Oxidative damage is implicated in glaucoma. Vitrectomy, when followed by cataract surgery, causes glaucoma. Vitrectomy and cataract surgery increase oxygen in the anterior chamber angle, most likely by increasing oxygen diffusion from the ciliary body stroma.
Abstract
Purpose.
Vitrectomy, when followed by cataract surgery, increases the risk of open-angle glaucoma. This study was conducted in patients to determine whether these procedures are associated with increased exposure of the trabecular meshwork to oxygen.
Methods.
Oxygen distribution was recorded with a fiberoptic probe in patients undergoing surgery for cataract, glaucoma, or retinal disease. pO2 was measured beneath the central cornea, in the mid-anterior chamber, and in the anterior chamber angle. In patients who were pseudophakic or were scheduled for cataract extraction, pO2 was also measured in the posterior chamber and near the lens.
Results.
Eyes with no previous cataract or vitrectomy surgery had steep oxygen gradients in the aqueous humor between the cornea and lens. pO2 was low in the posterior chamber and near the lens. Previous vitrectomy was associated with significantly increased pO2 in the posterior chamber. Eyes with previous cataract surgery had significantly elevated pO2 only in the posterior chamber and in front of the intraocular lens (IOL). Eyes that had both vitrectomy and previous cataract surgery had increased pO2 in the posterior chamber, anterior to the IOL, and in the anterior chamber angle. pO2 in the posterior chamber and the anterior chamber angle correlated strongly.
Conclusions.
Oxygen metabolism by the lens and cornea establishes oxygen gradients in the anterior segment. Vitrectomy and cataract surgery increase pO2 in the anterior chamber angle, potentially damaging trabecular meshwork cells. We propose that oxygen levels in the anterior chamber angle are strongly influenced by oxygen derived from the ciliary body circulation.
Several lines of evidence suggest that increased oxidative stress or the accumulation of oxidative damage contributes to the pathogenesis of glaucoma.1–7 Antioxidant protective mechanisms are decreased, and enzymes induced by oxidative stress are increased in the aqueous humor of glaucoma patients, compared with those undergoing cataract surgery.3,8 Oxidative damage to DNA increases in the trabecular meshwork cells of glaucoma patients, and these cells are more susceptible to oxidative DNA damage than are other cells in the anterior segment.9 In one study, the lymphocytes of glaucoma patients had pathogenic mutations in their mitochondrial DNA that were not present in control subjects.10 These alterations are consistent with oxidative damage. The mitochondria of glaucoma patients also had significantly lower oxidative activity than in control subjects.10 Despite the abundant evidence linking glaucoma's pathogenesis to oxidative damage, the source of the oxidative stress in these patients is not known. It is also not clear whether the increased oxidative damage in glaucoma patients is the result of increased exposure to oxidants, decreased antioxidative protection, or a combination of these factors.
Ocular surgery can increase the risk of developing glaucoma. Elevated intraocular pressure and glaucoma have long been associated with corneal transplantation (penetrating keratoplasty) or the implantation of an artificial cornea.11–19 Recently, two studies reported that vitrectomy is also associated with increased risk of elevated intraocular pressure and glaucoma. In both studies, the presence of the natural lens delayed the onset of glaucoma.20,21 In most older patients who undergo vitrectomy, nuclear sclerotic cataracts develop within 2 years.22–26 Nuclear cataract is associated with increased oxidative damage to the lens, and vitrectomy leads to increased exposure of the lens to oxygen.23,27,28 These observations led Chang20 to propose that, after vitrectomy, metabolites of oxygen, like hydrogen peroxide, may damage the tissues of the outflow pathway, contributing to the increased risk of glaucoma. Since the presence of the natural lens reduces the risk of glaucoma, he proposed that the lens protects the anterior segment from oxygen or oxygen metabolites.
The present study was designed to test Chang's hypothesis that oxygen is relatively low in the anterior segment of the non–surgically altered eye and that it increases after vitrectomy and cataract surgery. The distribution of oxygen in the anterior segment of the eye was measured in a reference group of patients who were undergoing surgery for glaucoma or cataract. Oxygen distribution in these patients was compared with that in patients who had had vitrectomy, cataract surgery, or both procedures. The results of these measurements supported the predictions of the Chang hypothesis, which suggest that increased exposure to oxygen or its metabolites causes open-angle glaucoma. These studies also revealed an unexpected physiologic mechanism that regulates the distribution of oxygen in the anterior chamber angle of the human eye.
Methods
Study Design
A cross-sectional study was designed to compare oxygen distribution in different regions of the eye in a reference group to the oxygen distribution in subjects who had undergone vitrectomy, cataract surgery, or both. The study was approved by the Human Resource Protection Office and the Institutional Review Board of the Washington University School of Medicine in compliance with the tenets of the Declaration of Helsinki. Informed consent was obtained before surgery after explanation of the nature and possible consequences of the study.
Patients and pO2 Measurements
Consecutive patients undergoing glaucoma and/or cataract surgery (CJS) or patients with previous cataract and vitrectomy surgery who were undergoing additional retina surgery (NMH) were eligible. Patients with corneal endothelial dysfunction, ischemic ocular disease, anterior chamber angle closure, inflammatory disease, ocular neoplasia, or monocular status were excluded. A complete general medical and ophthalmic history was obtained before surgery. Standard surgical procedures for glaucoma, retina, and cataract surgery were followed. Patients scheduled for cataract or vitreoretinal surgery received preoperative mydriatics. Supplemental oxygen was provided by nasal cannula. The nasal area was separated from the surgical field with an adhesive surgical drape to avoid exposure to oxygen of the surface of the eye. Oxygen saturation (SaO2) was monitored by continuous pulse oximetry. After intravenous sedation, anesthesia was administered by sub-Tenon's injection of 3 mL of 2% lidocaine and 0.375% bupivacaine, 50:50. Before the scheduled surgical procedure, a 30-gauge needle was used to fashion a peripheral corneal paracentesis, through which a 30-gauge pO2 optical oxygen sensor (Oxylab optode; Oxford Optronix, Oxford, UK) was introduced into the anterior chamber. The flexible fiberoptic probe was positioned for three measurements in all patients (Fig. 1): near the central corneal endothelium, in the mid-anterior chamber (AC), and in the AC angle. In patients who were pseudophakic or scheduled for cataract extraction, the optode was also positioned near the anterior lens surface and in the posterior chamber. To avoid possible lens damage, measurements were not made near the lens in phakic patients undergoing only glaucoma surgery. Instrument calibration was checked against solutions equilibrated to 0% and 5% oxygen. The patients were monitored postoperatively for complications.
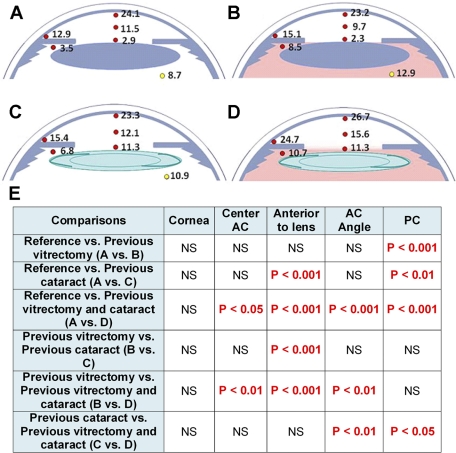
Figure 1.
Oxygen distribution in the anterior of the human eye. (A) Oxygen distribution in the reference group (no previous cataract surgery or vitrectomy), (B) after previous vitrectomy, (C) previous cataract surgery, (D) or previous vitrectomy and cataract surgery. Red dots: oxygen measurements made by corneal entry during glaucoma and/or cataract surgery; yellow dots: oxygen measurements made through pars plana entry at the beginning of vitrectomy surgery.23 In the table (E), the significant differences in pO2 are identified in different regions of the anterior segment when the reference group and the different surgical groups are compared. Statistical analysis was by ANOVA, with Bonferroni correction for multiple comparisons.
The pO2 reported by the optode used in this study varies with temperature. Probes supplied with a thermistor are thicker and, in preliminary studies in eye bank and pig eyes, were more likely to leak aqueous humor through the corneal wound. We therefore used thinner probes with no temperature compensation. On the basis of previous studies in rabbits, in which temperature from the inner surface of the cornea to the posterior chamber was 31°C to 34°C, we set the temperature compensation to a constant 32°C. At a constant pO2 of 38 mm Hg, a change of 1°C alters the apparent pO2 reported by the optode by 0.5 mm Hg. Therefore, over the temperature variation in the eye, pO2 was expected to be accurate to within ±1 mm Hg.
Statistical Analysis
The results are expressed as the mean ± SEM. ANOVA with Bonferroni correction for multiple comparisons was used to identify significant differences between groups. Multivariate regression was performed with adjustment for potential confounding variables (SPSS ver. 17.0; SPSS, Chicago, IL). P < 0.05 was considered statistically significant.
Results
Table 1 shows the characteristics of the patients. A total of 119 individuals participated. Seven eyes were excluded due to leakage of aqueous humor or malfunction of the optode, leaving a total of 112 eyes for the analysis. Patients with no history of cataract or vitrectomy surgery comprised a reference group of 72 individuals (72 eyes). Within this group, the surgical diagnosis (glaucoma, cataract, or both) was not related to pO2 at any location within the anterior segment (P > 0.1). This result suggests that the mydriatics used to dilate the iris of patients scheduled for cataract surgery, but not used in patients undergoing only glaucoma surgery, did not significantly alter the oxygen distribution at the locations measured. An additional 14 eyes had previous vitrectomy, 17 had previous cataract surgery, and 9 had previous vitrectomy and cataract surgery. The mean age of the groups was not significantly different. The reference group included patients with no previous ocular surgery (48 eyes) and glaucoma patients (24 eyes) who had undergone a glaucoma surgical procedure (laser trabeculoplasty, iridotomy, or trabeculectomy). When eyes with no previous surgery were compared with eyes with any glaucoma surgery, pO2 was significantly different only in the posterior chamber, where eyes with previous glaucoma surgery had slightly lower pO2 (P = 0.03). However, excluding eyes with any glaucoma surgery from the reference group had little effect on comparison of the reference group to eyes that had cataract surgery, vitrectomy surgery, or both (not shown). Because some of the eyes with previous cataract surgery also had undergone glaucoma surgery, we included eyes with glaucoma surgery in the reference group.
Table 1.
Patient Characteristics
| Group | n | Age* | Male | Female | Surgical Diagnosis |
Vitreo-retinal Disease | ||
|---|---|---|---|---|---|---|---|---|
| Cataract | Glaucoma | Combined | ||||||
| Reference (no previous cataract surgery or vitrectomy) | 72 | 69.9 ± 11.2 | 36 | 36 | 24 | 19 | 29 | 0 |
| Previous vitrectomy | 14 | 62.8 ± 17.3 | 9 | 5 | 12 | 0 | 2 | 0 |
| Previous cataract surgery | 17 | 72.4 ± 9.4 | 3 | 14 | 0 | 14 | 0 | 3 |
| Previous vitrectomy and cataract surgery | 9 | 64.3 ± 11.3 | 5 | 4 | 0 | 2 | 0 | 7 |
| Total | 112 | 53 | 59 | 36 | 35 | 31 | 10 | |
Data are shown as years ± SD. Age distribution was not significantly different in the four groups.
Steep oxygen gradients were present in the eyes of the reference group (Fig. 1; Table 2A). The approximate pO2 in air is 160 mm Hg, whereas the mean pO2 at the inner surface of the cornea was 24 mm Hg, a nearly sevenfold gradient. A similarly steep oxygen gradient was present in the anterior chamber between the inner surface of the cornea (24.1 mm Hg) and the anterior surface of the lens (2.8 mm Hg), with intermediate pO2 in the mid-anterior chamber (11.5 mm Hg). The mean pO2 measured in the posterior chamber was 3.5 mm Hg, whereas the pO2 behind the lens, measured in a previous study, was 8.7 mm Hg (Table 2B).23 Although the cornea overlying the anterior chamber angle is only slightly thicker than the central cornea, the pO2 in the anterior chamber angle (12.9 mm Hg) was approximately half of that beneath the central cornea (24.1 mm Hg).
Table 2.
Mean pO2
| A. Measurements at Five Locations in the Anterior of the Eye* | |||||
|---|---|---|---|---|---|
| Group | Cornea | Center AC | Lens | Angle | PC |
| Reference (no previous cataract surgery or vitrectomy) | 24.2 ± 0.9 | 11.5 ± 0.5 | 2.8 ± 0.3 | 12.9 ± 0.7 | 3.5 ± 0.4 |
| Previous vitrectomy | 23.2 ± 1.4 | 9.7 ± 0.9 | 2.3 ± 0.6 | 15.1 ± 1.2 | 8.5 ± 0.9 |
| Previous cataract surgery | 20.7 ± 1.8 | 11.9 ± 0.5 | 11.0 ± 1.2 | 14.9 ± 1.3 | 6.7 ± 0.7 |
| Previous vitrectomy and cataract surgery | 26.7 ± 1.4 | 15.6 ± 0.6 | 11.3 ± 1.6 | 24.7 ± 1.5 | 10.7 ± 0.7 |
| B. Measurements Near the Posterior Surface of the Lens† | |||
|---|---|---|---|
| Group | n | pO2 | P |
| Reference‡ | 16 | 8.7 ± 0.6 | |
| Previous vitrectomy‡ | 8 | 12.9 ± 0.5 | <0.001 |
| Previous cataract | 9 | 10.9 ± 0.8 | <0.05 |
AC, anterior chamber; PC, posterior chamber.
The measurements were obtained with a 30-gauge optode inserted through a clear corneal incision. Data are expressed as the mean pO2 (mm Hg ± SE).
The measurements were obtained with an optode inserted through the pars plana at the beginning of the vitrectomy. Data for eyes with previous cataract surgery were obtained by reanalysis of this study. Data are expressed as the mean pO2 (mm Hg ± SE).
These data have been reported elsewhere.23
Vitrectomy surgery chronically increases oxygen levels near the posterior surface of the human lens by an average of 48% (Table 2B).23 In the present study, vitrectomy was associated with a 2.4 fold increase in pO2 in the posterior chamber, compared with the reference group (Fig. 1, Table 2A; P < 0.001). Vitrectomy was also associated with increased pO2 in the anterior chamber angle, although the difference did not reach statistical significance.
Cataract surgery in the presence of an intact vitreous body was associated with an almost fourfold increase in pO2 anterior to the lens. Cataract surgery also increased the pO2 in the posterior chamber by a factor of two. Both levels were significantly different from those in the reference group (Fig. 1; P < 0.001 and 0.01, respectively). As in eyes with prior vitrectomy, cataract surgery increased pO2 in the anterior chamber angle by 2 to 3 mm Hg, a change that did not reach statistical significance when compared with the reference group.
In eyes that had previous vitrectomy and cataract surgery (Fig. 1), pO2 was significantly elevated in the posterior chamber (P < 0.001), anterior to the lens (P < 0.001), in the mid-anterior chamber (P < 0.05), and in the anterior chamber angle (P < 0.001).
To explore the relationship between oxygen levels in different regions of the eye, we performed multivariate regression analyses to determine how the pO2 in one part of the eye correlated with the pO2 in other areas (Table 3). In the reference group, the pO2 beneath the central cornea correlated strongly with the pO2 in the mid-anterior chamber, as would be expected if oxygen in the mid-anterior chamber were derived by diffusion through the cornea. pO2 near the anterior surface of the lens also correlated with that in the mid-anterior chamber, although the pO2 beneath the cornea did not correlate with that in front of the lens. Oxygen levels in the posterior chamber correlated significantly with pO2 anterior to the lens and in the anterior chamber angle. The association between pO2 in the posterior chamber and anterior to the lens seems logical, since oxygen levels in both locations would be affected by oxygen metabolism in the lens epithelium. However, it was not obvious why the pO2 in the posterior chamber correlated with pO2 in the anterior chamber angle.
Table 3.
Relationships between pO2 in Five Regions of the Eyes
| Cornea |
Center |
Lens |
Angle |
|||||
|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | |
| Reference group | ||||||||
| Cornea | — | — | ||||||
| Center | 1.118 | 2.5 × 10−5 | — | — | ||||
| Lens | 0.158 | 0.747 | 0.505 | 0.048 | — | — | ||
| Angle | 0.145 | 0.447 | −0.142 | 0.159 | 0.050 | 0.418 | — | — |
| PC | 0.451 | 0.217 | −0.155 | 0.429 | 0.334 | 0.003 | 0.800 | 0.005 |
| All eyes | ||||||||
| Cornea | — | — | ||||||
| Center | 1.135 | 2.3 × 10−7 | — | — | ||||
| Lens | −0.198 | 0.256 | 0.397 | 1 × 10−4 | — | — | ||
| Angle | 0.066 | 0.635 | 0.009 | 0.898 | 0.140 | 0.142 | — | — |
| PC | 0.193 | 0.382 | −0.139 | 0.203 | 0.323 | 0.030 | 0.784 | 9 × 10−6 |
β is the regression coefficient, the slope of the regression line. Statistically significant results are in italic.
We then expanded the analysis by combining the data from the reference group with those in the surgical groups (Table 3). As expected, pO2 in the mid-anterior chamber continued to correlate with pO2 beneath the central cornea. There was also a strong correlation between pO2 in the mid-anterior chamber and in front of the lens. Since the combined group included subjects with cataract surgery and IOL implantation, this result suggests that oxygen diffusing from the mid-anterior chamber contributes to the elevated pO2 in front of the IOL. In the combined group, the statistical significance of the association between the pO2 in the posterior chamber and anterior to the lens decreased, compared with that in the reference group. However, the strength of the association between pO2 in the posterior chamber and the anterior chamber angle increased markedly. These relationships strongly suggest that oxygen levels in the posterior chamber and anterior chamber angle are physiologically related.
Discussion
Control of Oxygen Distribution in the Human Eye
The oxygen distributions observed in the present study, together with published measurements of pO2 gradients in the vitreous,23 provide an overview of oxygen distribution in the human eye. We recognize that these measurements were made in eyes that were undergoing surgery for one or more eye diseases. With this limitation in mind, it is still reasonable that the gradients within the eye provide insight into the sources of oxygen and the tissues that consume it.
To a first approximation, the eye can be considered to be a sphere, with oxygen entering the ocular fluids at its periphery. In the posterior segment of the globe, oxygen diffuses from the retinal vasculature into the vitreous body.29–31 In the anterior segment of the eye, oxygen diffuses across the cornea.32–34 Data from the present study suggest that the major sites of oxygen consumption in the anterior segment of the human eye are the cornea, lens and ciliary epithelium. We recently found that ascorbate in the vitreous fluid also consumes oxygen.35 These “oxygen sinks” are revealed by the steep oxygen gradients across the cornea, between the inner surface of the cornea and the lens, by the low pO2 in the posterior chamber and by gradients of oxygen within the vitreous gel.23,35
Other investigators have measured and modeled oxygen consumption by the cornea and lens.32–34,36,37 Consistent with the results in these studies, we found that cataract surgery, which removes most of the lens tissue, was associated with increased oxygen levels immediately anterior to the lens, in the posterior chamber, and in the vitreous near the lens. However, cataract surgery did not lead to a significant increase in pO2 beneath the cornea, confirming an earlier study in rabbits, in which the researchers concluded that the oxygen supply to the cornea is independent of oxygen consumption by the lens.38 Multivariate regression revealed that the pO2 beneath the central cornea and close to the lens epithelium did not correlate, providing further evidence that the oxygen metabolism of the lens and cornea are independently regulated. The pO2 at both locations correlated with oxygen in the mid-anterior chamber, showing that oxygen consumption by the tissues surrounding the anterior chamber influences oxygen levels in the aqueous humor.
Our data demonstrate that, when the eyelids are open, oxygen levels near the inner surface of the cornea are set by the diffusion of oxygen from the air and by corneal oxygen consumption, not by oxygen delivered by the aqueous humor. In every patient in the reference group, pO2 was greater in the aqueous near the central corneal endothelium than at any other location in the anterior of the eye. Therefore, the diffusion of oxygen or the flow of aqueous humor in the anterior chamber can only decrease oxygen levels near the cornea. Stated another way, the pO2 within the cornea will always be higher than the pO2 in the aqueous near the corneal surface. These observations are consistent with measurements made in rabbits using an injected reporter molecule to remotely monitor oxygen levels in the anterior chamber.39 This study showed that pO2 decreased beneath the cornea and in the central anterior chamber when a contact lens with low oxygen permeability was placed on the eye. Studies that modeled corneal oxygen consumption and oxygen distribution within the cornea routinely set oxygen levels at the inner surface of the cornea to a constant value.34,40,41 However, in the present study, pO2 in the aqueous near the inner surface of the cornea ranged between 12 and 41 mm Hg. Therefore, in the eye with open lids, corneal oxygen permeability and metabolism determine pO2 in the aqueous; the aqueous does not set the pO2 for the corneal endothelium.
The steep gradients within the anterior chamber and the very low pO2 near the lens suggest that, even when the lids are closed, entry of oxygen at the epithelial surface is likely to be the primary source of corneal oxidative metabolism, making glycolysis an important energy source during sleep.42 The role of corneal oxygen consumption in setting pO2 in the anterior chamber is important, as we found that the pO2 beneath the central cornea was significantly different in Caucasian and African-American subjects, presumably reflecting racial differences in corneal oxygen metabolism (Siegfried CJ, manuscript submitted).
Previous measurements in rabbit, monkey, and human eyes, in which polarographic oxygen electrodes were used, can be interpreted to suggest that the iris vasculature is also a significant source of oxygen.43,44 However, the experimental design used in these studies did not exclude the possibility that, under normal breathing conditions, the oxygen measured anterior to the iris originated by diffusion through the cornea. Measurement of oxygen beneath the rabbit iris, using the same fiberoptic sensor as in the present study, showed that, in this species, the iris vasculature supplied oxygen to the posterior chamber.37 However, in the present study, oxygen levels in the posterior chamber, just beneath the iris, were very low: 3.5 mm Hg or about six times lower than in rabbits.37 These measurements suggest that, in the patients included in this study, relatively little oxygen was derived from the iris vasculature. Alternatively, the human iris is a source of oxygen, but the lens consumes substantially more oxygen than that of the rabbit.
In rabbits, monkeys, and the patients included in this study, the aqueous humor in the posterior chamber, which is produced by the ciliary epithelium, was relatively depleted of oxygen.37,43 This observation was initially surprising, since the ciliary body is richly vascularized. Presumably, oxidative metabolism in the ciliary epithelial cells, which produces the ATP needed to secrete aqueous humor, consumes most of the oxygen delivered to this tissue by the ciliary body vasculature (Fig. 2A).45–48
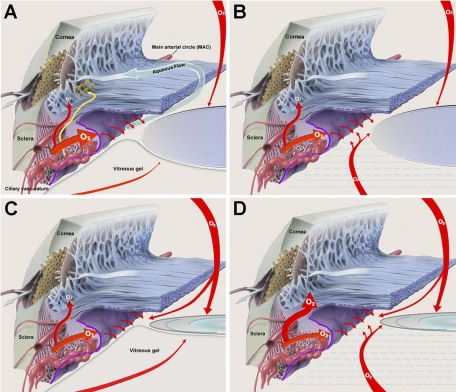
Figure 2.
Oxygen distribution in the nonsurgical eye and the proposed effect of vitrectomy and/or cataract surgery on oxygen delivery to the anterior chamber angle. (A) In the nonsurgical eye, oxygen enters from the retinal vasculature through the vitreous, across the ciliary epithelium from the ciliary body vasculature, and into the anterior chamber across the cornea. Oxygen is consumed by the lens and the ciliary epithelium. A small amount of oxygen enters the anterior chamber angle by diffusing across the ciliary body and iris stroma (curved red arrow). This is the same pathway as that of the plasma proteins (gold arrow). (B) After vitrectomy, more oxygen reaches the posterior chamber. This supplies more oxygen to the “aqueous surface” of the ciliary epithelium, reducing the amount of oxygen that the ciliary epithelium removes from the blood and slightly increasing the amount of oxygen available to enter the anterior chamber angle from the ciliary body stroma. (C) Cataract surgery reduces oxygen consumption by the lens, thereby increasing the pO2 anterior to the lens and in the posterior chamber. The increased oxygen on the aqueous surface of the ciliary epithelium reduces the amount of oxygen that the ciliary epithelium removes from the blood, thereby slightly increasing the amount of oxygen available to enter the anterior chamber angle from the ciliary body stroma. (D) After vitrectomy and cataract surgery, significantly more oxygen is available on the aqueous surface of the ciliary epithelium, resulting in the removal of significantly less oxygen from the blood. This process increases the amount of oxygen available to diffuse from the ciliary body stroma, across the iris stroma, and into the anterior chamber angle, exposing the outflow system to a large excess of oxygen and/or oxygen metabolites. (Diagrams modified with permission from an illustration by Keith Kasnot for Merck & Co., Inc. ©2010. Phoenix, AZ: Kasnot Medical Illustration, Inc. All rights reserved.)
The Effect of Vitrectomy on Oxygen Distribution in the Eye
We have shown in prior work that vitrectomy increases pO2 near the posterior surface of the lens.23 Since prolonged hyperbaric oxygen therapy and age-related liquefaction of the vitreous gel lead to nuclear cataract formation,49–51 we suggested that postvitrectomy cataracts are caused by the excessive exposure of the lens to oxygen.23,52 Subsequent investigations have found that vitrectomy also increases the risk of developing open-angle glaucoma, especially in eyes that also have had cataract surgery.20,21 It has been suggested that the increased risk of glaucoma after vitrectomy and cataract surgery is due to the increased exposure of the outflow tissues to oxygen or the metabolites of oxygen.20 The present study was designed to test this prediction by determining the effect of cataract surgery on oxygen levels in the eyes of patients with previous vitrectomy.
Like cataract surgery, previous vitrectomy was associated with a significant increase in pO2 in the posterior chamber. Each of these surgical procedures also increased oxygen levels in the anterior chamber angle, although these changes did not reach statistical significance in either case. In the eyes that had previous vitrectomy and cataract surgery, pO2 increased markedly in the posterior chamber and anterior to the lens. In contrast to the result of the individual surgeries, pO2 in the anterior chamber angle nearly doubled if both vitrectomy surgery and cataract surgery were performed. These data confirm the prediction that vitrectomy and cataract surgery increase exposure of the cells of the trabecular meshwork to oxygen and, possibly, metabolites of oxygen, such as hydrogen peroxide.20
Control of pO2 in the Anterior Chamber Angle: A Hypothesis
Statistical analysis of the pO2 in different regions of the eye in the reference group revealed a significant correlation between pO2 in the posterior chamber and in the anterior chamber angle. This relationship is puzzling, since the pO2 in the posterior chamber and anterior to the lens was approximately 3 to 4 mm Hg, whereas the pO2 in the angle was four times higher. Therefore, it is impossible for the oxygen in the anterior chamber angle to be derived from oxygen carried to the angle by the transpupillary flow of aqueous humor. In addition, under no circumstance did the pO2 in the anterior chamber angle correlate with the pO2 beneath the central cornea, suggesting that variations in oxygen consumption by the cornea did not significantly influence the pO2 in the angle. When eyes with previous ocular surgery were included in the regression analysis, the statistical significance of the association between oxygen in the posterior and anterior chambers increased, but the relationship between the pO2 in the posterior chamber and anterior to the lens decreased. If oxygen reached the anterior chamber angle by aqueous humor flow (Fig. 2), one would expect a stronger correlation between oxygen in the posterior chamber and anterior to the lens than between the posterior chamber and the anterior chamber angle. This paradox led us to consider an alternative explanation.
Studies conducted over the past 20 years by Freddo et al.53–55 in several species, including humans, revealed that most of the plasma proteins in the aqueous humor do not diffuse across the ciliary epithelium. Instead, they diffuse from the permeable vessels in the ciliary body,48 through the stromal tissue of the ciliary body and iris, across the anterior face of the iris and into the aqueous in the anterior chamber angle (Fig. 2A). In our previous studies of oxygen distribution in the rabbit eye, decreasing the oxygen saturation of the blood (SaO2) decreased pO2 in the anterior chamber angle, but not beneath the central cornea.37 From these data, we concluded that the pO2 in the angle was significantly influenced by oxygen from the blood. Combining these two observations, we postulate that a substantial amount of the oxygen in the anterior chamber angle follows the same route as that taken by the plasma proteins, diffusing from the ciliary body stroma into the aqueous at the root of the iris (Fig. 2A).
The ciliary body contains an abundant vasculature that delivers nutrients and oxygen to the ciliary body stroma and ciliary epithelium through fenestrated (permeable) capillaries.47,48 Since the pO2 in the posterior chamber of eyes in the reference group was low, most of the oxygen delivered to the ciliary epithelium by the ciliary body vasculature is likely to be consumed by oxidative metabolism in the ciliary epithelial cells, which have abundant mitochondria. We found that vitrectomy increased pO2 in the posterior chamber, thereby exposing the inner, or aqueous, surface of the ciliary epithelium to increased oxygen (Fig. 2B). This effect was associated with a small increase in the pO2 in the anterior chamber angle, but not anterior to the lens. After vitrectomy and cataract surgery, the pO2 in the posterior chamber was even higher, and the pO2 in the anterior chamber angle nearly doubled. We suggest that the increased oxygen at the aqueous surface of the ciliary epithelium, resulting from decreased oxygen utilization by the vitreous and lens, is used by the ciliary epithelial cells for oxidative metabolism. This process would decrease the amount of oxygen that these cells remove from the interstitial fluid of the ciliary body stroma. The excess oxygen remaining in the stroma would then diffuse, along with the extravascular plasma proteins, through the iris stroma and into the aqueous at the anterior chamber angle (Fig. 2D). In this way, the pO2 in the posterior chamber would indirectly regulate the pO2 in the anterior chamber angle, accounting for the strong correlation between the pO2 at these locations.
Oxidative Stress and Oxygen Adaptation
We found that vitrectomy and cataract surgery increased oxygen delivery to the outflow pathway. How might an increase in pO2 damage the cells of the aqueous outflow system? Stanley Chang20 suggested that increasing oxygen in the aqueous humor exposes the tissues of the outflow tract to oxygen metabolites, like hydrogen peroxide. Oxygen reacts with ascorbate in the ocular fluids to produce hydrogen peroxide, which is then converted to water by the action of catalase.35,56 When oxygen increases in the aqueous, its reaction with ascorbate may overwhelm the ability of catalase to remove peroxide, thereby exposing the outflow tissues to this toxic metabolite. Exposure of the cells of the outflow pathway to peroxide could damage or kill them, a result that would be consistent with the decreased cellularity of the trabecular meshwork in glaucoma patients.1,2,57–59
It is also possible that exposure of the tissues of the outflow pathway to increased molecular oxygen directly increases oxidative stress, just as exposure to excess molecular oxygen can damage other tissues in the body. For example, pulmonary epithelial cells are normally exposed to much higher oxygen than other cells in the body (∼21% O2). However, during oxygen therapy, prolonged exposure to 40% oxygen or more increases the levels of intracellular reactive oxygen species and causes pulmonary oxygen toxicity, a disease that involves fibrosis, the derangement of collagen and elastin fibrils in the lung parenchyma, and increased cell death.60–64 Therefore, it could be said that pulmonary epithelial cells are “adapted” to 21% oxygen and that significantly exceeding the level of oxygen to which they are adapted causes disease.
Our data show that oxygen levels in the eye are maintained within narrow limits by cellular metabolic activity. These normal levels are quite low: <10 mm Hg around the lens or 13 mm Hg (∼2% O2) in the anterior chamber angle. Vitrectomy chronically increases oxygen at the posterior of the lens by 48%, and increased oxygen exposure is likely to be the cause of nuclear cataracts.23,49 Vitrectomy plus cataract surgery nearly doubles the pO2 in the anterior chamber angle and increases the risk of open-angle glaucoma.20,21 If the cells of the outflow system are “adapted” to oxygen at 13 mm Hg, increasing the pO2 to 25 mm Hg could lead to oxygen toxicity, just as exposure to excess oxygen damages the lungs. If this also results in altered extracellular matrix accumulation, increased apoptosis, and decreased outflow facility, it could lead to ocular hypertension and increased risk of glaucoma. The potentially harmful effect of increased exposure to oxygen on the trabecular meshwork cells in vivo appears to be an area worthy of further study.
Acknowledgments
The authors thank Paul Kaufman for discussions and insight concerning the route of protein entry into the aqueous humor, Steven Kymes for statistical consultations, and Michael Kass for critical evaluation of the manuscript.
Footnotes
Supported by an American Health Assistance Foundation, National Glaucoma Research Grant (CJS), NIH Grant EY015863 (DCB), and by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences.
Disclosure: C.J. Siegfried, None; Y.-B. Shui, None; N.M. Holekamp, None; F. Bai, None; D.C. Beebe, None
References
- 1. Gabelt BAT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24:612–637 [DOI] [PubMed] [Google Scholar]
- 2. Izzotti A, Bagnis A, Sacca SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612:105–114 [DOI] [PubMed] [Google Scholar]
- 3. Izzotti A, Sacca SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003;114:638–646 [DOI] [PubMed] [Google Scholar]
- 4. Izzotti A, Sacca SC, Longobardi M, Cartiglia C. Sensitivity of ocular anterior-chamber tissues to oxidative damage and its relevance to glaucoma pathogenesis. Invest Ophthalmol Vis Sci. 2009;50:5251–5258 [DOI] [PubMed] [Google Scholar]
- 5. Kumar DM, Agarwal N. Oxidative stress in glaucoma: a burden of evidence. J Glaucoma. 2007;16:334–343 [DOI] [PubMed] [Google Scholar]
- 6. Saccà SC, Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Prog Brain Res. 2008;173:385–407 [DOI] [PubMed] [Google Scholar]
- 7. Sacca SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–463 [DOI] [PubMed] [Google Scholar]
- 8. Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF. Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol. 2004;137:62–69 [DOI] [PubMed] [Google Scholar]
- 9. Izzotti A, Sacca SC, Longobardi M, Cartiglia C. Sensitivity of ocular anterior chamber tissues to oxidative damage and its relevance to the pathogenesis of glaucoma. Invest Ophthalmol Vis Sci. 2009;50:5251–5258 [DOI] [PubMed] [Google Scholar]
- 10. Abu-Amero KK, Morales J, Bosley TM. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2533–2541 [DOI] [PubMed] [Google Scholar]
- 11. Banitt MR, Chopra V. Descemet's stripping with automated endothelial keratoplasty and glaucoma (review). Curr Opin Ophthalmol. 2010;21:144–149 [DOI] [PubMed] [Google Scholar]
- 12. Coleman AL, Miglior S. Risk Factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53:S3–S10 [DOI] [PubMed] [Google Scholar]
- 13. Foulks GN. Glaucoma associated with penetrating keratoplasty. Ophthalmology. 1987;94:871–874 [DOI] [PubMed] [Google Scholar]
- 14. Franca ET, Arcieri ES, Arcieri RS, Rocha FJ. A study of glaucoma after penetrating keratoplasty. Cornea. 2002;21:284–288 [DOI] [PubMed] [Google Scholar]
- 15. Karadag OMD, Kugu SMD, Erdogan GMD, et al. Incidence of and risk factors for increased intraocular pressure after penetrating keratoplasty. Cornea. 2010;29:278–282 [DOI] [PubMed] [Google Scholar]
- 16. Kirkness CM, Ficker LA. Risk factors for the development of postkeratoplasty glaucoma. Cornea. 1992;11:427–432 [DOI] [PubMed] [Google Scholar]
- 17. Simmons RB, Stern RA, Teekhasaenee C, Kenyon KR. Elevated intraocular pressure following penetrating keratoplasty. Trans Am Ophthalmol Soc. 1989;87:79–91, discussion 91–73 [PMC free article] [PubMed] [Google Scholar]
- 18. Zerbe BL, Belin MW, Ciolino JB. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006;113:1779, e1771–1777 [DOI] [PubMed] [Google Scholar]
- 19. Chew HF, Ayres BD, Hammersmith KM, et al. Boston keratoprosthesis outcomes and complications. Cornea. 2009;28:989–996 [DOI] [PubMed] [Google Scholar]
- 20. Chang S. LXII Edward Jackson Lecture: open angle glaucoma after vitrectomy. Am J Ophthalmol. 2006;141:1033. [DOI] [PubMed] [Google Scholar]
- 21. Luk FO, Kwok AK, Lai TY, Lam DS. Presence of crystalline lens as a protective factor for the late development of open angle glaucoma after vitrectomy. Retina. 2009;29:218–224 [DOI] [PubMed] [Google Scholar]
- 22. de Bustros S, Thompson JT, Michels RG, Enger C, Rice TA, Glaser BM. Nuclear sclerosis after vitrectomy for idiopathic epiretinal membranes. Am J Ophthalmol. 1988;105:160–164 [DOI] [PubMed] [Google Scholar]
- 23. Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139:302–310 [DOI] [PubMed] [Google Scholar]
- 24. Melberg NS, Thomas MA. Nuclear sclerotic cataract after vitrectomy in patients younger than 50 years of age. Ophthalmology. 1995;102:1466–1471 [DOI] [PubMed] [Google Scholar]
- 25. Novak MA, Rice TA, Michels RG, Auer C. The crystalline lens after vitrectomy for diabetic retinopathy. Ophthalmology. 1984;91:1480–1484 [DOI] [PubMed] [Google Scholar]
- 26. Van Effenterre G, Ameline B, Campinchi F, Quesnot S, Le Mer Y, Haut J. Is vitrectomy cataractogenic?—study of changes of the crystalline lens after surgery of retinal detachment (in French). J Fr Ophtalmol. 1992;15:449–454 [PubMed] [Google Scholar]
- 27. Truscott RJW. Age-related nuclear cataract–oxidation is the key. Exp Eye Res. 2004;80:709–725 [DOI] [PubMed] [Google Scholar]
- 28. Truscott RJ, Augusteyn RC. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim Biophys Acta. 1977;492:43–52 [DOI] [PubMed] [Google Scholar]
- 29. Alder VA, Cringle SJ. Vitreal and retinal oxygenation. Graefes Arch Clin Exp Ophthalmol. 1990;228:151–157 [DOI] [PubMed] [Google Scholar]
- 30. Alder VA, Niemeyer G, Cringle SJ, Brown MJ. Vitreal oxygen tension gradients in the isolated perfused cat eye. Curr Eye Res. 1986;5:249–256 [DOI] [PubMed] [Google Scholar]
- 31. Berkowitz BA, Wilson CA, Hatchell DL, London RE. Quantitative determination of the partial oxygen pressure in the vitrectomized rabbit eye in vivo using 19F NMR (published correction appears in Magn Reson Med. 1991;22(2):512). Magn Reson Med. 1991;21:233–241 [DOI] [PubMed] [Google Scholar]
- 32. Alvord LA, Hall WJ, Keyes LD, Morgan CF, Winterton LC. Corneal oxygen distribution with contact lens wear. Cornea. 2007;26:654–664 [DOI] [PubMed] [Google Scholar]
- 33. Bonanno JA, Stickel T, Nguyen T, et al. Estimation of human corneal oxygen consumption by noninvasive measurement of tear oxygen tension while wearing hydrogel lenses. Invest Ophthalmol Vis Sci. 2002;43:371–376 [PubMed] [Google Scholar]
- 34. Chhabra M, Prausnitz JM, Radke CJ. Diffusion and Monod kinetics to determine in vivo human corneal oxygen-consumption rate during soft contact-lens wear. J Biomed Mater Res. 2009;90:202–209 [DOI] [PubMed] [Google Scholar]
- 35. Shui Y-B, Holekamp NM, Kramer BC, et al. The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol. 2009;127(4):1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJW, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. J Physiol (Lond). 2004;559:883–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shui Y-B, Fu J-J, Garcia C, et al. oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest Ophthalmol Vis Sci. 2006;47:1571–1580 [DOI] [PubMed] [Google Scholar]
- 38. Kleinstein RN, Kwan M, Fatt I, Weissman BA. In vivo aqueous humor oxygen tension–as estimated from measurements on bare stroma. Invest Ophthalmol Vis Sci. 1981;21:415–421 [PubMed] [Google Scholar]
- 39. McLaren JW, Dinslage S, Dillon JP, Roberts JE, Brubaker RF. Measuring oxygen tension in the anterior chamber of rabbits. Invest Ophthalmol Vis Sci. 1998;39:1899–1909 [PubMed] [Google Scholar]
- 40. Brennan NA. Beyond flux: total corneal oxygen consumption as an index of corneal oxygenation during contact lens wear. Optom Vis Sci. 2005;82:467–472 [DOI] [PubMed] [Google Scholar]
- 41. Fatt I, Bieber MT. The steady-state distribution of oxygen and carbon dioxide in the in vivo cornea, I: the open eye in air and the closed eye. Exp Eye Res. 1968;7:103–112 [DOI] [PubMed] [Google Scholar]
- 42. Maurice DM. The Von Sallmann Lecture 1996: an ophthalmological explanation of REM sleep. Exp Eye Res. 1998;66:139–145 [DOI] [PubMed] [Google Scholar]
- 43. Hoper J, Funk R, Zagorski Z, Rohen JW. Oxygen delivery to the anterior chamber of the eye: a novel function of the anterior iris surface. Curr Eye Res. 1989;8:649–659 [DOI] [PubMed] [Google Scholar]
- 44. Helbig H, Hinz JP, Kellner U, Foerster MH. Oxygen in the anterior chamber of the human eye. Ger J Ophthalmol. 1993;2:161–164 [PubMed] [Google Scholar]
- 45. Braunagel SC, Yorio T. Aerobic and anaerobic metabolism of bovine ciliary process: effects of metabolic and transport inhibitors. J Ocul Pharmacol. 1987;3:141–148 [DOI] [PubMed] [Google Scholar]
- 46. Hoper J, Funk RH. Effect of epinephrine on local partial pressure of oxygen in the ciliary processes. Ophthalmic Res. 1993;25:265–272 [DOI] [PubMed] [Google Scholar]
- 47. Bill A. Blood circulation and fluid dynamics in the eye. Physiol Rev. 1975;55:383–417 [DOI] [PubMed] [Google Scholar]
- 48. Bill A, Tornquist P, Alm A. Permeability of the intraocular blood vessels. Trans Ophthalmol Soc U K. 1980;100:332–336 [PubMed] [Google Scholar]
- 49. Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984;68:113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Giblin FJ, Padgaonkar VA, Leverenz VR, et al. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp Eye Res. 1995;60:219–235 [DOI] [PubMed] [Google Scholar]
- 51. Harocopos GJ, Shui Y-B, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 2004;45:77–85 [DOI] [PubMed] [Google Scholar]
- 52. Holekamp NM. The vitreous gel: more than meets the eye. Am J Ophthalmol. 2010;149:32–36 [DOI] [PubMed] [Google Scholar]
- 53. Freddo TF, Bartels SP, Barsotti MF, Kamm RD. The source of proteins in the aqueous humor of the normal rabbit. Invest Ophthalmol Vis Sci. 1990;31:125–137 [PubMed] [Google Scholar]
- 54. Freddo TF. Shifting the paradigm of the blood-aqueous barrier. Exp Eye Res. 2001;73:581–592 [DOI] [PubMed] [Google Scholar]
- 55. Bert RJ, Caruthers SD, Jara H, et al. Demonstration of an anterior diffusional pathway for solutes in the normal human eye with high spatial resolution contrast-enhanced dynamic MR imaging. Invest Ophthalmol Vis Sci. 2006;47:5153–5162 [DOI] [PubMed] [Google Scholar]
- 56. Spector A, Ma W, Wang RR. The aqueous humor is capable of generating and degrading H2O2. Invest Ophthalmol Vis Sci. 1998;39:1188–1197 [PubMed] [Google Scholar]
- 57. Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond). 1987;1:204–210 [DOI] [PubMed] [Google Scholar]
- 58. Sacca SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007;84:389–399 [DOI] [PubMed] [Google Scholar]
- 59. Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21:714–727 [PubMed] [Google Scholar]
- 60. Fridovich I. Oxygen toxicity: a radical explanation. J Exp Biol. 1998;201:1203–1209 [DOI] [PubMed] [Google Scholar]
- 61. Cho H-Y, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006;8:76–87 [DOI] [PubMed] [Google Scholar]
- 62. Jackson RM. Pulmonary oxygen toxicity. Chest. 1985;88:900–905 [DOI] [PubMed] [Google Scholar]
- 63. Kazzaz JA, Xu J, Palaia TA, Mantell L, Fein AM, Horowitz S. Cellular oxygen toxicity: oxidant injury without apoptosis. J Biol Chem. 1996;271:15182–15186 [DOI] [PubMed] [Google Scholar]
- 64. Riley DJ, Berg RA, Edelman NH, Prockop DJ. Prevention of collagen deposition following pulmonary oxygen toxicity in the rat by cis-4-hydroxy-l-proline. J Clin Investig. 1980;65:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]