Abstract
Arc repressor forms a homodimer in which the subunits intertwine to create a single globular domain. To obtain Arc sequences that fold preferentially as heterodimers, variants with surface patches of excess positive or negative charge were designed. Several but not all oppositely charged sequence pairs showed preferential heterodimer formation. In the most successful design pair, α helix B of one subunit contained glutamic acids at positions 43, 46, 47, 48, and 50, whereas the other subunit contained lysines or arginines at these positions. A continuum electrostatic model captures many features of the experimental results and suggests that the most successful designs include elements of both positive and negative design.
Keywords: Arc repressor, continuum electrostatics, heterodimer, negative design, protein stability
A central challenge of protein science is to understand the relationship between amino acid sequence and the specification of tertiary structure (1–3). Attempting to design proteins that exhibit novel properties provides one means of testing and refining this understanding. Progress has been made in the design of coiled-coil and helical-bundle structures, and computational design algorithms have been applied successfully in several instances to the redesign of globular proteins (4–9). It is clear, nevertheless, that our understanding of the determinants of protein folding is far from complete (10–11).
In this paper, we use P22 Arc repressor, a member of the ribbon–helix–helix family of DNA-binding proteins, to probe the sequence determinants of tertiary and quaternary specificity. Arc is a small 53-residue protein that folds as a homodimer. The structure of the Arc dimer has been determined by solution NMR and x-ray crystallography (12–14). Unlike many dimers, subunits in Arc do not form independent domains but rather intertwine to form one domain with a single hydrophobic core (Fig. 1A). As a result, folding and dimerization of Arc are coupled, and tertiary and quaternary structure are formed concomitantly (15).
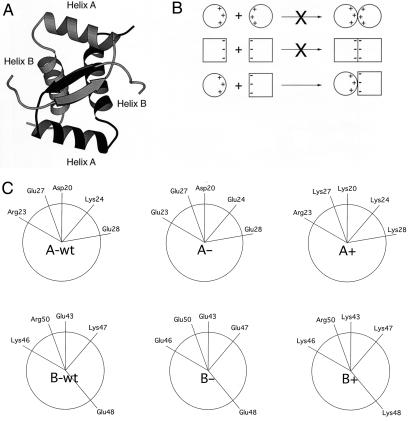
Figure 1.
(A) Ribbon representation of Arc-repressor dimer with one subunit shaded more darkly than the other subunit. The positions of α helices A and B are indicated. (B) Individual variants with excess negative or positive charge may form unstable homodimers alone but form a stable heterodimer in combination. (C) Helical wheel projections showing surface residues in α-helix A (Upper) and α-helix B (Lower) in wild-type Arc, and the A+, A−, B+, and B− variants.
Arc subunits interact with a dissociation constant of approximately 10 nM but do not form detectable heterodimers with related ribbon–helix–helix proteins that adopt the same tertiary fold. Clearly, the specificity of Arc dimerization depends on a set of detailed sequence-encoded interactions. We sought to design pairs of Arc variants that folded preferentially as heterodimers. Toward this end, we produced and characterized mutants with surface patches of positive or negative charge at high local densities. Some pairs of variants with complementary patterns of positive and negative charge exhibited preferential heterodimer formation that could be rationalized by using a simple method based on continuum electrostatics (16). The dimeric nature of this system allows certain positive- and negative-design issues to be quantified, because homodimers formed by individual sequences can be compared with heterodimers formed by sequence pairs.
Materials and Methods
Mutagenesis and Purification.
Mutations in the arc gene were constructed by cassette mutagenesis or by PCR mutagenesis, verified by DNA sequencing, and introduced into the pET800 vector for overexpression (17). Each Arc variant also contained an st11 (HHHHHHKNQHE) C-terminal extension to provide resistance to intracellular degradation (18). Arc-st11 variants were purified by chromatography on a Ni2+-nitrilotriacetic acid (NTA) column (Qiagen, Chatsworth, CA) and, where required, on SP-Sephadex (Amersham Pharmacia) to a final purity > 95% as judged by SDS/PAGE (18–19). For the column retention assay described below, a B− variant with an st5 (KNQHE) C-terminal extension was constructed and purified by ion-exchange chromatography on CM-Accell (Waters) and gel filtration (18).
Biophysical Analysis.
Except as indicated, experiments were performed at 25°C in a buffer containing 50 μM Tris⋅Cl (pH 7.5), 250 μM KCl, and 0.1 μM EDTA. Circular dichroism (CD) spectroscopy was performed by using a CD Spectrometer model 60DS (Aviv Associates, Lakewood, NJ) and a 1-cm path length. For far-UV and near-UV CD scans, the total protein concentrations in monomer equivalents were 5 and 100 μM, respectively. Thermal denaturation was monitored by changes in CD ellipticity at 222 nm with an averaging time of 30 sec and an equilibration time of 1.5 min for each temperature. Melting temperatures and free energies were determined by fitting as described (15, 20). Tryptophan fluorescence spectra were taken on an F4500 fluorescence spectrophotometer (Hitachi, Tokyo) with a total protein concentration of 10 μM.
Column-Binding Heterodimerization Assays.
Arc-st11 and Arc-st5 variants were mixed at concentrations of 25 μM, incubated at room temperature for 30 min, and Ni2+-NTA resin was added for 10 min. The resin was collected by centrifugation and the supernatant containing unbound protein was removed. The resin was washed once with buffer, and bound proteins were eluted with buffer plus 100 μM imidazole, separated by SDS/PAGE, and visualized by staining with Sypro orange.
Electrostatic Calculations.
The mutants described here all involved charge reversal at nonburied positions to create charged surface patches. The primary assumption used to compute the relative stabilities of the mutant homodimers and heterodimers was that charge reversal turned off individual protein interactions. Reversing two charges was assumed to leave their mutual interaction unchanged. The contribution of a single side chain to protein stability includes a desolvation contribution and pair-wise interactions with all other groups in the structure. A simple worst-case model of the stability of a mutant with charge reversal of a single side chain would retain the desolvation penalty of the wild-type side chain but change the sign of each of its pair-wise interactions while leaving the magnitude unchanged. This represents a worst case because it neglects relaxation that could relieve repulsion. A simple best-case model would retain the desolvation penalty but include each pair-wise interaction unchanged in sign or magnitude (i.e., the mutant has the same stability as wild type), implicitly assuming that the charge-reversal mutant could relax to produce a new set of interactions as favorable as that of the wild type. The model used here represents a compromise halfway between these pessimistic and optimistic extremes: the desolvation penalty was retained and changed interactions were zeroed in the mutant (halfway between entering with the opposite sign and the same sign as wild type). This method implicitly included the effect of some generalized relaxation in the mutants and neglected detailed structural differences between wild type and mutants. However, the approach is applicable broadly and does not depend on correct predictions of mutant structures.
Continuum electrostatic calculations were performed as described (21) by using the program DELPHI (16, 22–23); the methods will be described briefly here. The linearized Poisson–Boltzmann equation was solved by using a salt concentration of 0.145 M, a 2.0-Å Stern layer, and a water-probe radius of 1.4 Å. The value of the protein dielectric was 4 (except where noted) and the solvent dielectric was 80. A calculation with Debye–Hückel boundary conditions in which the molecule filled 23% of the grid was used to provide the boundary condition for a final calculation in which the molecule filled 92% of the 129 × 129 × 129 grid (adjacent grid points were 0.383 Å apart; tests at finer grid spacings showed no differences in computed results). CHARMM19 parameters were used for all calculations (24).
Interaction free energies were determined as the average from 17 wild-type homodimers, one from the x-ray crystal structure of the Arc tetramer bound to operator DNA [Protein Data Base (PDB) entry 1PAR; ref. 14], and 16 from solution NMR structures (PDB entry 1ARQ; ref. 13). Multiple wild-type conformations were used to account for the conformational flexibility of charged side chains on the surface of the protein. Because the E48 side chain was missing in the C chain of the CD dimer in the crystal structure, the AB dimer was chosen for the calculations. Hydrogens were added by using the HBUILD feature of the program CHARMM (24, 25).
Results
Design of Variants.
Our general strategy for obtaining Arc variants that show preferential heterodimerization is outlined in Fig. 1B. Basically, Arc mutants with local patches of increased positive or negative charge in α-helix A or α-helix B were designed, with the naive expectation that homodimers of like charge should be destabilized and heterodimers of opposite charge should be favored by electrostatic attraction. To create variants with excess negative charge, lysine and arginine residues were replaced with glutamic acid residues. Conversely, mutants with excess positive charge were obtained by replacing aspartic and glutamic acid residues with lysines. Four variants, designated A+ (D20K, E27K, and E28K), A− (R23E and K24E), B+ (E43K and E48K), and B− (K46E, K47E, and R50E), were constructed and purified. These sequence changes result in sets of five consecutive helical positions with the same charge, including residues 20, 23, 24, 27, and 28 in helix A and positions 43, 46, 47, 48, and 50 in helix B (Fig. 1C). The average solvent accessibilities of the charged portions of the wild-type side chains at these positions in the crystal structure are: D20, 43%; R23, 47%; K24, 54%; E27, 86%; E28, 36%; E43, 36%; K46, 52%; K47, 31%; E48, 38%; and R50, 25%.
CD spectra and stabilities to thermal denaturation were determined for each variant alone and for each pair-wise combination of variants with opposing charges (A+/A−; A+/B−; B+/A−; and B+/B−). A successful design was considered to be one in which the heterodimer folded stably, and the homodimers alone were destabilized significantly.
The B+/B− Heterodimer.
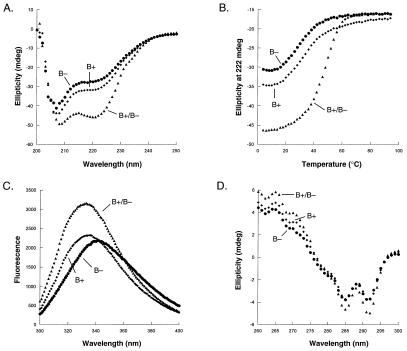
Fig. 2 shows experiments for the B+ and B− variants, which formed the most successful heterodimeric pair. An equal mixture of both proteins at 25°C gave a significantly stronger far-UV CD signal than either variant alone (Fig. 2A), indicating a higher level of α-helical structure. This result indicated that the mixture contained an increased number of native dimers and a decreased number of denatured monomers, which in turn suggested an energetic preference for the heterodimer relative to both homodimers. Thermal denaturation studies supported this conclusion (Fig. 2B). The B+/B− mixture displayed significantly greater stability than either B+ or B− alone, indicating clearly that the B+/B− heterodimer must be significantly more stable than either homodimer. The mixture had a tm of 45°C, whereas B+ or B− alone had tm between 23 and 25°C. Fitting of these data gave equilibrium constants for dimer denaturation and dissociation of 5.0 μM for the B+ homodimer, 5.9 μM for the B− homodimer, and 50 nM for the B+/B− mixture (Table 1). At a total protein concentration of 5 μM and 25°C, approximately 50% of the molecules are native homodimers when only B+ or B− is present by itself. In a mixture of 2.5 μM each of B+ and B−, approximately 90% of the molecules are native dimers, and heterodimers outnumber homodimers in this population by a factor of 55:1. This heterodimer preference value is listed in Table 1.
Figure 2.
(A) Far-UV CD spectra of 5 μM B+ (⧫), 5 μM B− (●), and 2.5 μM each of a B−/B+ mixture (▴). (B) Thermal denaturation curves of 5 μM B+ (⧫), 5 μM B− (●), and 2.5 μM each of a B−/B+ mixture (▴). (C) Tryptophan fluorescence spectra of 10 μM B+ (⧫), 10 μM B− (●), and 5 μM each of a B−/B+ mixture (▴). (D) Near-UV CD spectra of 100 μM B+ (⧫), 100 μM B− (●), and 50 μM each of a B−/B+ mixture (▴).
Table 1.
Properties of dimeric pairs
| tm, °C | Ku, M | ΔGu, kcal/mol | Heterodimer specificity | |
|---|---|---|---|---|
| B−/B− | 22.9 | 5.9 × 10−6 | 7.1 | |
| B+/B+ | 24.8 | 5.0 × 10−6 | 7.2 | |
| A+/A+ | 35.1 | 1.1 × 10−6 | 8.1 | |
| A−/A− | 40.4 | 4.7 × 10−7 | 8.6 | |
| A+/A− | 37.9 | 3.9 × 10−7 | 8.7 | 0.9 |
| B+/A− | 40.5 | 1.5 × 10−7 | 9.3 | 4.4 |
| B+/B− | 45.5 | 5.0 × 10−8 | 9.9 | 55 |
| A+/B− | 46.0 | 2.8 × 10−8 | 10.3 | 39 |
| Wild type | 54.0 | 8.0 × 10−9 | 11.0 |
tm values were determined at a total protein concentration of 5 μM for homodimers and 2.5 μM each for heterodimers. Ku values for dimer dissociation and denaturation were calculated at 25°C, 250 mM KCl, pH 7.5. ΔGu values were calculated under the same conditions using a standard-state concentration of 1 M. Heterodimer specificity was calculated as the ratio of heterodimer/homodimers at 25°C with 2.5 μM total concentrations of each variant in monomer equivalents. In comparing the stabilities of homodimers and heterodimers at the same total subunit concentration, homodimers have a statistical advantage. Data for wild-type Arc-st11 taken from ref. 17.
Near-UV CD and tryptophan fluorescence spectra confirmed that the B+/B− mixture had a higher degree of native structure than B+ or B− alone. The fluorescence spectrum of the B+/B− mixture was blue-shifted significantly relative to B+ or B− alone (Fig. 2C), indicating greater burial of Trp-14, which is the only tryptophan and part of the hydrophobic core in native Arc. Near-UV CD spectra were taken at protein concentrations of 100 mM, in which approximately 85% of the B+ and B− populations should be native homodimers and 97% of the B+/B− population should be native dimers. The near-UV CD spectra of the mixture (Fig. 2D) was nearly identical to that of wild-type Arc data with minima at 291 and 285 nm and a maximum at 265 nm (26). This result indicates that the packing of aromatic residues in the core of the heterodimer must be similar to the packing in wild-type Arc. The near-UV CD spectra of B+ or B− alone had slightly less signal (Fig. 2D), which is consistent with the reduced stability of the homodimers relative to the heterodimer but again had overall shapes similar to the spectrum of wild-type Arc.
To assess the degree of interaction between B+ and B− subunits directly, equal quantities of His6-tagged B+ and untagged B− were mixed and applied to a Ni2+-NTA column. After washing the resin, protein was eluted and quantified by SDS/PAGE. In the presence of His6-tagged B+, 87% of the untagged B− variant was retained by the Ni2+-NTA column (Fig. 3). By contrast, only trace amounts of untagged B− protein by itself were retained on the resin. Random assortment of B+ and B− would yield a 1:2:1 mixture of B−/B−:B+/B−:B+/B+ species and predict 50% maximum retention of the untagged B− subunit. Retention of the untagged protein at the higher level thus provided additional evidence that the B+/B− heterodimer was significantly more stable than the homodimers. Control experiments showed that only 35% of the untagged B− subunit was retained by an equal amount of wild-type Arc with a His6 tag, and only 8% was retained by an equal amount of His6-tagged B− (Fig. 3). These results showed that the Arc/B− heterodimer was less stable than the average of both homodimers and also confirmed that the B−/B− homodimer was rather unstable.
Figure 3.
Retention by Ni2+-NTA resin of untagged B− protein alone, in the presence of His6-tagged wild-type Arc, in the presence of His6-tagged B− protein, and in the presence of His6-tagged B+ protein.
Other Design Combinations.
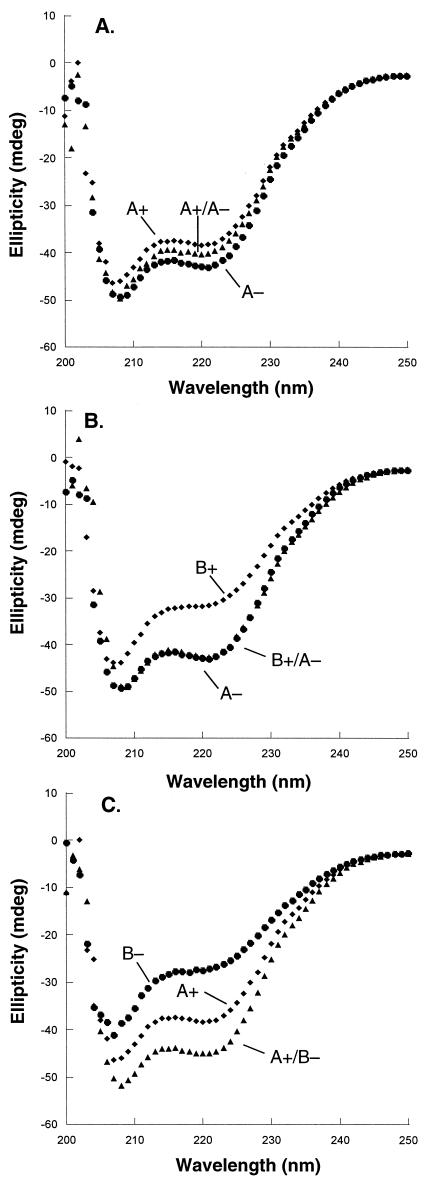
The A+/A− mixture produced a far-UV CD spectrum intermediate between those of A+ and A− alone (Fig. 4A), suggesting that the heterodimeric form, in this case, is not favored. The B+/A− combination had a CD spectrum roughly equivalent to that of A− alone but had more structure than B+ alone (Fig. 4B). This result shows some heterodimeric preference but also indicates that the heterodimer is not dramatically more stable than the best homodimer. The A+/B− mixture displayed significantly more secondary structure than either homodimer alone (Fig. 4C), indicating a substantial heterodimeric preference. Thermal-melt data supported the conclusion that the design variants form a series in which heterodimer preference decreases in the order B+/B− > A+/B− > B+/A− > A+/A− with heterodimer preferences of 55, 39, 4, and 1, respectively (Table 1).
Figure 4.
Far-UV CD spectra. (A) 5 μM A+ (⧫), 5 μM A− (●), and 2.5 μM each of A+ and A− (▴). (B) 5 μM A− (⧫), 5 μM B+ (●), and 2.5 μM each of A− and B+ (▴). (C) 5 μM A+ (⧫), 5 μM B− (●), and 2.5 μM each of A+ and B− (▴).
Electrostatic Modeling.
A simple model using continuum electrostatics was used to estimate the relative stability of each mutant homodimer and heterodimer. The energetics in each mutant were assumed equivalent to wild type except that charge reversal eliminated the interactions of a side chain. Reversing two charges left their mutual interaction unchanged. This model neglects detailed differences in the geometry and chemistry of wild-type and mutant residues and how it alters interaction energetics, other differences in conformation between the mutants and wild type, and changes to nonelectrostatic portions of the folding and dimerization free energy. Nevertheless, it is applicable broadly to a wide range of mutants and captures the main design criterion; namely it assesses the electrostatic rewards and penalties for producing extended surface charge patches. Results reported are averages of calculations carried out on each member on an ensemble of 16 NMR-derived structures of the wild-type Arc homodimer and one wild-type homodimer from the DNA-bound cocrystal structure (13–14).
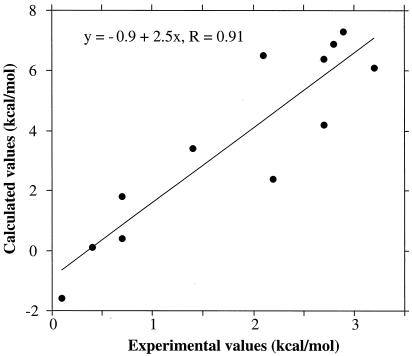
Comparison of the overall results of the calculations with the experiments (Table 2; Fig. 5) showed very good correlation (r > 0.9) for the relative stabilities of the homodimers and heterodimers, but the calculations overestimated the free-energy differences by a factor of 2 to 3. The calculations reproduced the experimentally observed heterodimer preferences for the B+/B− and A+/B− pairs, overestimated somewhat the modest B+/A− preference, and reproduced the absence of preference for the A+/A− pair. In addition, the calculated homodimer stabilities had the same ordering as that observed experimentally with A−/A− > A+/A+ > B+/B+ > B−/B− (Table 2). It was encouraging that such a simple model was capable of capturing, at least qualitatively, the experimentally observed trends. The dominance of electrostatics in these charge-reversal mutants is reasonable, although the effects of relaxation were underestimated by the approach taken. One computational technique for incorporation of uniform relaxation is to increase the internal dielectric constant. Calculations with a protein dielectric of 40 showed a consistent ordering but an even closer agreement with the experiments (heterodimer preferences of 0.3, 1.7, 2.9, and 3.5 kcal/mol for A+/A−, B+/A−, A+/B−, and B+/B−, respectively).
Table 2.
Comparison of experimental and calculated stabilities
| Heterodimer preference,
kcal/mol
|
Heterodimer stability over most/least
stable homodimer, kcal/mol
|
|||
|---|---|---|---|---|
| expt | calc | expt | calc | |
| A+/A− | 0.4 | 0.1 | 0.1/0.7 | −1.6/1.8 |
| B+/A− | 1.4 | 3.4 | 0.7/2.1 | 0.4/6.5 |
| A+/B− | 2.7 | 4.2 | 2.2/3.2 | 2.4/6.1 |
| B+/B− | 2.8 | 6.9 | 2.7/2.9 | 6.4/7.3 |
Heterodimer preference is the free energy of dissociation and denaturation of the heterodimer minus the average free energies of these reactions for the two homodimers. Stability over most/least stable homodimer is the difference in the free energies of dissociation and denaturation for the heterodimer and the most/least stable homodimer. Comparisons with the +/+ homodimer are indicated in bold.
Figure 5.
Correlation of differences in dimer stabilities determined experimentally and by electrostatic calculations. The data points are from Table 2.
Agreement between calculations and experiments was sufficiently good that the calculations were dissected to determine the source of the computed stability and specificity changes. The B+/B− pair had the highest specificity with the heterodimer more than 2.5 kcal/mol more stable than either homodimer. The energetics for this pair were dominated by positions 43 and 47, which were repulsive in the homodimers (K43, K47, K43′, and K47′ for B+/B+ and E43, E47, E43′, and E47′ for B−/B−) and attractive in the heterodimer (K43, K47, E43, E47 for B+/B−), accounting for 4.9 of the total 6.9 kcal/mol of calculated heterodimer specificity. The A+/B− design, which was slightly less successful in terms of heterodimer specificity but actually formed a more stable heterodimer, incorporated both an interaction between K28 of A+ and E50 of B− (1.2 kcal/mol in each of two occurrences) and the K47/K47′ repulsion that was relieved by the B− mutations (0.5 kcal/mol). Together these interactions account for about 70% of the calculated specificity. In the B+/A− heterodimer, which was only slightly more stable than the A−/A− homodimer, the only significant interaction favoring the heterodimer was relief of the E43/E43′ repulsion by the B+ mutations (70% of total computed specificity). There were no significant interactions favoring heterodimer formation for the A+/A− combination, which showed no heterodimer preference. Thus, the calculations suggest that the observed heterodimer preferences were dominated by a small number of specific, local electrostatic interactions rather than by more general, nonlocal charge effects.
Discussion
To test our understanding of the interactions that are important for both the structural specificity and stability of globular proteins, we have used the P22 Arc repressor system and asked a simple design question. Can we identify variants of Arc that fold stably as heterodimer combinations but fold unstably as homodimers? Variants with altered surface charges in the two α helices of Arc were constructed, and several combinations were shown to fulfill many of the criteria for a successful design. In the best case, both the B+/B+ and B−/B− homodimers were destabilized significantly relative to the B+/B− heterodimer. The B+/B− heterodimer had near-UV and far-UV CD spectra and a tryptophan fluorescence spectrum similar to wild-type Arc, strongly suggesting a native-like structure. Moreover, this heterodimer was only moderately less stable than the wild-type Arc homodimer (Table 1).
The mutant Arc variants were designed simply to create extended patches of positive or negative charge on the surfaces of α-helix A or α-helix B. Our expectation was that electrostatic repulsion would help destabilize the +/+ and −/− homodimers and electrostatic attraction would help stabilize the +/− heterodimer. Although this result occurs to some extent, it is clear that the detailed properties of specific variants also play significant roles in determining the stabilities of both the heterodimers and the homodimers. For example, the B−/B− homodimer was destabilized to a much greater extent than the A−/A− homodimer, consistent with the closer distance between the B helices than the A helices in the wild-type structure (Fig. 1A). An important result of these studies is that a continuum electrostatic analysis using a simplified model captured many aspects of the experimental results, providing an understanding of why some designs were more or less successful and a rational basis to plan improved designs.
For a given amino acid sequence to encode the folding of a protein, it is important that it can make interactions that will stabilize the native structure and not make interactions that would stabilize competing nonnative structures. Folding and dimerization are coupled processes in the Arc system, because both subunits intertwine to form a single hydrophobic core. In the design of Arc heterodimers, therefore, one can consider both positive interactions that will stabilize the heterodimer specifically and negative interactions that will destabilize the competing homodimers. Furthermore, for any combination of Arc sequences, it is possible to determine the stabilities of the homodimers alone and thus to study the interplay of positive and negative contributions to heterodimer stability. For instance, from the calculations it seems that a specific interaction between E43 in B− and K47 in B+ provides a positive interaction in the B+/B− heterodimer, but E43/E43′ and K47/K47′ interactions destabilize each homodimer. By contrast, in the A+/B− combination, which showed slightly less heterodimer specificity than B+/B−, the A+/A+ homodimer was found to have no strongly destabilizing interaction.
A simple model incorporating only the energetic effects of alterations to electrostatics was able to account reasonably for the observed specificity changes. This result supports the motivation for the designs, which postulated the dominance of electrostatics for charge-reversal mutants. Even so, it is interesting that experimental trends were reproduced without accounting for detailed packing interactions and conformational changes in the mutants. This finding suggests that the energetic consequences of relaxation were related simply to the strain introduced. Interestingly, the calculations indicate that a few essential interactions are responsible for the majority of the design effects, suggesting that preferential heterodimers could be constructed with fewer substitutions. This prediction can be tested experimentally. Moreover, determining the structures of the B+/B− or A+/B− heterodimers would also help to determine the molecular basis for the success of these designs.
Simple electrostatic interactions also seem responsible for the heterodimer specificity of the fos/jun coiled coil (27), and leucine-zipper heterodimers have been designed by using this principle (28). Core packing clearly also plays a role in determining the detailed structures of coiled coils (7). Changes in core packing in the p53 tetramerization domain, a four-helix bundle, result in variants that form homotetramers but do not form heterotetramers with a tumor-derived variant (29). We initially attempted to design alternative core arrangements that would stabilize Arc heterodimers preferentially by using different repacking algorithms (4, 6), but none of the variants that were predicted to have good heterodimer energies folded stably. Hence, although this approach has worked in other systems and remains a promising design route, it has yet to be implemented successfully for Arc heterodimers.
Acknowledgments
We thank Catherine Drennen for comments on the manuscript. Supported by National Institutes of Health Grants AI-15706 and GM-55758. M.J.N. was supported by a National Institutes of Health postdoctoral fellowship.
Abbreviations
- NTA
nitrilotriacetic acid
- CD
circular dichroism
References
- 1.Bryson J W, Betz S F, Lu H S, Suich D J, Zhou H X, O'Neil K T, DeGrado W F. Science. 1995;270:935–941. doi: 10.1126/science.270.5238.935. [DOI] [PubMed] [Google Scholar]
- 2.Cordes M H, Davidson A R, Sauer R T. Curr Opin Struct Biol. 1996;6:3–10. doi: 10.1016/s0959-440x(96)80088-1. [DOI] [PubMed] [Google Scholar]
- 3.Regan L. Curr Opin Struct Biol. 1999;9:494–499. doi: 10.1016/S0959-440X(99)80070-0. [DOI] [PubMed] [Google Scholar]
- 4.Desjarlais J R, Handel T M. Protein Sci. 1995;4:2006–2018. doi: 10.1002/pro.5560041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith C K, Regan L. Acc Chem Res. 1997;30:153–161. [Google Scholar]
- 6.Dahiyat B I, Mayo S L. Science. 1997;278:82–87. doi: 10.1126/science.278.5335.82. [DOI] [PubMed] [Google Scholar]
- 7.Harbury P B, Plecs J J, Tidor B, Alber T, Kim P S. Science. 1998;282:1462–1467. doi: 10.1126/science.282.5393.1462. [DOI] [PubMed] [Google Scholar]
- 8.Malakauskas S M, Mayo S L. Nat Struct Biol. 1998;5:470–475. doi: 10.1038/nsb0698-470. [DOI] [PubMed] [Google Scholar]
- 9.Shimaoka S M, Shifman J M, Jing H, Takagi J, Mayo S L, Springer T A. Nat Struct Biol. 2000;7:674–678. doi: 10.1038/77978. [DOI] [PubMed] [Google Scholar]
- 10.Venclovas C, Zemla A, Fidelis K, Moult J. Proteins. 1999;Suppl.3:231–237. doi: 10.1002/(sici)1097-0134(1999)37:3+<231::aid-prot30>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Mayo K H. Trends Biotechnol. 2000;18:212–217. doi: 10.1016/s0167-7799(00)01439-6. [DOI] [PubMed] [Google Scholar]
- 12.Breg J N, van Opheusden J H, Burgering M J, Boelens R, Kaptein R. Nature (London) 1990;346:586–589. doi: 10.1038/346586a0. [DOI] [PubMed] [Google Scholar]
- 13.Bonvin A M, Vis H, Breg J N, Burgering M J, Boelens R, Kaptein R. J Mol Biol. 1994;236:328–341. doi: 10.1006/jmbi.1994.1138. [DOI] [PubMed] [Google Scholar]
- 14.Raumann B E, Rould M A, Pabo C O, Sauer R T. Nature (London) 1994;367:754–757. doi: 10.1038/367754a0. [DOI] [PubMed] [Google Scholar]
- 15.Bowie J U, Sauer R T. Biochemistry. 1989;28:7139–7143. doi: 10.1021/bi00444a001. [DOI] [PubMed] [Google Scholar]
- 16.Sharp K A, Honig B. Annu Rev Biophys Biophys Chem. 1990;19:301–332. doi: 10.1146/annurev.bb.19.060190.001505. [DOI] [PubMed] [Google Scholar]
- 17.Milla M E, Brown B M, Sauer R T. Nat Struct Biol. 1994;1:518–523. doi: 10.1038/nsb0894-518. [DOI] [PubMed] [Google Scholar]
- 18.Milla M E, Brown B M, Sauer R T. Protein Sci. 1993;2:2198–2205. doi: 10.1002/pro.5560021219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown B M, Milla M E, Smith T L, Sauer R T. Nat Struct Biol. 1994;1:164–168. doi: 10.1038/nsb0394-164. [DOI] [PubMed] [Google Scholar]
- 20.Milla M E, Sauer R T. Biochemistry. 1995;34:3344–3351. doi: 10.1021/bi00010a025. [DOI] [PubMed] [Google Scholar]
- 21.Hendsch Z S, Tidor B. Protein Sci. 1999;8:1381–1392. doi: 10.1110/ps.8.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilson M K, Honig B H. Nature (London) 1987;330:84–86. doi: 10.1038/330084a0. [DOI] [PubMed] [Google Scholar]
- 23.Gilson M K, Sharp K A, Honig B H. J Comput Chem. 1988;9:327–335. [Google Scholar]
- 24.Brooks B R, Bruccoleri R E, Olafson B D, States D J, Swaminathan S, Karplus M. J Comput Chem. 1983;4:187–217. [Google Scholar]
- 25.Brünger A T, Karplus M. Proteins Struct Funct Genet. 1988;4:148–156. doi: 10.1002/prot.340040208. [DOI] [PubMed] [Google Scholar]
- 26.Cordes M H, Walsh N P, McKnight C J, Sauer R T. Science. 1999;284:325–328. doi: 10.1126/science.284.5412.325. [DOI] [PubMed] [Google Scholar]
- 27.O'Shea E K, Rutkowski R, Kim P S. Cell. 1992;68:699–708. doi: 10.1016/0092-8674(92)90145-3. [DOI] [PubMed] [Google Scholar]
- 28.O'Shea E K, Lumb K J, Kim P S. Curr Biol. 1993;3:658–667. doi: 10.1016/0960-9822(93)90063-t. [DOI] [PubMed] [Google Scholar]
- 29.Stavridi E S, Chehab N H, Caruso L C, Halazonetis T D. Protein Sci. 1999;8:1773–1779. doi: 10.1110/ps.8.9.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]