The EOMs are particularly adaptive to changes induced by recession and tenotomy surgery, responding with modulations in fiber remodeling and myosin expression and also with changes in antagonist and contralateral muscles. These results suggest the possibility that these processes are manipulated immediately after surgery to improve surgical success rates.
Abstract
Purpose.
Surgical recession of an extraocular muscle (EOM) posterior to its original insertion is a common form of strabismus surgery, weakening the rotational force exerted by the muscle on the globe and improving eye alignment. The purpose of this study was to assess myosin heavy chain (MyHC) isoform expression and satellite cell activity as defined by Pax7 expression in recessed EOMs of adult rabbits compared with that in muscles tenotomized but not recessed and with that in normal control muscles.
Methods.
The scleral insertion of the superior rectus muscle was detached and sutured either 7 mm posterior to its original insertion site (recession surgery) or at the same site (tenotomy). One day before euthanatization, the rabbits received bromodeoxyuridine (BrdU) injections. After 7 and 14 days, selected EOMs from both orbits were examined for changes in fast, slow, neonatal, and developmental MyHC isoform expression, Pax7 expression, and BrdU incorporation.
Results.
Recession and tenotomy surgery resulted in similar changes in the surgical EOMs. These included a decreased proportion of fast MyHC myofibers, an increased proportion of slow MyHC myofibers, and increased BrdU-positive satellite cells. Similar changes were seen in the non-operated contralateral superior rectus muscles. The ipsilateral inferior rectus showed reciprocal changes to the surgical superior rectus muscles.
Conclusions.
The EOMs are extremely adaptive to changes induced by recession and tenotomy surgery, responding with modulations in fiber remodeling and myosin expression. These adaptive responses could be manipulated to improve surgical success rates.
Strabismus is a common disorder of ocular alignment occurring in 2% to 5% of the children in the United States.1,2 Many patients can be managed medically with eye patching and glasses, but a large percentage require surgical intervention over the course of their lifetimes. Strabismus surgery attempts to restore binocular alignment by altering relative extraocular muscle (EOM) length and mechanical advantage. For example, resection surgery involves removal of a portion of what is considered an underacting muscle, and the shortened muscle is sutured to its original site of insertion. By contrast, recession surgery involves moving the insertion of what is considered an overacting muscle to a position posterior to its original site of attachment to the sclera.
A large proportion of strabismus surgeries fail. In companion studies of long-term follow-up of surgical outcomes in patients with either congenital esotropia or exotropia, more than 50% of both cohorts ultimately developed a misalignment of 10 prism diopters or more after the first surgery.3,4 In another study of surgical treatment for childhood esotropia, of the group that had been successfully aligned to within 10 prism diopters, 21% developed consecutive exotropia.5 In those patients who required multiple surgeries for successful alignment, the incidence of developing consecutive exotropia increased to 62%. The surgical failure rate is not surgeon or procedure based.6
One of the complexities in treating strabismus is that its etiology is not understood. Although risk factors associated with increased incidence of strabismus have been described,7 and several genes have been identified that cause rare forms of congenital cranial dysinnervation disorders,8 the underlying causes of the most common forms of strabismus are unknown. Recent studies examining muscles from patients with inferior oblique overaction, as defined by eye position in adduction, showed that the muscles had distinct histologic phenotypes. This finding suggests that there are likely to be multiple underlying causes of EOM dysfunction that are quite distinct from one another.9 Other studies demonstrate that the EOMs from strabismus patients appear to adapt to cues received from the ocular motor system in the brain that control their motor behavior,10,11 as their satellite cell populations are in a constant state of flux. These studies suggest that the EOMs react to surgical interventions and that these adaptations could be assessed and potentially manipulated with a goal of improved surgical outcomes.
In a recent study, we examined the effect of resection surgery on adult rabbit EOMs.12 We found that this muscle-shortening procedure resulted in a significant increase in satellite cell activation and their rapid fusion into existing myofibers. This result was present in both the actively stretched, resected muscle and in its passively stretched, antagonist muscle. In the present study, two muscle adaptations to surgery were examined in detail: the expression patterns of the myosin heavy chain (MyHC) isoforms and the reactions of the muscle regenerative cells, the myogenic precursor cells. Changes in MyHC isoforms were examined in both treatment arms (recession versus tenotomy), as these changes are associated with control of muscle shortening velocity and fatigability. In addition, as a means of assessing the rate of myofiber remodeling, both Pax7-positive muscle precursor cells and dividing cells in the satellite cell position were quantified; these are standard methods used to identify this muscle-regenerating population.10–12 An understanding of how a surgically altered EOM adapts may suggest potential interventions that could prolong the positive effects of surgery or alternatively could suggest ways that these changes may be prevented or magnified in an effort to maximize surgical effectiveness.
Materials and Methods
This study was performed on adult New Zealand White rabbits that were housed in an AALAC-approved facility at the University of Minnesota. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the animal care policy of the National Institutes of Health.
Rabbits were anesthetized with an intramuscular injection of ketamine and xylazine (1:1, 10 and 2 mg/kg, respectively). One set of 12 rabbits was subjected to recession surgery. One randomly selected superior rectus muscle in each animal was detached from the sclera. The muscle insertion was recessed 7 mm and sutured to the sclera with Vicryl 7-0 (polyglactin 910; Ethicon, Somerville, NJ). A second set of 10 rabbits was subjected to a tenotomy procedure, in which one randomly selected superior rectus muscle was detached from the sclera and then sutured directly to the site of its original insertion. A third set of six adult rabbit superior rectus muscles that had undergone no surgical interventions served as control muscles. Immediately after surgery, tobramycin-dexamethasone combination ointment (Alcon, Fort Worth, TX) was applied to the conjunctival cul-de-sac. One week after surgery, six recessed and six tenotomized rabbits each received intraperitoneal injections of BrdU in sterile isotonic saline every 2 hours for 12 hours, at a dose of 50 mg/kg body weight. Similarly, 2 weeks after surgery, BrdU injections were performed in the second group of rabbits. Twenty-four hours after the last injection, the animals were euthanatized by an overdose of barbiturate anesthesia. All superior and inferior rectus muscles were removed, embedded in tragacanth gum, frozen in 2-methylbutane chilled to a slurry on liquid nitrogen, and stored at −80°C until processed.
Cryostat sections were prepared at 12 μm, and serial sections were immunostained for one of the following molecules: fast (1:40), slow (1:40), developmental (1:20), and neonatal (1:20) MyHC isoforms (Vector Laboratories., Burlingame, CA); Pax7 (1:100; Hybridoma Bank, Iowa City, IA); BrdU (1:20,000; Sigma, St. Louis, MO); or dystrophin (1:20; Vector Laboratories). Immunostaining used standard methods.12 Briefly, the sections were blocked with 10% normal horse serum; incubated for 1 hour in primary antibody at room temperature, followed by sequential incubation in reagents from the ABC kit (VectaElite; Vector Laboratories), labeled with peroxidase; and developed with diaminobenzidine with heavy metals. In addition, sections were stained with hematoxylin and eosin and used for myofiber cross-sectional area measurements. The percentage of cells positive for each MyHC isoform as well as the mean cross-sectional areas were determined by manual tracing of myofibers with morphometry software (Nova Prime; Bioquant, Nashville, TN). We counted Pax7-positive satellite cells and BrdU-labeled satellite cells, defined positionally in relation to dystrophin double-staining, by using our standard methodology.13 Briefly, the proportion of labeled satellite cells, as defined by Pax7 staining or by positional localization of BrdU-labeling, as a percentage of the number of myofibers in a given microscopic field was calculated. For all measurements, data were collected in a minimum of four random fields in at least three separate cross sections within both the orbital and global layers of six rectus muscles and six control muscles for the recessed and four muscles each for the tenotomized muscles at each of the postsurgical survival times. Sections were chosen close to the insertional tendon and in the mid-belly region of the analyzed muscles. Thus, six slides were analyzed per muscle per staining parameter. All data are presented as the mean ± SEM. The percentage of positive cells was compared between the orbital and global layers and analyzed for statistical significance by using either an unpaired two-tailed t-test (if two groups were being compared) or an analysis of variance (ANOVA) and Dunn's multiple comparison tests (Prism and Statmate software; GraphPad, San Diego, CA) for multiple group comparisons. An F-test was used to verify that the variances were not significantly different. Data were considered significantly different if P < 0.05.
Results
Neither recession nor tenotomy surgery in adult rabbits resulted in any overt changes in eye alignment. This outcome was expected on the basis of multiple previous studies of recession or resection surgery performed in small laboratory animals.
Fast and Slow MyHC Isoform Adaptations
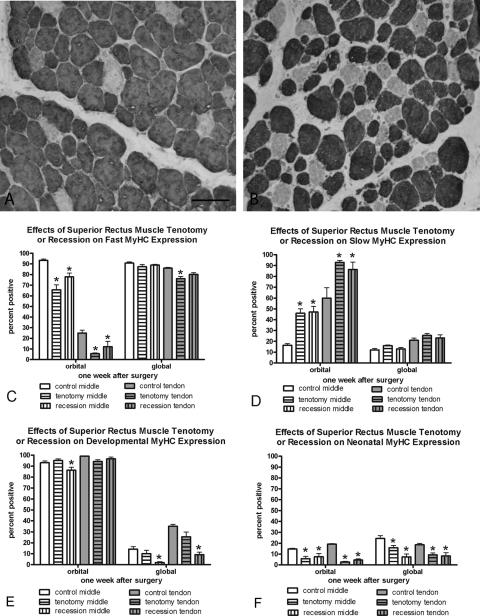
Examination of MyHC isoform expression demonstrated a significant adaptation to surgery in the orbital muscle layers after both tenotomy and recession surgery. With either procedure, there was a significant reduction in the percentage of myofibers expressing the fast MyHC isoform and a significant increase in the percentage of myofibers expressing the slow MyHC isoform (Figs. 1, 2, Table 1) at both 1 and 2 weeks. This result was seen in both the middle and tendon regions of the operated superior rectus muscles. There tended to be a lesser effect on fast and slow MyHC expression in the global layer at 1 week after surgery, but there were significant adaptations in fast and slow MyHC isoform expression in the global layer at 2 weeks after recession surgery.
Figure 1.
(A) Control superior rectus muscle global layer immunostained for the fast MyHC isoform. (B) Superior rectus muscle global layer 2 weeks after recession surgery immunostained for the fast MyHC isoform. Bar, 50 μm. (C–F) Morphometric analysis of the effect of recession and tenotomy surgery on the MyHC isoform expression patterns 1 week after surgery compared with normal untreated control superior rectus muscles. *Significantly different from the control. Data are expressed as the mean ± SEM.
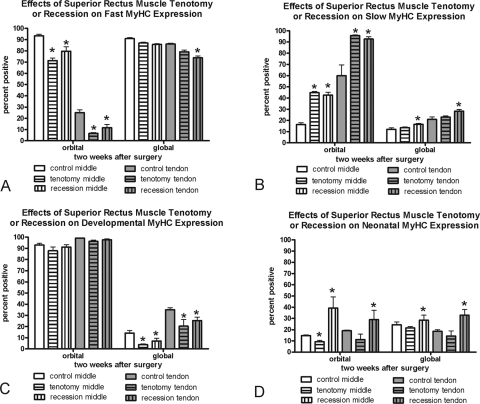
Figure 2.
Morphometric analysis of the effect of recession and tenotomy surgery on the MyHC isoform expression patterns 2 weeks after surgery compared with patterns in normal untreated control superior rectus muscles. *Significantly different from the control. Data are expressed as the mean ± SEM.
Table 1.
Summary of Changes from Surgery Compared to Controls
| Tenotomy |
Recession |
|||||||
|---|---|---|---|---|---|---|---|---|
| Orbital Middle | Global Middle | Orbital Tendon | Global Tendon | Orbital Middle | Global Middle | Orbital Tendon | Global Tendon | |
| One Week | ||||||||
| Fast |  |
 |
 |
 |
 |
|||
| Slow |  |
 |
 |
 |
||||
| Dev |  |
 |
 |
|||||
| Neo |  |
 |
 |
 |
 |
 |
 |
 |
| Pax7 |  |
 |
 |
 |
||||
| Brdu |  |
 |
 |
 |
 |
 |
 |
 |
| Two Weeks | ||||||||
| Fast |  |
 |
 |
 |
 |
|||
| Slow |  |
 |
 |
 |
 |
 |
||
| Dev |  |
 |
 |
 |
||||
| Neo |  |
 |
 |
 |
 |
|||
| Pax7 |  |
 |
 |
|||||
| Brdu |  |
 |
 |
 |
 |
 |
 |
 |
Developmental and Neonatal MyHC Isoform Adaptations
Developmental and neonatal MyHC isoform adaptations also occurred after recession and tenotomy surgery (Figs. 1, 2; Table 1). At 1 week, no change in the percentage of myofibers positive for developmental MyHC isoform was seen in the orbital layers except for a significant decrease in the mid region of the recessed muscles. In the global layer of the recessed superior rectus muscles, however, there were significant reductions in the proportion of myofibers positive for developmental MyHC in both middle and tendon regions. This reduction was seen after both 1 and 2 weeks. Tenotomy surgery also resulted in global layer reductions in developmental MyHC myofiber frequency, but only at 2 weeks after surgery. Neonatal MyHC isoform expression was highly adaptable over the 1 and 2 weeks after recession. One week after recession surgery all layers and all regions of the recessed muscles had decreased neonatal MyHC, whereas at 2 weeks all layers had significantly increased levels compared with those in normal control muscles (Figs. 1, 2). One week after tenotomy, all layers in all regions showed significantly decreased frequency of myofibers positive for the neonatal MyHC. However, by 2 weeks after tenotomy, all layers had returned to normal levels of expression of neonatal MyHC isoform except the orbital mid region (Fig. 2).
Mean Cross-Sectional Areas of Recessed and Tenotomized Superior Rectus Muscles
When examined at the distal tendon region, myofiber cross-sectional areas were fairly heterogeneous, and pockets of very large myofibers could be seen in some of the specimens (Fig. 3). However, when immunostained for dystrophin, at 1 week after recession surgery there were extensive areas at the very end of the tendon where the dystrophin ring of individual myofibers was reduced in thickness and intensity of staining compared with normal (Fig. 6A). By 2 weeks after recession surgery, the dystrophin returned to its normal staining intensity. Presumably, this is an effect of the initial loss of muscle tension due to unloading after recession.
Figure 3.
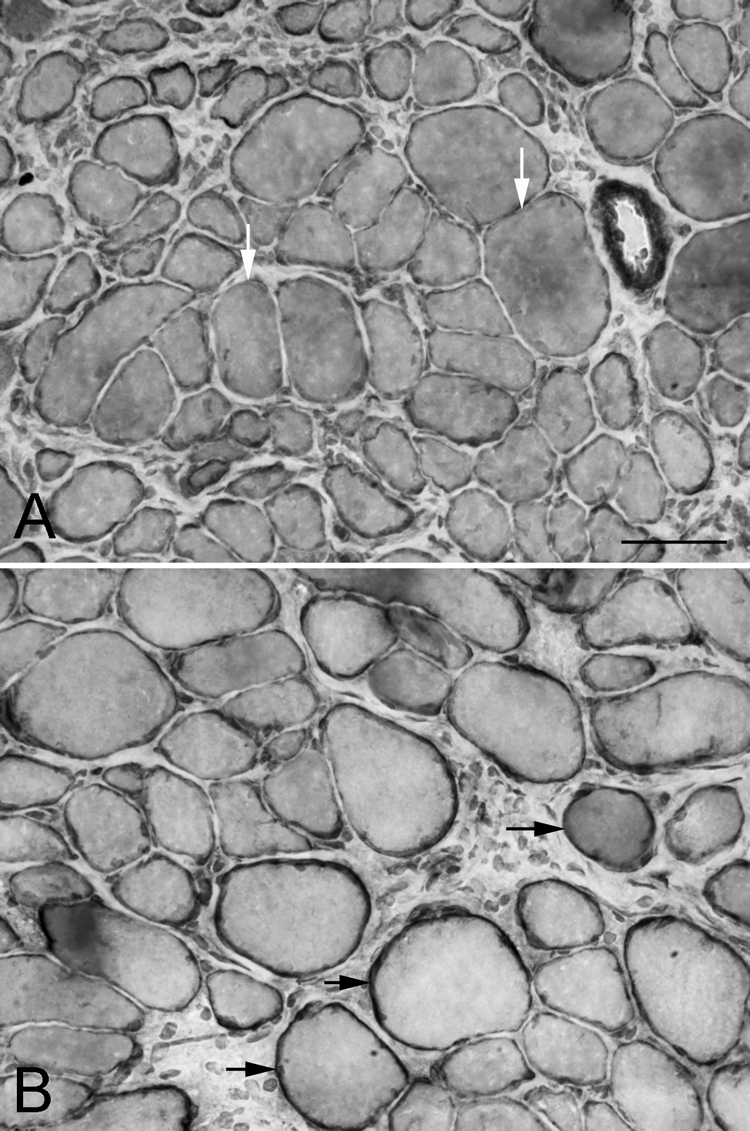
Global layer of the superior rectus (A) 1 and (B) 2 weeks after surgical recession, immunostained for dystrophin. White arrows: myofibers in cross-section with only a thin rim of dystrophin immunostaining; black arrows: myofibers with a normal rim of dystrophin staining. Bar, 50 μm.
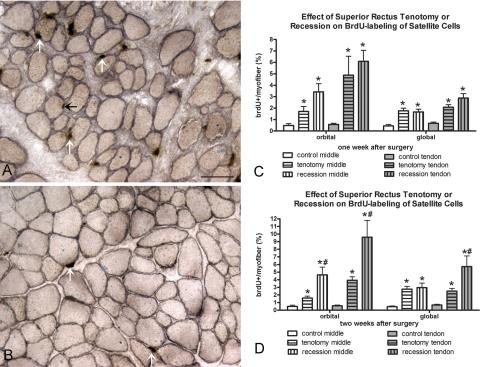
Figure 6.
(A) Recessed and (B) control superior rectus muscle immunostained for BrdU and dystrophin. Black arrow: a BrdU-positive myonucleus. White arrows: a BrdU-positive cell in the satellite cell position. Bar, 50 μm. Morphometric analysis of frequency of BrdU-positive cells 1 (C) and 2 (D) weeks after recession and tenotomy surgery compared with the number in normal control superior rectus muscles. *Significantly different from the control. Data are expressed as the mean ± SEM.
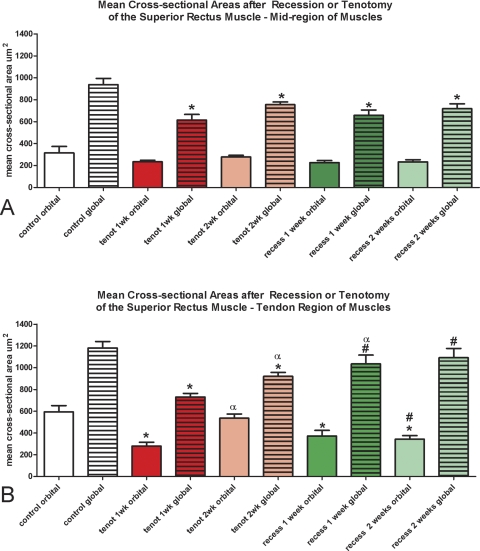
Mean cross-sectional areas were determined for all myofibers. No changes were seen in cross-sectional areas in the orbital layer in the middle of the muscles (Fig. 4A). In the mid region of the muscles, contrary to expectations, the myofibers in the global layer of the recessed muscles were significantly smaller in cross-sectional area than in the control muscles (Fig. 4A). The same was true of the tenotomized muscles (Fig. 4A). In the orbital layer in the tendon region of the muscles, myofibers were significantly smaller than in the control at 1 and 2 weeks after both the recession and the tenotomy surgeries except for 2 weeks after tenotomy (Fig. 4B). In the global layer in the tendon region of the muscles (Fig. 4B), only tenotomy resulted in a significantly decreased mean myofiber cross-sectional area after both 1 and 2 weeks. In contrast, after recession surgery there was no statistically significant difference from controls.
Figure 4.
Morphometric analysis of the effect of recession and tenotomy surgery on mean myofiber cross-sectional areas 1 and 2 weeks after surgery compared with normal untreated control superior rectus muscles. Red: tenotomized muscle; green: recessed muscles. *Significantly different from the control. #Significantly different from tenotomy. αSignificantly different compared with 1 week. Data are expressed as the mean ± SEM.
One striking difference between the mean myofiber cross-sectional areas after recession compared with tenotomy is that, in the tendon region, the decreases in myofiber size after recession surgery were stable over the 2 weeks after the surgery injection period. After tenotomy surgery, the myofibers were all significantly larger after 2 weeks than they were 1 week after the surgery.
Satellite Cells as Identified by Pax7
One week after tenotomy surgery, there was a significant increase in the number of myofibers with an associated Pax7-positive satellite cell in the orbital and global layers in the tendon region of the muscles (Fig. 5, Table 1). Only the tendon region of the orbital layer of tenotomized muscles showed an increase in Pax7-positive cells 2 weeks after surgery. In contrast, the recessed muscles showed an increased number of Pax7-positive cells only in the tendon regions of the orbital layer of the muscles after 1 week and the tendon region of both the orbital and global layers after 2 weeks. It should be noted that Pax7 is considered to be a marker of quiescent satellite cells, and thus this analysis does not include all myogenic precursor cell populations that are found in EOMs.
Figure 5.
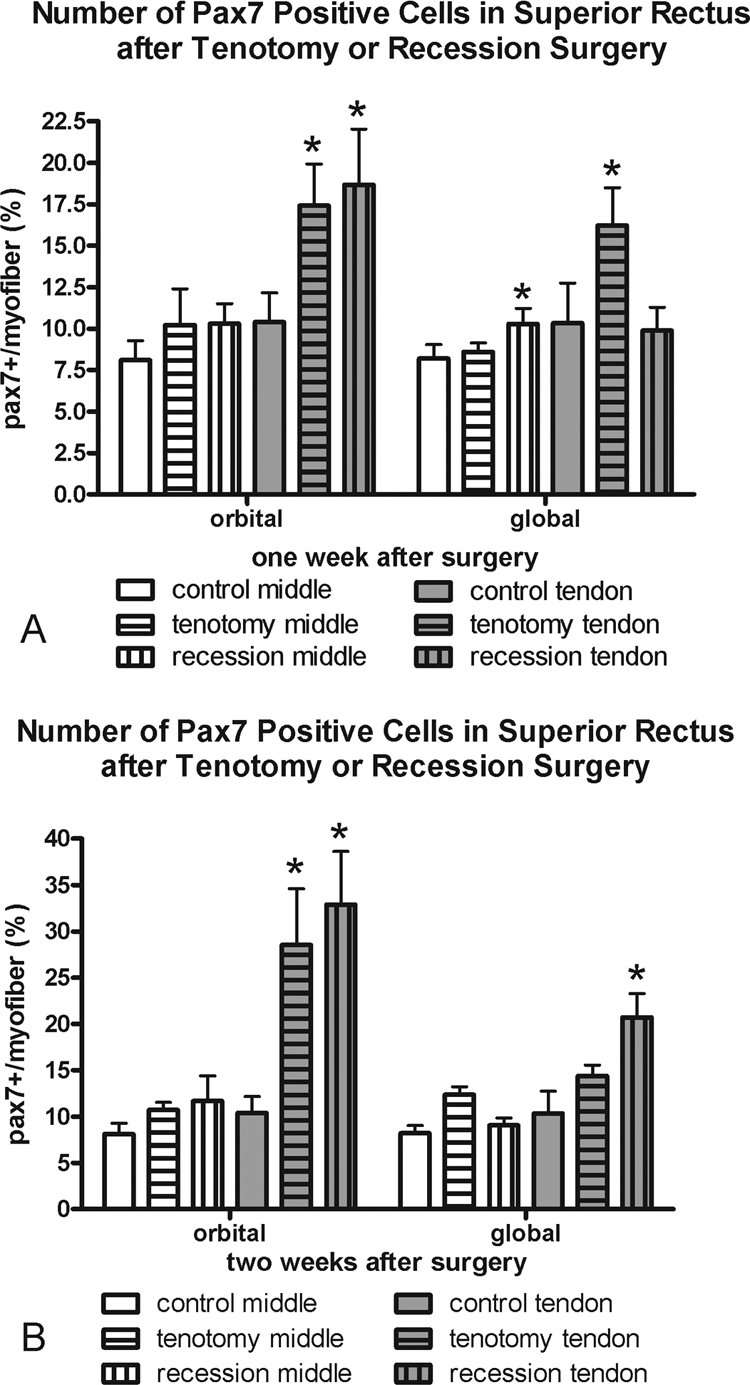
Morphometric analysis of the frequency of Pax7-positive cells (A) 1 and (B) 2 weeks after recession and tenotomy surgery compared with normal control superior rectus muscles. *Significantly different from the control. Data are expressed as the mean ± SEM.
BrdU-Labeled Satellite Cells
BrdU uptake demonstrates DNA replication during the period of systemic exposure. Any labeled cell had to incorporate the BrdU within the 24 hours before termination (Fig. 6, Table 1). Both the orbital and global layers in the middle and tendon regions of muscles after tenotomy or after recession surgery had a significantly elevated number of BrdU-positive cells in the satellite cell position of the muscles. Increases were seen at both 1 and 2 weeks after surgery (Fig. 6). Only after 2 weeks were there significantly more BrdU-positive satellite cells in the tendon end of both layers of the recessed muscles than in the tenotomized muscle.
Antagonist Muscle Alterations: Ipsilateral Inferior Rectus Muscles
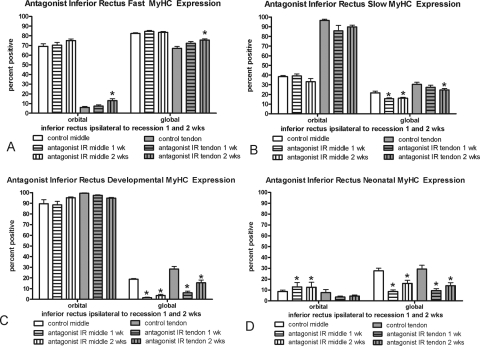
The effect of superior rectus muscle recession or tenotomy surgery on the inferior rectus (IR) of the surgical globe was also examined (Fig. 7). Similar to the surgical superior rectus muscles, there was a significant decrease in myofibers positive for developmental and neonatal MyHC in the global layer. However, in contrast to the adaptations of the operated superior rectus muscles, the antagonist IR expressed greater levels of fast MyHC in all layers, although this was significant only in the orbital and global tendon layers at 2 weeks, and fewer myofibers positive for slow myosin, particularly in the global layers at the 2-week point. No alterations in satellite cells positive for Pax7 or BrdU were seen in the IR muscles compared with the normal control muscles, except for the middle global layer at 1 week (Figs. 8, 9).
Figure 7.
Morphometric analysis of the MyHC isoform expression patterns in the layers of the antagonist inferior rectus muscle 1 and 2 weeks after surgical alteration of the ipsilateral superior rectus muscle compared with patterns in normal untreated control superior rectus muscles. *Significantly different from the control. Data are expressed as the mean ± SEM.
Figure 8.
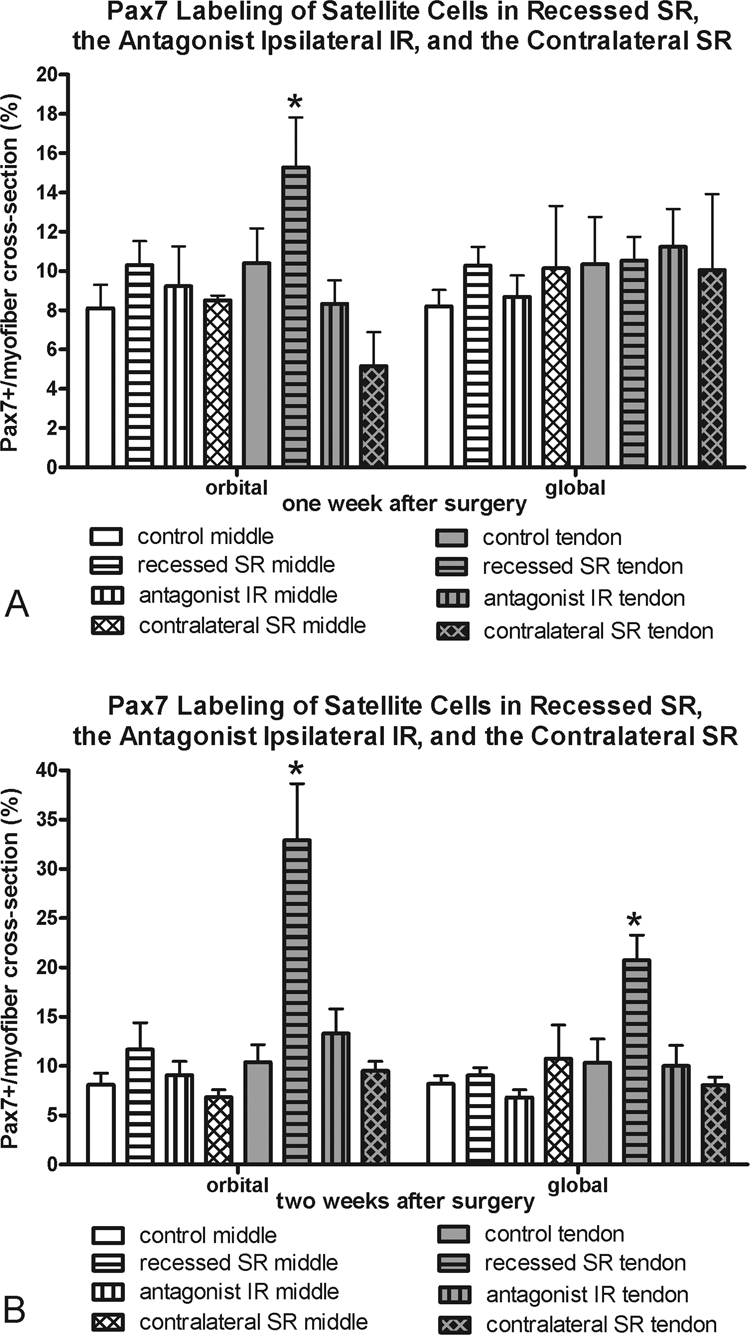
Morphometric analysis of frequency of Pax7-positive cells at (A) 1 and (B) 2 weeks in the recessed SR, the antagonist IR, and the contralateral SR compared with the frequency in normal control superior rectus muscles. *Significantly different from the control. Data are expressed as the mean ± SEM.
Figure 9.
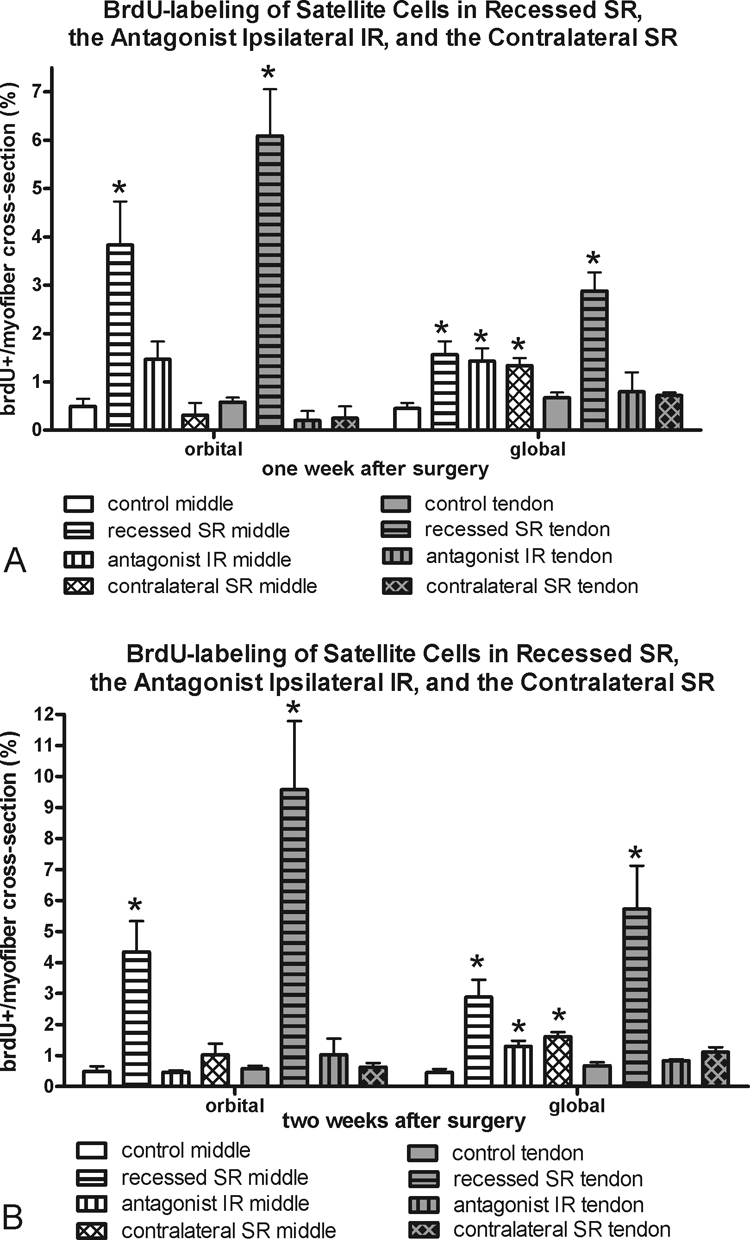
Morphometric analysis of frequency of BrdU-positive cells at (A) 1 and (B) 2 weeks in the recessed SR, the antagonist IR, and the contralateral SR compared with the frequency in normal control superior rectus muscles. *Significantly different from the control. Data are expressed as the mean ± SEM.
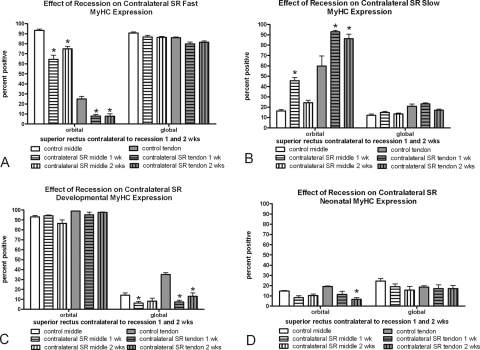
Contralateral Superior Rectus Muscle Alterations
The superior rectus contralateral to the recessed muscle was examined, and the MyHC isoform changes seen in the recessed superior rectus muscle were somewhat mirrored (Fig. 10). In the orbital layers only, significant decreases in fast-MyHC–positive myofibers and significant increases in slow-MyHC–positive myofibers were present at 1 and 2 weeks after surgery. No significant changes were seen in the fast and slow myofiber frequency in the global layers of these muscles. However, there was a decrease in myofibers positive for developmental MyHC in the global layer 1 week after the contralateral surgery. No statistically significant changes were seen in neonatal MyHC expression compared with normal control muscles, except in the orbital layer 2 weeks after surgery. Again, no changes in satellite cells as identified by Pax7 or BrdU were seen in the contralateral superior rectus muscles compared with the normal control specimens, except for the middle global layer at both 1 and 2 weeks (Figs. 8, 9).
Figure 10.
Morphometric analysis of the MyHC isoform expression patterns in the non-operated superior rectus muscle contralateral to a superior rectus muscle recession 1 and 2 weeks after surgery compared with the patterns in normal untreated control superior rectus muscles. *Significantly different from the control. Data are expressed as the mean ± SEM.
Discussion
The recessed and tenotomized superior rectus (SR) muscles showed rapid changes in expression of MyHC isoforms and in the myogenic precursor pool. In addition, the nonsurgically altered ipsilateral antagonist IR and contralateral SR showed coordinated changes compared with those seen in the altered SR muscles. These changes further support the view that EOMs rapidly adapt to perturbations.14,15 Recession surgery and tenotomy seem to be relatively minor perturbations of EOM homeostasis. As is clear in the present results, however, recession and tenotomy surgery caused acute changes in both myofiber MyHC isoform composition and satellite cell populations. These changes were seen across agonist–antagonist pairs and, remarkably, in the contralateral orbit, and they may provide insight into the high rate of failure of surgical treatment for strabismus.
Changes in Recessed or Tenotomized Superior Rectus Muscles
Recession involves moving the insertional tendon posteriorly, decreasing the resting tension on the muscle and its antagonist and decreasing the slack length of the muscle; this approach reduces its rotational force. By way of contrast, tenotomy, which involves transection of the insertional tendon followed by the resuturing of the tendon to the original scleral insertion site, should not alter the resting tension or lever arm of the muscle and, therefore, should not alter eye position. Tenotomy, however, would cause surgical trauma similar to that in recession surgery and should act as a surgical control for observed changes after recession surgery. Because MyHC composition is exquisitely sensitive to changes in resting tension on skeletal muscle, it is surprising that both recession and tenotomy surgery caused remarkably similar changes in MyHC isoform expression patterns after 1 week, decreasing fast and neonatal myofiber percentages and increasing slow myofiber frequency. Decreases in both fast and neonatal MyHC content would be predicted to result in a decrease in the shortening velocity of the affected muscles.16,17 Our findings suggest that surgical trauma alone is sufficient to induce changes in MyHC isoform composition. Changes unique to the recessed muscles may be the result of muscle unloading. Future studies will focus on whether the surgical muscles retain these changes or ultimately return to normal MyHC proportions over the months that follow surgery. Previous studies of skeletal muscle unloading support the reversibility of these MyHC changes.18 Normalization of MyHC composition with time is probably a consequence of muscle length adaptation.
The nonuniform changes in MyHC isoforms in the middle and tendon regions are quite evident from the data in Table 1. This effect has been described after load alterations in limb skeletal muscle.19 As the EOMs express significantly different MyHC isoforms in the middle and tendon ends relative to other skeletal muscles,20 the cell biological controls over localized gene and protein expression within individual myonuclei in muscle fibers would presumably control perturbation-related changes as well. This local control, even within myonuclear domains of individual myofibers, is evident when the timing of transcriptional activation of individual myonuclei is considered. Individual myofiber nuclei are not all active simultaneously but rather appear to be independently regulated.21 In the EOMs, with its extremely complex MyHC isoform expression patterns compared with limb skeletal muscle, this localized control appears to be even more dynamic.
Recession surgery is the unloading of the operated EOM. Experimental unloading of limb skeletal muscles, by hindlimb suspension or spaceflight, for example, causes significantly different changes in fast and slow muscles. Unloading generally causes predominantly slow muscles to shift toward increased percentages of myofibers expressing fast myosins,22 whereas predominantly fast muscles tend to shift toward increased percentages of myofibers that express the slower isoforms.23,24 Other muscles, such as extensor digitorum longus (EDL), showed no changes in MyHC isoform expression patterns after hindlimb suspension.25 These complex changes in MyHC phenotype with unloading suggest that each muscle is affected by different activity-independent influences.
Qualitative changes in dystrophin immunostaining were described in limb muscles after hindlimb suspension, again with muscle-specific alterations. In soleus, dystrophin levels significantly decrease after unloading, whereas in EDL these levels increase.25 Slow myofibers contain more dystrophin than do fast myofibers,26 leading to the hypothesis that unloading skeletal muscles shifts them toward characteristics midway between the fast and slow phenotypes. Biphasic EOM changes in dystrophin levels in the present study mimic those seen in unweighted limb skeletal muscles. Dystrophin is critically important during force transmission, both longitudinal and transverse, protecting the muscle fiber from contraction-induced injury. This suggests that the operated EOM would be prone to contraction-induced injury.
Changes in Satellite Cells in Tenotomized and Recessed Superior Rectus Muscles
Generally, significant increases in the number of satellite cells positive for Pax7 were seen after each surgical intervention, mainly focused at the tendon end. In contrast, an increased number of BrdU-positive cells in the satellite cell position were seen in all muscles regions after both surgeries. As with Pax7, the largest percentage changes were in the tendon regions, closest to the actual incision sites. Although finding increased BrdU-positive cells in the satellite position in the mid region of the superior rectus muscles was somewhat surprising, similar changes in satellite cell reactivity in proximal regions of EOM after localized growth factor treatment have been reported.27 In comparison to the effect of resection on rabbit superior rectus muscles,12 recession surgery resulted in fewer BrdU-positive myonuclei as well as delayed upregulation compared with the rapid increase after resection surgery.
It is interesting to note that botulinum toxin injections into adult rabbit superior rectus muscle, a treatment that causes functional denervation and muscle paralysis, also results in significant upregulation in the number of activated satellite cells.14 Although it does not produce a functional paralysis, recession increases the slack on the operated muscle, which should result in decreased muscle force generation when stimulated to contract. Although it may seem counterintuitive that muscle slack or paralysis would increase the processes involved in myofiber remodeling, in contrast to limb skeletal muscles, EOMs do not atrophy under these conditions.28,29 This suggests that the mechanisms that control EOM phenotype, whether intrinsic, from within the EOM, or extrinsic, from the ocular motor control system, play a strong role in maintaining myofiber homeostasis. Satellite cell proliferation in limb skeletal muscles is also altered by unloading.30 However, satellite cell proliferation quickly ends in unweighted limb muscles; the population is significantly reduced throughout the entire muscle length31; and significant muscle atrophy results. The ability of EOMs to maintain a proliferating satellite cell population after unloading and functional denervation helps explain both the maintenance of myofiber cross-sectional area after botulinum toxin and its quick restoration after recession surgery, as was seen in the present study. Even 15 months after an experimental trochlear nerve section in adult monkeys, the orbital layer is relatively spared.32 These studies suggest that strong intrinsic signals control myofiber maintenance in EOM.
In the present study, all the surgical manipulations were performed on otherwise normal EOMs. It may be that the effects of resection or recession surgery in the EOMs of a strabismic patient, whether the muscles are overacting or underacting, are completely different. The differential reactivity of satellite cell populations in surgical specimens from strabismus patients with the same diagnosis supports the hypothesis that individual EOM responses to perturbations differ from each other and from control EOM.10,11
In overacting inferior oblique muscles, there are elevated numbers of activated satellite cells.10 In overacting patient medial rectus muscles there are decreased numbers of activated satellite cells, and in underacting muscles, these numbers increase.11 Satellite cell reactivity in EOMs under all these conditions may reflect differences between patients that are significant and difficult to control: age at time of surgery, the duration of strabismus from time of onset to time of surgery, whether previous surgery had been performed, hormonal state,15 efferent tonus, and so forth. In addition, despite similarity in final eye position, the actual etiology in different patients may in fact be decidedly different,33 and may contribute to surgical failure after strabismus surgery. A recent study of inferior oblique muscles from patients diagnosed with “inferior oblique overaction” supports this hypothesis, where at least two distinct morphologic phenotypes were observed and correlated to two distinct diagnoses.9 Future studies will address this issue.
Changes in MyHC Isoforms in the Non-operated Inferior and Superior Rectus Muscles
It is interesting that the inferior rectus in the same orbit showed trends in fast and slow MyHC expression that were opposite those in the surgically treated superior rectus muscles. More striking is that the superior rectus on the side contralateral to the recession or tenotomy, where no surgical intervention occurred, showed changes similar to those in the recessed and tenotomized muscles. This demonstrates that alteration in the length/tension of a single EOM in one orbit substantially affects muscles in both orbits. The mechanisms for these changes are not understood and are a subject for conjecture. A previous physiologic study demonstrated that recession surgery results in decreased muscle tension in the nonsurgical antagonist muscle at 3 weeks, with a return to normal muscle tension 6 weeks after surgery.34 This outcome may be the result of muscle length adaptation and/or binocular and/or vestibular eye movement control mechanisms. Although rabbits have only a limited binocularity, the vestibular system also plays an important role in controlling eye position and coordinated eye movements.35 Conjugate eye movements necessitate the coordinated control of agonist/antagonist muscle pairs by the central nervous system. This interplay has been demonstrated experimentally. For example, microstimulation of the oculomotor nucleus in a region containing both motor neurons and interneurons results in a large twitch of the ipsilateral eye and a smaller but concomitant twitch of the contralateral eye.36 Relative to vertical eye movements, burst neurons within the rostral interstitial nucleus of the medial longitudinal fasciculus project to motor neurons such that they are unilateral to depressor muscles but bilateral to elevator muscles.37 Most abducens motor neurons responsible for driving the lateral rectus muscle are tuned to movement of the ipsilateral eye, but the other 40% are tuned to movement of the contralateral eye.38 However, the feedback signal that controls eye position is a matter of some debate. Evidence suggests that correct eye position is the result of visual processing, vestibular input, and learning,39 but consists of nonretinal feedback as well.35,40 Despite a lack of clear understanding of the feedback systems that allow the brain to “sense” changes in the effector organ, concomitant changes in the ipsilateral but unaltered inferior rectus muscles and in the contralateral but unaltered superior rectus muscles suggest that these feedback systems are sensitive and rapid. It also appears that patterns of coordinated oculomotor control are conserved, even in animals that are not binocular, such as the rabbit.
Changes in Satellite Cells in the Non-operated Inferior and Superior Rectus Muscles
In contrast to an increased number of Pax7-positive satellite cells in the tendon region of the recessed and tenotomized muscles, no changes in the Pax7 satellite cell frequency were seen in either the antagonist inferior rectus muscle or the contralateral superior rectus muscle at either posttreatment time point. BrdU-labeled satellite cells were elevated in the global middle region of both antagonist IR and contralateral SR, but interestingly not in the tendon region. Thus, whatever signal causes satellite cell activation is mainly directed at the mid region of the nonsurgical muscles, suggesting that it is related to specific neural influences. In a previous study examining the effect of resection surgery in rabbits,12 significant increases in activated satellite cells were evident in the antagonist IR; this effect was not seen in the present study.
EOM Tendons
The similarity between changes after recession and tenotomy surgery suggest that they may be due to signaling from tissues directly injured at the incision site. This could include the tendon itself, the muscle fiber ends, blood vessels, sensory nerves, and specialized endings within this region. Results in most studies agree that the scleral insertions of EOMs do not contain muscle fibers and are connective tissue only, so signaling due to release of factors from directly injured muscle fibers is unlikely. Rabbit EOMs do not have Golgi tendon organs, nor do they have muscle spindles. Palisade endings, which are found at the site of myofiber ends within the proximal part of the insertional tendon, are motor and not sensory,41 suggesting that these proprioceptive endings are not a source of eye position signals to the brain. Primary afferents from the trigeminal ganglion project to individual EOMs,42 yet bilateral deafferentation in normal adult primates has no effect on eye alignment or movements, including conjugacy, either acutely or chronically.43
Recent studies suggest that fibroblasts in EOM differ from those in limb muscles.44 It may be that the injured tendon releases signaling factors that play a role in EOM adaptations after recession and tenotomy surgery. Hindlimb suspension studies support this notion as, after unloading, both insulin growth factor-I and mechanogrowth factor increase in the muscle tendons.45 Increased expression of either factor would result in significant alterations in EOM properties.47 Further studies are needed to determine the molecular control of EOM adaptations to tenotomy and recession surgery.
Conclusions
In summary, recession surgery causes several adaptive changes in the operated superior rectus muscle after 1 and 2 weeks. Tenotomy surgery, similar to the recession surgery except that the muscle insertion is not moved, causes adaptive changes similar to those in the operated superior rectus muscle, despite the presumed maintenance of normal muscle length–tension parameters. Relative to MyHC adaptations, coordinated changes were seen in the antagonist IR and contralateral SR muscles. MRI demonstration of hypertrophy of the inferior rectus ipsilateral and superior rectus contralateral to a superior oblique palsy provides further support for these types of coordinated changes in the EOM in disease and after surgery.48,49 These studies reinforce the concept that the EOMs are highly adaptable to the changing demands on them, whether through innervational changes or surgical interventions. The success rates for the treatment of strabismus may be improved by a method or methods that modulate the strong signals to retain the status quo within the EOMs.
Footnotes
Supported by Grants EY15313 and EY11374 from the National Eye Institute, the Minnesota Lions and Lionesses, a Lew Wasserman Mid-Career Merit Award from Research to Prevent Blindness (RPB), an unrestricted grant to the Department of Ophthalmology from RPB Inc., and Grant FAPESP 2007-05260-0 (RSA-F).
Disclosure: S.P. Christiansen, None; R.S. Antunes-Foschini, None; L.K. McLoon, None
References
- 1. Govindan M, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood exotropia: a population-based study. Ophthalmology. 2005;112:104–108 [DOI] [PubMed] [Google Scholar]
- 2. Greenberg AR, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood esotropia: a population-based study. Ophthalmology. 2007;114:170–174 [DOI] [PubMed] [Google Scholar]
- 3. Ekwadi NS, Nusz KJ, Diehl NN, Mohney BG. Postoperative outcomes in children with intermittent exotropia from a population-based cohort. J AAPOS. 2009;13:4–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louwagie CR, Diehl NN, Greenberg AE, Mohney BG. Long-term follow-up of congenital esotropia in a population-based cohort. J AAPOS. 2009;13:8–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ganesh A, Pirouznia S, Ganguly SS, Fagerholm P, Lithander J. Consecutive exotropia after surgical treatment of childhood esotropia: a 40-year follow-up study. Acta Ophthalmol. Published online November 19, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Polling JR, Eijkemans MJC, Esser J, et al. A randomized comparison of bilateral recession versus unilateral recession-resection as surgery for infantile esotropia. Br J Ophthalmol. 2009;93:954–957 [DOI] [PubMed] [Google Scholar]
- 7. Chew E, Remaley NA, Tamboli A, Zhao J, Podgor MJ, Klebanoff M. Risk factors for esotropia and exotropia. Arch Ophthalmol. 1994;112:1349–1355 [DOI] [PubMed] [Google Scholar]
- 8. Engle EC. The genetic basis of complex strabismus. Pediatr Res. 2006;59:343–348 [DOI] [PubMed] [Google Scholar]
- 9. McLoon LK, Felius J, Stager DR. Inferior oblique muscles from patients with inferior oblique overaction: examination of myosin heavy chain isoforms. J AAPOS. 2010;14:e23 [Google Scholar]
- 10. Antunes-Foschini RS, Ramalho FS, Ramalho LN, Bicas HE. Increased frequency of activated satellite cells in overacting inferior oblique muscles from humans. Invest Ophthalmol Vis Sci. 2006;47:3360–3365 [DOI] [PubMed] [Google Scholar]
- 11. Antunes-Foschini RS, Miyashita D, Bicas HE, McLoon LK. Activated satellite cells in medial rectus muscles of patients with strabismus. Invest Ophthalmol Vis Sci. 2008;49:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christiansen SP, McLoon LK. The effect of resection on satellite cell activity in rabbit extraocular muscles. Invest Ophthalmol Vis Sci. 2006;47:605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seale P, Sabourin LA, Girgis-Gabardo A, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786 [DOI] [PubMed] [Google Scholar]
- 14. Ugalde I, Christiansen SP, McLoon LK. Botulinum toxin treatment of extraocular muscles in rabbits results in increased myofiber remodeling. Invest Ophthalmol Vis Sci. 2005;46:4114–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harrison AR, Lee MS, McLoon LK. Effects of elevated thyroid hormone on adult rabbit extraocular muscles. Invest Ophthalmol Vis Sci. 2010;51:183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harridge SDR. Plasticity in human skeletal muscle: gene expression and in vivo function. Exp Physiol. 2007;92:783–797 [DOI] [PubMed] [Google Scholar]
- 17. Nelson AG, Thompson WJ. Contractile properties and myosin phenotype of single motor units from neonatal rats. Am J Physiol. 1994;266:C919–C924 [DOI] [PubMed] [Google Scholar]
- 18. Gupta M, Zak R. Reversibility of load-induced changes in myosin heavy chain gene expression. Am J Physiol. 1992;262:R346–R349 [DOI] [PubMed] [Google Scholar]
- 19. Alway SE. Stretch induced non-uniform isomyosin expression in quail anterior latissimus dorsi muscle. Anat Rec. 1993;237:1–7 [DOI] [PubMed] [Google Scholar]
- 20. McLoon LK, Rios L, Wirtschafter JD. Complex three-dimensional patterns of myosin isoform expression: differences between and within specific extraocular muscles. J Muscle Res Cell Motil. 1999;20:771–783 [DOI] [PubMed] [Google Scholar]
- 21. Newlands S, Levitt LK, Robinson CS, et al. Transcription occurs in pulses in muscle fibers. Genes Dev. 1998;12:2748–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Talmadge RJ, Roy RR, Edgerton VR. Distribution of myosin heavy chain isoforms in non-weight bearing rat soleus muscle fibers. J Appl Physiol. 1996;2540–2546 [DOI] [PubMed] [Google Scholar]
- 23. Zhong H, Roy RR, Woo J, Kim JA, Edgerton VR. Differential modulation of myosin heavy chain phenotype in an inactive extensor and flexor muscle of adult rats. J Anat. 2007;210:19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ohira Y, Yoshinaga T, Ohara M, et al. The role of neural and mechanical influences in maintaining normal fast and slow muscle properties. Cell Tissues Organs. 2006;182:129–142 [DOI] [PubMed] [Google Scholar]
- 25. Chopard A, Pons F, Marini JF. Cytoskeletal protein contents before and after hindlimb suspension in a fast and slow rat skeletal muscle. Am J Physiol Regul Integrat Comp Physiol. 2001;280:R323–R330 [DOI] [PubMed] [Google Scholar]
- 26. Ho-Kim MA, Rogers PA. Quantitative analysis of dystrophin in fast- and slow-twitch mammalian skeletal muscle. FEBS Lett. 1992;304:187–191 [DOI] [PubMed] [Google Scholar]
- 27. Li T, Wiggins LM, von Bartheld CS. Insulin-like growth factor-1 (IGF1) and cardiotrophin 1 (CT1) increase strength and mass of extraocular muscle in juvenile chicken. Invest Ophthalmol Vis Sci. 2010;51:2479–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Christiansen SP, Harral RL, Brown H. Extraocular muscle fiber morphometry following combined recession-resection procedures in rabbits. J Pediatr Ophthalmol Strabismus. 1996;33:247–250 [DOI] [PubMed] [Google Scholar]
- 29. Spencer RF, McNeer KW. Botulinum toxin paralysis of adult monkey extraocular muscle. Structural alterations in orbital, singly innervated muscle fibers. Arch Ophthalmol. 1987;105:1703–1711 [DOI] [PubMed] [Google Scholar]
- 30. Ferreira R, Neuparth MJ, Ascensao A, et al. Skeletal muscle atrophy increases cell proliferation in mice gastrocnemius during the first week of hindlimb suspension. Eur J Appl Physiol. 206;97:340–346 [DOI] [PubMed] [Google Scholar]
- 31. Wang XD, Kawano F, Matsuoka Y, et al. Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am J Physiol Cell Physiol. 2006;290:C981–C989 [DOI] [PubMed] [Google Scholar]
- 32. Demer JL, Poukens V, Ying H, Shan X, Tian J, Zee DS. Effects of intracranial trochlear neurectomy on the structure of the primate superior oblique muscle. Invest Ophthalmol Vis Sci. 2010;51:3486–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kushner BJ. Multiple mechanisms of extraocular muscle “overaction”. Arch Ophthalmol. 2006;124:680–688 [DOI] [PubMed] [Google Scholar]
- 34. Christiansen SP, Soulsby ME, Seifen EE. Effect of antagonist weakening on developed tension in cat extraocular muscle. Invest Ophthalmol Vis Sci. 1995;36:2547–2550 [PubMed] [Google Scholar]
- 35. Roy JE, Cullen KE. Brain stem pursuit pathways: dissociating visual, vestibular, and proprioceptive inputs during combined eye-head gaze tracking. J Neurophysiol. 2003;90:271–290 [DOI] [PubMed] [Google Scholar]
- 36. Clendaniel RA, Mays LE. Characteristics of antidromically identified oculomotor internuclear neurons during vergence and versional eye movements. J Neurophysiol. 1994;71:1111–1127 [DOI] [PubMed] [Google Scholar]
- 37. Bhidayasiri R, Plant GT, Leigh RJ. A hypothetical scheme for the brainstem control of vertical gaze. Neurology. 2000;54:1985–1993 [DOI] [PubMed] [Google Scholar]
- 38. Van Horn MR, Cullen KE. Dynamic characterization of agonist and antagonist oculomotoneurons during conjugate and disconjugate eye movements. J Neurophysiol. 2009;102:28–40 [DOI] [PubMed] [Google Scholar]
- 39. Trommershauser J, Glimcher PW, Gegenfurtner KR. Visual processing, learning and feedback in the primate eye movement system. Trends Neurosci. 2009;32:583–590 [DOI] [PubMed] [Google Scholar]
- 40. Krommenhoek KP, Van Gisbergen JAM. Evidence for nonretinal feedback in combined version-vergence eye movements. Exp Brain Res. 1994;102:95–109 [DOI] [PubMed] [Google Scholar]
- 41. Blumer R, Konakci KZ, Pomikal C, Wieczorek G, Lukas JR, Streicher J. Palisade endings: cholinergic sensory organs or effector organs? Invest Ophthalmol Vis Sci. 2009;50:1176–1186 [DOI] [PubMed] [Google Scholar]
- 42. Aigner M, Lukas RJ, Denk M, Ziya-Ghazvini F, Kaider A, Mayr R. Somatotopic organization of primary afferent perikarya of the guinea-pig extraocular muscles in the trigeminal ganglion: a post-mortem DiI-tracing study. Exp Eye Res. 2000;70:411–418 [DOI] [PubMed] [Google Scholar]
- 43. Lewis RF, Zee DS, Hayman MR, Tamargo RJ. Oculomotor function in the rhesus monkey after deafferentation of the extraocular muscles. Exp Brain Res. 2001;141:349–358 [DOI] [PubMed] [Google Scholar]
- 44. Kusner LL, Young A, Tjoe S, Leahy P, Kaminski HJ. Perimysial fibroblasts of extraocular muscle, as unique as the muscle fibers. Invest Ophthalmol Vis Sci. 2010;51:192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heinemeier KM, Olesen JL, Haddad F, Schjerling P, Baldwin KM, Kjaer M. Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J Appl Physiol. 2009;106:178–186 [DOI] [PubMed] [Google Scholar]
- 46. Anderson BC, Christiansen SP, Grandt S, Grange RW, McLoon LK. Increased extraocular muscle strength with direct injection of insulin-like growth factor-I. Invest Ophthalmol Vis Sci. 2006;47:2461–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiang L, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior rectus muscle in superior oblique palsy. Ophthalmology. 2008;115:2079–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clark RA, Demer JL. Magnetic resonance imaging (MRI) demonstrates enhanced vertical rectus contractility in superior oblique palsy. J AAPOS. 2010;14:e1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]