The authors have developed a new method to isolate Schlemm's canal endothelial cells from pig eyes.
Abstract
Purpose.
The authors sought to develop a technique for isolating and culturing angular aqueous plexus (AAP) cells from more plentiful porcine eyes. AAP is an analogue of Schlemm's canal.
Methods.
Cells were differentially selected with puromycin, a toxin often used to select brain microvascular endothelial cells based on the expression of P-glycoprotein (P-gp), a multidrug resistance efflux pump. Trabecular meshwork containing AAP was dissected and pooled from fresh porcine eyes, digested in collagenase I, washed, filtered, and cultured for 8 days in a gelatin-coated plastic flask. Cells were then selected by exposure to 4 μg/mL puromycin for 2 days in the culture medium. Cells were fixed and immunostained for P-gp, ICAM II, von Willebrand factor (vWF), VE-cadherin, and α-smooth muscle actin (α-SMA).
Results.
Histology of the limbus showed that the dissection was limited to the trabecular meshwork region, including the AAP. Before puromycin treatment, cells appeared heterogeneous and polygonal, suggestive of a mixed population. More than 90% of the cells were removed by puromycin, leaving a population that appeared uniformly cobblestone-like when grown to confluence and that was contact inhibited. Puromycin-selected cells stained positively for the endothelial markers ICAM II, vWF, and VE-cadherin but negatively for α-SMA, consistent with staining patterns in whole tissue.
Conclusions.
Based on marker expression, morphology, and behavior in culture, puromycin-selected cells from porcine outflow tissues are AAP endothelial cells. Thus, porcine eyes can provide a plentiful alternative cell source for studying Schlemm's canal biology related to ocular hypertension.
The endothelium of Schlemm's canal is a continuous cell monolayer, and all aqueous humor must cross this endothelium as it drains through the conventional outflow pathway. Direct pressure measurements1,2 and other studies suggest that the inner wall of Schlemm's canal (SC) and the underlying juxtacanalicular tissues are the primary sites of outflow resistance.3,4 These and other findings5,6 have motivated studying SC cells in culture, with the goal of understanding their role in ocular hypertension, the major risk factor for primary open angle glaucoma.
In vitro studies of Schlemm's canal endothelial cells have provided insight into the barrier function7 and signaling mechanisms8 of this endothelium. Unfortunately, because of the relatively small number of cells per eye and their physical location, it is difficult to isolate and culture SC endothelial cells, making in vitro studies of these cells challenging. Human SC cells have been harvested by inserting sutures into SC and subsequently culturing the attached cells9 or by immunopanning to select CD31+ SC cells from mixed trabecular meshwork cultures.8 These techniques are not straightforward and have not been used in nonhuman eyes. Unfortunately, there is a decline in the availability of human donor eyes,10 and donors are often advanced in age, which limits the amount of cells available for experiments. Because of this low supply of human donor eyes and the availability of transgenic animals, it would be advantageous to be able to isolate and culture SC cells from the eyes of other species.
In the commonly used nonhuman, nonprimate eyes (e.g., in ungulates), the morphology of the duct that collects aqueous humor is different from that in humans. In animals it is more tortuous and discontinuous and is known properly as the angular aqueous plexus (AAP). This tortuosity and discontinuity make the insertion of a suture into the AAP extremely technically demanding. However, the AAP is functionally similar to SC; for example, both drain aqueous humor and the lining cells of both demonstrate pressure-dependent giant vacuoles. The goal of this study was, therefore, to isolate and culture AAP cells as a surrogate cell source for understanding SC cellular physiology. We present an approach involving the selection of AAP cells based on their ability to resist the toxic effects of puromycin.
Puromycin is an aminonucleoside antibiotic produced by Streptomyces alboniger. Cells that express p-glycoprotein (P-gp), an adenosine triphosphate–binding cassette transporter, show resistance to puromycin toxicity. Microvascular endothelial cells express P-gp; thus, puromycin exposure has been successfully used to select microvascular endothelial cells from mixed populations.11,12 Because AAP endothelial cells have similarities to microvascular endothelial cells,13 we hypothesized that they could be isolated from surrounding cells using puromycin treatment.
Materials and Methods
Primary mixed cultures of porcine trabecular meshwork (TM) cells (including AAP cells) were established after an enzymatic digestion approach similar to that described by Karl et al.8 Then the mixed populations of cells were treated with puromycin to select AAP endothelial cells.
Porcine eyes from 4- to 6-month-old pigs obtained from a local abattoir were stored at 4°C to 8°C in Hanks balanced salt solution supplemented with 200 U/mL penicillin, 200 μg/mL streptomycin, 5 μg/mL amphotericin B, and 100 μg/mL gentamicin and were used within approximately 24 hours of death. Dulbecco's modified Eagle's medium (DMEM), endothelial cell growth factor, newborn calf serum, heparin, penicillin, streptomycin, amphotericin, gentamicin, 2% gelatin solution, phosphate-buffered saline (PBS), sterile Hanks balanced salt solution with sodium bicarbonate, 1× trypsin EDTA solution, Triton X-100, and Hoechst 33258 were all purchased from Sigma (Poole, UK). Collagenase I was obtained from Worthington (Reading, UK). The cell strainer (40 μm; Falcon) was from Becton Dickinson (Oxford, UK), UK. Platelet-poor plasma-derived serum was sourced from Biomedical Technologies, Inc. (Stoughton, MA). Puromycin was from InvivoGen (San Diego, CA). Antiseptic solution was from Adams Healthcare (Videne; Leeds, UK). von Willebrand factor (vWF) rabbit polyclonal antibody, VE-cadherin rabbit polyclonal antibody for cell labeling, and α-smooth muscle actin rabbit polyclonal antibody were from Abcam (Cambridge, UK). ICAM II rabbit polyclonal antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). CD31 mouse anti-pig monoclonal antibody was from AbD Serotec (Kidlington, UK). VE-cadherin monoclonal rabbit for whole mount staining was from Cell Signaling Technology (Danvers, MA). P-gp antibody was from Abcam (Cambridge, MA). Nucleic acid stain (Sytox) was from Invitrogen (Burlington, ON, Canada).
Primary Cell Culture
Porcine eyes were cleaned of extraocular fat, muscles, and connective tissue and were then briefly disinfected in antiseptic solution (Videne; Adams Healthcare). The eyes were hemisected and the vitreous humor, lens, and iris were removed by gentle traction. The corneal endothelium and iris root were scraped away using a surgical blade under a dissecting microscope. The trabecular meshwork tissue was freed by gentle grasping with fine forceps and by cutting circumferentially around the eye along the external aspect of the meshwork with fine curved scissors. Freed tissue from six eyes (four quadrants per eye) was pooled to create a large enough population of cells for subsequent selection. In several eyes, the tissue was fixed either before or after the dissection and was then processed for paraffin sectioning and hematoxylin and eosin staining.
The pooled tissue was incubated with 1 mg/mL collagenase I for 1 hour at 37°C in complete culture medium, consisting of DMEM supplemented with 5% fetal bovine serum, 5% newborn calf serum, 5 mM l-glutamine, 10 μg/mL endothelial cell growth factor, 90 μg/mL heparin, 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL amphotericin B, and 50 μg/mL gentamicin. The preparation was then centrifuged at 300g for 5 minutes, the supernatant was aspirated, and the cell pellet was resuspended in complete culture medium. This procedure was repeated, and then cells were filtered through a 40-μm cell strainer and plated into a 2% gelatin-coated T25 flask. Cells were then allowed to multiply until they were nearly confluent (typically 8 days), with media changes every 2 days. After the primary cells were near confluence, we selected AAP cells using the protocol described below.
Isolation of AAP Cells by Puromycin Treatment
Mixed cultures were exposed to puromycin to select for AAP cells. In preliminary studies, we investigated a range of puromycin concentrations (0.1–10 μg/mL) and exposure durations (1–4 days) and a range of starting cell densities (corresponding to 1 to 12 days in primary culture) to optimize the viability and purity of the selected cells. Too high a concentration or duration of puromycin treatment or too short a culture time before puromycin treatment reduced cell viability and inhibited cell growth. The following conditions were found to be optimal: after 8 days of mixed primary culture, media were removed from the cells and replaced by media with added 4 μg/mL puromycin for 2 days. During this step, the media were as described except that the fetal bovine serum was replaced with platelet poor plasma-derived serum. In our hands, this reduced contaminating cells and helped keep endothelial cells alive. After 2 days, the media were removed and replaced by complete culture medium.
Characterization of Selected Cells
Cells were characterized by immunolabeling for ICAM II, VE-cadherin, vWF, CD31, and α-smooth muscle actin. Cells were seeded onto glass coverslips that were precoated with 2% gelatin solution. After reaching confluence, the cells were fixed with 4% formaldehyde overnight and then washed three times with PBS. Cells were blocked in goat serum for 1 hour at room temperature and permeabilized with 0.2% Triton X-100 for 5 minutes. Cells were then incubated with antibodies against vWF (1:1000), ICAM II (1:400), VE-cadherin (1:100), or α-smooth muscle actin (1:100) overnight at 4°C. Porcine aortic endothelial cells (PAEC) were used as a positive control for endothelial-specific markers, whereas porcine corneal fibroblasts (PCFs) were used as a negative control. These cells were isolated according to established methods.14,15 For P-gp labeling, calf liver was used as a positive control. For a secondary antibody negative control, the primary antibody was omitted and replaced by a blocking solution containing 20% goat serum. Cells were washed in PBS and incubated with Alexa488-conjugated goat anti-rabbit IgG solution (1:400). In some preparations, Hoechst 33258 was also added to label nuclei. After incubation for 2 hours at room temperature, cells were washed three times with PBS and observed with a confocal microscope (Leica, Milton Keynes, UK).
The root of the iris is located near the trabecular meshwork. Because it contains vascular endothelial cells that could be selected by the techniques described here, we were concerned that iris vascular cells could contaminate our AAP culture. To test this possibility, we repeated the puromycin selection using a single iris that had been removed from the eye by gentle traction. Cells from the mixed iris culture were treated with puromycin in the same way APP cells were selected. We also stained an iris culture that had not been exposed to puromycin for the markers listed.
In addition to immunostaining of cultured cells, we labeled anterior segments of porcine eyes using endothelial-specific IgG, which served as a reference for cell culture labeling patterns. Fresh eyes were received from the abattoir and opened at the equators. Anterior segments were fixed by immersion in 4% formaldehyde, and then small wedges of outflow tissue were dissected. Thick sections through the outflow track were cut with a razor blade, stained for the markers listed, and imaged with a confocal microscope using techniques similar to those previously described.16
Culture Purity
Confocal images of vWF and Hoechst-stained cells were analyzed using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). The number of nuclei in an image was quantified by an established method,17 but vWF-positive cells were counted manually. In each image, the percentage of AAP cells was equal to the number of vWF-positive cells divided by nuclei counts and multiplied by 100%.
Results
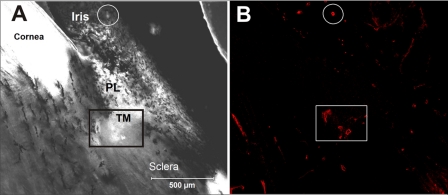
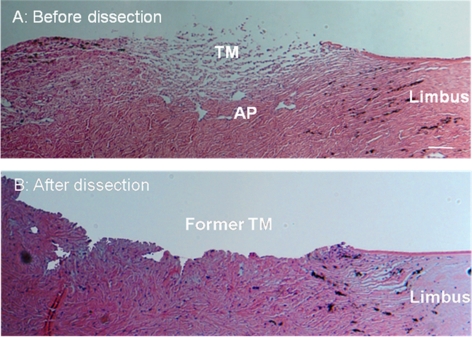
Because the anatomy of the porcine outflow tract is different from that of the human, we first carried out histology to verify that endothelial-specific immunolabeling (VE-cadherin) was localized to the expected region of the outflow tract (Fig. 1). We observed labeling of individual vessels adjacent to and within the trabecular meshwork; these vessels had irregularly shaped lumens consistent with previous descriptions of the porcine AAP18 (see also Movies S1–S3, http://www.iovs.org/cgi/content/full/51/11/5744/DC1). Labeling of oval lumens, as expected for blood vessels, was also observed within the iris, ciliary body, and sclera. In our microdissection, we were, therefore, very careful to remove vascularized tissues and selectively isolate the trabecular meshwork. Histologic examination showed that our dissection procedure successfully harvested trabecular meshwork and AAP tissue (Fig. 2) without extending deeply into the sclera.
Figure 1.
Overview of porcine anterior segment structures, imaged from a thick sagittal section of outflow tissues. (A) Transmitted light image through thick section. Box: region in which AAP lumens were identified. Circle: iridial vessel. (B) Immunolabeled image of same field of view stained for an endothelial-specific marker (VE-cadherin). AAP lumens can be clearly seen adjacent to and within the trabecular meshwork region. Image is representative of five examined. PL, pectinate ligaments; TM, trabecular meshwork.
Figure 2.
Hematoxylin and eosin–stained paraffin-embedded sagittal sections of the angle region from porcine eyes. (A) Sample with the iris removed and with the corneal endothelium and iris root scraped but with the trabecular meshwork and aqueous plexus region untouched. (B) Sample after harvesting the trabecular meshwork and aqueous plexus tissue for cell culture, showing tissues that were cut away during dissection. Scale bar, 100 μm.
After enzymatic digestion, freshly isolated cells from the tissue explants attached to the gelatin-coated cell culture flask within several hours of seeding. Cultures initially contained a mixture of cells with various morphologies and grew rapidly in vitro, reaching confluence within 12 days.
Puromycin Exposure to Select AAP Cells
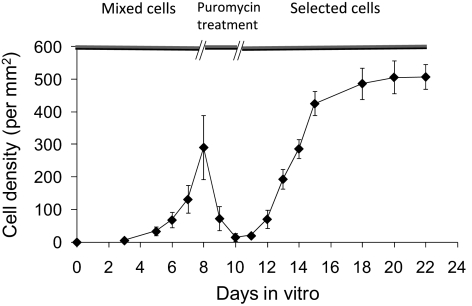
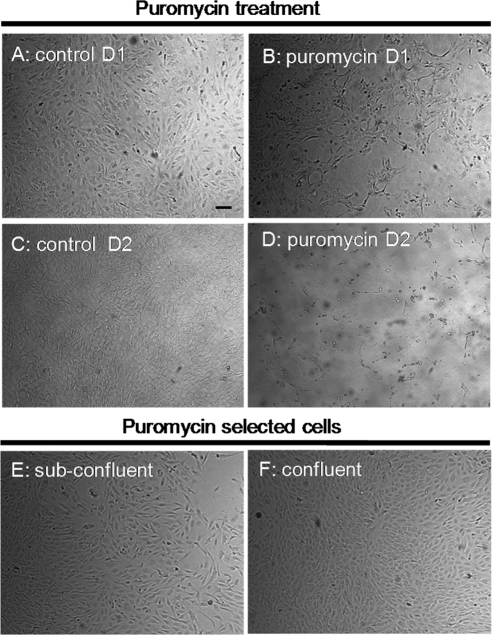
Compared with control conditions (no puromycin added to media), approximately two-thirds of the cells died after 1 day of puromycin exposure (Figs. 3, 4A, 4B); after 2 days of exposure, most of the cells were dead (Figs. 3, 4C, 4D). The surviving cells started to repopulate flasks when the puromycin was removed and appeared healthy, becoming confluent approximately 10 days after puromycin exposure was stopped. Similar to that expected for endothelial cells, selected cells were contact inhibited (Fig. 3) with a polygonal morphology, forming a cobblestone-like monolayer (Figs. 4E, 4F). Interestingly, cells selected by puromycin strongly expressed P-gp (Fig. 5).
Figure 3.
Growth curve (mean ± SD) for primary mixed culture of trabecular meshwork/angular aqueous plexus porcine cells before, during, and after puromycin treatment (n = 3 replicates of experiment).
Figure 4.
Phase-contrast microscopy of porcine cells from outflow tissues after exposure to puromycin. Approximately two-thirds of the cells died on treatment with puromycin (B) for 1 day (D1) compared with control (A). After 2 days (D2) of exposure, the number of dead cells increased significantly (D) compared with control cells (C). After the selection process, the remaining cells appeared healthy, both at 6 days (E) and at 10 days (F) after the removal of puromycin. At confluence, the selected cells displayed a cobblestone appearance (F). Shown are images from 1 of 6 experiments. All images are shown at the same magnification. Scale bar, 50 μm.
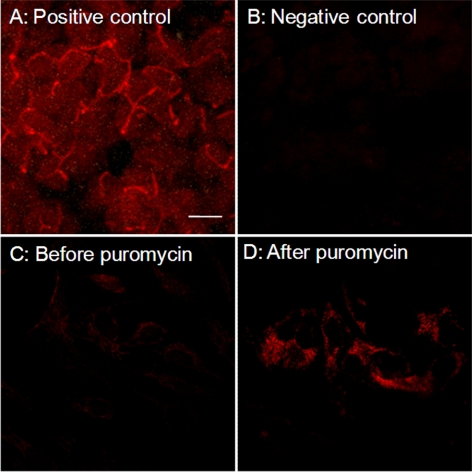
Figure 5.
After puromycin treatment, P-gp expression was observed in the cells. Positive control shows P-gp expression in calf liver (A). Negative control shows background fluorescence in the absence of primary antibody (B). The mixed population of cultured cells before puromycin treatment did not express P-gp at detectable levels (C). In contrast, immediately after puromycin treatment for 2 days, the selected cells strongly expressed P-gp (D). Shown are images of 1 of 3 experiments. All images are shown at the same magnification. Scale bar, 20 μm.
Characterization of Selected Cells
Puromycin-selected cells expressed the endothelial marker proteins ICAM II, VE-cadherin, and vWF (Fig. 6); these markers were also expressed by AAP cells in sections through the outflow tissues of porcine eyes (Fig. 7). Importantly, vWF and VE-cadherin expression in whole outflow tissue was specific to AAP cells (Fig. 7). α-Smooth muscle actin was not expressed in the selected cells, nor was CD31 (data not shown). Based on vWF and Hoechst staining, puromycin selection resulted in cultures with extremely high purities of 98.6% ± 1.57% (n = 6).
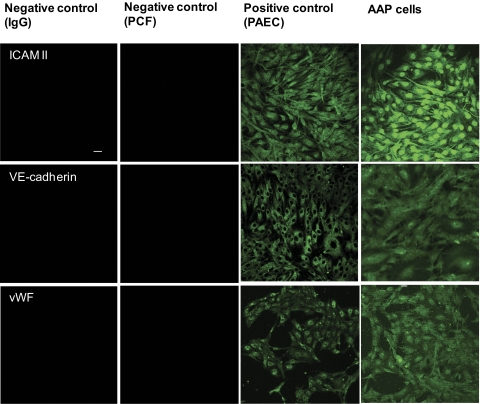
Figure 6.
Expression of endothelial marker proteins by puromycin-selected AAP cells. Selected cells express the typical endothelial markers ICAM II, VE-cadherin, and vWF. For comparison, we used PAECs as positive control and PCFs as negative control. In addition, background labeling was monitored by omission of the primary antibody (left column). Shown are representative images of 1 of 6 experiments. All the images are shown at the same magnification. Scale bar, 50 μm.
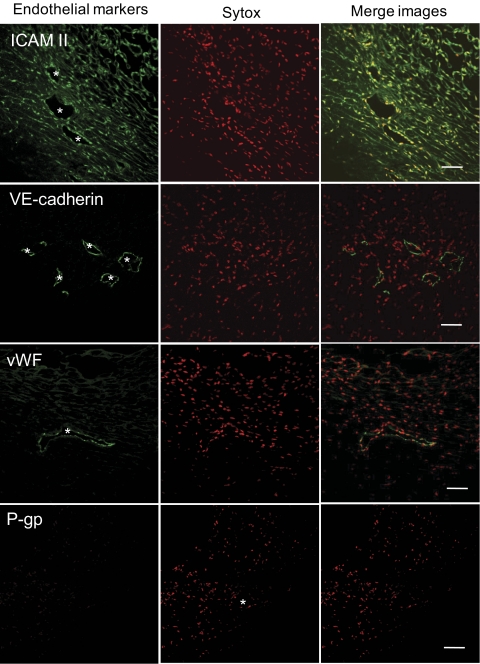
Figure 7.
Expression of endothelial marker proteins in confocal sections through porcine aqueous plexus region. Confocal immunofluorescence microscopy shows expression of ICAM II, VE-cadherin, and vWF by aqueous plexus lining cells. Asterisks: AP lumen. We were unable to detect P-gp expression using this methodology. Endothelial marker protein (top left of each image) is shown in green, and nuclei are shown in red. Data are representative images from three porcine eyes analyzed. Scale bars, 50 μm.
Because of pectinate ligament connections with outflow tissues, we were concerned that purified cultures might be contaminated by iris endothelial cells given the inadvertent inclusion of iris remnants during the dissection process. However, cells from iris cultures had a morphology that was very different from that of puromycin-selected AAP cells; iris cell cultures were more pigmented and displayed a mixture of cell shapes (Fig. 8). In addition, after 2 days of puromycin treatment, almost all the cells died, and the iris culture could not be recovered. For the iris culture that had not been exposed to puromycin, we did not observe expression of the endothelial-specific markers vWF, VE-cadherin, or ICAM II (Fig. 8).
Figure 8.
Phase-contrast and confocal microscopy of the isolated iris cells that were not exposed to puromycin. Iris cells appear more pigmented and display a mixture of morphologic features (left). Moreover, we were unable to detect any of the endothelial markers expressed by AAP cells (i.e., vWF). Shown are representative images of 1 of 3 experiments. All images are shown at the same magnification. Scale bar, 100 μm.
Discussion
In this study, we describe a puromycin-based selection method to produce high-purity cultures of AAP cells from the porcine eyes. Multiple lines of evidence indicate that for several reasons, the puromycin-selected cells were in fact AAP cells.
First, selected cells exhibited a polygonal morphology in subconfluent cultures, similar to that reported by Karl et al.8 for human SC cells. At confluence, however, selected cells displayed a cobblestone appearance (Fig. 4). This is different from the fusiform morphology reported of human SC cells at confluence,9 which could be due to differences in species or age.
Second, selected cells exhibited contact inhibition on reaching confluence (Fig. 3), consistent with the behavior reported for human SC cells.9
Third, selected cells expressed the same surface markers as those seen in cultured human SC cells and in whole porcine tissue. Specifically, selected cells expressed the typical endothelial markers VE-cadherin, vWF and ICAM II (Fig. 6). This marker profile is the same as reported in cultured human SC cells,19,20 including the observation that CD31 is downregulated in primary cultures of human SC cells,9 (although this latter observation is consistent with the possibility that CD31 is not expressed in the porcine AAP). It is also broadly consistent with in situ expression profiles of AAP cells (Fig. 7), particularly the finding that vWF and VE-cadherin are specifically expressed by porcine AAP cells.
Fourth, by definition, selected cells survived puromycin treatment, which is consistent with other microvascular endothelial cells (e.g., brain capillary) that are regularly selected using puromycin.11,12 In addition, after puromycin treatment, cell P-gp levels were higher than in untreated cells (Fig. 5) and native porcine tissue (Fig. 7).
Fifth, control experiments effectively ruled out contamination by iris microvascular endothelial cells and corneal fibroblasts (keratocytes), two obvious sources of unwanted cells that did not tolerate puromycin, express the same marker profile, or exhibit the same morphology as AAP cells.
Both microvascular endothelial cells and APP cells locate in the region where the exchange of fluids takes place: the former lines the blood-brain barrier and the latter lines the blood-aqueous barrier.13 Therefore, we hypothesized that we could select AAP cells using techniques similar to those for selecting cerebral microvascular endothelial cells. Indeed, similar to the selection of microvascular endothelial cells, our titration experiments showed that 2 days of 4 μg/mL puromycin treatment allowed the survival of AAP endothelial cells while effectively killing other cell populations.11,12 The technique was aided by the use of platelet-poor plasma-derived serum instead of fetal bovine serum; the former has been shown to increase endothelial culture purity by suppressing the growth of smooth muscle cells.11,21,22
It is interesting that we could not observe P-gp staining either in the outflow tissue or in cultured cells before selection, yet the cells surviving after puromycin treatment strongly expressed P-gp. There are at least two possible explanations. Puromycin might have selected for a subpopulation of AAP cells with initially higher P-gp expression,12,23 but, because these cells were rare in the mixed culture, we could not observe any significant P-gp staining before puromycin treatment. Alternatively, this observation was consistent with puromycin induction of P-gp expression.24 P-gp acts as an efflux pump that confers drug resistance; as a result, the high expression levels or upregulated expression of P-gp prevented the entry and accumulation of toxic puromycin into the cytoplasm of endothelial cells.
Puromycin-based selection of AAP cells overcomes several limitations of existing methods by successfully and reliably isolating large numbers of high-purity cells in a relatively short amount of time. The current gold standard for SC cell isolation in human eyes involves culturing a gelatin-coated suture threaded through the lumen of SC.9 Although this method yields adequate cell numbers, culture times may exceed several weeks and 50% or more of the attempts may be unsuccessful. Alternatively, an immunopanning technique against CD31 achieves rapid yields but limited cell numbers.8 In contrast, puromycin-based selection of porcine AAP cells, in our hands, yielded viable cell strains in almost all attempts, and each attempt yielded an adequate number of cells for further culture and experimentation (one 25-cm2 flask) in 2 to 3 weeks. The endothelial-specific markers were examined on cells from passages 1 and 2, during which time cell morphology and labeling profiles were consistent. We have not tested labeling profiles beyond passage 2, though we observed no significant changes in cell morphology up to passage 4. For experiments, we did not use cells beyond passage 2 because porcine eyes were plentiful and this procedure had a good yield so that it was possible to obtain many cells even at low passage numbers. Future work should examine whether puromycin-based selection can be used to improve the yield of human SC cells.
Several factors motivated our choice to use porcine tissue as a source of AAP cells. The pig is phylogenetically close to the human, and porcine eyes share similarities with human eyes, including physical size and magnitude of outflow facility.18,25 Porcine eyes have been widely used as a model for studying glaucoma,25–28 aqueous humor outflow physiology,28–30 and chronic intraocular pressure elevation,18,25 and porcine eyes exhibit physiological similarities to human eyes (e.g., reduced facility in response to TGF-β229). At the ultrastructural level, the porcine aqueous plexus exhibits an elastic cribriform network in the juxtacanalicular region, a discontinuous endothelial basement membrane, and giant vacuoles that appear functionally similar to those structures in primate eyes.29 Although supplies of human eye tissue are dwindling,10 fresh porcine tissue is readily available, and porcine eyes already serve as the primary source of nonhuman trabecular meshwork cells for research.31–33 To our knowledge, this is the first method to isolate nonhuman AAP cells.
In summary, we have developed a reliable and efficient method to isolate pure populations of AAP cells from porcine eyes using a puromycin-based selection procedure. This method overcomes time and cell-yield limitations of previous techniques and exploits fresh porcine tissue, a plentiful alternative to human donor tissue. Given that AAP cells (together with TM cells) are responsible for regulating aqueous humor outflow and intraocular pressure, this method provides a valuable model for ongoing research in glaucoma.
Supplementary Material
Acknowledgments
The authors thank Lorraine Lawrence (Imperial College London) for histology support and Patrick Turowski (Institute of Ophthalmology, London) for stimulating discussions leading up to this project.
Footnotes
Supported by a Royal Society Wolfson Foundation Research Excellence Award (CRE), National Institutes of Health Grant R01 EY17007 (WDS, CRE), American Health Assistance Foundation (DRO), and Research to Prevent Blindness (WDS).
Disclosure: Y. Lei, None; D.R. Overby, None; A.T. Read, None; W.D. Stamer, None; C.R. Ethier, None
References
- 1. Maepea O, Bill A. The pressures in the episcleral veins, Schlemm's canal and the trabecular meshwork in monkeys: effects of changes in intraocular pressure. Exp Eye Res. 1989;49:645–663 [DOI] [PubMed] [Google Scholar]
- 2. Maepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res. 1992;54:879–883 [DOI] [PubMed] [Google Scholar]
- 3. Ethier CR, Coloma FM, de Kater AW, Allingham RR. Retroperfusion studies of the aqueous outflow system, 2: studies in human eyes. Invest Ophthalmol Vis Sci. 1995;36:2466–2475 [PubMed] [Google Scholar]
- 4. Ethier CR. The inner wall of Schlemm's canal. Exp Eye Res. 2002;74:161–172 [DOI] [PubMed] [Google Scholar]
- 5. Johnson M, Chan D, Read AT, Christensen C, Sit A, Ethier CR. The pore density in the inner wall endothelium of Schlemm's canal of glaucomatous eyes. Invest Ophthalmol Vis Sci. 2002;43:2950–2955 [PubMed] [Google Scholar]
- 6. Allingham RR, de Kater AW, Ethier CR, Anderson PJ, Hertzmark E, Epstein DL. The relationship between pore density and outflow facility in human eyes. Invest Ophthalmol Vis Sci. 1992;33:1661–1669 [PubMed] [Google Scholar]
- 7. Burke AG, Zhou W, O'Brien ET, Roberts BC, Stamer WD. Effect of hydrostatic pressure gradients and Na2EDTA on permeability of human Schlemm's canal cell monolayers. Curr Eye Res. 2004;28:391–398 [DOI] [PubMed] [Google Scholar]
- 8. Karl MO, Fleischhauer JC, Stamer WD, et al. Differential P1-purinergic modulation of human Schlemm's canal inner-wall cells. Am J Physiol Cell Physiol. 2005;288:C784–C794 [DOI] [PubMed] [Google Scholar]
- 9. Stamer WD, Roberts BC, Howell DN, Epstein DL. Isolation, culture, and characterization of endothelial cells from Schlemm's canal. Invest Ophthalmol Vis Sci. 1998;39:1804–1812 [PubMed] [Google Scholar]
- 10. Curcio CA. Declining availability of human eye tissues for research. Invest Ophthalmol Vis Sci. 2006;47:2747–2749 [DOI] [PubMed] [Google Scholar]
- 11. Calabria AR, Weidenfeller C, Jones AR, de Vries HE, Shusta EV. Puromycin-purified rat brain microvascular endothelial cell cultures exhibit improved barrier properties in response to glucocorticoid induction. J Neurochem. 2006;97:922–933 [DOI] [PubMed] [Google Scholar]
- 12. Perriere N, Demeuse P, Garcia E, et al. Puromycin-based purification of rat brain capillary endothelial cell cultures: effect on the expression of blood-brain barrier-specific properties. J Neurochem. 2005;93:279–289 [DOI] [PubMed] [Google Scholar]
- 13. Ramos RF, Hoying JB, Witte MH, Daniel Stamer W. Schlemm's canal endothelia, lymphatic, or blood vasculature? J Glaucoma. 2007;16:391–405 [DOI] [PubMed] [Google Scholar]
- 14. Beales MP, Funderburgh JL, Jester JV, Hassell JR. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999;40:1658–1663 [PubMed] [Google Scholar]
- 15. Bogle RG, Baydoun AR, Pearson JD, Mann GE. Regulation of L-arginine transport and nitric oxide release in superfused porcine aortic endothelial cells. J Physiol. 1996;490(pt 1):229–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Read AT, Chan DW, Ethier CR. Actin structure in the outflow tract of normal and glaucomatous eyes. Exp Eye Res. 2007;84:214–226 [DOI] [PubMed] [Google Scholar]
- 17. Lei Y, Garrahan N, Hermann B, et al. Topography of neuron loss in the retinal ganglion cell layer in human glaucoma. Br J Ophthalmol. 2009;93:1676–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McMenamin PG, Steptoe RJ. Normal anatomy of the aqueous humour outflow system in the domestic pig eye. J Anat. 1991;178:65–77 [PMC free article] [PubMed] [Google Scholar]
- 19. Heimark RL, Kaochar S, Stamer WD. Human Schlemm's canal cells express the endothelial adherens proteins, VE-cadherin and PECAM-1. Curr Eye Res. 2002;25:299–308 [DOI] [PubMed] [Google Scholar]
- 20. Krohn J. Expression of factor VIII-related antigen in human aqueous drainage channels. Acta Ophthalmol Scand. 1999;77:9–12 [DOI] [PubMed] [Google Scholar]
- 21. Bowman PD, Betz AL, Goldstein GW. Primary culture of microvascular endothelial cells from bovine retina: selective growth using fibronectin coated substrate and plasma derived serum. In Vitro. 1982;18:626–632 [DOI] [PubMed] [Google Scholar]
- 22. Abbott NJ, Hughes CC, Revest PA, Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci. 1992;103(pt 1):23–37 [DOI] [PubMed] [Google Scholar]
- 23. Male DK. Expression and induction of p-glycoprotein-1 on cultured human brain endothelium. J Cereb Blood Flow Metab. 2009;29:1760–1763 [DOI] [PubMed] [Google Scholar]
- 24. Demeuse P, Fragner P, Leroy-Noury C, et al. Puromycin selectively increases mdr1a expression in immortalized rat brain endothelial cell lines. J Neurochem. 2004;88:23–31 [DOI] [PubMed] [Google Scholar]
- 25. Ruiz-Ederra J, Garcia M, Hernandez M, et al. The pig eye as a novel model of glaucoma. Exp Eye Res. 2005;81:561–569 [DOI] [PubMed] [Google Scholar]
- 26. Shaarawy T, Wu R, Mermoud A, Flammer J, Haefliger IO. Influence of non-penetrating glaucoma surgery on aqueous outflow facility in isolated porcine eyes. Br J Ophthalmol. 2004;88:950–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liton PB, Lin Y, Luna C, Li G, Gonzalez P, Epstein DL. Cultured porcine trabecular meshwork cells display altered lysosomal function when subjected to chronic oxidative stress. Invest Ophthalmol Vis Sci. 2008;49:3961–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci. 1999;40:74–81 [PubMed] [Google Scholar]
- 29. Bachmann B, Birke M, Kook D, Eichhorn M, Lutjen-Drecoll E. Ultrastructural and biochemical evaluation of the porcine anterior chamber perfusion model. Invest Ophthalmol Vis Sci. 2006;47:2011–2020 [DOI] [PubMed] [Google Scholar]
- 30. Yan DB, Trope GE, Ethier CR, Menon IA, Wakeham A. Effects of hydrogen peroxide-induced oxidative damage on outflow facility and washout in pig eyes. Invest Ophthalmol Vis Sci. 1991;32:2515–2520 [PubMed] [Google Scholar]
- 31. Bradley JM, Kelley MJ, Rose A, Acott TS. Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest Ophthalmol Vis Sci. 2003;44:5174–5181 [DOI] [PubMed] [Google Scholar]
- 32. Obazawa M, Mashima Y, Sanuki N, et al. Analysis of porcine optineurin and myocilin expression in trabecular meshwork cells and astrocytes from optic nerve head. Invest Ophthalmol Vis Sci. 2004;45:2652–2659 [DOI] [PubMed] [Google Scholar]
- 33. Vittal V, Rose A, Gregory KE, Kelley MJ, Acott TS. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2005;46:2857–2868 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.