Scholar]
- 24. Li Z, Rumbaut RE, Burns AR, Smith CW. Platelet response to corneal abrasion is necessary for acute inflammation and efficient re-epithelialization. Invest Ophthalmol Vis Sci. 2006;47:4794–4802 [DOI] [PubMed] [Google Scholar]
- 25. Li Z, Burns AR, Smith CW. Lymphocyte function-associated antigen-1-dependent inhibition of corneal wound healing. Am J Pathol. 2006;169:1590–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharma GD, Ottino P, Bazan NG, Bazan HE. Epidermal and hepatocyte growth factors, but not keratinocyte growth factor, modulate protein kinase Calpha translocation to the plasma membrane throughout 15(S)-hydroxyeicosatetraenoic acid synthesis. J Biol Chem. 2005;280:7917–7924 [DOI] [PubMed] [Google Scholar]
- 27. Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607 [DOI] [PubMed] [Google Scholar]
- 28. Dovi JV, He LK, DiPietro LA. Accelerated wound closure in neutrophil-depleted mice. J Leukoc Biol. 2003;73:448–455 [DOI] [PubMed] [Google Scholar]
- 29. Ueno M, Lyons BL, Burzenski LM, et al. Accelerated wound healing of alkali-burned corneas in MRL mice is associated with a reduced inflammatory signature. Invest Ophthalmol Vis Sci. 2005;46:4097–4106 [DOI] [PubMed] [Google Scholar]
- 30. Wang HQ, Smart RC. Overexpression of protein kinase C-alpha in the epidermis of transgenic mice results in striking alterations in phorbol ester-induced inflammation and COX-2, MIP-2 and TNF-alpha expression but not tumor promotion. J Cell Sci. 1999;112:3497–3506 [DOI] [PubMed] [Google Scholar]
- 31. Cataisson C, Joseloff E, Murillas R, et al. Activation of cutaneous protein kinase C alpha induces keratinocyte apoptosis and intraepidermal inflammation by independent signaling pathways. J Immunol. 2003;171:2703–2713 [DOI] [PubMed] [Google Scholar]
- 32. Lin N, Bazan HE. Protein kinase C substrates in corneal epithelium during wound healing: the phosphorylation of growth associated protein-43 (GAP-43). Exp Eye Res. 1995;61:451–459 [DOI] [PubMed] [Google Scholar]
- 33. Lin N, Bazan HE. Protein kinase C subspecies in rabbit corneal epithelium: increased activity of alpha subspecies during wound healing. Curr Eye Res. 1992;11:899–907 [DOI] [PubMed] [Google Scholar]
- 34. Chandrasekher G, Bazan NG, Bazan HE. Selective changes in protein kinase C (PKC) isoform expression in rabbit corneal epithelium during wound healing. Inhibition of corneal epithelial repair by PKCalpha antisense. Exp Eye Res. 1998;67:603–610 [DOI] [PubMed] [Google Scholar]
- 35. Cataisson C, Pearson AJ, Tsien MZ, et al. CXCR2 ligands and G-CSF mediate PKCalpha-induced intraepidermal inflammation. J Clin Invest. 2006;116:2757–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]