This is the first report of the ability of azithromycin to inhibit the production of inflammatory mediators by human corneal epithelial cells. The authors demonstrated that the fungal component zymosan induces proinflammatory responses through TLR2 and NF-κB signaling pathways, whereas azithromycin suppresses its stimulation by blocking NF-κB activation in human corneal epithelial cells, suggesting the potential efficacy of this antibiotic for treating ocular surface inflammatory disorders.
Abstract
Purpose.
In addition to its antibiotic effects, azithromycin has been noted to have anti-inflammatory activity, particularly in the context of microbial infections. This study was conducted to explore the suppressive effects of azithromycin on the production of proinflammatory mediators by human corneal epithelial cells (HCECs) stimulated by a fungal component, zymosan.
Methods.
Primary HCECs were cultured from donor corneal limbal explants and grown to subconfluence. The cells were treated with toll-like receptor (TLR) 2 agonist zymosan (1–50 μg/mL) for 4 to 48 hours, with or without preincubation with azithromycin (1–50 μg/mL), TLR2 antibody, or NF-κB activation inhibitor quinazoline (NF-κB-I). The cells were subjected to total RNA extraction, reverse transcription (RT), and real-time PCR using gene expression assays. Cells treated for 48 hours were used for immunofluorescence staining and Western blot analysis, and their medium supernatants were collected for protein quantitation by immunobead assays.
Results.
The mRNA expression and protein production of proinflammatory cytokines (TNF-α and IL-1β), chemokines (IL-8 and RANTES), and matrix metalloproteinases (MMP-1, -3, and -9) by HCECs were stimulated by zymosan in a concentration-dependent manner, with peak levels noted at 4 hours. These stimulated levels of proinflammatory mediators by zymosan were significantly inhibited by TLR2 antibody, NF-κB-I, or azithromycin, which blocked zymosan-induced NF-κB activation as determined by p65 protein nuclear translocation.
Conclusions.
These findings demonstrated that the fungal component zymosan induces proinflammatory responses through TLR2 and NF-κB signaling pathways, whereas azithromycin suppresses its stimulation by blocking NF-κB activation in HCECs, suggesting the potential efficacy of this antibiotic for treating ocular surface inflammatory disorders.
Azithromycin is a macrolide antibiotic used to treat or prevent certain bacterial infections, such as otitis media, tonsillitis, sinusitis, pharyngitis, laryngitis, bronchitis, pneumonia, typhoid, dysentery, chlamydia, and urethritis. Azithromycin has also been used topically to treat ocular surface infections including bacterial and inclusion conjunctivitis and posterior blepharitis.1,2 In addition to antibiotic effects, azithromycin has been noted to have anti-inflammatory effects, particularly in the context of microbial infections. Azithromycin has been shown to reduce mRNA expression and protein production of tumor necrosis factor (TNF-)-α3 and interleukin (IL)-84 in cystic fibrosis airway epithelial cells; prevent the upregulation of MUC5AC and MMP-9, triggered by the inflammatory stimulus, in human airway epithelia5; and reduce TNF-α–induced IL-6 and IL-8 levels in tracheal aspirate cells.6 Azithromycin also suppresses IL-12 expression in lipopolysaccharide (LPS)- and interferon (IFN)-γ–stimulated macrophages.7 Inhibition of NF-κB activation was identified as one mechanism for the anti-inflammatory effects of azithromycin in these cells.4,7–9 The anti-inflammatory properties of azithromycin on ocular surface inflammation have not been elucidated. The purpose of this study was to evaluate the effectiveness of azithromycin on inhibiting the production of proinflammatory mediators in human corneal epithelial cells (HCECs) stimulated by a toll-like receptor (TLR) agonist. The TLR agonist zymosan was chosen because of its recognized proinflammatory effects on the corneal epithelium.
Zymosan is a carbohydrate-rich cell wall preparation derived from the yeast Saccharomyces cerevisiae, which is a well-recognized TLR2 ligand and has been widely used as a phagocytic stimulus. Zymosan can upregulate leukotriene production by monocytes, increase lysosomal enzyme secretion, and stimulate the production and release of proinflammatory cytokines. For example, zymosan has triggered the production of the proinflammatory cytokines TNF-α and IL-6,10,11 chemokine IL-8, monocyte chemoattractant protein-1 (MCP-1) and CXCL1,12–14 and matrix metalloproteinase 9.15 This study was performed to explore the potential suppressive effects of azithromycin on the production of proinflammatory mediators in cultured HCECs stimulated by TLR2 ligand.
Materials and Methods
Materials and Reagents
Cell culture dishes, plates, centrifuge tubes, and other plastic ware were purchased from Becton Dickinson (Lincoln Park, NJ). Dulbecco modified Eagle medium (DMEM), Ham F-12, amphotericin B, and gentamicin were from Invitrogen (Grand Island, NY). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). The TLR2 ligand zymosan from yeast was purchased from InvivoGen (San Diego, CA). TLR2 mouse antibody and p65 rabbit antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). Purification kit was from Qiagen (RNeasy Mini kit; Valencia, CA). Gene expression assays (TaqMan) and real-time master mix (PCR Master Mix) were from Applied Biosystems (Foster City, CA). Azithromycin was provided by Inspire Pharmaceuticals, Inc. (Durham, NC).
HCEC Cultures for Zymosan Induction
Fresh human corneoscleral tissues (<72 hours after death) not suitable for clinical use, from donors aged 19 to 67 years, were obtained from the Lions Eye Bank of Texas (Houston, TX). Primary HCECs were cultured in 12-well plates using explants from corneal limbal rims in a supplemented hormonal epidermal medium containing 5% FBS (SHEM) using our previous methods.16 Confluent corneal epithelial cultures were switched to serum-free SHEM and treated for different time periods (4, 8, 16, 24, or 48 hours) with TLR2 ligand zymosan (1–50 ng/mL) alone or in the presence of azithromycin (1, 10, 50, μg/mL), which was added to the cultures 1 hour before or 4 hours after the inflammatory stimulus. Each experiment was repeated at least three times. The cells treated for 4 to 24 hours were lysed for total RNA extraction and mRNA expression. The conditioned media of cultures treated for 48 hours were collected and stored at −80°C for immunobead assays (Luminex; Upstate-Millipore, Billerica, MA), whereas these cells were lysed in RIPA buffer for Western blot analysis.
TLR2 and NF-κB Signaling Pathway Assay
HCECs were preincubated with azithromycin, specific TLR2 mouse antibody (10 μg/mL), or NF-κB activation inhibitor quinazoline (NF-κB-I, 10 μM) for 1 hour before the addition of zymosan for periods of 4 to 48 hours. The cells treated for 4 hours were lysed for RNA extraction and quantitative real-time PCR analysis, or cytoplasmic and nuclear proteins were extracted to detect the change of p65 intracellular location between cytoplasm and nuclei by Western blot analysis. The cells treated for 48 hours were lysed and stored at −80°C for microbead assays (Luminex; Upstate-Millipore). For immunostaining, primary HCECs were passaged into eight-chamber slides and treated for 4 or 48 hours.
Total RNA Extraction, Reverse Transcription, and Quantitative Real-time PCR
Total RNA was isolated from cells using a purification kit (RNeasy Mini kit; Qiagen) according to the manufacturer's protocol and was quantified by spectrophotometer (ND-1000; NanoDrop, Wilmington, DE) and stored at −80°C. First-strand cDNA was synthesized by RT from 1 μg total RNA using first-strand beads (Ready-To-Go You-Prime; GE Healthcare, Piscataway, NJ), as previously described.17 Real-time PCR was performed in a Stratagene Mx3005P system (Mx3005P; Stratagene, La Jolla, CA) using a 20-μL reaction volume containing 5 μL cDNA, 1 μL primers (TaqMan Gene Expression Assay; Applied Biosystems) and probes for TNF-α, IL-1β, IL-8, RANTES, MMP-1, MMP-3, MMP-9 or GAPDH, and 10 μL master mix (TaqMan Gene Expression Master Mix; Applied Biosystems). The thermocycler parameters were 50°C for 2 minutes and 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. A nontemplate control was included to evaluate DNA contamination. The results were analyzed by the comparative Ct method and normalized for GAPDH, as previously described.18
Immunofluorescence Staining
HCECs cultured on eight-chamber slides were fixed in acetone at −30°C for 5 minutes. Cell cultures were permeabilized with 0.2% nonionic surfactant (Triton X-100; Sigma-Aldrich, St. Louis, MO) in PBS at RT for 10 minutes. Indirect immunostaining was performed with our previous methods19 using primary mouse monoclonal antibodies against human MMP-1, MMP-3, or MMP-9 (1:50; R&D Systems, Minneapolis, MN) or rabbit antibody against human p65 (1:50). Species-specific AlexaFluor488-conjugated secondary antibodies were applied, and propidium iodide (PI) was used for nuclear counterstaining. Secondary antisera alone (without primary antisera), mouse isotype IgG1 control (for 3 MMPs), or normal rabbit IgG (for p65) was used as negative control. The staining was imaged with a laser scanning confocal microscope (LSCM510META; Zeiss, Thornwood, NY).
Immunobead Assay
Concentrations of TNF-α, IL-1β, and RANTES in HCEC media obtained from various experimental groups in three independent experiments were measured using bead assay (Luminex; Upstate-Millipore). Samples were pipetted into a 96-well plate and incubated overnight with 25 μL of 1× beads coupled to specific antibodies for TNF-α, IL-1β, IL-8, or RANTES. Serial dilutions of these cytokines were added to wells in the same plate to generate a standard curve. The next day, 25 μL biotinylated secondary cytokine antibody mixture was applied for 1.5 hours in the dark at room temperature. The reactions were detected with streptavidin-phycoerythrin (Luminex 100 IS 2.3 system; Austin, TX). Results of samples from the three experiments were averaged.
Western Blot Analysis
Western blot analysis was performed using a previously reported method.20 In brief, the concentration of cytoplasmic and nuclear protein samples was measured by a BCA protein assay kit, and 30 μg each sample protein was mixed with 6× SDS reducing sample buffer and boiled for 5 minutes before loading. Proteins were separated by SDS-PAGE gel and transferred electronically to PVDF membranes. The membranes were blocked with 5% nonfat milk in TTBS (50 mM Tris [pH 7.5], 0.9% NaCl, and 0.1% Tween-20) for 1 hour at room temperature and incubated with primary antibodies against MMP-1, -3, or -9 overnight at 4°C and then with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. Signals were detected with an ECL chemiluminescence reagent (GE Healthcare), and images were acquired by an image station (2000R; Eastman Kodak, Rochester, NY).
Statistical Analysis
Statistical analyses were performed (Prism v5.01; GraphPad Software, Inc., La Jolla, CA). Data were expressed as mean ± SD. The production of inflammatory mediators was compared between two groups using Student's t-test and among three or more groups by ANOVA. P < 0.05 was considered statistically significant.
Results
Stimulatory Effects of Zymosan on Production of Inflammatory Mediators by HCECs
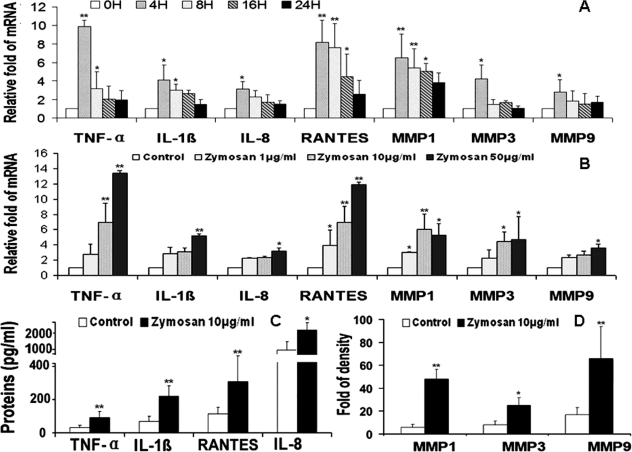
Zymosan, a yeast cell wall component, is a well-recognized TLR2 agonist.10 We hypothesized that zymosan could stimulate the production of proinflammatory mediators by corneal epithelial cells through a TLR2-mediated response. When confluent primary HCECs were treated with 10 μg/mL zymosan for different time periods (4, 8, 16, and 24 hours), the mRNA levels of proinflammatory cytokines TNF-α and IL-1β, chemokines IL-8 and RANTES, and MMPs (MMP-1, -3, and -9) increased significantly, with the peak levels observed at 4 hours, as evaluated by RT and quantitative real-time PCR (Fig. 1A). When the cells were treated with different concentrations (1, 10, and 50 μg/mL) of zymosan for 4 hours, levels of transcripts for these inflammatory mediators were found to be stimulated in a concentration-dependent fashion as shown in Figure 1B. The stimulatory effects of zymosan on production of these inflammatory mediators were confirmed at the protein levels. As shown in Figure 1C, concentrations of TNF-α, IL-1β, IL-8, and RANTES were significantly increased in the media of HCECs exposed to 10 μg/mL zymosan for 48 hours, as measured by immunobead assays (Luminex; Upstate-Millipore). The same pattern was observed for MMP-1, -3, and -9 when their Western blot band densities were measured (Fig. 1D; see Fig. 4B).
Figure 1.
Zymosan induces proinflammatory cytokines TNF-α and IL-1β, chemokines IL-8 and RANTES, and MMP-1, -3, and -9, in HCECs. (A) The time course (0, 4, 8, 16, and 24 hours) of zymosan (10 μg/mL) on the induction of mRNA expression of these seven inflammatory mediators, evaluated by RT and real-time PCR. (B) Concentration-dependent responses of mRNA levels of these seven inflammatory mediators by HCECs exposed to zymosan (1, 10, and 50 μg/mL) for 4 hours. (C) Concentration of TNF-α, IL-1β, IL-8, and RANTES in supernatants of HCECs exposed to zymosan (10 μg/mL) for 48 hours, measured by immunobead assay. (D) Levels of MMP-1, -3, and -9 by HCECs exposed to zymosan (10 μg/mL) for 48 hours, evaluated by relative density of MMP bands in Western blot analyses. Experiments were repeated three times. Values represent mean ± SD. *P < 0.05 and **P < 0.01 compared with the control group.
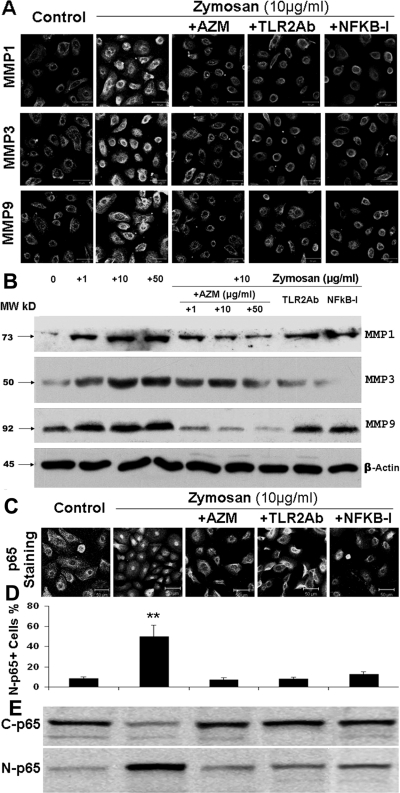
Figure 4.
Azithromycin suppresses zymosan-induced MMP production by blocking NF-κB activation. (A) Representative images of immunofluorescence staining showing stimulated production of MMP-1, -3, and -9 in HCECs exposed to 10 μg/mL zymosan for 48 hours, and suppression in the presence of TLR2Ab (10 μg/mL), NF-κB-I (5 μM), or azithromycin (AZM; 10 μg/mL). (B) Western blot analysis showing the concentration-dependent stimulation (by zymosan) and suppression (by AZM) of MMP-1, -3, and -9 with β-actin as an internal control in the condition described in (A) and (C). Representative images of immunofluorescence staining showing that NF-κB activation through p65 protein translocation from cytoplasm to nuclei was stimulated in HCECs exposed to 10 μg/mL zymosan for 4 hours and was suppressed by preincubation with TLR2Ab (10 μg/mL), NF-κB-I (5 μM), or AZM (10 μg/mL). (D) Percentages of nuclear p65-positive cells in all p65-positive cells, calculated from data in C in triplicate experiments. **P < 0.01. (E) Cells in six-well plates treated for 4 hours were subjected to cytoplasm and nuclear protein extraction for NF-κB p65 activation by Western blot analysis with 30 μg total proteins per lane. C-p65, cytoplasmic p65; N-p65, nuclear p65.
Suppressive Effects of Azithromycin on Zymosan-Stimulated Production of Proinflammatory Mediators in HCECs
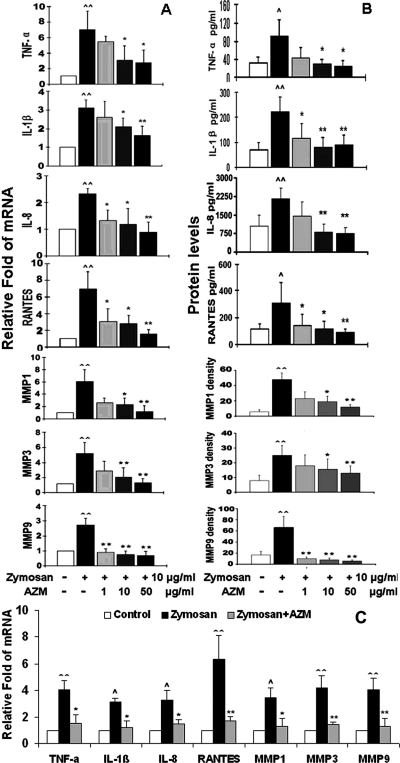
To investigate whether azithromycin, a macrolide antibiotic with immune regulatory properties, suppresses the zymosan-induced innate inflammatory response, primary cultured HCECs were pretreated with azithromycin in concentrations ranging from 1 to 50 μg/mL for 1 hour before they were challenged by zymosan 10 μg/mL for 4 and 48 hours. Interestingly, azithromycin displayed a concentration-dependent inhibitory effect on zymosan-stimulated mRNA expression of inflammatory cytokines (TNF-α and IL-1β), chemokines (IL-8 and RANTES), and MMPs (MMP-1, -3, and -9), as shown in Figure 2A and evaluated by RT and real-time PCR. This suppressive effect was further confirmed at the protein level by immunobead assay (Luminex; Upstate-Millipore) and Western blot analysis, as shown in Figure 2B (and see Fig. 4B). Furthermore, the anti-inflammatory effect of azithromycin was demonstrated after the induction of inflammation with zymosan. As shown in Figure 2C, the zymosan-stimulated mRNA levels of seven inflammatory mediators were significantly inhibited by azithromycin added to the cultures 4 hours after zymosan stimulation.
Figure 2.
Azithromycin concentration dependently suppressed zymosan-induced production of proinflammatory cytokines (TNF-α and IL-1β), chemokines (IL-8 and RANTES), and MMPs (MMP-1, -3, and -9) by HCECs. (A) Levels of mRNA transcripts, as evaluated by RT and real-time PCR, after stimulation by 10 μg/mL zymosan for 4 hours with or without preincubation of azithromycin (AZM; 1, 10, and 50 μg/mL) for 1 hour. (B) Protein levels, as evaluated by immunobead assay (TNF-α, IL-1β, IL-8, and RANTES) and relative density of MMP bands in Western blot analysis, after stimulation by 10 μg/mL zymosan for 48 hours in the absence and presence of azithromycin (AZM; 1, 10, and 50 μg/mL). (C) Levels of mRNA transcripts after stimulation by 10 μg/mL zymosan for 8 hours in the absence or presence of 10 μg/mL azithromycin in the last 4 hours of incubation. Experiments were repeated three times. Values represent mean ± SD. ∧P < 0.05 and ∧∧P < 0.01 compared with control group. *P < 0.05 and **P < 0.01 compared with zymosan-stimulated group.
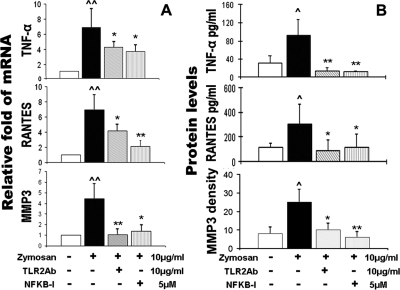
Suppressive Effects of Azithromycin on Zymosan-Stimulated Inflammation via NF-κB Signaling Pathway
NF-κB is one of the key transcription factors regulating the expression of proinflammatory genes and is known to be involved in mediating TLR2 innate responses by zymosan.10,21 To study the pathways involved in the inflammatory response stimulated by the TLR2 ligand zymosan, corneal epithelial cells were preincubated with TLR2 monoclonal antibodies (TLR2 mAb) or the NF-κB activation inhibitor quinazoline (NF-κB-I) before they were challenged by zymosan. Interestingly, the zymosan-induced increases of proinflammatory cytokine (TNF-α), chemokine (RANTES), and MMP-3, at both the mRNA (Fig. 3A) and the protein levels (Fig. 3B), were dramatically blocked by TLR2 antibody or NF-κB-I, as evaluated by real-time PCR, immunobead assay, and Western blot analysis. A similar pattern was observed in the regulation of all other proinflammatory mediators studied (data not shown). We further investigated the signaling pathway for zymosan-induced MMP stimulation. As shown in Figure 4A, immunofluorescence staining displayed the strongest fluorescence intensity for MMP-1, -3, and -9 in cells exposed to zymosan for 48 hours. The zymosan-stimulated immunoreactivity of MMPs was dramatically inhibited by TLR2 mAb, NF-κB-I, and azithromycin. Western blot analysis further confirmed that these MMP proteins were indeed stimulated by zymosan in a concentration-dependent manner, and azithromycin concentration dependently suppressed zymosan-simulated MMP production with a magnitude similar to that achieved with TLR2 mAb and NF-κB-I (Fig. 4B). Furthermore, the NF-κB activation by zymosan was demonstrated by p65 protein translocation from cytoplasm to nuclei, a phenomenon that was blocked by TLR2 mAb or NF-κB-I and by azithromycin (Fig. 4C). This result was quantitatively shown as the percentages of the nuclear p65-positive cells in all p65-positive cells in each condition, calculated from triplicate staining experiments (Fig. 4D). Western blot analysis further confirmed that NF-κB was activated by zymosan as p65 protein translocated from cytoplasm to nuclei, a process that was blocked by azithromycin, TLR2 mAb, or NF-κB-I (Fig. 4E). All these findings suggest that azithromycin suppresses the zymosan-stimulated production of proinflammatory cytokines, chemokines, and MMPs through an NF-κB signaling pathway.
Figure 3.
TLR2 neutralizing mAb (TLR2Ab) and NF-κB activation inhibitor quinazoline (NF-κB-I) blocked the zymosan-induced production of cytokine (TNF-α), chemokine (RANTES), and MMP-3 by HCECs. (A) mRNA transcript levels, as evaluated by RT and real-time PCR, after stimulation by 10 μg/mL zymosan for 4 hours with or without preincubation of TLR2Ab (10 μg/mL) or NF-κB-I (5 μM) for 1 hour. (B) Protein levels, as evaluated by immunobead assay (TNF-α and RANTES) and relative density of MMP-3 Western blot bands in HCECs, stimulated by 10 μg/mL zymosan for 48 hours in the absence and presence of TLR2Ab (10 μg/mL) or NF-κB-I (5 μM). Experiments were repeated three times. Values represent mean ± SD. ∧P < 0.05 and ∧∧P < 0.01 compared with control group. *P < 0.05 and **P < 0.01 compared with zymosan-stimulated group.
Discussion
Mucosa epithelial cells have been recently recognized to play a vital role in the initiation and regulation of immune responses. Innate immunity, the first line of defense against microorganisms, can detect the pathogen and start a rapid defensive response through pattern recognition receptors (PRRs), which detect pathogens through the recognition of pathogen-associated molecular patterns.22,23 The discovery of the TLRs, the most important family among the PRRs, is a breakthrough in the understanding of the ability of the innate immune system to rapidly recognize pathogens. In addition to innate immune cells, an array of TLRs is expressed by epithelial cells. HCECs have been shown to express functional TLR1 to TLR9, except for TLR8,24 and to be capable of recognizing a variety of microbial ligands from bacteria, fungi, and viruses. Activation of TLRs leads to the activation and recruitment of neutrophils and macrophages to sites of infection and enhances the production of antimicrobial factors. TLR-mediated induction and stimulation of human corneal epithelium-derived proallergic cytokine thymic stromal lymphopoietin and antibacterial protein peptidoglycan recognition proteins has been observed by our recent studies.25,26
Zymosan, a cell wall preparation from yeast, has been observed to trigger inflammatory responses through TLR2 in a variety of tissues and different types of cells. Production of several proinflammatory cytokines, chemokines, and MMP-9 has been found to be stimulated by zymosan.10–15 However, the effects by zymosan on the ocular surface have not been investigated. In the present study, we demonstrated that zymosan is a strong stimulator that promotes the expression and production of major proinflammatory mediators, including cytokines TNF-α and IL-1β, chemokines IL-8 and RANTES, and MMP-1, -3, and -9, in HCECs. When the HCECs were exposed to different concentrations of zymosan for increasing time periods, levels of mRNA transcripts for these inflammatory mediators were found to be stimulated at a concentration-dependent manner in a range of 1 to 50 μg/mL with peak levels reached at 4 hours. The stimulated expression of these cytokines, chemokines, and MMPs was confirmed at the protein level by immunobead assay, immunofluorescence staining, and Western blot analysis. Interestingly, the stimulation of these seven proinflammatory mediators by zymosan was significantly suppressed by preincubation with azithromycin in concentration-dependent fashion ranging from 1 to 50 μg/mL.
This is the first report of the ability of azithromycin to inhibit the production of inflammatory mediators by HCECs. TLR-mediated innate immune responses have been known to occur through activation of the NF-κB signaling pathway.24–26 In the present study, we observed that when these cells were pretreated with TLR2 antibody or the NF-κB activation inhibitor quinazoline, these stimulatory effects of zymosan on inflammatory mediators were dramatically blocked. As shown in Figures 3 and 4, evaluated by immunofluorescence staining and Western blot analysis, NF-κB was dramatically activated with p65 protein nuclear translocation in corneal epithelial cells exposed to zymosan for 4 hours. This p65 nuclear translocation was markedly blocked by TLR2 antibody and NF-κB inhibitor and by azithromycin. These findings confirmed that the stimulation of these proinflammatory mediators by zymosan in HCECs occurred through TLR and NF-κB signaling pathways. Furthermore, azithromycin was noted to be capable of suppressing NF-κB activation and suppressing zymosan-induced inflammatory responses by HCECs.
Our findings in HCECs were consistent with previous reports demonstrating that the anti-inflammatory effect of azithromycin was mediated by its NF-κB inhibitor activity in A549 human alveolar8and cystic fibrosis airway3 epithelial cells and in macrophages.7 Azithromycin was reported to inhibit the binding of activator protein-1, nuclear factor of activated T cells, and interferon consensus sequence binding protein to the DNA-binding site in the promoter site.3,7 Azithromycin was found to restrain TNF-α production by monocytes by suppressing the HSp-70- and p38-related signaling pathways to LPS stimulation.27 However, the molecular mechanisms and binding sites by which azithromycin exhibited anti-inflammatory activity have not been fully elucidated. For its antibacterial activity, azithromycin is known to inhibit bacterial protein synthesis by binding within the peptide exit tunnel of the 50S ribosomal subunit, and species-specific structural differences may account for the apparent discrepancies between the antibiotic binding modes obtained for different organisms.28,29 Anti-inflammatory activity of azithromycin may involve the interaction between bacteria and host cells, which requires further investigation.
In summary, this study provides the first evidence that the fungal component zymosan induces proinflammatory responses in the corneal epithelium through TLR2 and NF-κB signaling pathways. Our findings demonstrated that azithromycin suppresses the zymosan-stimulated production of proinflammatory cytokines TNF-α and IL-1β, chemokines IL-8 and RANTES, and MMP-1, -3, and -9, in HCECs, which may suggest the potential efficacy of this antibiotic on certain TLR-mediated ocular surface inflammatory conditions such as blepharitis, conjunctivitis, and keratitis.
Footnotes
Supported in part by National Institutes of Health Grant EY11915 (SCP), Department of Defense CDMRP PRMRP Grant FY06 PR064719 (DQL), an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation, the William Stamps Farish Fund, and Inspire Pharmaceuticals, Inc.
Disclosure: D.-Q. Li, Inspire Pharmaceuticals, Inc. (F); N. Zhou, Inspire Pharmaceuticals, Inc. (F); L. Zhang, Inspire Pharmaceuticals, Inc. (F); P. Ma, Inspire Pharmaceuticals, Inc. (F); S.C. Pflugfelder, Inspire Pharmaceuticals, Inc. (F)
References
- 1. Friedlaender MH, Protzko E. Clinical development of 1% azithromycin in DuraSite, a topical azalide anti-infective for ocular surface therapy. Clin Ophthalmol. 2007;1:3–10 [PMC free article] [PubMed] [Google Scholar]
- 2. Luchs J. Efficacy of topical azithromycin ophthalmic solution 1% in the treatment of posterior blepharitis. Adv Ther. 2008;25:858–870 [DOI] [PubMed] [Google Scholar]
- 3. Cigana C, Assael BM, Melotti P. Azithromycin selectively reduces tumor necrosis factor alpha levels in cystic fibrosis airway epithelial cells. Antimicrob Agents Chemother. 2007;51:975–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cigana C, Nicolis E, Pasetto M, Assael BM, Melotti P. Anti-inflammatory effects of azithromycin in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun. 2006;350:977–982 [DOI] [PubMed] [Google Scholar]
- 5. Ribeiro CM, Hurd H, Wu Y, et al. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well-differentiated human airway epithelia. PLoS One. 2009;4:e5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aghai ZH, Kode A, Saslow JG, et al. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of proinflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr Res. 2007;62:483–488 [DOI] [PubMed] [Google Scholar]
- 7. Yamauchi K, Shibata Y, Kimura T, et al. Azithromycin suppresses interleukin-12p40 expression in lipopolysaccharide and interferon-gamma stimulated macrophages. Int J Biol Sci. 2009;5:667–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheung PS, Si EC, Hosseini K. Anti-inflammatory activity of azithromycin as measured by its NF-kappaB, inhibitory activity. Ocul Immunol Inflamm. 2010;18:32–37 [DOI] [PubMed] [Google Scholar]
- 9. Tsai WC, Hershenson MB, Zhou Y, Sajjan U. Azithromycin increases survival and reduces lung inflammation in cystic fibrosis mice. Inflamm Res. 2009;58:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sato M, Sano H, Iwaki D, et al. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are downregulated by lung collectin surfactant protein A. J Immunol. 2003;171:417–425 [DOI] [PubMed] [Google Scholar]
- 11. Utsunomiya I, Ito M, Oh-ishi S. Generation of inflammatory cytokines in zymosan-induced pleurisy in rats: TNF- induces IL-6 and cytokine-induced neutrophil chemoattractant (CINC) in vivo. Cytokine. 1998;10:956–963 [DOI] [PubMed] [Google Scholar]
- 12. Friedland JS, Constantin D, Shaw TC, Stylianou E. Regulation of interleukin-8 gene expression after phagocytosis of zymosan by human monocytic cells. J Leukoc Biol. 2001;70:447–454 [PubMed] [Google Scholar]
- 13. Takahashi M, Galligan C, Tessarollo L, Yoshimura T. Monocyte chemoattractant protein-1 (MCP-1), not MCP-3, is the primary chemokine required for monocyte recruitment in mouse peritonitis induced with thioglycollate or zymosan A. J Immunol. 2009;183:3463–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conte FP, Barja-Fidalgo C, Verri WA, Jr, et al. Endothelins modulate inflammatory reaction in zymosan-induced arthritis: participation of LTB4, TNF-alpha, and CXCL-1. J Leukoc Biol. 2008;84:652–660 [DOI] [PubMed] [Google Scholar]
- 15. Kolaczkowska E, Chadzinska M, Scislowska-Czarnecka A, Plytycz B, Opdenakker G, Arnold B. Gelatinase B/matrix metalloproteinase-9 contributes to cellular infiltration in a murine model of zymosan peritonitis. Immunobiology. 2006;211:137–148 [DOI] [PubMed] [Google Scholar]
- 16. Kim HS, Jun SX, de Paiva CS, Chen Z, Pflugfelder SC, Li D-Q. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoon KC, de Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48:2561–2569 [DOI] [PubMed] [Google Scholar]
- 18. de Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535 [DOI] [PubMed] [Google Scholar]
- 19. Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li D-Q. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luo L, Li D-Q, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301 [DOI] [PubMed] [Google Scholar]
- 21. Ikeda Y, Adachi Y, Ishibashi K, Miura N, Ohno N. Activation of toll-like receptor-mediated NF-kappa beta by zymosan-derived water-soluble fraction: possible contribution of endotoxin-like substances. Immunopharmacol Immunotoxicol. 2005;27:285–298 [DOI] [PubMed] [Google Scholar]
- 22. Kumar A, Zhang J, Yu FS. Innate immune response of corneal epithelial cells to Staphylococcus aureus infection: role of peptidoglycan in stimulating proinflammatory cytokine secretion. Invest Ophthalmol Vis Sci. 2004;45:3513–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Unterholzner L, Bowie AG. The interplay between viruses and innate immune signaling: recent insights and therapeutic opportunities. Biochem Pharmacol. 2008;75:589–602 [DOI] [PubMed] [Google Scholar]
- 24. Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006;6:327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma P, Bian F, Wang Z, et al. Human corneal epithelium-derived thymic stromal lymphopoietin links the innate and adaptive immune responses via TLRs and Th2 cytokines. Invest Ophthalmol Vis Sci. 2009;50:2702–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ikegaya S, Inai K, Iwasaki H, Naiki H, Ueda T. Azithromycin reduces tumor necrosis factor-alpha production in lipopolysaccharide-stimulated THP-1 monocytic cells by modification of stress response and p38 MAPK pathway. J Chemother. 2009;21:396–402 [DOI] [PubMed] [Google Scholar]
- 28. Garza-Ramos G, Xiong L, Zhong P, Mankin A. Binding site of macrolide antibiotics on the ribosome: new resistance mutation identifies a specific interaction of ketolides with rRNA. J Bacteriol. 2001;183:6898–6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petropoulos AD, Kouvela EC, Starosta AL, Wilson DN, Dinos GP, Kalpaxis DL. Time-resolved binding of azithromycin to Escherichia coli ribosomes. J Mol Biol. 2009;385:1179–1192 [DOI] [PubMed] [Google Scholar]