The authors describe the isolation and characterization of a novel conditionally immortalized Müller cell line from the postnatal mouse retina. This cell line expresses both classical Müller glial and retinal stem cell genes and should prove valuable for in vitro analysis of the physiology and stem cell characteristics of Müller glia.
Abstract
Purpose.
Müller glia have multiple functions in the retina, including synthesis of neurotrophic factors, uptake and metabolism of neurotransmitters, spatial buffering of ions, maintenance of the blood-retinal barrier, and response to injury. A population of Müller glia has some stem cell-like characteristics both in vivo and in vitro. The purpose of this study was to generate and characterize novel Müller glial cell lines from the postnatal mouse retina.
Methods.
Cells were cultured from postnatal day (P) 10 double heterozygous transgenic (H-2Kb-tsA58/+; HRhoGFP/+) or C57BL/6 mice after papain dissociation. Interferon gamma (IFNγ) induction of the SV40 T-antigen (TAg) was assayed by immunohistochemistry and Western blot analysis. Proliferation was assayed by BrdU uptake and cell counts of calcein AM/ethidium bromide–stained cells. Gene expression was analyzed by RT-PCR and immunohistochemistry.
Results.
Conditionally immortalized (ImM10 [Immortmouse Müller P10]) and spontaneously immortalized (C57M10 [C57BL/6 Müller P10]) Müller glial cell lines were selected by differential adherence to laminin; both consisted of adherent flat cells with large, diffusely staining nuclei and an epithelial morphology. TAg induction stimulated BrdU uptake by Müller glia in mixed retinal cultures from H-2Kb-tsA58/+; HRhoGFP/+ mice and increased the proliferation of ImM10 cells. ImM10 and C57M10 cells expressed genes characteristic of Müller glia but not genes characteristic of differentiated retinal neurons. ImM10 cells also expressed retinal stem cell genes.
Conclusions.
The ImM10 cell line is a novel, conditionally immortalized Müller glial cell line isolated from the P10 mouse retina that expresses genes characteristic of Müller glial and retinal stem cells.
Müller glia, the radial glia of the neural retina, function to maintain retinal homeostasis through synthesis of neurotrophic factors, uptake and metabolism of neurotransmitters, spatial buffering of ions during retinal activity, and maintenance of the blood-retinal barrier.1,2 In the mature retina in mammals, Müller glia proliferate in the context of retinal injury or disease, contributing to gliosis and the formation of glial scars.3 In addition, a subset of Müller glia show some stem cell characteristics and upregulate stem cell-associated genes, although their inherent regenerative ability is extremely limited in vivo.3–5 In contrast, in teleost fish Müller glia are the source of retinal stem cells in the inner retina that generate rod photoreceptors during normal growth and regenerate all types of retinal neurons after injury.6–8 The robust neurogenic capacity of Müller glia in fish and, to a limited extent, in birds9 has sparked research to understand the stem cell properties and neurogenic potential of Müller glia in the mammalian retina both in vitro and in vivo.9–15
Cell culture is useful for studying basic cellular and molecular processes including cell-cell interactions, cell movements, and regulation of gene expression. The ability to generate large numbers of cells in vitro has utility for high-throughput assays for drug development and tissue engineering applications. For studies of differentiation and regeneration, in vitro culture systems enable precise control of the cellular environment and remove cells from inhibitory constraints that may limit their regenerative capacity in vivo. The use of primary cells for studying basic cellular and molecular processes is hampered by the low number of cells that can be isolated, difficulty in obtaining pure populations of cells, and lack of inherent proliferative capacity of adult mammalian retinal cells. To overcome these problems, immortalized cell lines have been generated from tumors (e.g., Y79 and Weri-Rb1 retinoblastoma cell lines16,17), by constitutive overexpression of exogenous oncogenes in primary cells18–20 or by spontaneous immortalization.21 The increased proliferative capacity of transformed cells enables the generation of large numbers of cells. However, permanent immortalization alters the innate characteristics of primary cells; if used for in vivo transplantation, there are concerns that unregulated proliferation could generate tumors.
Conditional immortalization using inducible oncogenes enables precise control over the timing of immortalization.22 H-2Kb-tsA58 transgenic mice (Immortomouse; Jackson Laboratories, Wilmington, MA; hereafter designated tsA58) carry a transgene encoding an inducible, thermolabile simian virus 40-large T-antigen (TAg) under control of the mouse major histocompatibility complex H-2kb class 1 promoter.23 Induction by interferon-gamma (IFNγ) upregulates TAg expression and immortalizes cells cultured at the permissive temperature (33°C). Removal of IFNγ represses transgene expression, and incubation at physiological temperatures (37°C-39°C) inactivates residual TAg, permitting differentiation. Conditionally immortalized cell lines have been generated from both neuronal and nonneuronal cells isolated from tsA58 mice and rats. Conditionally immortalized retinal cell lines described to date include rat Müller glia,24 retinal capillary endothelial cells25 and pericytes,26 and mouse retinal endothelial cells27 and astrocytes.28
To generate novel cell lines from the postnatal mouse retina, we isolated retinal cells from transgenic mice heterozygous for the tsA58 and HRhoGFP transgenes and from C57BL/6 mice. The HRhoGFP transgene encodes a human rhodopsin-GFP fusion protein under the control of the endogenous rhodopsin promoter29 and is expressed specifically in postmitotic rod photoreceptors. This enables longitudinal imaging of living photoreceptors in culture and could prove useful in future studies of the neurogenic potential of conditionally immortalized Müller glia. We report the isolation and characterization of two novel Müller glia cell lines from the postnatal day (P) 10 mouse retina: conditionally immortalized ImM10 (Immortmouse Müller postnatal day 10) and spontaneously immortalized C57M10 (C57BL/6 Müller postnatal day 10).
Methods
Mice
All mice were handled and euthanatized according to the animal care and use policies of the University of Houston and Johns Hopkins University School of Medicine and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The following strains were used: C57BL/6 (Jackson Laboratories), HRhoGFP29 (generous gift of Theodore Wensel), and H-2 Kb-tsA58 transgenic (tsA58; Immortomouse; Charles River Laboratories).23 Homozygous tsA58 males were bred to homozygous HRhoGFP females to generate double heterozygous mice (tsA58/+; HRhoGFP/+) used for retinal dissociation and cell culture.
Retinal Dissociation
P10 pups were euthanatized by halothane or CO2 inhalation, eyeballs were enucleated, and retinas were dissected free of the retinal pigment epithelium and dissociated by incubation in phosphate-buffered saline (PBS) containing 16.5 U/mL activated papain (Worthington, Lakewood, NJ) and 124 U/mL DNase (Sigma-Aldrich, St. Louis, MO) for 30 minutes at 37°C with gentle trituration. Digestion was stopped by the addition of ovomucoid (Worthington, Lakewood, NJ); cells were pelleted by centrifugation (800 rpm, 8 minutes) and resuspended in plating medium (Neurobasal containing B27 supplement, 20 mM l-glutamine, and penicillin/streptomycin antibiotics [Pen/Strep]). Cell culture media, supplements, and reagents were obtained from Gibco/Invitrogen (Carlsbad, CA) unless otherwise indicated.
Cell Culture
Dissociated primary retinal cells were plated on poly-d-lysine/laminin–coated multiwell plates or 100-mm culture dishes and maintained in cell culture incubators at 5.5% CO2. To select Müller glial cells from mixed cultures, papain-dissociated retinal cells were allowed to settle on 12-well dishes coated with poly-d-lysine/laminin for 3 hours; subsequently, nonadherent cells were transferred to empty poly-d-lysine/laminin coated wells and, after overnight incubation, remaining nonadherent cells were transferred to empty wells. After plating, adherent cells were maintained for 7 days at 37°C in plating medium under nonimmortalizing conditions and subsequently were transferred to immortalizing conditions in growth medium (GM; Neurobasal with 2% fetal bovine serum [FBS]; Gibco/Invitrogen) and incubated at 33°C with 100% media changes at 3- to 5-day intervals. For growth under immortalizing conditions, GM was supplemented with mouse recombinant IFNγ (PeproTech; Rocky Hill, NJ) at 50 U/mL, unless otherwise indicated. To eliminate tsA58 transgene expression (nonimmortalizing conditions), primary cultures of mixed retinal cells and ImM10 Müller cells were cultured in GM without IFNγ and incubated from 37°C to 39°C. Primary cells from C57BL/6 were isolated according to the same method but were maintained at 37°C in DMEM/F12 medium containing 10% FBS and antibiotics (Pen/Strep; Gibco/Invitrogen). During the initial isolation of both ImM10 and C57Ml0 Müller cells, we used poly-d-lysine/laminin–coated plates. For maintenance and subsequent analyses, cells were grown on uncoated tissue culture dishes.
BrdU Assay
Cells were plated in GM+IFNγ containing 10 μM BrdU and incubated at 33°C for 17 hours. Cells were washed in PBS, fixed in 10% formalin for 10 minutes, washed in PBS, and incubated in 2 N HCl in PBS for 30 minutes before immunostaining.
Immunohistochemistry
For immunostaining, tissues or cells were fixed in 4% paraformaldehyde or 10% formalin (for BrdU immunostaining) for 30 minutes. Samples for PAX2 immunostaining were fixed for 5 minutes in 4% paraformaldehyde. Samples were washed in PBS, incubated in 1% sodium borohydride (2 minutes), and blocked with either PBS containing 20% normal goat serum/0.5% Triton X-100 or PBS containing 10% normal goat serum/0.5% Triton X-100/1% fish gelatin/5% bovine serum albumin for 2 hours. Primary antibodies (Supplementary Table S1, http://www.iovs.org/cgi/content/full/51/11/5991/DC1) were applied overnight at 4°C. Secondary antibodies conjugated to AlexaFluor488, AlexaFluor543, AlexaFluor633 (Molecular Probes, Eugene, OR) or TRITC (Sigma, St. Louis, MO) were diluted 1:200 and incubated for 1 to 2 hours at room temperature. For BrdU labeling, a Cy3-conjugated primary antibody was used. Cells were counterstained with Hoechst 33342 or 4′, 6-diamidino-2-phenylindole (DAPI). Specificity of labeling was confirmed by omitting primary antibody or by substituting normal serum for the species used to generate the primary antibody. Immunostained cells were imaged with an inverted microscope (IX71; Olympus, Tokyo, Japan) with a monochrome, cooled CCD digital camera (Rolera-XR; Q-Imaging, Surrey, BC, Canada). Images were subsequently pseudocolored and adjusted for contrast using image editing software (Photoshop; Adobe, San Jose, CA). Images used for comparison of staining intensity (BrdU, SV40TAg, PAX2 immunostaining) were photographed at a uniform exposure setting and prepared as a montage with brightness and color adjusted simultaneously for all images.
Western Blot Analysis
ImM10 cells in GM containing 0 to 200 U/mL IFNγ were grown on 100-mm plates for 6 days at 33°C, with 100% media changes every 2 days. Cells were harvested in PBS and sonicated. Cell lysates were quantified by bicinchoninic acid assay (BCA-1; Sigma-Aldrich), diluted in Laemmli buffer (2× final concentration) and were loaded at 15 μg/lane onto duplicate polyacrylamide gels (Bio-Rad, Hercules, CA). After electrophoresis at 100 V, proteins were transferred onto nitrocellulose (NT8017; Sigma-Aldrich) by electroblotting and processed for immunodetection using anti-TAg antibodies (RDI; Fitzgerald Industries, Concord, MA) at 1:250 and enhanced chemiluminescence (Amersham Biosciences, Pittsburgh, PA). Duplicate gels run in parallel and stained (Gel-Code Blue; Pierce, Rockford, IL) were used to compare protein loading.
Cell Counts
Proliferation assays used on-chip flow cytometry (Bioanalyzer 2100; Agilent, Foster City, CA). ImM10 cells were plated in 12-well dishes at 1 × 105 cells per well on duplicate plates and were cultured at 33°C in GM+ IFNγ and at 39°C in GM without IFNγ. For cell counts, three individual wells for each condition were stained with 2.5 μM calcein AM and 2.5 μM ethidium bromide homodimer (Live-Dead Stain; Invitrogen) in PBS for 30 minutes. Cells were pelleted and resuspended in 30 μL cell buffer (cell kit #5067–1519; Agilent), and 10 μL resuspended cells from each sample was loaded into individual wells of a cell chip. Fluorescence on the two channels (calcein AM: excitation 470 nm/emission 535 nm; ethidium: excitation 630 nm/emission 680 nm) was read for 6 minutes per well. Wells that gave low counts in the calcein channel were examined microscopically, and those with bubbles or blockage in the microfluidics channels were repeated on a second chip using the rest of the sample. The threshold for calcein fluorescence intensity for a “live cell” (>0.7 × 101) was determined empirically using calcein staining of methanol-killed cells and unstained cells. For passages 4 to 7, the entire experiment was repeated twice; for passages 14 to 17, the entire experiment was repeated three times.
RNA Isolation and RT-PCR
RNA was harvested from ImM10 and C57M10 cells and whole mouse retina using affinity columns (RNeasy; Qiagen, Valencia, CA) according to the manufacturer's instructions. Briefly, cells were washed in RNase/DNase-free PBS, lysed in a guanidinium thiocyanate containing buffer, and sheared using a 20-gauge needle before loading onto an RNA affinity spin column. Total RNA was eluted in RNase-free water and quantified by spectrophotometry (Nanodrop; Thermo Scientific, Wilmington, DE), and quality was assessed (RNAnano Chip on a Bioanalyzer 2100; Agilent). RNA samples with quality scores >9.5 were used for analysis.
For analysis of gene expression by quantitative RT-PCR, total RNA (200 ng) was reverse transcribed using oligo-dT primers (Affinityscript; Stratagene, La Jolla, CA). Primers (IDT, Coralville, IA) were designed to target the 3′ end of genes and to span introns when possible (Supplementary Table S2, http://www.iovs.org/cgi/content/full/51/11/5991/DC1). Primers were optimized using total retinal RNA and were analyzed by melting curve analysis and gel electrophoresis to verify amplification of a single product of the predicted size. Standard curves were generated to determine amplification efficiency and the linear range of amplification. PCR amplification (Brilliant II SYBR Green; MX3005p instrument; Stratagene) consisted of a 10-minute incubation at 95°C followed by 40 cycles of 30 seconds 95°C (denature), 30 seconds 60°C (anneal), and 30 seconds 72°C (extension). Reactions were conducted in triplicate on three independent RNA samples per condition. Fold-differences in gene expression were calculated using the ΔΔCt method, corrected for amplification efficiency. Statistical analysis was performed with pairwise fixed reallocation randomization using (Relative Expression Software Tool [http://www.gene-quantification.de/rest.html]).30 Additional samples amplified without the melting curve analysis were separated by gel electrophoresis (1.0% agarose, 0.5× TBE), stained with ethidium bromide, and photographed.
Results
Mixed Retinal Cultures from tsA58/Rho-GFP Transgenic Mice
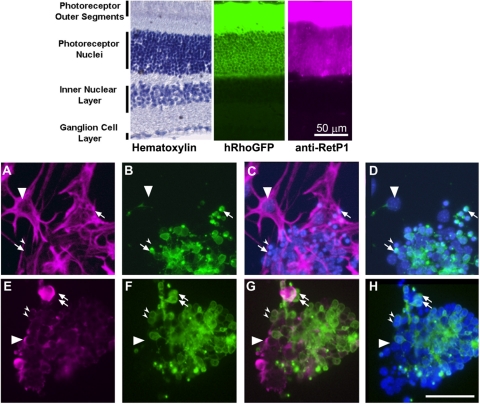
Under immortalizing conditions (plating medium at 33°C, with 50 U/mL IFNγ), cultures of total retinal cells from PN10 retina of tsA58/+; HRhoGFP/+ mice consisted of multiple, morphologically distinct populations. One population consisted of large, flat, adherent cells that were vimentin positive and contained large nuclei containing diffuse, lightly stained chromatin and prominent nucleoli (Figs. 1A, 1C). The second population consisted of abundant small, spherical cells with intensely staining nuclei that, by 4 days in culture, were almost exclusively clustered on the surfaces of the adherent cells (Figs. 1A–D). The spherical cells were vimentin negative, and most were HRhoGFP+ rod photoreceptors, as evidenced by the fluorescence of the GFP-tagged rhodopsin in their cell membranes and the polarized accumulation of HRhoGFP at the cell surface (Fig. 1B, small arrows). A subpopulation of the HRhoGFP-negative spherical cells was immunostained by antibodies against PKC (Figs. 1E–H, large arrowhead), consistent with a rod bipolar cell identity. In mixed cultures, rare pigmented epithelial cells were present at the initial plating and were identifiable as adherent cells containing distinct melanin granules. Melanin-containing cells were never associated with the large flat cells and were not observed in long-term cultures (data not shown).
Figure 1.
Immunostaining of mixed retinal cultures from P10 tsA58/+; HRhoGFP/+ mice. Top: cryosections of the retina of an adult HRhoGFP mouse showing hematoxylin staining (left), HRhoGFP epifluorescence of the eGFP-tagged human rhodopsin transgene in the rod photoreceptors (center), and immunostaining for rhodopsin (right). Prominent fluorescence of the rhodopsin-GFP and rhodopsin immunostaining is present in the outer segments and plasma membranes of the rod photoreceptor cell bodies in the outer nuclear layer. Bottom: epifluorescence photomicrographs of mixed retinal cultures from dissociated retinas of PN10 tsA58/+; HRhoGFP/+ double heterozygous mice. (A) Vimentin immunostaining. (B) Same field as (A) but showing HRhoGFP fluorescence of rod photoreceptors. (C) Overlay of (A) and Hoechst nuclear staining. (D) Overlay of (B) and Hoechst nuclear staining. (A–D, large arrowheads) Nuclei of vimentin-positive adherent cells. Arrows: HRhoGFP-positive rod photoreceptors. Small arrowheads: vimentin-negative, HRhoGFP-negative cells. (E) Protein kinase C alpha (PKC) immunostaining. (F) Similar to (E) showing HRhoGFP fluorescent rod photoreceptors. (G) Overlay of (E) and (F). (H) Overlay of (E) and Hoechst nuclear staining. (E–G, large arrowheads) PKC-positive; HRhoGFP-negative cells. Arrows: PKC-negative; HRhoGFP-positive cells. Small arrowheads: cells positive for both PKC and HRhoGFP. Scale bars, 50 μm.
The adherent flat cells and small spherical cells survived up to 50 days in mixed cultures. Over time, the flat cells covered increasing areas of the substrate, whereas the spherical cells did not appear to increase in number. This was confirmed using BrdU incorporation to assay DNA synthesis. After 17 hours of BrdU exposure in mixed retinal cultures (tsA58/+; HRhoGFP/+) grown in the presence of IFNγ at 33°C, BrdU immunoreactivity was detected exclusively in the large nuclei of the adherent cells (Figs. 2A–C, arrowheads).
Figure 2.
BrdU labeling and TAg expression in conditionally immortalized retinal cells. (A–F) BrdU immunostaining of primary, mixed retinal cultures in growth medium with 50 U/mL IFNγ at 33°C showing (A, D) BrdU, (B, E) Hoechst, and (C, F) overlay. (A–C) BrdU (10 μM) added 17 hours before fixation and immunostaining. Large arrowheads: large BrdU-positive nuclei with typical diffuse Hoechst staining. Arrows: small BrdU-negative nuclei with uniform and intense Hoechst staining. (D–F) Control wells grown in parallel without BrdU. (G–L) Photomicrographs of mixed retinal cultures showing (G, J) immunostaining for SV40-TAg and (H, K) green fluorescence protein (HRhoGFP) expression. (I, L) Overlay. (G–I) Cells cultured with 50 U/mL IFNγ at 33°C for 4 days. Arrows: TAg-positive, HRhoGFP-negative (nonphotoreceptor) cells. Large arrowheads: TAg-positive, HRhoGFP-positive (rod photoreceptors) cells. Small arrowheads: TAg-negative, HRhoGFP-positive cells. (J–L) Cells cultured with 0 U/mL IFNγ at 39°C for 4 days. Scale bars, 50 μm. (M) Western blot analysis of TAg expression in ImM10 cells cultured in growth medium containing 0 to 200 U/mL IFNγ for 4 days in the presence of 0 to 200 U/mL IFNγ and in HEK293 cells (negative control). (N) Longer exposure of blot shown in (A) reveals trace levels of TAg expression in ImM10 cells cultured without IFNγ but none in HEK293. Increased intensity of TAg expression at 100 U/mL reflects increased loading of total proteins in that lane.
To examine TAg expression, mixed retinal cells were cultured at 33°C for 4 days with or without 50 U/mL mouse recombinant IFNγ and analyzed by immunohistochemistry (Figs. 2G–L). TAg immunoreactivity was detected in cells cultured in the presence of IFNγ in both GFP-negative cells (non-rod photoreceptors; Figs. 2G–I, small arrows) and HRhoGFP-expressing rod photoreceptors (Figs. 2G–I, large arrowheads), although some rod photoreceptors lacked distinct TAg immunoreactivity (Figs. 2G–I, small arrowheads).
Purification of ImM10 Cell line
We used preferential adhesion to laminin-coated substrates to enrich for the adherent cell population in primary cultures (Supplementary Fig. S1, http://www.iovs.org/cgi/content/full/51/11/5991/DC1). After 26 days in culture, the adherent cells reached confluence. Only low numbers of HRhoGFP-positive cells were present after the initial enrichment and were rarely detected after the first passage. By the third passage, all cells in the culture were GFP-negative, adherent cells with large nuclei containing prominent nucleoli typical of Müller glia (Figs. 3A–H).19,31 This new cell line, named ImM10, has been maintained for up to 50 passages under immortalizing conditions.
Figure 3.
Photomicrographs of representative cultures of ImM10 and C57M10 cell lines in culture. (A) Calcein-stained ImM10 cells, passage 14, cultured under immortalizing conditions (GM with 50 U/mL IFNγ, 33°C). (B) Calcein-stained ImM10 cells, passage 14, plated in parallel cultured under nonimmortalizing conditions (GM without IFNγ, 39°C). (C, D) DIC images of ImM10 cells, passage 5, cultured under immortalizing conditions. (E, F) ImM10 cells, passage 5, cultured under nonimmortalizing conditions. (G) ImM10 cells, passage 37, cultured under immortalizing conditions. (H) ImM10 cells, passage 37, plated in parallel cultured under nonimmortalizing conditions. (I) Spontaneously immortalized Müller cells from retinas of PN10 C57Bl/6 mice, passage 47, cultured in DMEM with 10% FBS. Scale bars: 500 μm (A, B); 200 μm (C–I).
Using the same approach, we also generated a cell line from nontransgenic C57BL/6 mice (P10) for comparison of gene expression with the conditionally immortalized cell line. Adherent cells from C57BL/6 mice showed similar morphology (Fig. 3I), but to obtain sufficient numbers of cells for RNA isolation, we expanded the primary cultures in F12/DMEM supplemented with 10% FBS. After an initial period of slow growth, the adherent cells began to proliferate robustly. Because of their transition from slow to robust proliferation, we consider them to be spontaneously immortalized, rather than true primary cultures. The C57Bl/6 Müller cell line, named C57M10 (C57BL/6 Müller cells from P10), has been maintained for more than 50 passages.
TAg Induction and Growth Characteristics of ImM10 Cells
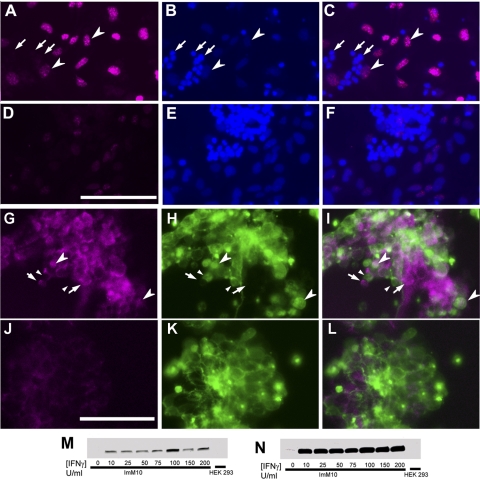
On Western blot analysis, TAg induction by IFNγ in ImM10 cells (passage 6) was detected at all concentrations of IFNγ tested (25–200 U/mL) and did not show an obvious dose response (Figs. 2M–N). Only minimal expression of the TAg was detected in the absence of IFNγ (Fig. 2N). Based on these results and previously published methods,23 we used 50 U/mL IFNγ to induce TAg expression in all subsequent experiments.
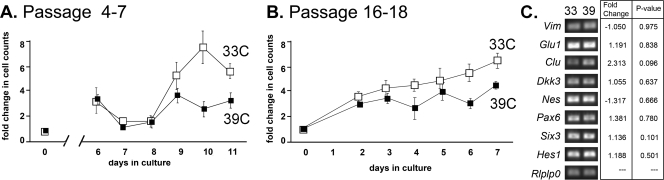
To test the effects of conditional immortalization on cell proliferation, we compared proliferation of ImM10 cells grown in GM under TAg-inducing conditions (33°C with 50 U/mL IFNγ) and noninducing conditions (39°C without IFNγ; Fig. 4). ImM10 cells at early passages (passages 4–7) cultured under immortalizing conditions (33°C with 50 U/mL IFNγ) showed minimal changes in cell numbers during the first week in culture, followed by a rapid increase in cell numbers, with cell counts increased nearly eightfold (three population doublings) by 10 days in culture. Early-passage ImM10 cells cultured under nonimmortalizing conditions (39°C without IFNγ), showed a similar lag in growth during the first week but achieved only two population doublings after 10 days in culture (Fig. 4A). Overall proliferation rates increased at later passages (Fig. 4B; passages 16–18), although ImM10 cells cultured under immortalizing conditions consistently grew more rapidly than those under nonimmortalizing conditions.
Figure 4.
Comparison of proliferation of Tag-induced and noninduced ImM10 cells in culture. (A) Passages 4 to 7 and (B) passages 16 to 18 cultured under immortalizing (open squares; 33°C, GM+IFNγ) or non-immortalizing (closed squares; 39°C, GM without IFNγ) conditions showing mean fold-change in live cells (calcein AM positive, ethidium bromide homodimer negative). Error bars show SEM. Early-passage cells were not counted on days 1 to 6 because visual observations and pilot studies showed minimal changes in cell number before 7 days in culture. (C) Quantitative RT-PCR comparing mRNA expression between cells cultured under immortalizing conditions (33°C) and nonimmortalizing conditions (39°C). Image shows ethidium bromide–stained agarose gels of amplicons. Table shows fold-change and P-values.
Gene Expression in ImM10 Cells
Quantitative RT-PCR using a panel of primer pairs for eight genes expressed in Müller cells showed that ImM10 cells expressed vimentin, glutamine synthetase, clusterin, Dkk3, nestin, Pax6, Six3, and Hes1, with no significant differences in gene expression between immortalizing and nonimmortalizing conditions (Fig. 4C). We also analyzed the expression of additional genes characteristic of Müller glial or other retinal cell types in ImM10 cells grown under immortalizing conditions, cultures of spontaneously immortalized Müller glia from C57BL/6 mice (DMEM/F12; 10% FBS, 37°C) using RT-PCR (Fig. 5P). Both ImM10 and C57M10 adherent cell cultures expressed the Müller cell genes vimentin, glutamine synthetase, retinaldehyde binding protein 1 (Rlbp1/CRALBP), GLAST, and cyclin D3. There were some differences in gene expression patterns between the ImM10 and C57M10 Müller cell lines. Primers for glial fibrillary acidic protein (Gfap) amplified a faint band from reverse-transcribed RNA from ImM10 cells, but no visible product was amplified from C57M10 cells. Although transcripts for carbonic anhydrase 14 were detected in C57M10 cells (passage 5), no expression was detected in ImM10 cells or in later passages of C57M10 cells (passage 20).
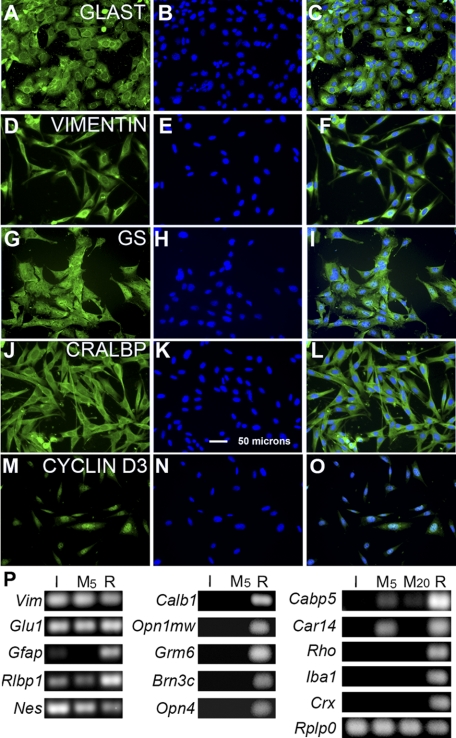
Figure 5.
Immunostaining and quantitative RT-PCR analysis of gene expression in ImM10 cells cultured in GM with 50 U/mL IFNγ at 33°C. In each row, the first panel shows antibody staining, the second panel shows 4′, 6-diamidino-2-phenylindole (DAPI) staining of nuclei, and the third panel shows the overlay of the first two images. (A–C) Glial glutamate aspartate transporter (GLAST). (D–F) Vimentin. (G–I) Glutamine synthetase (GS). (J–L) Cellular retinaldehyde binding protein (CRALBP). (M–O) Cyclin D3. Scale bar, 50 μm for all panels. (P) Ethidium bromide–stained agarose gels showing amplicons from quantitative RT-PCR analysis of (I) ImM10 cells, C57M10 cells at (M5) passage 5 and (M20) passage 20, and [R] whole adult C57BL/6 mouse retina. Vimentin (Vim); glutamine synthetase (Glu1); glial fibrillary acidic protein (Gfap); cellular retinaldehyde binding protein (Rlbp1); calbindin (Calb1); medium wavelength cone opsin (Opn1mw); metabotropic glutamate receptor mGluR6 (Grm6); POU domain, class 4 transcription factor 3 (Pou4f3; Brn3c); melanopsin (Opn4); calcium-binding protein 5 (Cabp5); carbonic anhydrase 14 (Car14) rhodopsin (Rho); inflammatory factor-1 (Iba1); cone-rod homeobox (Crx); acidic ribosomal phosphoprotein P0 (Rplp0) was used as normalizing gene.32
Immunostaining of ImM10 cells confirmed the RT-PCR results and revealed uniform expression of Müller glial proteins in all cells. Antibodies against glial glutamate aspartate transporter (GLAST) revealed a typical punctate pattern throughout the cytoplasm, with the most intense staining in the perinuclear region (Figs. 5A–C). Antibodies against the intermediate filament protein vimentin stained a characteristic network of filaments throughout the cytoplasm with strong perinuclear staining (Figs. 5D–F).33 Antibodies against glutamine synthetase labeled the entire cell, including processes and nuclei (Figs. 5G–I), similar to what is observed in histologic sections of the retina.33 Antibodies against cellular retinaldehyde binding protein (CRALBP) showed relatively diffuse cytoplasmic staining, with some perinuclear enrichment (Figs. 5J–L) as typical for Müller glia.19,34 Antibodies against cyclin D3 showed predominantly nuclear staining (Figs. 5M–O), characteristic of Müller glia in the adult retina.3
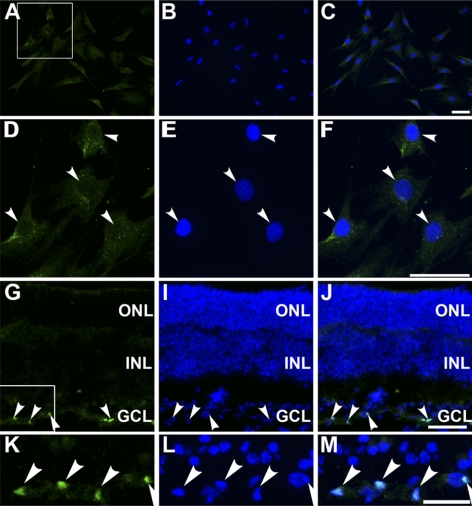
In addition to expressing genes characteristic of Müller glia, neither ImM10 nor C57M10 cell lines expressed genes characteristic of other differentiated retinal cell types (Fig. 5P). By RT-PCR, both cell lines were negative for genes expressed in photoreceptors (rhodopsin/Rho, medium wavelength cone opsin/Opn1mw, cone-rod homeobox/Crx), horizontal cells (calbindin/Calb1), bipolar cells (metabotropic glutamate receptor mGluR6/Grm6, calcium binding protein 5, Cabp5), ganglion cells (Pou4f3/Brn3c), and photosensitive ganglion cells (melanopsin/Opn4). Both cell lines were also negative for the microglial marker Iba1. Antibodies against PAX2 showed no nuclear staining in ImM10 cells (Figs. 6A–F), though some cytoplasmic immunoreactivity was present. In contrast, strong nuclear staining was present in retinal astrocytes in the inner retina (Figs. 6G–M). We cannot attribute the Pax2 immunoreactivity in ImM10 cells solely to nonspecific staining because, by quantitative RT-PCR, low levels of Pax2 mRNA were detected in ImM10 cells (mean Ct values 31.11 ± 1.18 SEM) under immortalizing conditions and in C57M10 (mean Ct values 34.38 ± 1.10 SEM). By comparison, the mean Ct values for Six3 (24.65 ± 0.08 SEM) and Pax6 (24.63 ±1.35 SEM) were 6.5 cycles lower for ImM10 cells under immortalizing conditions, corresponding to approximately 90-fold higher expression of Six3 and Pax6 compared with Pax2.
Figure 6.
PAX2 immunostaining in ImM10 cells and mouse retina. In each row, the first panel shows PAX2 antibody staining, the second panel shows 4′, 6-diamidino-2-phenylindole (DAPI) staining of nuclei, and the third panel shows the overlay of the first two images. (A–C) PAX2 immunostaining of ImM10 cells cultured at 33°C shows cytoplasmic, but not nuclear, staining. (D–F) Higher magnification of boxed area in (A). Arrowheads: nuclei. (G, I, J) PAX2 immunostaining of adult C57BL/6 mouse retina shows nuclear staining in retinal astrocytes in the inner retina (arrowheads). (K–M) Higher magnification of boxed area in (G). Scale bars, 50 μm (A–F), 100 μm (G–M).
Expression of Retinal Stem Cell Genes in ImM10 Cell Line
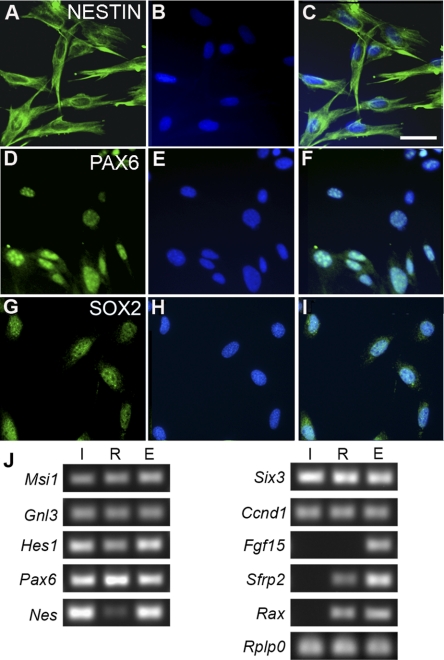
Using RT-PCR, we showed that ImM10 cells expressed mRNA for Nestin and the transcription factors Pax6, Six3, and Hes1 (Fig. 7). We confirmed the expression of Nestin and PAX6 using immunohistochemistry (Figs. 7A–F). By immunostaining, ImM10 cells also expressed SOX2 (Figs. 7G–I). As expected, PAX6 and SOX2 were localized primarily in the nucleus. By RT-PCR, ImM10 cells expressed other genes characteristic of retinal progenitors, including the RNA-binding protein musashi-1 (Msi1), nucleostemin (Gnl3), and cyclin D1 (Ccnd1) (Fig. 7J). In contrast, secreted frizzled related protein 2 (Sfrp2), fibroblast growth factor 15 (Fgf15), and retinal and anterior neural fold homeobox (Rax) are expressed in retinal progenitors during development, but not in mature Müller glia,35 and expression of these three genes was not detected by RT-PCR in RNA isolated from ImM10 cells.
Figure 7.
Immunostaining and RT-PCR analysis of retinal stem cell–associated gene expression in ImM10 cells cultured in GM with 50 U/mL IFNγ at 33°C. (A–I) In each row, the first panel shows antibody staining, the second panel shows 4′, 6-diamidino-2-phenylindole (DAPI) staining of nuclei, and the third panel shows the overlay of the first two images. (A–C) Nestin. (D–F) PAX6. (G–I) SOX2. Scale bar, 50 μm. (J) Ethidium bromide–stained agarose gels showing PCR amplicons generated from reverse-transcribed total RNA from (I) ImM10 cells, passage 14, cultured in GM with IFNγ at 33°C, (R) C57BL/6 adult mouse retina, and (E) whole mouse embryo at embryonic day 12.5. The following genes were analyzed: musashi 1 (Msi1); nucleostemin (guanine nucleotide binding protein-like 3; Gnl3); hairy and enhancer of split homolog 1 (Hes1); Clusterin (Clu); paired box gene 6 (Pax6); nestin (Nes); sine oculis-related homeobox homolog 3 (Six3); cyclin D1 (Ccnd1); fibroblast growth factor 15 (Fgf15); secreted frizzled related protein 2 (Sfrp2); retinal and anterior neural fold homeobox (Rax). Acidic ribosomal phosphoprotein P0 (Rplp0) was used as a normalizing gene.32
Discussion
We report the isolation and characterization of two novel cell lines isolated from the P10 mouse retina: ImM10, a conditionally immortalized Müller glial cell line generated from tsA58/+; HRhoGFP/+ mice, and C57M10, a spontaneously immortalized cell line from C57BL/6 mice. Several mammalian Müller glial cell lines have been previously described, including a conditionally immortalized Müller glia from rat retina (TR-MUL5),24 nonconditional, SV40 TAg immortalized cell lines from rat (rMC-1),19 and spontaneously immortalized Müller glia from postmortem human retinas.11,21 Untransformed mouse Müller glia have been reported to survive in vitro for at least 7 passages.36 However, this is the first report of a conditionally immortalized Müller glial cell line from mouse that maintains Müller glial characteristics for more than 50 passages in vitro. Moreover, consistent with recent evidence that Müller cells in vivo express retinal stem cell genes,35,37,38 the ImM10 cell line is the first with demonstrated expression of multiple retinal stem cell genes when cultured as adherent monolayers.
ImM10 and C57M10 cells show the typical morphology of cultured Müller glia: large adherent cells with large, diffusely staining nuclei containing prominent nucleoli and an epithelioid morphology with multiple broad processes with distinct lamellipodia.31 This is consistent with the morphology of primary and immortalized Müller glia from other species, including rat,12,19,39 mouse,36 and human.21 ImM10 and C57M10 cells express a combination of genes typical of mature Müller glia, including Vim, Rlbp1, Dkk3, Clu, and Glu1, and we found no significant differences in gene expression between ImM10 cells cultured under immortalizing or nonimmortalizing conditions. Carbonic anhydrase 14 (Car14), a gene typically expressed in Müller glia in vivo, was not detected in mRNA from ImM10 cells, whereas C57M10 cells expressed low levels of Car14 mRNA at early passages but not at later passages. Downregulation of proteins involved glial physiological functions has previously been reported in cultured Müller glia and is thought to reflect adaptation to in vitro conditions.40 Thus, the absence of Car14 expression in ImM10 cells is most likely associated with adaptation to in vitro culture rather than a consequence of conditional immortalization.
The ImM10 and C57M10 cell lines were not clonally derived, and, although we cannot completely exclude the possibility that they consist of mixed populations, no heterogeneity was observed in the patterns of immunostaining. However, both Müller glial and retinal astrocytes express many of the same genes and show similar morphologies in culture. CRALBP expression is characteristic of Müller glia and has been used in by other groups to identify cultured Müller glia.11,12,21,36,40–42 Although developing retinal astrocytes transiently express CRALBP as they migrate from the optic nerve head, they downregulate expression in the postnatal retina with the protein no longer detectable by P7,43 prior to the age when we isolated these cell lines. Consistent with a Müller glial identity, Rlbp1 mRNA was expressed in both ImM10 and C57M10 cell lines, and immunostaining revealed uniform and robust expression of CRALBP protein in 100% of the ImM10 cells. Furthermore, ImM10 cells also uniformly expressed cyclin D3, which is specifically expressed by Müller glia in the mature retina.3
PAX2 is a marker of retinal astrocytes, and previous reports describing conditionally immortalized retinal astrocytes from the transgenic mice show distinct nuclear staining for PAX2.28 In ImM10 cells, PAX2 immunoreactivity was present but consistently showed cytoplasmic rather than nuclear localization (Fig. 6). Although cytoplasmic accumulation of PAX2 protein has been previously reported in mammary gland,44 the significance of cytoplasmic PAX2 is unclear. This may reflect nonspecific staining because we have previously observed nonspecific staining of nerve fibers in mouse embryos using these antibodies.45 However, multiple dilutions of the PAX2 antibodies all resulted in similar staining patterns. Similarly, omission of the primary antibodies revealed no nonspecific staining by the secondary antibodies. Because ImM10 cells expressed low levels of Pax2 mRNA by qRT-PCR, we cannot exclude the possibility that the cytoplasmic staining may reflect the presence of PAX2 protein. PAX2 expression is not detected in Müller glia in the intact retinas of mammals, including mice, though a few PAX2-positive glial cells have been observed in the peripheral retinas in older dogs.46 In contrast, in the chick retina, FGF2 treatment followed by excitotoxic injury upregulates PAX2 expression in Müller glia that reenter the cell cycle, suggesting a potential role in the glial-stem cell switch in Müller glia.46 To our knowledge, expression patterns of PAX2 have not been reported for other Müller glial cell lines; therefore, it is not apparent whether a low level of cytoplasmic PAX2 is unique to this cell line or is a more general property of Müller glia in culture.
The low expression level of GFAP expression in ImM10 and C57M10 cells is also consistent with identification as Müller glia rather than as retinal astrocytes. In the retina, Müller glia express low levels of Gfap in the absence of injury or disease, whereas retinal astrocytes constitutively express high levels of Gfap.47 Consistent with this, the SAGE tag ratio of vimentin to Gfap in two human Müller glial cell lines was 164:148; by immunohistochemistry, human Müller glial cell lines21 and primary rat Müller glial cultures31 also expressed very low levels of GFAP. In contrast to ImM10 and C57M10 cells, mouse Müller cells (mMCI and mMCII) cultured from P5 to 12 mice express high levels of Gfap and low levels vimentin by RT-PCR.36 The rat rMC-1 cell line19 and some primary human Müller cell cultures49 show robust immunoreactivity for the GFAP protein. Although the basis for these differences is unclear, upregulation of GFAP is characteristic of Müller glial activation after injury and in disease.50,51 The rMC-1 cell line was generated from light-damaged retinas; therefore, injury-induced upregulation of Gfap before cell isolation is one possibility. Because only some of the previously characterized human Müller cell cultures have elevated Gfap expression, preexisting injury or disease in human donor eyes might have contributed to higher than expected Gfap expression in these particular cultures.49
ImM10 cells also express multiple genes characteristic of both embryonic retinal stem cells and Müller glia in vivo, including the transcriptional regulators Pax6, Hes1, and Sox2.35,37,38 However, ImM10 cells did not express Sfrp2, Fgf15, or Rax, which are characteristically expressed in early retinal progenitors. These results are consistent with detailed gene expression profiling of single Müller glial cells that showed coexpression of Pax6, Hes1, and Sox2 in ImM10 cells but no significant expression of Sfrp2 or Rax.37 Although overexpression of Rax can promote differentiation of Müller glia,52 activity of the Rax promoter in Müller glia is downregulated as Müller glia differentiate53 and is absent in Müller glia in the mature retina.35 Sfrp2 and Fgf15 are also downregulated as differentiation proceeds and, by SAGE tag counts and in situ hybridization, both Sfrp2 and Fgf15 are undetectable in the adult retina.35 The absence of Sfrp2, Fgf15, and Rax expression in ImM10 cells appears to be consistent with their identification as Müller glia, despite the expression of multiple genes characteristic of retinal stem cells.
Expression of retinal stem cell-associated genes in ImM10 cells differs from the previously described mMCI and mMCII mouse Müller cells,36 which expressed only low levels of retinal stem cell genes when cultured as adherent cultures. Interestingly, mMCI cells upregulate nestin when induced to generate neurospheres by culturing in serum-free, FGF2/EGF-supplemented medium.36 Similarly, after growth factor stimulation, human MIO-M1 cells11 and primary Müller glia from rat13 and mouse retinas54 generate neurospheres that express stem cell genes, including Pax6, Sox2, and nestin. However, many published reports do not describe gene expression patterns in Müller glial cultures grown under nonneurosphere conditions. Thus, it is unclear whether the expression of retinal stem cell genes in neurospheres generated using other Müller cell lines represents de novo upregulation or persistent expression of genes that were expressed before neurosphere induction.
Multiple immortalized retinal cell lines characterized as neuronal in origin have been generated from embryonic or fetal tissue.55–57 In contrast, few have been generated from postmitotic retinal neurons, suggesting that retinal progenitors are more amenable to immortalization. We observed that postmitotic neurons from the transgenic mice survived in mixed cultures. However, cells with neuronal morphologies did not incorporate BrdU and did not increase in number over time in culture, even with the induction of TAg expression. Müller glial cell lines from multiple species have been generated from a variety of ages by both spontaneous immortalization and overexpression of viral oncogenes.11,19,21,24,48 This likely reflects the inherent proliferative ability of Müller glia in vivo and potentially the limited stem cell-like properties of some Müller glia, both in vivo and in vitro.10
We conclude that the morphologic and gene expression profiles of the ImM10 and C57M10 cell lines are consistent with their identification as Müller glia. These mouse cell lines will facilitate research on the physiology and function of Müller glia and will complement the numerous genetic mouse models of retinal disease in use. In light of the growing evidence that some Müller glia may have stemlike characteristics both in vivo and in vitro, evaluation of the neurogenic capacity of ImM10 cells is under way.
Supplementary Material
Acknowledgments
The authors thank Mary Guirguis, Krista Beach, and Laila Pillai for expert technical assistance, Theodore Wensel (Baylor College of Medicine) for the HRhoGFP mice, and Donald J. Zack (Johns Hopkins University School of Medicine) for valuable insights provided during the development of this cell line and in manuscript preparation.
Footnotes
Presented in part at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2008.
Supported by the University of Houston and unrestricted funds from the University of Houston College of Optometry (DCO); National Institutes of Health/NEIT32 Grant EY07024 (MJP); Ezell Fellowship; American Optometric Foundation (MJP); and University of Houston College of Optometry Core Grant PN30 EY07751.
Disclosure: D.C. Otteson, None; M.J. Phillips, None
References
- 1. de Melo Reis RA, Ventura AL, Schitine CS, de Mello MC, de Mello FG. Müller glial as an active compartment modulating nervous activity in the vertebrate retina: neurotransmitters and trophic factors. Neurochem Res. 2008;33:1466–1474 [DOI] [PubMed] [Google Scholar]
- 2. Bringmann A, Pannicke T, Grosche J, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424 [DOI] [PubMed] [Google Scholar]
- 3. Dyer MA, Cepko CL. Control of Müller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000;3:873–880 [DOI] [PubMed] [Google Scholar]
- 4. Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A. 2008;105:19508–19513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wohl SG, Schmeer CW, Kretz A, Witte OW, Isenmann S. Optic nerve lesion increases cell proliferation and nestin expression in the adult mouse eye in vivo. Exp Neurol. 2009;219:175–186 [DOI] [PubMed] [Google Scholar]
- 6. Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Müller glial that function as retinal stem cells. J Neurosci. 2007;27:7028–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Otteson DC, D'Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232:62–76 [DOI] [PubMed] [Google Scholar]
- 8. Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44:289–307 [DOI] [PubMed] [Google Scholar]
- 9. Fischer AJ, Reh TA. Potential of Müller glial to become neurogenic retinal progenitor cells. Glia. 2003;43:70–76 [DOI] [PubMed] [Google Scholar]
- 10. Jadhav AP, Roesch K, Cepko CL. Development and neurogenic potential of Müller glial cells in the vertebrate retina. Prog Retin Eye Res. 2009;28:249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lawrence JM, Singhal S, Bhatia B, et al. MIO-M1 cells and similar Müller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25:2033–2043 [DOI] [PubMed] [Google Scholar]
- 12. Monnin J, Morand-Villeneuve N, Michel G, Hicks D, Versaux-Botteri C. Production of neurospheres from mammalian Müller cells in culture. Neurosci Lett. 2007;421:22–26 [DOI] [PubMed] [Google Scholar]
- 13. Das AV, Mallya KB, Zhao X, et al. Neural stem cell properties of Müller glial in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302 [DOI] [PubMed] [Google Scholar]
- 14. Kubota A, Nishida K, Nakashima K, Tano Y. Conversion of mammalian Müller glial cells into a neuronal lineage by in vitro aggregate-culture. Biochem Biophys Res Commun. 2006;351:514–520 [DOI] [PubMed] [Google Scholar]
- 15. Walcott JC, Provis JM. Müller cells express the neuronal progenitor cell marker nestin in both differentiated and undifferentiated human foetal retina. Clin Exp Ophthalmol. 2003;31:246–249 [DOI] [PubMed] [Google Scholar]
- 16. Reid TW, Albert DM, Rabson AS, et al. Characteristics of an established cell line of retinoblastoma. J Natl Cancer Inst. 1974;53:347–360 [DOI] [PubMed] [Google Scholar]
- 17. McFall RC, Sery TW, Makadon M. Characterization of a new continuous cell line derived from a human retinoblastoma. Cancer Res. 1977;37:1003–1010 [PubMed] [Google Scholar]
- 18. Seigel GM. The golden age of retinal cell culture. Mol Vis. 1999;5:4. [PubMed] [Google Scholar]
- 19. Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Müller cell line. Invest Ophthalmol Vis Sci. 1998;39:212–216 [PubMed] [Google Scholar]
- 20. Krishnamoorthy RR, Agarwal P, Prasanna G, et al. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001;86:1–12 [DOI] [PubMed] [Google Scholar]
- 21. Limb GA, Salt TE, Munro PM, Moss SE, Khaw PT. In vitro characterization of a spontaneously immortalized human Müller cell line (MIO-M1). Invest Ophthalmol Vis Sci. 2002;43:864–869 [PubMed] [Google Scholar]
- 22. Hodges H, Pollock K, Stroemer P, et al. Making stem cell lines suitable for transplantation. Cell Transplant. 2007;16:101–115 [PubMed] [Google Scholar]
- 23. Jat PS, Noble MD, Ataliotis P, et al. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci U S A. 1991;88:5096–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tomi M, Funaki T, Abukawa H, et al. Expression and regulation of L-cystine transporter, system xc-, in the newly developed rat retinal Müller cell line (TR-MUL). Glia. 2003;43:208–217 [DOI] [PubMed] [Google Scholar]
- 25. Hosoya K, Tomi M, Ohtsuki S, et al. Conditionally immortalized retinal capillary endothelial cell lines (TR-iBRB) expressing differentiated endothelial cell functions derived from a transgenic rat. Exp Eye Res. 2001;72:163–172 [DOI] [PubMed] [Google Scholar]
- 26. Kondo T, Hosoya K, Hori S, et al. Establishment of conditionally immortalized rat retinal pericyte cell lines (TR-rPCT) and their application in a co-culture system using retinal capillary endothelial cell line (TR-iBRB2). Cell Struct Funct. 2003;28:145–153 [DOI] [PubMed] [Google Scholar]
- 27. Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis. 2003;9:171–178 [PubMed] [Google Scholar]
- 28. Scheef E, Wang S, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal astrocytes. Mol Vis. 2005;11:613–624 [PubMed] [Google Scholar]
- 29. Chan F, Bradley A, Wensel TG, Wilson JH. Knock-in human rhodopsin-GFP fusions as mouse models for human disease and targets for gene therapy. Proc Natl Acad Sci U S A. 2004;101:9109–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hicks D, Courtois Y. The growth and behaviour of rat retinal Müller cells in vitro, 1: an improved method for isolation and culture. Exp Eye Res. 1990;51:119–129 [DOI] [PubMed] [Google Scholar]
- 32. Simpson DA, Feeney S, Boyle C, Stitt AW. Retinal VEGF mRNA measured by SYBR green I fluorescence: a versatile approach to quantitative PCR. Mol Vis. 2000;6:178–183 [PubMed] [Google Scholar]
- 33. Wang MH, Frishman LJ, Otteson DC. Intracellular delivery of proteins into mouse Müller glial cells in vitro and in vivo using Pep-1 transfection reagent. J Neurosci Methods. 2009;177:403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bunt-Milam AH, Saari JC. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J Cell Biol. 1983;97:703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blackshaw S, Harpavat S, Trimarchi J, et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:E247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Florian C, Langmann T, Weber BH, Morsczeck C. Murine Müller cells are progenitor cells for neuronal cells and fibrous tissue cells. Biochem Biophys Res Commun. 2008;374:187–191 [DOI] [PubMed] [Google Scholar]
- 37. Roesch K, Jadhav AP, Trimarchi JM, et al. The transcriptome of retinal Müller glial cells. J Comp Neurol. 2008;509:225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bhatia B, Singhal S, Lawrence JM, Khaw PT, Limb GA. Distribution of Müller stem cells within the neural retina: evidence for the existence of a ciliary margin-like zone in the adult human eye. Exp Eye Res. 2009;89:373–382 [DOI] [PubMed] [Google Scholar]
- 39. Wan J, Zheng H, Xiao HL, She ZJ, Zhou GM. Sonic hedgehog promotes stem-cell potential of Müller glial in the mammalian retina. Biochem Biophys Res Commun. 2007;363:347–354 [DOI] [PubMed] [Google Scholar]
- 40. Hauck SM, Suppmann S, Ueffing M. Proteomic profiling of primary retinal Müller glial cells reveals a shift in expression patterns upon adaptation to in vitro conditions. Glia. 2003;44:251–263 [DOI] [PubMed] [Google Scholar]
- 41. Geller SF, Ge PS, Visel M, Flannery JG. In vitro analysis of promoter activity in Müller cells. Mol Vis. 2008;14:691–705 [PMC free article] [PubMed] [Google Scholar]
- 42. Lupien CB, Bolduc C, Landreville S, Salesse C. Comparison between the gene expression profile of human Müller cells and two spontaneous Müller cell lines. Invest Ophthalmol Vis Sci. 2007;48:5229–5242 [DOI] [PubMed] [Google Scholar]
- 43. Johnson PT, Geller SF, Lewis GP, Reese BE. Cellular retinaldehyde binding protein in developing retinal astrocytes. Exp Eye Res. 1997;64:759–766 [DOI] [PubMed] [Google Scholar]
- 44. Silberstein GB, Van Horn K, Hrabeta-Robinson E, Compton J. Estrogen-triggered delays in mammary gland gene expression during the estrous cycle: evidence for a novel timing system. J Endocrinol. 2006;190:225–239 [DOI] [PubMed] [Google Scholar]
- 45. Otteson DC, Shelden E, Jones JM, Kameoka J, Hitchcock PF. Pax2 expression and retinal morphogenesis in the normal and Krd mouse. Dev Biol. 1998;193:209–224 [DOI] [PubMed] [Google Scholar]
- 46. Stanke J, Moose HE, El-Hodiri HM, Fischer AJ. A comparative study of Pax2 expression in glial cells in the retina and optic nerve of birds and mammals. J Comp Neurol. 2010;518:2316–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarthy PV, Fu M, Huang J. Developmental expression of the glial fibrillary acidic protein (GFAP) gene in the mouse retina. Cell Mol Neurobiol. 1991;11:623–637 [DOI] [PubMed] [Google Scholar]
- 48. Lupien C, Brenner M, Guerin SL, Salesse C. Expression of glial fibrillary acidic protein in primary cultures of human Müller cells. Exp Eye Res. 2004;79:423–429 [DOI] [PubMed] [Google Scholar]
- 49. Aotaki-Keen AE, Harvey AK, de Juan E, Hjelmeland LM. Primary culture of human retinal glia. Invest Ophthalmol Vis Sci. 1991;32:1733–1738 [PubMed] [Google Scholar]
- 50. Chen H, Weber AJ. Expression of glial fibrillary acidic protein and glutamine synthetase by Müller cells after optic nerve damage and intravitreal application of brain-derived neurotrophic factor. Glia. 2002;38:115–125 [DOI] [PubMed] [Google Scholar]
- 51. Okada M, Matsumura M, Ogino N, Honda Y. Müller cells in detached human retina express glial fibrillary acidic protein and vimentin. Graefes Arch Clin Exp Ophthalmol. 1990;228:467–474 [DOI] [PubMed] [Google Scholar]
- 52. Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Müller glial by postnatal retinal progenitor cells. Neuron. 2000;26:383–394 [DOI] [PubMed] [Google Scholar]
- 53. Matsuda T, Cepko CL. Controlled expression of transgenes introduced by in vivo electroporation. Proc Natl Acad Sci U S A. 2007;104:1027–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nickerson PE, Da Silva N, Myers T, Stevens K, Clarke DB. Neural progenitor potential in cultured Müller glial: effects of passaging and exogenous growth factor exposure. Brain Res. 2008;1230:1–12 [DOI] [PubMed] [Google Scholar]
- 55. Dutt K, Cao Y. Engineering retina from human retinal progenitors (cell lines). Tissue Eng Part A. 2009;15:1401–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Seigel GM. Establishment of an E1A-immortalized retinal cell culture. In Vitro Cell Dev Biol Anim. 1996;32:66–68 [DOI] [PubMed] [Google Scholar]
- 57. Pessac B, Girard A, Romey G, Crisanti P, Lorinet AM, Calothy G. A neuronal clone derived from a Rous sarcoma virus-transformed quail embryo neuroretina established culture. Nature. 1983;302:616–618 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.