Abstract
Carisbamate (CRS) exhibits broad acute anticonvulsant activity in conventional anticonvulsant screens, genetic models of absence epilepsy and audiogenic seizures, and chronic spontaneous motor seizures arising after chemoconvulsant-induced status epilepticus. In add-on phase III trials with pharmacoresistant patients CRS induced <30% average decreases in partial-onset seizure frequency. We assessed the antiepileptogenic and antiepileptic performance of subchronic CRS administration on posttraumatic epilepsy (PTE) induced by rostral parasaggital fluid percussion injury (rpFPI), which closely replicates human contusive closed head injury. Studies were blind and randomized, and treatment effects were assessed on the basis of sensitive electrocorticography (ECoG) recordings. Antiepileptogenic effects were assessed in independent groups of control and CRS-treated rats, at 1 and 3 months postinjury, after completion of a 2-week prophylactic treatment initiated 15 min after injury. The antiepileptic effects of 1-week CRS treatments were assessed in repeated measures experiments at 1 and 4 months postinjury. The studies were powered to detect ∼50 and ∼40% decreases in epilepsy incidence and frequency of seizures, respectively. Drug/vehicle treatment, ECoG analysis, and [CRS]plasma determination all were performed blind. We detected no antiepileptogenic and an equivocal transient antiepileptic effects of CRS despite [CRS]plasma comparable with or higher than levels attained in previous preclinical and clinical studies. These findings contrast with previous preclinical data demonstrating large efficacy of CRS, but agree with the average effect of CRS seen in clinical trials. The data support the use of rpFPI-induced PTE in the adolescent rat as a model of pharmacoresistant epilepsy for preclinical development.
Introduction
Carisbamate [CRS; (S)-2-O-carbamoyl-1-o-chlorophenyl-ethanol)] is an investigational neuromodulator that has a broad spectrum of anticonvulsant activity in numerous preclinical tests (Table 1). In comparison with eight marketed antiepileptic drugs (AEDs), CRS's anticonvulsant potency ranked fourth, first, second, and first for seizures evoked in the mouse by maximal electroshock, pentylenetetrazole, bicuculline, and picrotoxin, respectively (Novak et al., 2007). CRS has also demonstrated anticonvulsant activity in kindling, lamotrigine-resistant kindled rat, and genetic models of absence epilepsy and audiogenic seizures and suppresses the chronic spontaneous motor seizures that arise after chemoconvulsant-induced status epilepticus (SE) (Novak et al., 2007). Grabenstatter and Dudek (2008) rigorously examined the effects of acute administration of CRS on chronic spontaneous motor seizures after kainate-induced SE. Seizure frequency was reduced by ∼75%, and CRS seemed more efficacious than topiramate (Grabenstatter et al., 2005). Similarly encouraging results were obtained in a study with prolonged lithium-pilocarpine-induced SE in which a 1-week course of twice-daily injections of a fixed dose of CRS begun after the onset of SE resulted in a delay or prevention of the appearance of spontaneous motor seizures in the chronic period. This has been interpreted as indicative of a possible antiepileptogenic, or disease-modifying, effect of CRS (André et al., 2007). Thus, CRS has demonstrated a broader spectrum of activity in preclinical tests than other marketed AEDs (Rogawski, 2006).
TABLE 1.
Preclinical evaluation of carisbamate
| Test | Mouse | Rat |
|---|---|---|
| Maximal electroshock | +a | +b |
| Pentylenetetrazole | +a | −b |
| Bicuculline | +a | +b |
| Picrotoxin | +a | +b |
| Corneal kindling | N.D. | +b |
| Hippocampal kindling | N.D. | +b |
| Lamotrigine-resistant amygdale kindled rat | N.A. | +b |
| 6-Hz seizures | N.D. | +b |
| Li+-pilocarpine SE | N.D. | +b |
| Audiogenic seizure | +b | +c |
| Genetic generalized spike-wave seizure (GAERS rat) | N.A. | +c |
| Chronic spontaneous seizure (Li+-pilocarpine SE) | N.D. | +d |
| Chronic spontaneous seizure (kainate SE) | N.D. | +e |
N.D., not determined; N.A., not applicable.
However, the translation of encouraging preclinical findings to the clinic has been an incomplete success. Although the introduction of numerous AEDs over the past 30 years has provided a wider range of therapeutic options and improved the tolerability of pharmacotherapy, the proportion of patients with poor seizure control has not substantially changed (Duncan et al., 2006; French, 2007), and no agent has been identified to cure epilepsy (Temkin, 2009). Thus, the preclinical investigation of putative AEDs is not ideal and could benefit from several refinements. First, more comprehensive studies could be conducted using chronic spontaneous seizure models, including acquired epilepsy models based on etiologically realistic insults (Meldrum, 2002; Schmidt and Rogawski, 2002; Stables et al., 2002; White, 2002, 2003; Kwan and Brodie, 2003; Löscher and Schmidt, 2004; D'Ambrosio and Miller, 2010a,b). Second, the antiepileptic assessment could be obtained for prolonged drug exposure. The acute effects of AED administration may not adequately reflect the performance of drugs administered chronically in the clinical setting (Löscher, 2007). They also cannot detect antiepileptic effects that develop slowly, over days to weeks, as observed with valproate (Löscher and Hönack, 1995; Eastman et al., 2010). Third, preclinical studies should probe the entire spectrum of epileptic seizures by electrocorticography (ECoG) and not be exclusively based on convulsive behavior. In humans, many epileptic seizures are not accompanied by overt or distinctive behavioral output such as convulsions, and human nonconvulsive seizures are more often pharmacoresistant than tonic-clonic convulsions (Juul-Jensen, 1986; Mattson et al., 1996; Semah et al., 1998). Finally, blinded and randomized studies would help ensure an unbiased preclinical workup (Crossley et al., 2008; Philip et al., 2009).
Thus, we have assessed the antiepileptic and antiepileptogenic effects of subchronically administered CRS on chronic spontaneous seizures in an etiologically realistic model of pharmacoresistant acquired epilepsy in which chronic spontaneous seizures were detected and quantified by ECoG. Posttraumatic epilepsy (PTE) was induced by rostral parasaggital fluid percussion injury (rpFPI) in the rat, which is mechanically identical to human contusive head injury and reproduces many of its pathophysiological sequelae (Thompson et al., 2005), including an epileptic syndrome featuring neocortical and limbic seizures (D'Ambrosio et al., 2004, 2005, 2009) that are fully blocked by halothane, partially responsive to valproate, but resistant to carbamazepine (Eastman et al., 2010). These studies were conducted in a blind and randomized fashion, using dosing protocols designed to maintain blood levels of CRS in a therapeutic range for the duration of the on-drug phase of each study. Despite statistical power adequate to detect at least ∼40% reduction in seizure frequency and ∼50% reduction in incidence of epilepsy, these studies did not demonstrate significant antiepileptic or antiepileptogenic activity.
Materials and Methods
Animals.
Outbred 32- to 36-day-old male Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) were housed in a specific-pathogen-free facility two to three per cage to allow socialization, under controlled conditions of temperature and humidity and a 12-h light/12-h dark cycle. Water and food were available ad libitum. All experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Fluid Percussion Injury.
Rostral parasaggital fluid percussion injury was performed as detailed previously (D'Ambrosio et al., 2009; Eastman et al., 2010) with care to ensure consistent procedure. In brief, animals were anesthetized (4% halothane), intubated, and mechanically ventilated (1–1.5% halothane, 30% O2, and air). Core temperature was maintained at 37°C with a heat pad. A 3-mm burr hole was drilled 2 mm posterior to bregma and 3 mm from the midline over the right convexity. Animals were disconnected from the ventilator for injury, and an 8-ms, 3.4- to 3.7-atm pressure pulse was delivered through the FPI device (Scientific Instruments, University of Washington) and measured by a transducer (Measurement Specialties, Hampton, VA). To standardize posttraumatic hypoxia, the mechanical ventilation was resumed 10 s after injury. Injured animals had righting times in excess of 10 min, and acute mortality from posttraumatic complications was ∼11%.
Electrode Implantation and Video-ECoG.
Epidural electrodes were implanted as detailed previously (D'Ambrosio et al., 2009). In brief, epidural electrodes (stainless-steel screws of Φ = 1 mm) were implanted through guiding craniotomies (∼ Φ = 0.75 mm) with care to avoid brain compression or damage to the underlying dura that sometimes induces artifactual epileptiform discharges. The ECoG montage consisted of five epidural electrodes: a reference electrode placed midline in the frontal bone and two electrodes per parietal bone, placed at coordinates bregma 0 mm and −6.5 mm, 4 mm from the midline. All electrodes were connected through insulated wire to gold-plated pins in a plastic pedestal, and the entire assembly was cemented onto the skull with dental acrylic (Jet; Lang Dental Manufacturing Co., Wheeling, IL). Because of stresses peculiar to these experiments initial batches of animals showed a higher incidence of headset loosening than in our previous studies, which resulted in noisy recordings over time. Thus, acrylic headsets were more securely adhered with VetBond (World Precision Instruments, Inc., Sarasota, FL) in later batches, which improved their mechanical resilience and the proportion of useful recordings. Rats were housed separately after implantation to protect headsets. When possible, lost headsets were reimplanted once. Electrical brain activity was amplified (×5000) and filtered (0.3-Hz high-pass, 100-Hz low-pass) using a Neurodata 12 or a M15 amplifier (Grass Instruments, Quincy, MA), acquired at 512 Hz per channel, stored, and analyzed on computers equipped with SciWorks and Experimenter V3 software (Datawave Technologies Inc., Longmont, CO), and DT3010 acquisition boards (DataTranslation Inc., Marlboro, MA). One-day ECoG recordings were performed two or three times weekly. Videos were recorded using digital cameras connected to videocassette recorders. Each camera was used to monitor a maximum of two cages (one animal per cage) to permit detection of subtle changes in behavior.
Quality Control of Surgical Procedures.
As in previous work, we took precautions to ensure that chronic epileptiform ECoG events were induced by FPI and not by cortical damage caused by substandard drilling or by heating or compression during epidural electrode implantation. Thus, the skull and drill bit were cooled during drilling with room-temperature sterile saline, and we took care never to deform the skull or the dura with the drill bit. In addition, the depth of epidural electrodes was carefully measured to avoid brain compression (D'Ambrosio et al., 2009).
Identification of Seizures.
Random samples of both video and ECoG of awake and sleep patterns were first examined in drug-treated animals to ensure CRS did not affect the typical ECoG patterns of normal rat brain activity and our capability to recognize seizures. ECoG was then manually scrolled offline as described previously (D'Ambrosio et al., 2004, 2005, 2009). Seizures were characterized by epileptiform ECoG patterns with trains of 150- to 250-ms-long spikes, initial amplitudes exceeding twice the previous 2-s baseline with ictal behavioral changes as described previously. These seizures are seen only in FPI and not in sham-injured animals. Consistent with our previous demonstration of the existence of short clinical seizures in posttraumatic epileptic rat and humans (D'Ambrosio et al., 2009), observed seizure duration ranged from 1 s to 10 min. Seizures occurring within 5 s of each other were defined as one seizure event. Because all FPI animals were at risk of developing PTE, we diagnosed epilepsy upon their first proven ictal electroclinical event (Beghi et al., 2005; Fisher et al., 2005; Fisher and Leppik, 2008). ECoG seizures were categorized as: 1) grade 1 (G1), if appearing to be limited to a cortical focus; 2) grade 2 (G2), when appearing first in a limited cortical area and then spread to other cortical areas; and 3) grade 3 (G3), if appearing bilateral at their cortical onset. G1 and G2 seizures are generated by a neocortical focus, whereas G3 events have a likely limbic origin (D'Ambrosio et al., 2004, 2005, 2009). In accord with previous work (D'Ambrosio et al., 2004, 2005), age-dependent idiopathic seizures, typically 1- to 10 s long, bilateral in onset, with a sharp-wave pattern that was larger in amplitude in the parietal-occipital cortex, were not seen in this study because they typically appear after ∼5 to 6 months of age.
Plasma CRS Determination.
Blood (0.3–0.5 ml) for plasma CRS determination was withdrawn from the tail vein into a microtainer tube with K2EDTA (BD Biosciences, San Jose, CA). Blood samples were spun at 3000g for 10 min in a Marathon 6K tabletop centrifuge (Thermo Fisher Scientific, Waltham, MA). Plasma supernatant was separated, frozen, coded for blind analysis, and shipped on dry ice to Keystone Analytical (North Wales, PA), which measured CRS levels by high-performance liquid chromatography.
Carisbamate Dosing.
Pilot studies were conducted to identify oral dosing regimens capable of stably maintaining CRS plasma levels. For this purpose, naive rats age- and gender-matched to our experimental animals were administered CRS (Johnson and Johnson Pharmaceutical Research and Development, Raritan, NJ) suspended in a condensed milk-based vehicle. Early studies showed a steady decline from targeted steady-state plasma levels during administration of 90 mg/kg CRS three times daily (Fig. 1A), presumably because of auto-induction of its metabolism. However, [CRS]plasma showed no similar decline from target levels in rats administered increasing doses of CRS (Fig. 1B). The first 7 days of this regimen were used for the antiepileptic study, whereas the antiepileptogenesis study used a modified regimen (Table 2) that included a higher dose in the first days of treatment to boost possible antiepileptogenesis (Fig. 1B). All oral doses of CRS were delivered by syringe in a volume of ∼0.2 ml in 75% Borden's Eagle Brand sweetened condensed milk in sterile water, a vehicle in which control and drug preparations were visually indistinguishable. For intraperitoneal injection, CRS was solubilized in 10% Solutol (generously provided by BASF, Ludwigshafen, Germany) according to the manufacturer's instructions.
Fig. 1.
Pilot carisbamate pharmacokinetic studies. A, steady-state plasma CRS levels decline during administration of a fixed dose of 90 mg/kg three times daily (n = 9–10 per time point). B, the decline in steady-state plasma CRS levels is prevented by administration of increasing doses of CRS three times daily as shown (n = 3–10 per time point). In both studies, doses were delivered at 7 AM, 3 PM, and 11 PM daily.
TABLE 2.
Dosing protocols for the antiepileptic and antiepileptogenic studies
| Day | CRS Dosea |
|
|---|---|---|
| Antiepileptic Studyb | Antiepileptogenesis Studyc | |
| mg/kg | ||
| 1 | 45, 70 80 | 60d, 100 |
| 2 | 80 | 107 |
| 3 | 90 | 114 |
| 4 | 95 | 122 |
| 5 | 105 | 129 |
| 6 | 115 | 136 |
| 7 | 130 | 143 |
| 8 | 151 | |
| 9 | 158 | |
| 10 | 165 | |
| 11 | 165 | |
| 12 | 165 | |
| 13 | 165 | |
| 14 | 165 | |
CRS was administered orally each day at 7AM, 3 PM, and 11 PM at the indicated doses.
CRS administration began on postinjury week 5 and/or 17.
CRS administration began 15 min after FPI.
Administered intraperitoneally.
Antiepileptic Assay.
The study protocol is shown in Fig. 2C. Because different seizure types develop with different time courses after rpFPI (D'Ambrosio et al., 2004, 2005), antiepileptic testing was conducted at 4 to 6 weeks and 16 to 18 weeks after rpFPI (Fig. 2C) to assess the efficacy of CRS in controlling G1 seizures and G2 + G3 seizures, respectively. Assigned treatments were administered on postinjury weeks 5 or 17. CRS was administered in increasing doses according to the schedule determined by the pilot pharmacokinetic study (Table 2). To ensure adequate and stable blood levels of CRS during on-drug data acquisition, video-ECoG recordings commenced after a 2-day wash-on period. Blood samples were collected at least 12 h before the first week 5 on-drug ECoG recording and at the end of a 2-day washoff period (week 6). On-drug blood samples were collected at most 2 h before CRS administration to sample trough levels of CRS. Each animal served as its own control. The effect of CRS on neocortical seizures was assessed 5 weeks after injury in two groups of 10 animals that all met a three seizures per baseline week criterion (Eastman et al., 2010) on postinjury week 4. The effect of CRS on limbic (G3) and spreading neocortical (G2) seizures was assessed in a cohort of seven rats, three of which had been used to test the antiepileptic effects of CRS at 4 to 6 weeks. Thus four animals received CRS on week 17 only. Because kindling in the presence of CRS did not induce CRS resistance (Klein et al., 2007), it is unlikely that week 5 CRS exposure affected CRS responsiveness at week 17.
Fig. 2.
Study design and power analyses. A, antiepileptogenesis study. Immediately after rpFPI, animals were randomly assigned to treatment with either vehicle or CRS with equal probability. Treatment commenced 15 min after injury and continued for 2 weeks. Epidural electrodes were implanted 2 weeks after injury, and 48 h of ECoG recordings were obtained from each rat on postinjury weeks 4 and 12. B, nonparametric power analyses for antiepileptogenesis study. Graph shows group sizes required to provide 80% power to detect the indicated antiepileptic effects (one-tailed α = 0.05). ▴ indicate group sizes required to detect uniform decreases in seizure frequency. ○ indicate group sizes required to detect increases in the incidence of seizure-freedom. C, antiepileptic study. Rats received rpFPI, and epidural electrodes were implanted 3 weeks after injury, allowing 1 week for recovery before predrug recording. Animals were randomized to treatment groups in a 1:1 proportion before their first ECoG recordings. Each 3-week testing sequence consisted of three 1-day ECoG recordings on the first week (Baseline I) before drug exposure, on the second week of testing (Drug on) during which the animal was on the drug after a 48-h wash-on period, and on the third week (Baseline II) during which the animal was off the drug after the washoff period. Thus, 72 h of ECoG recordings were acquired from each rat during each week of the 3-week test sequence (∼216 h total per rat). D, nonparametric power analysis for antiepileptic study. Graph shows group size required to provide 80% power to detect uniform decreases in seizure frequency (one-tailed α = 0.05). Data are from Eastman et al. (2010).
Antiepileptogenesis Assay.
The study protocol is shown in Fig. 2A. CRS or vehicle dosing was as in Table 2. Blood for [CRS]plasma determination was drawn from all animals 15 min to 2 h before the afternoon dose on the second and ninth day after the start of CRS administration. Measured [CRS]plasma thus approximate trough levels of the drug.
Blinding Measures.
Antiepileptic and antiepileptogenesis studies both were fully masked. Rats were randomized to treatment with drug or vehicle before the first ECoG recordings (antiepileptic) or the start of drug treatment (antiepileptogenesis). Treatment suspensions of vehicle or drug were supplied in visually indistinguishable vials labeled only with coded identifiers. Thus, treatments were administered by investigators who were blind to both the contents of treatment suspensions and the group assignments of individual subjects. All ECoG data were analyzed by investigators who were blind to the subject, treatment group, or collection date of data files. The decision to discard a noisy ECoG file was made blind to file identity. Data file identifiers remained hidden until all data had been collected and verified. Blood samples were collected and coded, and CRS levels were measured blind to subject, treatment, and dosing protocol.
Data Analysis.
In all experiments, video-ECoG data were analyzed off-line, in random sequence, by trained investigators. Seizures of grades 1, 2, and 3 were identified, and seizure frequencies (events/h), mean seizure durations (s), and times spent seizing (s/h), were determined. To facilitate comparison with previous studies in which seizures were arbitrarily defined based on duration, parallel analyses of all seizures versus seizures lasting ≥5 s are presented. Seizure frequencies and times spent seizing were logarithmically transformed before comparison using t tests.
Nonparametric power analyses have shown this strategy to be more sensitive to detect antiepileptic effects of investigational drugs and robust to the confounding effect of nonresponders (Eastman et al., 2010). Because the logarithm of zero is −∞ and the occurrence of no seizures in a finite observation period only suggests a seizure frequency of less than one per observation period (72 h per time point for the antiepileptic study and 48 h per time point for the antiepileptogenic study), observations of zero seizure frequency or time seizing were assigned conservative floor values (Eastman et al., 2010) of 1/72 and 1/48 in the antiepileptic and antiepileptogenic studies, respectively. For determination of the antiepileptic effect of CRS, decreases from predrug (week 4 or 16) levels of seizure frequency and time seizing were evaluated for the corresponding on-drug (weeks 5 or 17) and posttreatment (weeks 6 or 18) periods using paired t tests (Eastman et al., 2010). Paired decreases in seizure duration were evaluated using Wilcoxon's matched pairs signed ranks test. Antiepileptic effects on seizure frequency or time seizing shown in Figs. 4 and 6 were computed as mean differences between log-transformed pretreatment and posttreatment frequencies or times seizing (e.g., effect = log(frequencyt1/frequencyt2), in which t1 is pretreatment baseline, and t2 is the week either on treatment or post-treatment). We have shown previously (Eastman et al., 2010) that optimal analysis of seizure frequency data from FPI rats (paired t test using logarithmically transformed data) of a group of just eight rats is sufficient to provide at least 80% power to detect a drug-induced ∼45% decrease in seizure frequency in the absence of nonresponders. In the antiepileptogenesis study, seizure frequencies (events/h), mean seizure durations (s), and times spent seizing (s/h) were determined 4 and 12 weeks after rpFPI. These data were compared between groups of rats that had been treated with vehicle or CRS. Antiepileptogenesis was assessed at postinjury weeks 4 and 12 on the basis of unpaired comparisons of vehicle- and CRS-treated log-transformed seizure frequency and time spent seizing (unpaired t test). Untransformed seizure durations were evaluated using the Mann–Whitney test. Because logarithms of values less than one are negative, log-transformed frequencies and times seizing were plotted as log(frequency) − log(floor) such that increases in frequency are represented as positive deflections from a zero floor. Treatment randomizations were carried out with the aid of Excel 2002 (Microsoft Inc., Redmond, WA) and Statistics101 (http://www.statistics101.net) resampling statistics software. Data were compiled and analyzed in Excel 2002, and statistical analyses were conducted using SPSS (version 10; SPSS Inc., Chicago, IL). In keeping with our focus on identifying antiepileptic or antiepileptogenic activity, statistical tests were one-tailed. All results are presented as mean ± S.E.M.
Fig. 4.
CRS had no effect on neocortical seizures at 5 weeks post-FPI. A and B, frequencies of all seizures recorded from individual vehicle-treated (A) and CRS-treated (B) rats before, during, and after week 5 treatment are shown on logarithmic scale. C and D, log-transformed frequencies of G1, G2, and G3 seizures in the vehicle-treated (C) and CRS-treated (D) groups. There was no significant decrease in any seizure type. E and F, log-transformed times spent in G1, G2, and G3 seizure in the vehicle-treated (E) and CRS-treated (F) group. There was no significant decrease in the neocortical seizures that predominate at this stage of the evolution of FPI-induced PTE. Decreases, with respect to week 4, in the times spent in rare G3 seizures seemed significant at both weeks 5 and 6 (*, p ≤ 0.047). G, log-transformed frequency of all seizures in the vehicle- and CRS-treated groups. The frequency of seizure was not significantly decreased with respect to the week 4 pretreatment baseline at any time point in either group. H, log-transformed seizure duration was stable in both treatment groups over the course of the study. I, the antiepileptic effects determined during (week 5) and after (week 6) CRS treatment showed no significant correlation with plasma CRS levels. Effect is computed as log(frequencyweek 4/frequencyweek x).
Fig. 6.
Antiepileptic and antiepileptogenic properties of CRS on seizures of different duration. A and B, histograms showing the frequency of seizures of differing durations before, during, and after CRS treatment at 4, 5, and 6 weeks post-FPI (A) and at 16, 17, and 18 weeks (B). C and D, antiepileptic effects based on all detectable clinical seizures (C) or using only clinical seizures lasting longer than 5 s (D). In either case, no significant antiepileptic effect was detected. E and F, histograms showing the frequency of seizures of differing durations in vehicle-treated (CON) and CRS-treated rats at 4 (E) and 12 (F) weeks after injury in the antiepileptogenic study. Seizure frequency was not affected by prophylactic treatment, and both the shortest and longest seizures appeared equally after vehicle or CRS. G and H, log-transformed seizure frequencies determined on the basis of all detectable clinical seizures (G) or just those lasting longer than 5 s (H) at 4 and 12 weeks postinjury in the antiepileptogenic study. To display log-transformed frequencies as positive values with a zero minimum, they are plotted as log(frequencyobserved/frequencyfloor).
Nonparametric Power Analysis.
To estimate the experimental group size required to detect reductions in seizure frequency in the antiepileptogenesis study, a nonparametric power analysis was conducted as described previously (Eastman et al., 2010). In brief, two samples of size N were randomly drawn from a database containing seizure frequencies previously measured at 4 weeks postinjury from identically injured rats. Treatment was simulated in one sample by uniformly reducing the sampled frequencies by a set percentage (effect size), and both samples were logarithmically transformed and compared using a one-tailed unpaired Welch's t test (α = 0.05). This operation was repeated 105 times for each specified N and effect size, correct detections of the specified treatment effects were counted, and the statistical power was computed as proportion of the 105 comparisons yielding correct (statistically significant) detections of the simulated treatment effects. The seizure frequency database included 102 frequencies (one per animal) obtained from injured rats that were recorded for 48 h 4 weeks after injury. In 23 of these rats, no seizures were observed in the 48 h of recording. The mean seizure frequency (n = 102) was 1.9 ± 0.4 events/h, and the median was 0.34 events/h.
Results
Antiepileptogenesis Study.
At 4 weeks postinjury, 2 weeks after cessation of CRS treatment, there was no detectable prophylactic effect on FPI-induced PTE in 22 control and 27 CRS rats (Fig. 3). The incidence of epilepsy was 82 and 85% and the frequency of seizures was 1.4 ± 0.6 and 1.5 ± 0.4 seizures/h in vehicle- and CRS-treated animals, respectively. Both groups are consistent with our historical data (D'Ambrosio et al., 2004, 2005). The mean log-transformed frequencies of (Fig. 3A) and times spent in (Fig. 3E) G1, G2, G1 + G2 (neocortical), G2 + G3 (spreading), and G1 + G2 + G3 (all) seizures in CRS-treated animals all were nominally elevated with respect to controls, and no seizure type was significantly decreased by CRS treatment (all p > 0.35, unpaired t test). The mean duration of seizures (Fig. 3C) also was not decreased significantly in the CRS-treated group (Mann–Whitney test; all p > 0.3). At 12 weeks postinjury, 10 weeks after cessation of CRS treatment, the log-transformed frequency (Fig. 3B), time spent seizing (Fig. 3F), and the duration (Fig. 3D) still did not differ between control (n = 13) and CRS-treated (n = 17) animals (all p > 0.25; unpaired t test). The mean duration of seizure was also undiminished in the CRS-treated group (all p > 0.2; Mann–Whitney).
Fig. 3.
CRS did not decrease FPI-induced epileptogenesis. A, log-transformed frequencies of G1, G2, G3, neocortical (G1 + G2), spreading (G2 + G3), and all (G1–3) seizures in vehicle-treated (CON) and CRS-treated rats at 4 weeks post-FPI. B, log-transformed frequencies of seizure, as in A at 12 weeks postinjury. C and D, duration of seizures at 4 (C) and 12 (D) weeks after injury. E and F, log-transformed times spent seizing at 4 (E) and 12 (F) weeks post-FPI. Both vehicle- and CRS-treated groups had seizure frequency and duration and time spent seizing consistent with historical data from untreated animals. G, the mean [CRS]plasma remained above 10 μg/ml through day 9 of the 14-day protocol. The low, but detectable, levels of CRS seen in some vehicle-treated (CON) rats were attributed to coprophagous ingestion of CRS excreted by CRS-treated cage mates. H, despite the wide range of [CRS]plasma measured in individual rats, there was no apparent relationship between [CRS]plasma and seizure frequency at either 4 or 12 weeks after injury. Plasma CRS levels are the means of measurements at days 2 and 9.
In CRS-treated rats, mean plasma CRS (Fig. 3G) was 29 ± 6 μg/ml 2 days after the start of treatment and 11 ± 2 μg/ml on the ninth day of treatment. In vehicle-treated rats, plasma CRS was 0.24 ± 0.10 μg/ml on day 2 of the treatment period and increased to 1.90 ± 0.40 μg/ml on day 9. The detection of CRS in untreated controls was unexpected and was traced to the fact that, in contrast to the pilot study, CRS-treated animals were caged together with controls. The CRS-treated animals created a fecal source of CRS that was consumed by controls. There was no indication of a relationship between the [CRS]plasma measured during prophylactic treatment and the frequency of seizure at either 4 or 12 weeks postinjury (Fig. 3H). The highest log-transformed seizure frequencies (>0.5 ≈ 3.16 events/h) were observed in control and CRS rats that had mean [CRS]plasma ranging from 0.7 to 46 μg/ml during prophylactic treatment. There was also no significant correlation between plasma CRS and time spent seizing (not shown). It is therefore unlikely that an antiepileptogenic effect of CRS would have been observed at a higher dose.
Antiepileptic Study.
The antiepileptic efficacy of CRS was first assessed at 4 to 6 weeks postinjury, when seizures predominantly originate from a perilesional neocortical focus and most of them do not spread (G1). Thus, limbic seizures (G3) and spreading neocortical seizures (G2) were rare compared with nonspreading neocortical seizures (G1; Fig. 4, C and D). As reported previously (Eastman et al., 2010), seizure frequencies varied widely among the individual rats in each treatment group (Fig. 4, A and B). The mean pretreatment baseline frequencies of seizure were comparable in control versus CRS group after logarithmic transformation (−0.01 ± 0.16 in controls; 0.03 ± 0.24 in CRS group). Also the pretreatment duration of seizure was comparable in the two groups (6.7 ± 1.1 s in controls; 5.8 ± 0.9 s in the CRS group). The mean log-transformed frequency of seizure (all types) did not decrease during week 5 treatment in either treatment group, and the nominal decrease, with respect to week 4, observed in CRS-treated rats in the week after cessation of treatment (Fig. 4G) did not reach significance (paired t test; p = 0.10). We also detected no significant decreases in the mean log-transformed frequency of any type of seizure either during (all p > 0.3; paired t test) or after (all p > 0.1; paired t test) week 5 CRS treatment (Fig. 4D). Likewise, no significant time- or treatment-related decreases were detected in the duration of all seizures (all p ≥ 0.25; Wilcoxon) in either vehicle- or CRS-treated rats (Fig. 4H). Log-transformed times spent seizing were nominally elevated during week 5 treatment in both vehicle and CRS groups, and the post-treatment decreases in time seizing did not approach significance (both p > 0.25; paired t test) in either group (not shown).
Although CRS did not significantly affect G1 or G2 neocortical seizures, there was limited evidence pointing to a possible effect on the much rarer G3 events that originate from a subcortical focus. There were no significant time-dependent (Fig. 4C) or treatment-dependent (Fig. 4D) decreases in log-transformed frequency of G3 seizures (p > 0.3; paired t test), but log-transformed times spent seizing seemed to be significantly decreased during (week 5) and after (week 6) CRS treatment (both p ≤ 0.047; Fig. 4F). It is worth noting, however, that only 6/10 rats exhibited G3 seizures at 1 month postinjury, and G3 frequency was very low in those that did: 0.027 ± 0.005 events/h versus 2.2 ± 0.7 events/h and 0.19 ± 0.07 events/h for seizures of grades 1 and 2, respectively in the 20 (control + CRS) rats recorded at week 4 postinjury. Thus, this study was not designed to accurately and confidently assess decreases in the frequency of G3 seizures.
CRS levels in plasma samples obtained ∼48 h after start of treatment and before week 5 recordings were 14.80 ± 3.7 μg/ml (range 6.40–28.30 μg/ml). Data from CRS-treated animals revealed no significant correlation (rho = 0.45; p = 0.26; Spearman) between [CRS]plasma and the observed antiepileptic effect of CRS as indicated by week 4 minus week 5 difference in log-transformed seizure frequencies (Fig. 4I). CRS effects at week 6 were also not significantly correlated with [CRS]plasma (rho = 0.21; p = 0.63; Spearman). Thus, there is no evidence that higher plasma CRS levels may have been more effective.
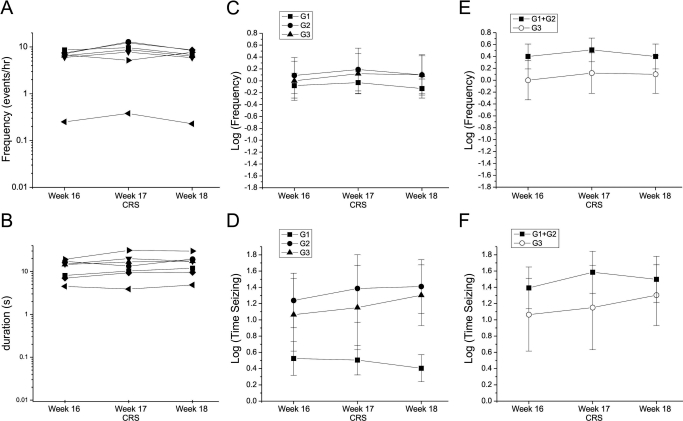
To better examine the possibility that CRS may particularly affect limbic seizures, a cohort of seven animals was challenged with CRS at 16 to 18 weeks post-FPI. At that time spreading seizures (G2 and G3) predominated (Fig. 5, C-F) and limbic seizures (G3) accounted for 30 to 40% of observed seizures. The mean log-transformed frequency of and time spent seizing in G3 seizures were nominally elevated over predrug levels both during (week 17) and after (week 18) CRS treatment (Fig. 5, C and D), and there was no decreases in overall seizure frequency or in the frequency of any type of seizure (all p > 0.33).
Fig. 5.
CRS decreased neither neocortical nor limbic seizures at 17 weeks postinjury. A and B, frequencies (A) and durations (B) of all seizures recorded from individual rats before, during, and after week 17 CRS administration are shown on a logarithmic scale. C and D, mean log-transformed frequencies of (C) and times spent in (D) G1, G2, and G3 seizures before, during, and after week 17 treatment with CRS. There was no significant decrease in any seizure type. E and F, mean log-transformed frequencies of (E) and times spent in (F) neocortical (G1 + G2) and limbic (G3) seizures before, during, and after week 17 treatment with CRS. There was no significant decrease in either seizure class.
Evaluation of CRS Effects on Seizures of Different Durations.
To facilitate comparison with previous studies that define seizures as lasting at least 5 s, the data presented above were reanalyzed after breaking down the seizures in classes based on their duration: ≥1 to <2 s, ≥2 to <5 s, ≥5 to <15 s, ≥15 to <30 s, and ≥30 s. The resulting seizure duration histograms indicate no antiepileptic effect of CRS on seizures of any duration either at 5 weeks (Fig. 6A) or 17 weeks (Fig. 6B) postinjury and no antiepileptogenic effect of the prophylactic CRS treatment at either 4 weeks (Fig. 6E) or 12 weeks (Fig. 6F) post-FPI. Exclusion of the shorter (1–5 s) clinical seizures resulted in a 67 ± 2% decrease in the apparent frequency of seizure, a 107 ± 6% increase in the apparent duration of seizure, and a 41 ± 2% decrease in time spent seizing, but led to similar inferences regarding both the antiepileptic (Fig. 6, C and D) and antiepileptogenic (Fig. 6, G and H) activities of CRS.
Discussion
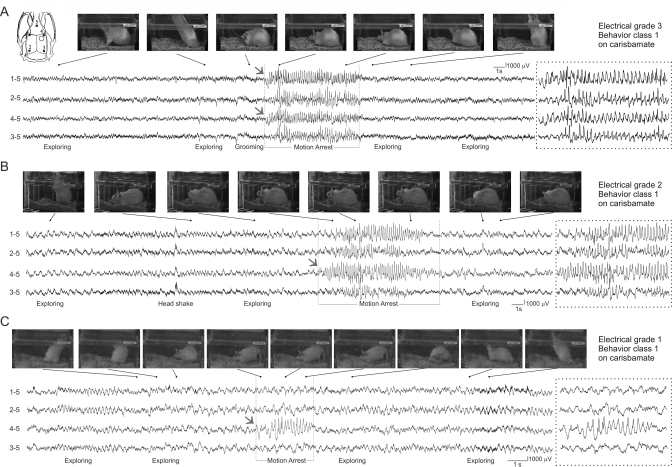
We report the first assessment of the effects of CRS on neocortical and limbic partial seizures induced by head injury in the rat. The study incorporates several strategies intended to facilitate translation to the clinical setting: 1) the epileptogenic insult is etiologically realistic to better capture mechanisms operating in human PTE, 2) ECoG is used to sensitively detect and comprehensively examine the epileptic syndromes; 3) the antiepileptic effect on spontaneous seizures is evaluated during a subchronic treatment that maintains stable blood levels of the drug, to better simulate the clinical conditions under which AEDs have to perform, and 4) the study is conducted in a blind and randomized fashion. The main findings of this study, that CRS demonstrated no meaningful antiepileptogenic or antiepileptic effects on FPI-induced PTE, are in contrast to previous preclinical studies performed with different models and convulsive endpoints. Examples of seizures observed during CRS treatment are shown in Fig. 7.
Fig. 7.
Electrographic and behavioral features of G1, G2, and G3 seizures recorded during CRS administration. A, a typical G3 seizure. Active exploration is evident in frames captured before the onset of electrographic activity that is first detected simultaneously by perilesional (4–5) and contralateral (1–5) electrodes. Static posture is evident in two frames captured during the electrographic discharge, and movement resumes after termination of the G3 seizure. B, a typical G2 seizure. Postural changes are evident in frames taken before onset of an electrographic discharge that is first detected by the perilesional electrode (4–5) and spreads both caudally and contralaterally to be detected by all four epidural electrodes. Animal's posture is static in frames captured during the electrographic discharge and movement resumes after termination of the G2 seizure. A head-shaking artifact detected by all four electrodes is clearly distinguishable. C, a typical short G1 seizure. Active exploratory behavior is evident in changes in the animal's position in frames captured shortly before the onset of a brief G1 seizure detected only by the perilesional electrode (4–5). Ictal motion arrest is indicated by the animal's static posture in frames captured (arrest) during the electrographic discharge. Resumption of exploration is evident after termination of the discharge. Electrode montage in A applies to A to C. Numbers shown to the left of ECoG traces indicate recording and reference electrodes as shown in the Inset. In A to C, dotted boxes show electrographic activity during motion arrest on an expanded time scale. Gray arrows indicate ECoG seizure onset.
Lack of Antiepileptogenic Effect of Carisbamate after FPI.
Although a possible antiepileptogenic activity has been inferred based on the efficacy of CRS in kindling models (Novak et al., 2007) and a report that the emergence of chronic spontaneous motor seizures after lithium-pilocarpine-induced status epilepticus was delayed after prophylactic CRS treatment (André et al., 2007), we found no evidence for an antiepileptogenic effect of CRS after head injury. Mean log-transformed seizure frequencies and times seizing all were nominally higher in CRS-treated rats than in controls at both 1 and 3 months postinjury (Fig. 3). The lack of correlation between [CRS]plasma and seizure frequency in individual subjects (Fig. 3H) provides additional evidence that inadequate dosing does not account for the apparent inactivity. The [CRS]plasma maintained for the first week of prophylactic treatment in this study was probably higher than that attained (but not measured) in a study reporting CRS to have an antiepileptogenic effect after a weeklong treatment begun during pilocarpine-induced SE (André et al., 2007). Given the steady decline in [CRS]plasma achieved during repeated administration of CRS at a fixed dose (Fig. 1A) and the good absorption of orally administered CRS (Mamidi et al., 2007), our extended (2 week) regimen of escalating doses (160–495 mg/kg per day) that reached 430 mg/kg per day by day 7 probably produced higher [CRS]plasma for a longer period than the 7 days of twice-daily 120 mg/kg intraperitoneal dose that delayed or prevented chronic seizures after pilocarpine. Although it cannot be ruled out that the apparent antiepileptogenic activity of CRS administered before or during pilocarpine induction of status epilepticus may reflect an antiepileptic effect on the status epilepticus that serves as an epileptogenic insult in that model, the discrepant results may also reflect significant differences in the mechanisms of epileptogenesis induced by head injury versus pilocarpine-induced SE.
Lack of Substantial Antiepileptic Effects of Carisbamate on FPI-Induced Epilepsy.
Previous studies have shown CRS to have potent antiepileptic activity in genetic models of audiogenic and absence epilepsies and on the spontaneous motor seizures that arise after kainate-evoked SE. CRS, at doses of just 20 and 30 mg/kg i.p., completely suppressed sound-evoked seizures in Wistar AS rats, and classic spike and wave discharges in GAERS rats could be completely suppressed after administration of 60 mg/kg CRS (François et al., 2008). Using a kainate model of acquired epilepsy, Grabenstatter and Dudek (2008) reported that the frequency of spontaneous convulsive seizures in epileptic rats decreased by ∼75% during 6-h monitoring periods after acute CRS administration. These results were obtained with CRS doses producing ∼11μg/ml CRS in plasma at the midpoint of each acute monitoring period.
In contrast, CRS maintained at comparable or greater [CRS]plasma for 1 full week demonstrated no convincing antiepileptic activity in FPI-induced PTE. Neither the neocortical nor the overall frequency of seizure and time spent seizing were significantly diminished by CRS at 1 month (Fig. 4, G and H) or 3 months (Fig. 5, D and F) postinjury. The observed effect on limbic seizures (Fig. 4F) may be unreliable because of their rarity at this time point (D'Ambrosio et al., 2004, 2005). The effect is also time-limited, because CRS administered at 17 weeks did not control them (Fig. 5E). Thus, it would probably be of little clinical significance.
Differences from Previous Preclinical Studies.
These data show FPI-induced PTE to be resistant to CRS. Our results differ starkly from previous preclinical studies, including those focused on spontaneous seizures. This disparity is not attributable to differences in dosing, statistical power, or seizure detection. The [CRS]plasma maintained in the present studies were comparable with, or greater than, those achieved in preclinical studies demonstrating robust antiepileptic or antiepileptogenic effects (Rogawski, 2006) and in clinical trials (Sperling et al., 2010). Power analyses (Fig. 2, B and D) indicate the study designs to be adequate to detect effects comparable with the antiepileptogenic 45% incidence of seizure freedom reported by André et al. (2007) in the pilocarpine model and substantially smaller than the antiepileptic ∼75% decrease in frequency of motor seizure reported by Grabenstatter et al. (2008) in the kainate model. The poor performance of CRS in rpFPI-PTE is also not attributable to the inclusion, in our analysis, of short (<5 s) clinical seizures that have been ignored in previous studies (D'Ambrosio et al., 2009; D'Ambrosio and Miller, 2010b; Eastman et al., 2010) because longer seizures are not more responsive to CRS (Fig. 6).
Thus, the disparities between this and previous preclinical work must depend on other features that distinguish this experimental approach (Eastman et al., 2010), which we now refine to include blind and randomized experimental design. First, the prolonged (1 week) continuous AED exposure used in our studies are more likely to be affected by the time-dependent functional or metabolic tolerance that occur during prolonged exposure to therapeutic levels of AEDs (Löscher, 2007) than the acute (hours) exposures used in previous preclinical studies. Second, previous work has focused exclusively on behaviorally observed convulsive seizures, whereas we monitored all seizures, and there is little reason to expect that etiologically and phenotypically distinct seizures should be similarly responsive to treatment. Human complex partial seizures, which are characterized by altered cognition and motor output depending on regions of onset and spread and are often nonconvulsive, represent a much greater challenge for pharmacological treatment than tonic-clonic convulsions (Juul-Jensen, 1986; Mattson et al., 1996; Semah et al., 1998). The relationship between etiology and pharmacoresponsiveness is not well explored, but acquired epilepsies tend to be pharmacoresistant (Semah et al., 1998; Callaghan et al., 2007; Hitiris et al., 2007), and human PTE, in particular, seemed more resistant to treatment in a clinical trial in which outcomes were evaluated by etiology (Lu et al., 2009). Thus, our study of acquired and predominantly nonconvulsive clinical seizures may contribute to the overall resistance to CRS.
rpFPI in Adolescent Rat as Model of Pharmacoresistant PTE.
Chronic recurrent spontaneous seizures induced by rpFPI in the adolescent rat are fully blocked by halothane in all animals and by valproate in those animals with few and/or exclusively nonspreading seizures. Carbamazepine, in contrast, is ineffective in all animals tested (Eastman et al., 2010). The poor efficacy of CRS we now report further demonstrates that rpFPI induces a pharmacoresistant PTE in most animals. Although the mechanisms of this pharmacoresistance remain to be elucidated, the realistic contusive closed head injury and ensuing PTE syndrome probably recruit mechanisms similar to those operating in human pharmacoresistant PTE. This model's record of pharmacoresistance is unique and contrasts with the broad responsiveness to virtually all AEDs of chronic seizures induced in SE models. Indeed, with the exception of ethosuximide, all AEDs tested have been found to have large effects against spontaneous motor seizures after pilocarpine, kainate, or amygdalar stimulation-induced SE (Glien et al., 2002; Löscher, 2002; Grabenstatter et al., 2005; Grabenstatter and Dudek, 2008).
Despite its disparity with previous preclinical work, the equivocal antiepileptic effect of CRS we found in rpFPI-PTE was not inconsistent with that observed in populations of partial-onset pharmacoresistant epilepsy patients in add-on clinical trials (Faught et al., 2008; Sperling et al., 2010), because large effects of CRS were not seen in either case. No clinical data are available to assess the performance of CRS on patients with pharmacoresistant epilepsy caused by head trauma, and neither our study nor the add-on clinical trials of CRS in pharmacoresistant epilepsies may reflect the performance of CRS on pharmacoresponsive epilepsy patients. It remains possible that CRS may exhibit exemplary performance in clinical trials conducted with newly diagnosed epilepsy patients suffering from tonic-clonic convulsions, as suggested by the bulk of previous preclinical work.
Conclusions
FPI-induced PTE in the adolescent rat is resistant to carbamazepine, but can be fully blocked by halothane in all animals and by valproate in some animals (Eastman et al., 2010). The failure of CRS to prevent or reduce epileptic seizures in this study further supports rpFPI-PTE as a model of pharmacoresistant PTE. In spite of very promising preclinical studies using other animal models, clinical trials showed CRS had limited efficacy as add-on treatment for most pharmacoresistant patients. Thus, FPI-PTE may prove useful for identifying agents effective in the ∼30% of epilepsy patients who are now pharmacoresistant.
Acknowledgments
We thank Drs. Steve White and Brian Klein for helpful discussion and service as liaisons with Johnson and Johnson Pharmaceutical Research and Development, LLC, and Dr. Nancy Temkin for helpful discussion and statistical consultation.
This work was supported by Johnson and Johnson Pharmaceutical Research and Development, LLC, and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS053928] (to R.D.).
Disclosure: In 2006, R.D. served as a paid consultant to Johnson and Johnson.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.175133.
Abbreviations:
- CRS
- carisbamate
- AED
- antiepileptic drug
- ECoG
- electrocorticography
- G1
- grade 1
- G2
- grade 2
- G3
- grade 3
- PTE
- posttraumatic epilepsy
- rpFPI
- rostral parasagittal fluid percussion injury
- SE
- status epilepticus.
Authorship Contributions
Participated in research design: Eastman, Verley, Fender, and D'Ambrosio.
Conducted experiments: Eastman, Verley, Fender, Stewart, Nov, Curia, and D'Ambrosio.
Performed data analysis: Eastman, Verley, Fender, Curia, and D'Ambrosio.
Wrote or contributed to the writing of the manuscript: Eastman and D'Ambrosio.
References
- André V, Dubé C, François J, Leroy C, Rigoulot MA, Roch C, Namer IJ, Nehlig A. (2007) Pathogenesis and pharmacology of epilepsy in the lithium-pilocarpine model. Epilepsia 48:41–47 [DOI] [PubMed] [Google Scholar]
- Beghi E, Berg A, Carpio A, Forsgren L, Hesdorffer DC, Hauser WA, Malmgren K, Shinnar S, Temkin N, Thurman D, Tomson T. (2005) Comment on epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46:1698–1699 [DOI] [PubMed] [Google Scholar]
- Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA. (2007) Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol 62:382–389 [DOI] [PubMed] [Google Scholar]
- Crossley NA, Sena E, Goehler J, Horn J, van der Worp B, Bath PM, Macleod M, Dirnagl U. (2008) Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke 39:929–934 [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Miller JW. (2010a) Point. Epilepsy Curr 10:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R, Miller JW. (2010b) What is an epileptic seizure? Unifying definitions in clinical practice and animal research to develop novel treatments. Epilepsy Curr 10:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. (2004) Post-traumatic epilepsy following fluid percussion injury in the rat. Brain 127:304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, Miller JW. (2005) Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain 128:174–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R, Hakimian S, Stewart T, Verley DR, Fender JS, Eastman CL, Sheerin AH, Gupta P, Diaz-Arrastia R, Ojemann J, et al. (2009) Functional definition of seizure provides new insight into post-traumatic epileptogenesis. Brain 132:2805–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. (2006) Adult epilepsy. Lancet 367:1087–1100 [DOI] [PubMed] [Google Scholar]
- Eastman CL, Verley DR, Fender JS, Temkin NR, D'Ambrosio R. (2010) ECoG studies of valproate, carbamazepine and halothane in frontal-lobe epilepsy induced by head injury in the rat. Exp Neurol 224:369–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faught E, Holmes GL, Rosenfeld WE, Novak G, Neto W, Greenspan A, Schmitt J, Yuen E, Reines S, Haas M. (2008) Randomized, controlled, dose-ranging trial of carisbamate for partial-onset seizures. Neurology 71:1586–1593 [DOI] [PubMed] [Google Scholar]
- Fisher RS, Leppik I. (2008) Debate: When does a seizure imply epilepsy? Epilepsia 49 (Suppl 9):7–12 [DOI] [PubMed] [Google Scholar]
- Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J., Jr (2005) Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46:470–472 [DOI] [PubMed] [Google Scholar]
- French JA. (2007) Refractory epilepsy: clinical overview. Epilepsia 48 (Suppl 1):3–7 [DOI] [PubMed] [Google Scholar]
- François J, Boehrer A, Nehlig A. (2008) Effects of carisbamate (RWJ-333369) in two models of genetically determined generalized epilepsy, the GAERS and the audiogenic Wistar AS. Epilepsia 49:393–399 [DOI] [PubMed] [Google Scholar]
- Glien M, Brandt C, Potschka H, Löscher W. (2002) Effects of the novel antiepileptic drug levetiracetam on spontaneous recurrent seizures in the rat pilocarpine model of temporal lobe epilepsy. Epilepsia 43:350–357 [DOI] [PubMed] [Google Scholar]
- Grabenstatter HL, Dudek FE. (2008) A new potential AED, carisbamate, substantially reduces spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia 49:1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenstatter HL, Ferraro DJ, Williams PA, Chapman PL, Dudek FE. (2005) Use of chronic epilepsy models in antiepileptic drug discovery: the effect of topiramate on spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia 46:8–14 [DOI] [PubMed] [Google Scholar]
- Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. (2007) Predictors of pharmacoresistant epilepsy. Epilepsy Res 75:192–196 [DOI] [PubMed] [Google Scholar]
- Juul-Jensen P. (1986) Epidemiology of intractable epilepsy, in Intractable Epilepsy (Schmidt D, Morselli P. eds) pp 5–11, Raven Press, New York [Google Scholar]
- Klein BD, Smith MD, White HS. (2007) The novel neuromodulator carisbamate delays the acquisition of rat amygdala kindling and maintains acute antiepileptic activity when evaluated in post-kindled rats. Epilepsia 48 (Suppl 6):367–368 [Google Scholar]
- Kwan P, Brodie MJ. (2003) Clinical trials of antiepileptic medications in newly diagnosed patients with epilepsy. Neurology 60 (Suppl 4):S2–S12 [DOI] [PubMed] [Google Scholar]
- Löscher W. (2002) Animal models of epilepsy for the development of antiepileptogenic and disease-modifying drugs. A comparison of the pharmacology of kindling and post-status epilepticus models of temporal lobe epilepsy. Epilepsy Res 50:105–123 [DOI] [PubMed] [Google Scholar]
- Löscher W. (2007) The pharmacokinetics of antiepileptic drugs in rats: consequences for maintaining effective drug levels during prolonged drug administration in rat models of epilepsy. Epilepsia 48:1245–1258 [DOI] [PubMed] [Google Scholar]
- Löscher W, Hönack D. (1995) Comparison of anticonvulsant efficacy of valproate during prolonged treatment with one and three daily doses or continuous (“controlled release”) administration in a model of generalized seizures in rats. Epilepsia 36:929–937 [DOI] [PubMed] [Google Scholar]
- Löscher W, Schmidt D. (2004) New horizons in the development of antiepileptic drugs: the search for new targets. Epilepsy Res 60:77–159 [DOI] [PubMed] [Google Scholar]
- Lu Y, Yu W, Wang X. (2009) Efficacy of topiramate in adult patients with symptomatic epilepsy: an open-label, long-term, retrospective observation. CNS Drugs 23:351–359 [DOI] [PubMed] [Google Scholar]
- Mamidi RN, Mannens G, Annaert P, Hendrickx J, Goris I, Bockx M, Janssen CG, Kao M, Kelley MF, Meuldermans W. (2007) Metabolism and excretion of RWJ-333369 [1,2-ethanediol,1-(2-chlorophenyl)-,2-carbamate,(S)-] in mice, rats, rabbits, and dogs. Drug Metab Dispos 35:566–575 [DOI] [PubMed] [Google Scholar]
- Mattson RH, Cramer JA, Collins JF. (1996) Prognosis for total control of complex partial and secondarily generalized tonic clonic seizures. Department of Veterans Affairs Epilepsy Cooperative Studies No. 118 and No. 264 Group. Neurology 47:68–76 [DOI] [PubMed] [Google Scholar]
- Meldrum B. (2002) Do preclinical seizure models preselect certain adverse effects of antiepileptic drugs? Epilepsy Res 50:33–40 [DOI] [PubMed] [Google Scholar]
- Novak GP, Kelley M, Zannikos P, Klein B. (2007) Carisbamate (RWJ-333369). Neurotherapeutics 4:106–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip M, Benatar M, Fisher M, Savitz SI. (2009) Methodological quality of animal studies of neuroprotective agents currently in phase II/III acute ischemic stroke trials. Stroke 40:577–581 [DOI] [PubMed] [Google Scholar]
- Rogawski MA. (2006) Diverse mechanisms of antiepileptic drugs in the development pipeline. Epilepsy Res 69:273–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Rogawski MA. (2002) New strategies for the identification of drugs to prevent the development or progression of epilepsy. Epilepsy Res 50:71–78 [DOI] [PubMed] [Google Scholar]
- Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M. (1998) Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology 51:1256–1262 [DOI] [PubMed] [Google Scholar]
- Sperling MR, Greenspan A, Cramer JA, Kwan P, Kälviäinen R, Halford JJ, Schmitt J, Yuen E, Cook T, Haas M, et al. (2010) Carisbamate as adjunctive treatment of partial onset seizures in adults in two randomized, placebo-controlled trials. Epilepsia 51:333–343 [DOI] [PubMed] [Google Scholar]
- Stables JP, Bertram EH, White HS, Coulter DA, Dichter MA, Jacobs MP, Loscher W, Lowenstein DH, Moshe SL, Noebels JL, et al. (2002) Models for epilepsy and epileptogenesis: report from the NIH workshop, Bethesda, Maryland. Epilepsia 43:1410–1420 [DOI] [PubMed] [Google Scholar]
- Temkin N. (2009) Preventing and treating posttraumatic epilepsy: the human experience. Epilepsia 50 (Suppl 2):10–13 [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. (2005) Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma 22:42–75 [DOI] [PubMed] [Google Scholar]
- White HS. (2002) Animal models of epileptogenesis. Neurology 59 (Suppl 5):S7–S14 [DOI] [PubMed] [Google Scholar]
- White HS. (2003) Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia 44 (Suppl 7):2–8 [DOI] [PubMed] [Google Scholar]
- White H, Srivastava A, Klein B, Zhao B, Choi YM, Gordon R, Lee JS. (2006) The novel investigational neuromodulator RWJ-333369 displays a broad-spectrum anticonvulsant profile in rodent seizure and epilepsy models. Epilepsia 47:200–201 [Google Scholar]