Abstract
This study was designed to evaluate further a prototypical ranitidine analog, JWS-USC-75-IX, [(3-[[[2-[[(5-dimethylaminomethyl)-2-furanyl]methyl]thio]ethyl]amino]-4-nitropyridazine, JWS], for neuropharmacologic properties that would theoretically be useful for treating cognitive and noncognitive behavioral symptoms of neuropsychiatric disorders. JWS was previously found to inhibit acetylcholinesterase (AChE) activity, serve as a potent ligand at muscarinic M2 acetylcholine receptors, and elicit positive effects on spatial learning, passive avoidance, and working memory in rodents. In the current study, JWS was evaluated for binding activity at more than 60 neurotransmitter receptors, transporters, and ion channels, as well as for inhibitory activity at AChE and butyrylcholinesterase (BChE). The results indicate that JWS inhibits AChE and BChE at low (micromolar) concentrations and that it is a functional antagonist at M2 receptors (KB = 320 nM). JWS was subsequently evaluated orally across additional behavioral assays in rodents (dose range, 0.03–10.0 mg/kg) as well as nonhuman primates (dose range, 0.05–2.0 mg/kg). In rats, JWS improved prepulse inhibition (PPI) of the acoustic startle response in nonimpaired rats and attenuated PPI deficits in three pharmacologic impairment models. JWS also attenuated scopolamine and (−)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801)-related impairments in a spontaneous novel object recognition task and a five-choice serial reaction time task, respectively. In monkeys, JWS elicited dose-dependent improvements of a delayed match-to-sample task as well as an attention-related version of the task where randomly presented (task-relevant) distractors were presented. Thus, JWS (potentially via effects at several drug targets) improves information processing, attention, and memory in animal models and could potentially treat the cognitive and behavioral symptoms of some neuropsychiatric illnesses.
Introduction
It is now well documented that life expectancies are increasing worldwide and that this phenomenon is resulting in an unprecedented growth of elderly populations (U.S. CDC, 2003; United Nations, 2007). However, the optimism surrounding this important trend is in many ways attenuated in older individuals by the fear of declining memory function and the specter of dementia. Although there are therapies available to treat dementia, such as acetylcholinesterase inhibitors (AChEIs) and the NMDA antagonist memantine, these agents are limited by their modest levels of efficacy and/or adverse side effects. Furthermore, the cognitive deficits in those with dementia are often accompanied by other adverse behavioral symptoms (e.g., agitation, aggression, psychosis) that are, for the most part, left untreated by the currently available primary therapies (Minger et al., 2000). These “noncognitive behavioral symptoms” significantly increase caregiver burden and the direct costs of care as well as result in earlier institutionalization of patients (see Eustace et al., 2002 for a review). Unfortunately, to control these behavioral symptoms, patients are often treated with potent antipsychotic medications that are associated with movement disorders and/or a wide variety of metabolic abnormalities (e.g., weight gain, hyperglycemia, hyperlipidemia; see Miyamoto et al., 2005 for a review) as well as increased mortality in older patients with dementia (Ballard et al., 2009).
In diseases such as AD, it can be argued that a multiple-drug target approach to therapy is necessary to address the varied pathological aspects of the disease and its diverse symptoms. However, even if the strategy of combining drugs with different therapeutic targets is feasible, the development of multifunctional compounds would circumvent the challenge of administering multiple drugs with potentially different degrees of bioavailability, pharmacokinetics, and metabolism (see Youdim and Buccafusco, 2005 for a review). An additional advantage to single drugs with multiple actions is the simplification of the therapeutic regimen and improved compliance (an important consideration, especially for individuals who suffer from disorders such as AD).
Several years ago, we synthesized a series of ranitidine analogs for the purpose of creating nontoxic AChEIs. The impetus for this work was the prior observation that ranitidine (a histamine H2 antagonist commonly used to treat duodenal ulcers) had mild AChEI properties in vitro (Gwee and Cheah, 1986). Several of the compounds from this series of ranitidine analogs were subsequently found to possess potent AChEI properties as well as low toxicity profiles (Valli et al., 1992). Of this series, one compound (i.e., compound 26), JWS-USC-75-IX [3-[[[2-[[(5-dimethylaminomethyl)-2-furanyl]methyl]thio]ethyl]amino]-4-nitropyridazine, JWS], was found to inhibit AChE activity in vitro (IC50, ∼470 nM) and to bind M2 muscarinic acetylcholine receptors with relative high affinity in rodent cerebral cortex (IC50, ∼60 nM). It is noteworthy that it has been hypothesized that in the therapy of AD, an M2-selective (autoreceptor) antagonist might be very useful given in conjunction with an AChEI to prevent the acetylcholine from decreasing its own release (Mash et al., 1985; Quirion, 1993). JWS, thus, combined both features in a single molecule and offered a significant potential for improved reliability over presently available compounds for the treatment of diseases where cholinergic function is impaired (e.g., AD).
Later, we evaluated JWS for effects in memory-related tasks in rodents (Terry et al., 1999) and observed JWS-related improvements in spatial learning, inhibitory avoidance, and working memory. One objective of the current study was to determine whether JWS has the potential to improve memory-related function in additional cognitive domains in rodents (e.g., attention, recognition memory) as well as to evaluate the compound in higher animals that more closely resemble humans (i.e., nonhuman primates). It is noteworthy that there are some data to suggest that M2 receptor levels are elevated in the frontal and temporal cortex of patients with AD who suffer from psychotic symptoms (i.e., compared with those without these symptoms; Lai et al., 2001). Such data suggest that M2 antagonists might have a role in ameliorating psychotic as well as cognitive symptoms in these patients with AD. Thus, another objective of this study was to evaluate JWS for potential antipsychotic-like activity in rodents. Antipsychotic and procognitive properties of JWS (if detected) would also suggest potential indications for neuropsychiatric disorders beyond AD (e.g., schizophrenia).
Because JWS was derived from the ranitidine molecule (a histaminic H2 antagonist), it was conceivable that the compound might have activity at histaminic receptors (which had not previously been assessed) as well as additional drug targets. JWS was thus screened at a single concentration (10 μm) across more than 60 neurotransmitter receptors, transporters, ion channels, and the enzyme acetylcholinesterase. Subsequent ligand binding studies and functional assays were performed with multiple JWS concentrations to confirm activity at the targets identified in the screen.
Materials and Methods
All procedures used during this study that involved animals were reviewed and approved by the Medical College of Georgia Institutional Animal Care and Use Committee and are consistent with Association for Assessment and Accreditation of Laboratory Animal Care guidelines. Measures were taken to minimize pain or discomfort in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). Significant efforts were also made to minimize the total number of animals used while maintaining statistically valid group numbers.
Drugs
All drug doses were calculated based on the free base weight. JWS-USC-75-IX was synthesized as described under Synthesis of JWS-USC-75-IX. Apomorphine, MK-801, scopolamine hydrobromide, d-amphetamine sulfate, and haloperidol were obtained from Sigma-Aldrich (St. Louis, MO); donepezil was obtained from A&A Pharmachem (Ottawa, ON, Canada); risperidone and galantamine were obtained from Janssen Pharmaceutica (Beerse, Belgium); and rivastigmine was obtained from AK Scientific, Inc. (Mountain View, CA). With the exception of JWS and the antipsychotic drugs risperidone and haloperidol, the vehicle for all drugs was normal (0.9%) saline. JWS was dissolved in microliter amounts of dimethylsulfoxide (DMSO) and then diluted in distilled water to the desired concentration. Risperidone was dissolved in 0.1 N acetic acid and diluted to the desired concentration, and the pH of the solution was adjusted to 5.0 with 0.1 N NaOH. Haloperidol was dissolved in a mixture of 0.1 N acetic acid and 0.03% DMSO and then diluted to the desired concentration, and the pH of the solution was adjusted to 6.0 with 0.1 N NaOH.
Synthesis of JWS-USC-75-IX
JWS-USC-75-IX was synthesized as described previously (Valli et al., 1992). In brief, commercially available 2-[(2-aminoethylthio)methyl]-5-[(N,N-dimethylamino)methyl]furan was reacted with 1,1-bis(methylthio)-2-nitroethylene to yield the monothiomethyl intermediate. This remaining thiomethyl group was displaced by hydrazine, and the resulting adduct cyclized with aqueous glyoxal to form the nitropyridazine JWS-USC-75-IX. The structural characterization and purity of JWS-USC-75-IX was assessed using 400 MHz 1H NMR, thin-layer chromatography, and elemental analysis (Atlantic Microlab, Norcross, GA).
Pharmacological Activity of JWS-USC-75-IX (In Vitro)
JWS was screened at a single concentration (10 μm) across more than 60 neurotransmitter receptors, transporters, ion channels, and the enzyme acetylcholinesterase by NovaScreen/Caliper Life Sciences (Hanover, MD). Details of each assay condition can be accessed through Caliper's web site (http://www.caliperls.com). Subsequent ligand binding studies were performed by NovaScreen/Caliper Life Sciences with three JWS concentrations to confirm activity at the targets identified in the screen (defined as ≥70% inhibition of binding). A functional assay was also conducted by Cerep (Celle l'Evesault, France) using standard protocols and procedures described on their web site (http://www.cerep.com). In brief, to identify agonist/antagonist activity of JWS-USC-75-IX at M2 muscarinic receptors, a modification of the method of Michal et al. (2001) was used. Experiments were performed on Chinese hamster ovary cells stably transfected with the human gene for muscarinic M2 receptor subtype, and agonist/antagonist activity of JWS was determined by monitoring the synthesis of cyclic AMP in response to 1.0 μM acetylcholine in the presence of various concentrations of JWS. The IC50 values (concentration causing a half-maximal inhibition of the control-specific agonist response) were determined by nonlinear regression analysis of the concentration-response curves generated with mean replicate values using Hill equation curve fitting [Y = D + [(A − D)/(1 + (C/C50)]nH, where Y is the specific response, D is the minimum specific response, A is the maximum specific response, C is the compound concentration, C50 is the IC50, and nH is the slope factor]. This analysis was performed using Hill software (developed at Cerep) and validated by comparison with data generated by the commercial software SigmaPlot 4.0 for Windows (SPSS Inc., Chicago, IL). The apparent dissociation constants (KB) were calculated using the modified Cheng Prusoff equation [KB = IC50/[1 + (A/EC50A)], where A is the concentration of reference agonist in the assay, and EC50A is the EC50 value of the reference agonist].
The inhibitory activity of JWS (compared with currently prescribed AChEIs galantamine, donepezil, and rivastigmine) on AChE and butyrylcholinesterase was determined using a modification of the method of Ellman et al. (1961) in a 24-well plate format at 37°C. Electric eel cholinesterase and equine butyrylcholinesterase purified from serum were purchased from Sigma-Aldrich. Assays were performed in 0.1 M sodium phosphate buffer, pH 8.0, containing 200 μM substrate (acetylthiocholine or butyrylthiocholine), 100 μM dithiobisnitrobenzoic acid, and 0.005 units of enzyme in a final volume of 3000 μl. After an 8-min preincubation with inhibitor and enzyme, the reaction was initiated by the addition of substrate. The 24-well plate was shaken for 30 s using a Jitterbug plate shaker (Boekel Scientific, Feasterville, PA) before it was placed in a μQuant microplate spectrophotometer (BioTek Instruments, Winooski, VT). The formation of a reaction product (yellow in color) was monitored by measuring absorbance at 412 nm. Velocity was expressed as micromoles of substrate hydrolyzed per minute for every milligram of protein. All assays were performed at least two or three times. The IC50 values (concentration causing a half-maximal inhibition of the control response) were determined by nonlinear regression analysis of the concentration-response curves generated.
Rodent Behavioral Studies
All rat behavioral experiments were conducted in rooms equipped with white noise generators (San Diego Instruments, San Diego, CA), set to provide a constant background level of 70 db, and ambient lighting of approximately 25 to 30 lux (lumens per square meter). Animals were transferred (in their home cages) to the behavioral testing rooms each morning approximately 30 min before the beginning of experiments.
Test Subjects (Rats)
Male albino Wistar rats (2–3 months old; Harlan, Indianapolis, IN) were housed in pairs in a temperature-controlled room (25°C), maintained on a reverse 12-h light/dark cycle with free access to food (Teklad Rodent Diet 8604 pellets; Harlan Teklad, Madison, WI).
Amphetamine-Induced Locomotor Activity
Rat open-field activity monitors (43.2 × 43.2 cm; Med Associates, St. Albans, VT) were used for amphetamine-induced locomotor experiments, and photobeam breaks (ambulatory counts) were assessed. Rats were habituated in the test chambers for 30 min, and then vehicle, a dose of JWS, or risperidone (as a positive control) was administered by oral gavage. At 60 min, all rats were injected with 1.0 mg/kg s.c. amphetamine and then monitored for an additional 120 min.
Catalepsy
Catalepsy was assessed via the bar test as described in Sanberg et al. (1988). The front limbs of the rats were placed over a 2-cm high horizontal bar. Catalepsy was measured by the time the rats remained in this position on three consecutive trials (maximum, 120 s). An average of the three trials was used for the catalepsy score.
Rotarod
Rats were given 3 days of rotarod training consisting of three to four trials per day on an accelerating rod (0–13 rpm over 60 s) with a trial duration of 120 s; the intertrial interval was 30 min. On the test day (day 4), rats were given three additional training trials to ensure learned task performance. Only animals that remained on the accelerating rod for two of the three trials were used for testing. Test compounds were then administered (again by oral gavage), and at the appropriate pretreatment interval, rotarod performance was assessed during two rotarod test trials.
Conditioned Avoidance Responding
Conditioned avoidance training was conducted using commercially available shuttle boxes (GEMINI Avoidance System; San Diego Instruments). The Plexiglas shuttle boxes were divided into two equally sized compartments by a guillotine door. The shuttle boxes were fitted with a stainless steel grid rod floor and wired for presentation of an electric foot shock. In addition, each side of the chamber was equipped with a house light, a speaker for delivering a tone, and multiple infrared photobeam detectors that tracked the location of the subject within the chamber. Training sessions consisted of a 2-min acclimation period followed by 11 trials presented on a variable interval schedule (intertrial interval of 25–40 s). Each trial consisted of a 10-s warning tone (85 db) and illumination of the house light (conditioned stimulus) followed by a 10-s foot shock (0.7 mA; unconditioned stimulus) presented through the grid floor on the side where the animal was located. The shock was terminated when the animal crossed over to the other compartment or after 10 s had lapsed. Active avoidance was defined as crossing over into the opposite compartment during the 10-s conditioning stimuli (light and tone pairing), thereby preventing the delivery of the shock. Crossing over to the other compartment after the delivery of the shock terminated shock delivery and was recorded as an escape response. If the animal failed to cross over to the other compartment after termination of the shock, the response was recorded as an escape failure. Training was continued until stable performance was obtained and a criterion of 80% avoidance responding for 3 consecutive days was achieved. Drug testing occurred twice a week with training trials interspersed among the test trials, and the animals had to maintain an 80% avoidance response accuracy to be included in the test.
Prepulse Inhibition
To assess the effects of JWS on sensorimotor gating, a PPI procedure was conducted as described in detail previously (Hohnadel et al., 2007). In brief, four startle chambers (San Diego Instruments) were used, the background white noise was set at 70 db, and the PPI trials consisted of a prepulse (20-ms burst of white noise with intensities of 75, 80, or 85 db) followed, 100 ms later, by a startle stimulus (120 db, 20-ms white noise). PPI was calculated according to the formula 100 − (startle amplitude on prepulse-pulse trials/startle amplitude on pulse-alone trials) × 100. The mean level of PPI (i.e., averaged across the three prepulse intensities) was also analyzed. Rats were administered vehicle, PPI-impairing agents, JWS, or reference doses of risperidone or donepezil (as positive controls) plus the PPI-impairing agents before being evaluated in the PPI procedure. For the apomorphine and MK-801 reversal studies, test subjects were administered vehicle, JWS, or risperidone dissolved in vehicle by oral gavage 30 min before testing followed by either saline, MK-801 (0.1 mg/kg), or apomorphine (0.5 mg/kg s.c.) 10 min before testing. For the scopolamine reversal studies, 0.33 mg/kg i.p. scopolamine hydrobromide was administered 40 min before testing followed by vehicle, JWS, or donepezil (by oral gavage) 20 min before testing. The compounds used to disrupt PPI (and their doses) were based on earlier studies (Mansbach et al., 1988; Mansbach and Geyer, 1989) and recent work in our laboratory (Hohnadel et al., 2007).
Spontaneous Novel Object Recognition Test
Spontaneous novel object recognition (NOR) tests were conducted as described in detail previously (Terry et al., 2007). In brief, habituation to the test apparatus consisted of two daily 10-min sessions in which the animals were allowed to freely explore the open field box. Video-recorded object recognition (OR) testing began on the 3rd day and ended on day 5. Each test day began with a 3-min information session (i.e., the A/A session with identical objects) followed by a 1-, 15-, or 60-min delay period (administered in a pseudorandom order) and a subsequent 3-min dissimilar stimuli (A/B) session. The objects discriminated were made of glass, ceramic, clay, or plastic. The total exploration time that the subjects spent investigating the each object was recorded. A discrimination index (d2) was calculated on each A/B trial and was defined as the difference in time spent exploring the novel and familiar objects divided by the total exploration time for both objects: d2 index = (novel − familiar)/(novel + familiar). This measure is considered an index of recognition memory and takes into account individual differences in the total amount of object exploration time. For the evaluation of JWS in the scopolamine impairment model, scopolamine hydrobromide was administered subcutaneously 40 min before the A/A session; donepezil, as a positive control, or JWS was administered by oral gavage 20 min before testing.
Five-Choice Serial Reaction Time Task
Five-choice serial reaction time task (5C-SRTT) training and testing was conducted using six ventilated, sound-attenuated operant chambers (Med Associates) as we have described previously (Middlemore-Risher et al., 2010). Each operant chamber consisted of nine nose pokes/apertures, four of which were closed off with metal inserts, leaving every other nose poke available (2.5 cm wide, 4 cm deep). The apertures were arranged on a curved panel 2 cm above the floor and were equipped with a photocell beam to detect nose pokes. There was a lamp (2.8 W) on the rear wall of each aperture that could be illuminated randomly and for varying durations. Food pellets were delivered automatically to a magazine on the opposite wall to the nose pokes. A light inside the food magazine was also turned on to indicate that a pellet (45 mg of chow pellet; BioServ, Frenchtown, NJ) had been dispensed. The food magazine was equidistant from all nose-poke apertures. There was a house light that remained on for the entire session unless an error or omission occurred; the light was located toward the roof of the operant chamber above the magazine. The apparatus was controlled using MedPC software (Med Associates).
Week 1 consisted of handling animals to reduce stress and anxiety in preparation for training and testing. Week 2 consisted of handling and food restriction. Week 3 consisted of food restriction, habituation to the apparatus, and preparation for training. In detail, on days 1 and 2 of week 3, subjects were placed in the operant chambers for 15 min with the house light and magazine light on and 10 pellets in the magazine dispenser. On days 3 to 5 of week 3, animals performed a nonspatial training program. In brief, the animals were placed in the 5C-SRTT operant chamber with the house light illuminated. A pellet was released into the food magazine, which was simultaneously illuminated for a maximum of 5 s or until the animal collected the pellet. After a 10-s interval, another pellet was released from the simultaneously illuminated food magazine. This continued until the 15-min habituation period expired.
Spatial training in the task began on day 1 of week 4. Animals began with the stimulus duration of 10 s, each session being 100 trials or 30 min in duration. An initial pellet was delivered to the magazine to facilitate the start of each session. One of the five nose-poke apertures was illuminated randomly for 10 s, after which the light was extinguished. The animal was then required to respond correctly by nose-poking the previously illuminated aperture within 5 s of the light being extinguished. A correct response in the previously established time frame (5 s) resulted in a pellet being dispensed into the magazine that was simultaneously illuminated for a maximum of 5 s or until the animal retrieved the pellet. Collection of this pellet initiated the intertrial interval, a delay of 5 s, before the next trial began. An incorrect response, premature response, or failure to respond (omission) resulted in a 5-s time-out, marked by the extinction of the house light for 5 s and no food reward, after which the animal initiated the next trial by a nose poke into the magazine. Animals were trained 5 days a week until they reached stable performance levels at the 10-s stimulus duration (stable performance criterion defined as 3 consecutive days at >80% accuracy, <20% omissions, and completion of all 100 trials). Once this criterion was achieved, the animals were moved to the next, more challenging stimulus duration, and the same performance criterion was applied before the next progression. The following stimulus durations were used: 5 → 2.5 → 1.25 → 1.0 → 0.8 → 0.6 → 0.5 s. The animals were required to meet criterion for a minimum of 3 days at the 0.5-s stimulus duration before being moved to the testing groups. Reaching criterion at the 0.5-s stimulus duration required, on average, 42 ± 1.36 sessions.
The following parameters were measured to assess performance: % correct = [number correct/(number correct + number incorrect)] ×100; % omissions = [number omissions/(number of trials completed)] × 100; % premature responses (impulsivity) = [(premature responses/trials initiated)] × 100 (specifically, the percentage of responses made after the trial began but before the onset of the light stimulus, i.e., during the 5-s intertrial interval); and % perseverative responses (compulsivity) = [(perseverative responses/correct responses)] × 100 (specifically, the percentage of nose pokes made after the correct response was made but before collecting the reward). Responding could occur in the aperture where the responding had just earned a food reward or at another location. Trials completed is the number correct + number incorrect + number of omissions; Latency to correct is the time elapsed from the onset of the light stimulus to making the correct nose-poke response; Latency to incorrect is the time elapsed from the onset of the light stimulus to making the incorrect nose-poke response; and Latency to reward is the time elapsed from making a correct nose-poke response to retrieving the food reward from the magazine.
Nonhuman Primate Studies
Test Subjects.
Nonhuman primate subjects were five male and four female pigtail macaques (Macaca nemestrina). Subject information is summarized in Table 1. Each animal was well trained (>100 individual sessions) in the delayed matching-to-sample (DMTS) task. The animals were maintained on tap water (unlimited) and standard laboratory monkey chow (Harlan Teklad Laboratory monkey diet; Madison, WI) supplemented with fruits and vegetables. Food was removed from cages at approximately 6:30 AM and replaced after the completion of testing of all subjects for the day (at approximately 4:30 PM). Additional nourishment was derived from 300 mg of reinforcement food pellets (commercial composition of standard monkey chow and banana flakes, Noyes Precision food pellets; P.J. Noyes Co., Lancaster, NH) obtained during experimental sessions. On weekends, animals were fed without time restrictions. Room temperature and humidity were maintained at 22 ± 0.6°C and 52 ± 2%, respectively.
TABLE 1.
Monkey subject information
| Subject ID | Sex | Age | Weight | Delay Intervals |
||
|---|---|---|---|---|---|---|
| Short | Medium | Long | ||||
| years | kg | s | ||||
| 119 | Female | 20 | 8.6 | 5 | 10 | 20 |
| 146 | Male | 25 | 9.6 | 5 | 15 | 30 |
| c8r | Male | 11 | 12.2 | 10 | 15 | 45 |
| p18 | Female | 14 | 7.8 | 15 | 40 | 80 |
| pa1 | Male | 17 | 17.0 | 30 | 30 | 45 |
| tp8 | Female | 19 | 7.2 | 10 | 15 | 35 |
| 797 | Male | 19 | 15.2 | 10 | 20 | 30 |
| V6t | Male | 11 | 17.0 | 5 | 10 | 20 |
| Mean | 17.00 | 11.83 | 11.25 | 19.38 | 38.13 | |
| S.E.M. | 1.70 | 1.45 | 2.95 | 3.71 | 6.88 | |
Each test subject had previously participated in one or more short-term studies assessing the effects of reversible drugs on DMTS performance. Prior drug experience produced no observable untoward effects in the animals, and each subject received at least a 4-week washout period (with continued weekday DMTS testing) before the start of this study.
Drug Administration.
JWS was dissolved in microliter amounts of DMSO, subsequently diluted in commercial fruit juice, and administered orally 30 min before behavioral testing. Five doses of JWS—0.05, 0.10, 0.50, 1.0, and 2.0 mg/kg—were evaluated. Control sessions consisted of oral administration of fruit juice/DMSO only.
Delayed Match-to-Sample Testing
DMTS testing was conducted using a modification of the procedure we have described previously (Terry et al., 2005). Test panels attached to each animal's home cage presented the task by using a computer-automated system. The test system included a touch-sensitive screen (15-inch AccuTouch LCD panel-mount touchmonitor; Elo TouchSystems, Menlo Park, CA) and pellet dispenser units (Med Associates) mounted in lightweight aluminum chasses. The stimuli included red, blue, and yellow rectangles presented against a black background. A trial was initiated by presentation of a sample stimulus composed of one of the three colors. The sample stimulus (located above and centered between the two choice stimuli) remained in view until the monkey touched the screen within the borders of the sample rectangle to initiate a preprogrammed delay interval. Touching a stimulus provided the illusion that the figure was actually depressed. After the delay interval, the two choice stimuli were presented. One of the two choice colors was presented so that the color of one stimulus matched the color of the sample stimulus. A correct (matching) choice was reinforced. Nonmatching choices were neither reinforced nor punished. The intertrial interval was 5 s, and each session consisted of 96 trials. The presentation of stimulus color, choice colors, and choice position were fully counterbalanced so as to relegate nonmatching (mediating) strategies to chance levels of accuracy. Three to five different presentation sequences were rotated through each daily session to prevent the subjects from memorizing the first several trials. Delay (memory retention) intervals were established during several nondrug or vehicle sessions before initiating the study. The duration for each delay interval was adjusted for each subject until three levels of group performance accuracy were approximated: zero delay (85–100% of trials answered correctly), short delay interval (75–84% correct), medium delay interval (65–74% correct), and long delay interval (55–64% correct). For this subject population, the average delay intervals for each category are provided in Table 1. The assignment of delay intervals was necessary to avoid ceiling effects in the most proficient animals during drug studies and to ensure that each animal began testing at relatively the same level of task difficulty. Failure to respond during a trial initiated the next trial in the sequence. Task accuracy (the percentage of trials correct) was determined only from the total number of trials actually completed.
Delayed Match-to-Sample with Distractor Testing
In a separate experimental series, distractor stimuli (interference trials) were presented in 24 of the 96 trials completed during distractor DMTS (DMTS-D) sessions. The stimuli were presented simultaneously on the sample and choice keys for 3 s, and they consisted of a random pattern of the three colored rectangles flashing in an alternating manner. The distractor rectangles were composed of the same three colors used for sample and choice stimuli presentation. The total duration of presentation for a given colored light was 0.33 s. Distractor stimuli were presented an equal number of times on trials with short, medium, and long delay intervals. The distractor sequence was initiated 1 s into the delay interval. Two response latencies were also measured: the “sample latency,” which is the time between presentation of the sample color and the animal pressing in sample rectangle; and the “choice latency,” which is the time between presentation of the choice colors and the animal pressing one of the choice rectangles. Choice latencies were divided into those associated with correct and incorrect responses.
Statistical Analyses
For one- and two-factor comparisons, analysis of variance (with repeated measures when indicated) was used, followed by the Student-Newman-Keuls or Dunnett's method (for comparisons with vehicle controls only) for post hoc analysis (SigmaPlot 4.0 for Windows; Systat Software, Inc., San Jose, CA). When multiple factors were analyzed (NOR), a multifactorial analysis of variance (ANOVA) with repeated measures (JMP statistical software package; SAS Institute, Cary, NC) was used. For post hoc comparisons, an orthogonal multicomparison t test (Bonferroni-corrected) was used to compare individual means. For each figure, presented error values denoted by the plus/minus sign indicate the standard error of the mean. Differences between means from experimental groups were considered significant at p < 0.05. Trends toward significance were considered at p < 0.10.
Results
Pharmacological Activity of JWS-USC-75-IX (In Vitro)
The results of the initial pharmacological screen for JWS activity across more than 60 neurotransmitter receptors, transporters, ion channels, and the enzyme AChE are provided in Table 2. Significant activity at any of these sites was defined as the inhibition of ligand binding (or AChE activity) by ≥70% (indicated by the bold text in Table 2). Using this criterion significant activity was observed at muscarinic M1, M2, and M4 receptors; at serotonin 5HT4 receptors; and at σ1 receptors. To investigate further activity at the targets identified in the initial screen, additional ligand binding experiments were conducted using three concentrations of JWS (100.0 nM and 1.0 and 10.0 μM). The results of these experiments are provided in Tables 3 and 4. The most notable observation from these experiments was the >96% inhibition of ligand binding at M2 receptors by 1.0 μM JWS. Based on these results, IC50 and KI values were determined in pharmacological displacement assays, and subsequent functional assays were conducted to determine agonist/antagonist activity (and the KB value). The results of these assays indicate that JWS is a functional antagonist at M2 receptors with a KB value of approximately 320 nM. Inhibitory activity of JWS at AChE was also observed in the initial pharmacological screen (see Table 2). Subsequent enzyme assays using multiple concentrations of JWS indicate that JWS inhibits both AChE and BChE at low (micromolar) concentrations (see Table 4). The commonly prescribed AChEIs galantamine, rivastigmine, and donepezil were also evaluated in these assays as positive controls. The IC50 values determined for galantamine, rivastigmine, and donepezil agree well with previously published values (Schott et al., 2006; Zhang et al., 2009) and indicate that donepezil is the most potent AChEI, followed by galantamine and rivastigmine, and that rivastigmine is the most potent inhibitor of BChE. In these experiments, JWS was found to be a modest AChEI with an IC50 value that is similar to galantamine.
TABLE 2.
In vitro binding profile of JWS-USC-75-IX
Values are expressed as the percentage inhibition of specific binding and represent the average of replicate tubes at each of the concentrations tested. Bold text highlights ≥70% inhibition.
| Target | Inhibition (10.0 μM) |
|---|---|
| % | |
| Adenosine transporter | −11.25 |
| A1 (h) | 4.25 |
| A2A (h) | 0.09 |
| α1A | 55.89 |
| α1B | 59.63 |
| α2A (h) | 16.65 |
| α2B | 53.20 |
| α2C (h) | 22.12 |
| β1 (h) | 15.80 |
| β2 (h) | 13.88 |
| DA transporter | 26.35 |
| D1 (h) | 28.40 |
| D2S (h) | 13.16 |
| D3 (h) | 59.10 |
| D4.4 (h) | −3.00 |
| GABAA, agonist site | −15.06 |
| GABAA, BDZ, α1 site | 38.74 |
| GABAB | 20.94 |
| Glutamate, AMPA site | −0.90 |
| Glutamate, kainate site | 5.50 |
| Glutamate, MK-801 site | −7.92 |
| Glutamate, NMDA agonist site | −11.47 |
| Glutamate, NMDA, PCP site | 11.83 |
| Glutamate, NMDA, glycine (strychnine-insensitive site) | 8.06 |
| Glycine, strychnine-sensitive | 6.47 |
| Histamine, H1 | 10.48 |
| Histamine, H2 | 92.72 |
| Histamine, H3 | 63.43 |
| Muscarinic, M1 (h, r) | 80.24 |
| Muscarinic, M2 (h) | 96.69 |
| Muscarinic, M3 (h) | 32.00 |
| Muscarinic, M4 (h) | 73.80 |
| Muscarinic, M5 (h) | 44.33 |
| Nicotinic, neuronal (a-BnTx-insensitive) | 52.02 |
| NE transporter (h) | 7.73 |
| Opioid, δ2 (h) | −0.80 |
| Opioid, μ (h) | 14.66 |
| 5-HT transporter | 67.83 |
| 5-HT1A (h) | 21.51 |
| 5-HT1D | 37.59 |
| 5-HT2A (h) | 16.67 |
| 5-HT2C (h) | 14.12 |
| 5-HT3 (h) | 17.59 |
| 5-HT4 | 77.81 |
| 5-HT5A (h) | 1.41 |
| 5-HT6 (h) | 25.36 |
| 5-HT7 (h) | 35.19 |
| σ1 | 90.01 |
| σ2 | 19.92 |
| Ca2+ channel (L-type, dihydropyridine site) | 2.30 |
| Ca2+ channel (N-type) | −10.36 |
| GABA, chloride, TBOB site | 12.98 |
| K+ channel, ATP-sensitive | 9.41 |
| K+ channel, Ca2+-activated VI | 19.82 |
| K+ channel, I[Kr] (HERG) (h) | 20.02 |
| Na+ channel (site 2) | 6.50 |
| NO, NOS (neuronal binding) | 8.17 |
| Leukotriene, LTB4 (BLT) | 13.83 |
| Leukotriene, LTD4 (CysLT1) | −8.58 |
| Thromboxane A2 (h) | −7.54 |
| Angiotensin II, AT1 (h) | 0.25 |
| Bradykinin, BK2 | 24.55 |
| Endothelin, ETA (h) | −11.04 |
| Neurokinin, NK1 | 21.31 |
| Neuropeptide, NPY2 (h) | 0.10 |
| Acetylcholinesterase (h) | 87.98 |
5-HT, 5-hydroxytryptamine; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BDZ, benzodiazepine; DA, dopamine; h, human; HERG, human ether-a-go-go-related gene; NE, norepinephrine; NO, nitric oxide; NOS, nitric-oxide synthase; PCP, phencyclidine; r, rat; TBOB, [3H]t-butylbicycloorthobenzoate.
TABLE 3.
Additional in vitro pharmacology of JWS-USC-75-IX
| Receptor Binding Target | Inhibition |
||
|---|---|---|---|
| 100.0 nM | 1.0 μM | 10.0 μM | |
| % | |||
| Muscarinic, M1 (h, r) | 1.00 | 64.80 | 86.71 |
| Muscarinic, M2 (h) | 49.50 | 96.28 | 99.62 |
| Muscarinic, M4 (h) | 1.57 | 46.39 | 90.55 |
| Serotonin, 5-HT4 | −5.68 | 32.83 | 94.82 |
| σ1 | 12.59 | 54.23 | 97.89 |
5-HT, 5-hydroxytryptamine; h, human; r, rat.
TABLE 4.
Additional in vitro pharmacology of JWS-USC-75IX (functional assays)
| Drug | Muscarinic, M2 (h) |
IC50 |
||||
|---|---|---|---|---|---|---|
| IC50 | KI | Slope | KB | AChE | BChE | |
| nM | nM | μM | ||||
| IC50/KI determination | 166.0 | 56.3 | −1.2 | |||
| JWS-USC-75-IX | 1.2 | 0.41 | −1.0 | |||
| (−)-Scopolamine MeBr | ||||||
| Functional agonist/antagonist determination | ||||||
| JWS-USC-75-IX | 3300.0 | 320.0 | ||||
| Methoctramine | 210.0 | 20.0 | ||||
| Cholinesterase inhibitor properties | ||||||
| JWS-USC-75-IX | 1.67 | 4.78 | ||||
| Galantamine | 1.12 | 8.29 | ||||
| Rivastigmine | 12.75 | 1.01 | ||||
| Donepezil | 0.05 | 6.43 | ||||
h, human.
Rodent Behavioral Studies
Effects of JWS-USC-75-IX (JWS) on Locomotor Activity and Motor Function.
Amphetamine-induced locomotor activity.
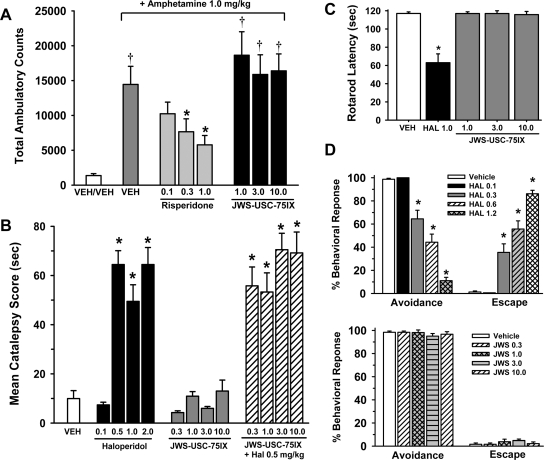
Figure 1A illustrates the dose-effect relationship for risperidone and JWS (compared with vehicle) in the amphetamine-induced locomotor activity test. There was a highly significant difference between the study groups [main effect for treatment, F(7,80) = 7.9, p < 0.001]. Post hoc analysis indicates (as expected) that 1.0 mg/kg amphetamine produced a robust increase in locomotor activity (p < 0.001 versus the vehicle response) and that risperidone attenuated the effects of amphetamine in a dose-dependent manner (p < 0.05 for the 0.3 and 1.0 mg/kg doses compared with amphetamine alone). In contrast, the amphetamine-induced increase in locomotor activity was not attenuated by any of the JWS doses that were evaluated (1.0–10.0 mg/kg).
Fig. 1.
A, effects of JWS on locomotor activity and motor function. A, effects of oral administration of JWS (compared with the second-generation antipsychotic risperidone) on amphetamine (1.0 mg/kg s.c.)-induced locomotor activity (total photobeam breaks). B, effects of oral administration of the first-generation antipsychotic haloperidol (HAL), JWS alone, and JWS combined with haloperidol on the mean catalepsy score. C, effects of oral administration of JWS (compared with haloperidol) in the rotarod task. D, effects of oral administration of haloperidol compared with JWS (n = 11–14) on conditioned avoidance responding. Decreases in avoidance responses and increases in response failures produced by haloperidol and the lack of effects of JWS in rats where behavior was maintained under a discrete-trial avoidance schedule are illustrated. VEH, vehicle.
Catalepsy.
Figure 1B illustrates the dose-effect relationship for haloperidol and JWS (compared with vehicle) as well as the combination of JWS with 0.5 mg/kg haloperidol in the catalepsy test. There was a highly significant difference between the study groups [main effect for treatment, F(12,156) = 26.2, p < 0.001]. Post hoc analysis indicates that haloperidol dose-dependently increased the mean catalepsy score, that JWS (when administered alone) did not significantly affect the catalepsy score (compared with vehicle), and finally, that JWS (at the doses evaluated) was unable to attenuate the catalepsy response induced by 0.5 mg/kg haloperidol.
Rotarod.
Figure 1C illustrates the dose-effect relationship for JWS (compared with a reference dose of haloperidol and vehicle) in the rotarod test. There was a highly significant difference between the study groups [main effect for treatment, F(4,25) = 24.1, p < 0.001]. Post hoc analysis indicates that latencies (to fall off the rotarod) in rats administered haloperidol were significantly lower than that associated with vehicle administration (p < 0.001) and that animals administered JWS were not significantly different from vehicle controls.
CAR.
Two separate vehicle-controlled studies were conducted to evaluate the effects of oral administration of haloperidol and JWS on CAR. The results of these experiments are presented in Fig. 1D. In the haloperidol experiments, there was a highly significant main effect of dose [F(4,48) = 20.9, p < 0.001], behavioral response (avoidance, escape, or escape failure) [F(2,96) = 187.8, p < 0.001], and a significant dose × behavioral response interaction [F(8,96) = 61.9, p < 0.001]. Post hoc comparisons indicate that haloperidol significantly reduced avoidance responding and increased escape responses at doses of 0.3, 0.6, and 1.2 mg/kg (p < 0.001 for these three doses). Haloperidol did not elicit escape failure responding (p > 0.05 for all doses compared with vehicle). In the JWS experiments, there was a highly significant behavioral response interaction [F(2,80) = 423.3, p < 0.001] (most subjects exhibited avoidance responding and very few exhibited escape responses or escape failures); however, there was neither a significant main effect of dose of JWS nor a significant dose × behavioral response interaction (p > 0.05).
PPI Experiments
In all of the PPI studies described below, there was a highly significant reduction in the startle response that was dependent on the magnitude of the prepulse stimulus (i.e., prepulse level difference, p < 0.001 in all studies; see the open bars in the A insets of Figs. 2–5).
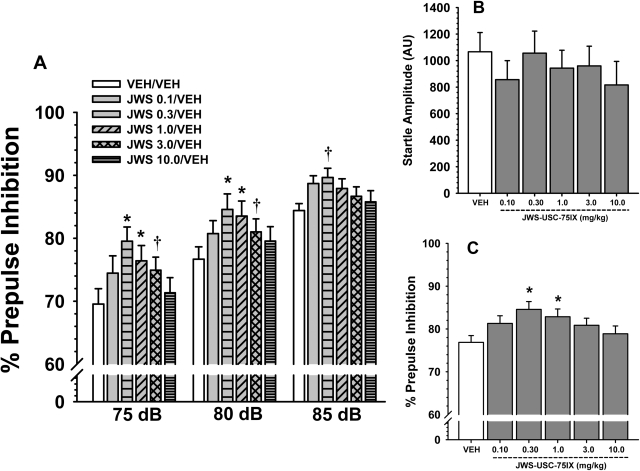
Fig. 2.
A, effects of oral administration of JWS on the percentage of PPI in rats for three prepulse intensities (5, 10, and 15 db above background). B, JWS effects on the mean startle amplitude to a 120-db, 20-ms noise burst. C, JWS effects on the percentage of prepulse inhibition averaged across the three prepulse intensities. Bars represent mean ± S.E.M. for each treatment. VEH, vehicle. *, p < 0.05, significantly different from vehicle controls; †, p < 0.09. n = 12 to 18 rats per group.
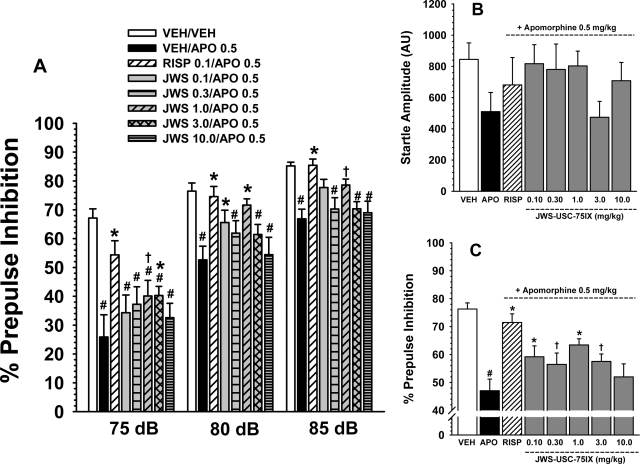
Fig. 3.
A, effects of apomorphine (0.5 mg/kg s.c.) and several oral doses of JWS on apomorphine-induced deficits in prepulse inhibition in rats associated with three prepulse intensities (75, 80, and 85 db). A reference dose of risperidone (0.3 mg/kg) was included as a positive control for attenuating the effects of apomorphine. B, effects of apomorphine and JWS combined with apomorphine on startle amplitude. C, effects of 0.5 mg/kg apomorphine and several doses of JWS on apomorphine-induced deficits in prepulse inhibition averaged across prepulse levels. Bars represent mean ± S.E.M. for each treatment (n = 8–10). VEH, vehicle; APO, apomorphine; RISP, risperidone. #, p < 0.05, significantly different from the vehicle-associated response. *, p < 0.05, significantly different from the apomorphine-associated response. †, nearly significantly (p < 0.09) different from the apomorphine-associated response.
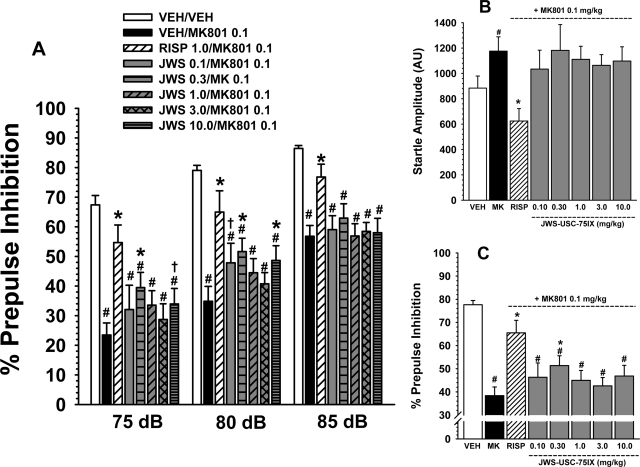
Fig. 4.
A, effects of MK-801 (0.1 mg/kg s.c.) and several oral doses of JWS on MK-801-induced deficits in prepulse inhibition in rats associated with three prepulse intensities (75, 80, and 85 db). A reference dose of risperidone (0.3 mg/kg) was included as a positive control for attenuating the effects of MK-801. B, effects of MK-801 and JWS combined with MK-801 on startle amplitude. C, effects of JWS on MK-801-induced deficits in prepulse inhibition averaged across prepulse levels. Bars represent mean ± S.E.M. for each treatment (n = 10). VEH, vehicle; MK, MK-801; RISP, risperidone. #, p < 0.05, significantly different from the vehicle-associated response. *, p < 0.05, significantly different from the MK-801-associated response; †, p < 0.08, nearly significantly different from the MK-801-associated response.
Fig. 5.
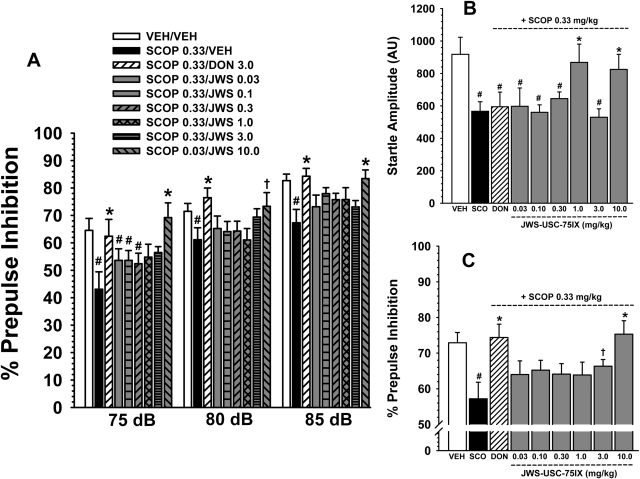
A, effects of scopolamine (0.33 mg/kg i.p.) and several oral doses of JWS on scopolamine-induced deficits in prepulse inhibition in rats associated with three prepulse intensities (75, 80, and 85 db). A reference (oral) dose of donepezil (2.0 mg/kg) was included as a positive control for attenuating the effects of scopolamine. B, effects of scopolamine and JWS combined with scopolamine on startle amplitude. C, effects of JWS on scopolamine-induced deficits in prepulse inhibition averaged across prepulse levels. Bars represent mean ± S.E.M. for each treatment (n = 8–10). VEH, vehicle; SCOP, scopolamine; DON, donepezil. #, p < 0.05, significantly different from the vehicle associated response. *, p < 0.05, significantly different from the scopolamine-associated response; †, p < 0.08, nearly significantly different from the scopolamine-associated response.
Effects of JWS Alone on Startle and PPI.
As indicated in Fig. 2A, JWS improved the PPI response compared with vehicle [main effect for treatment, F(5,112) = 2.5, p = 0.034]; however, the treatment × prepulse level interaction was not significant. Post hoc analyses indicate that 0.3 and 1.0 mg/kg JWS significantly (p < 0.05) improved PPI at the 75- and 80-db levels. The positive effect of JWS was also apparent when the data were averaged across the prepulse levels (see Fig. 2C). There were no significant effects of JWS on startle amplitude (Fig. 2B).
Effects of the JWS on Pharmacological Inhibitors of PPI.
Attenuation of apomorphine.
As indicated in Fig. 3A, there were significant differences in responses to the various drug treatments in the apomorphine reversal study [main effect for treatment, F(7,107) = 6.3, p = < 0.001], and the treatment × prepulse level interaction was statistically significant as well [F(2,214) = 2.4, p = 0.004]. Post hoc analyses indicate that 0.5 mg/kg apomorphine significantly (p < 0.05) diminished PPI at all three prepulse levels when the effect was compared with the vehicle-associated response. Risperidone (0.1 mg/kg) (as a positive control) significantly antagonized the effects of apomorphine on PPI at all three prepulse levels. The effect of risperidone on the apomorphine-associated response was also significant when the data were averaged across prepulse intensity (Fig. 3C). Post hoc analyses further indicate that doses of JWS ranging from 0.1 to 3.0 mg/kg (depending on the prepulse level) significantly (p < 0.05) or nearly significantly (p < 0.09) attenuated the deficits in PPI produced by apomorphine (Fig. 3A). The positive effects of JWS on PPI were also apparent when the data were averaged across the prepulse levels (see Fig. 3C). There were no significant effects of apomorphine, risperidone, or the JWS-apomorphine combinations on startle amplitude (Fig. 3B).
Attenuation of MK-801.
As indicated in Fig. 4A, there were significant differences in response to the various drug treatments in the MK-801 reversal study [main effect for treatment, F(7,128) = 13.8, p < 0.001], and the treatment × prepulse level interaction was nearly significant [F(2,256) = 1.6, p < 0.08]. Post hoc analyses indicate that 0.1 mg/kg MK-801 significantly (p < 0.05) diminished PPI (at all prepulse levels) compared with the vehicle-associated response. Risperidone (0.1 mg/kg) (as a positive control) significantly antagonized the effects of MK-801 on PPI at all three prepulse levels. Post hoc analyses further indicate that doses 0.1, 0.3, and 10.0 mg/kg (primarily at the 75- and 80-db prepulse level) significantly (p < 0.05) or nearly significantly (p < 0.07) attenuated the deficits in PPI produced by MK-801. The positive effects of JWS on PPI were limited to the 0.3 mg/kg dose of JWS when the data were averaged across the prepulse levels (see Fig. 4C). There were significant treatment-related effects on startle amplitude in the MK-801 reversal study (Fig. 4B). In detail, MK-801 was associated with a significant increase in startle amplitude, an effect that was reversed by risperidone. There were no significant effects of the JWS-MK-801 combination on startle amplitude compared with the other treatment groups.
Attenuation of scopolamine.
As indicated in Fig. 5A, there were also significant differences in response to the various drug treatments in the scopolamine reversal study [main effect for treatment, F(8,37) = 2.6, p = 0.01]; however, the treatment × prepulse level interaction was not significant. Post hoc analyses indicate that 0.33 mg/kg scopolamine significantly (p < 0.05) diminished PPI (at all prepulse levels) compared with the vehicle-associated response. Donepezil (3.0 mg/kg) (as a positive control) significantly antagonized the effects of scopolamine on PPI at all three prepulse levels. Post hoc analyses further indicate that only the 10 mg/kg dose of JWS significantly (p < 0.05) attenuated the deficits in PPI produced by scopolamine. When the data were averaged across the prepulse levels (see Fig. 5C), there was also a trend (p < 0.07) toward attenuation of the scopolamine-related response associated with the 3.0 mg/kg dose. There were also significant treatment-related effects on startle amplitude in the scopolamine reversal study (Fig. 5B). In detail, in all groups in which scopolamine was administered (with the exception of the subjects also administered the 1.0 and 10.0 mg/kg doses of JWS), a significant (p < 0.05) decrease in startle amplitude was observed compared with the vehicle control group.
Spontaneous Novel Object Recognition Test.
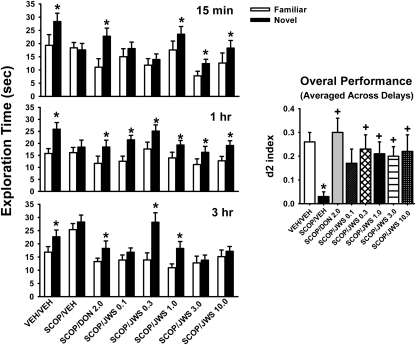
Figure 6 illustrates the effects of JWS treatment on performance in the OR task in a scopolamine impairment model (only the A/B sessions are illustrated). In statistical analyses the most notable results were the significant main effects of treatment [F(7,297) = 10.2, p < 0.0001], object type (i.e., novel versus familiar) [F(1,297) = 89.2, p < 0.0001], the treatment × delay interaction [F(14,297) = 2.5, p < 0.003], and the treatment × object type interaction [F(7,297) = 3.6, p < 0.001]. Post hoc analysis indicates that the significant preference for the novel object was lost in animals treated with scopolamine at all three delay intervals (i.e., no asterisk over the black-filled bars) and that 2.0 mg/kg donepezil (as a positive control) restored the preference (note the asterisk over the black-filled bars) at all three delay intervals. In addition, depending on the dose and delay interval, JWS restored the preference for the novel object. The positive effects of donepezil and JWS were further exemplified by the analysis of the d2 index for the overall response (averaged across delay interval; see Fig. 6 inset).
Fig. 6.
Effects of scopolamine (0.33 mg/kg i.p.) and several oral doses of JWS on scopolamine-induced deficits in the performance of a spontaneous novel object recognition task. A reference (oral) dose of donepezil (2.0 mg/kg) was included as a positive control for attenuating the effects of scopolamine. The illustrations at the left indicate the preference for the novel object compared with the familiar object (*, p < 0.05) at each of the three delays. The inset at right illustrates drug effects (averaged across delays) on the “discrimination index” (d2), which refers to the proportion of the total exploration time the animal spent investigating the novel object (see Materials and Methods). VEH, vehicle; SCOP, scopolamine; DON, donepezil. +, p < 0.01, significantly different from vehicle control performance. Data are expressed as the mean ± S.E.M. n = 12 to 18 rats per group.
5C-SRTT.
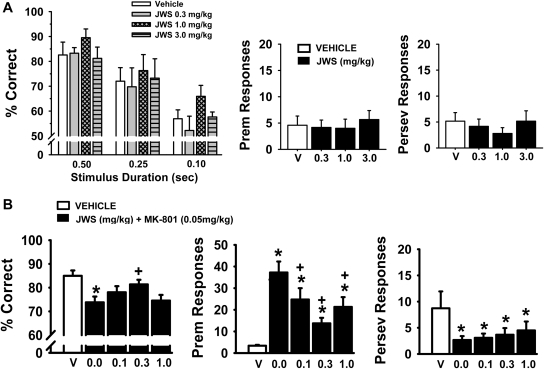
In the variable stimulus duration version of the 5C-SRTT (Fig. 7A), JWS was associated with improvements in accuracy [main effect of dose F(3,15) = 5.0, p = 0.013], stimulus duration [F(2,29) = 30.90, p < 0.001], and dose by stimulus duration interaction [F(6,29) = 0.33, p = 0.92]. Post hoc analysis indicates (as expected) that accuracy in all subjects was reduced with each decrease in stimulus duration, and furthermore, the 1.0 mg/kg dose of JWS was associated with an increase in accuracy compared with vehicle controls (p = 0.035). This improvement in accuracy was not dependent on stimulus duration (p < 0.05 versus control for all three SDs). In addition, there were no significant effects of JWS on the number of premature or perseverative responses (Fig. 7A, middle and far right) or on the mean latencies associated with correct responses, incorrect responses, and reward collection (Table 5). Likewise, there were no significant effects on the number of omissions or the number of trials completed.
Fig. 7.
A, effects of JWS on the performance of a variable stimulus duration version of the 5C-SRTT. Rats (n = 6) were trained to meet specific performance criterion (described under Materials and Methods) at SD of 0.5 s. Then, shorter SDs (0.10 and 0.25 s) were presented pseudorandomly along with the 0.5-s SD. Vehicle (V) and three doses of JWS (administered orally 30 min before testing) were evaluated for effects on task accuracy (percentage correct), the percentage of premature (Prem) responses, and the percentage of perseverative (Perserv) responses. B, effects of JWS on MK-801-related impairments in the standard version of 5C-SRTT with a 0.5-s stimulus duration (n = 12). Vehicle and several doses of JWS (administered orally 30 min before testing) were evaluated for their ability to attenuate the negative effects of MK-801 (administered subcutaneously 10 min before testing). Each bar represents the mean ± S.E.M. for each test group. *, p < 0.05, significantly different compared with vehicle-associated performance level. +, p < 0.05, significantly different compared with MK-801-associated performance level (ANOVA).
TABLE 5.
Effects of JWS USC-75-IX in the 5C-SRTT on latencies, omissions, and trials completed in the variable stimulus duration version of the 5C-SRTT (n = 6)
| Treatment & Stimulus Duration | Latency Correct | Latency Incorrect | Magazine Latency | Omissions | Trials Completed |
|---|---|---|---|---|---|
| s | % | ||||
| Vehicle | |||||
| 0.10 | 0.82 ± 0.08 | 1.56 ± 0.15 | 1.59 ± 0.12 | 2.67 ± 0.99 | |
| 0.25 | 0.70 ± 0.06 | 1.64 ± 0.15 | 1.41 ± 0.16 | 3.50 ± 0.80 | (Total) |
| 0.5 | 0.73 ± 0.05 | 1.83 ± 0.18 | 1.43 ± 0.14 | 2.58 ± 0.89 | 100.0 ± 0.0 |
| JWS-USC-75-IX (0.3 mg/kg) | |||||
| 0.10 | 0.86 ± 0.11 | 1.55 ± 0.06 | 1.37 ± 0.11 | 2.42 ± 1.08 | |
| 0.25 | 0.74 ± 0.08 | 1.67 ± 0.08 | 1.36 ± 0.14 | 2.58 ± 0.96 | (Total) |
| 0.5 | 0.82 ± 0.11 | 1.83 ± 0.20 | 1.34 ± 0.12 | 1.75 ± 0.76 | 100.0 ± 0.0 |
| JWS-USC-75-IX (1.0 mg/kg) | |||||
| 0.10 | 0.82 ± 0.10 | 1.35 ± 0.29 | 1.48 ± 0.12 | 3.83 ± 0.87 | |
| 0.25 | 0.64 ± 0.04 | 1.26 ± 0.26 | 1.43 ± 0.12 | 3.17 ± 1.01 | (Total) |
| 0.5 | 0.72 ± 0.06 | 1.34 ± 0.42 | 1.56 ± 0.15 | 3.17 ± 1.47 | 100.0 ± 0.0 |
| JWS-USC-75-IX (3.0 mg/kg) | |||||
| 0.10 | 0.81 ± 0.11 | 1.58 ± 0.18 | 1.39 ± 0.20 | 1.83 ± 1.17 | |
| 0.25 | 0.69 ± 0.07 | 1.38 ± 0.15 | 1.30 ± 0.14 | 2.17 ± 1.33 | (Total) |
| 0.5 | 0.64 ± 0.07 | 1.31 ± 0.27 | 1.34 ± 0.18 | 2.00 ± 0.82 | 100.0 ± 0.0 |
In the standard version of the 5C-SRTT (single 0.5-s SD), JWS was evaluated for its ability to attenuate impairments induced by the glutamate NMDA antagonist MK-801. The results of these experiments are provided in Fig. 7B and Table 6. In the accuracy assessment, there was a significant main effect of treatment [F(4,44) = 6.5, p < 0.001]. Post hoc analyses indicate that MK-801 impaired accuracy of the task (p < 0.05 versus vehicle) and the 0.3 mg/kg dose of JWS attenuated this impairment (p < 0.05 versus MK-801). Likewise, MK-801 increased the number of premature responses, an effect that was attenuated by all three doses of JWS [main effect of treatment, F(4,44) = 13.8, p < 0.001, post hoc results; p < 0.05 for JWS versus MK-801 for all three doses]. The other notable observation was that MK-801 decreased the number of trials completed, and again, all three doses of JWS attenuated this effect [F(4,44) = 5.4, p = 0.001, post hoc results; p < 0.05 for JWS versus MK-801 all three doses] (see Table 6). MK-801 also slightly (but significantly) decreased magazine latencies and the number of perseverative responses (see Table 6 and Fig. 7B, respectively), but this outcome was not affected by JWS.
TABLE 6.
Effects of MK-801 alone and MK-801 combined with JWS-USC-75-IX on latencies, omissions, and trials completed in the standard version of the 5C-SRTT (0.5-s stimulus duration; n = 12)
Data are presented as mean ± S.E.M. The MK-801 dose was 0.05 mg/kg.
| Treatment | Latency |
Omissions | Trials Completed | ||
|---|---|---|---|---|---|
| Correct | Incorrect | Magazine | |||
| s | % | ||||
| Vehicle + vehicle | 0.71 ± 0.04 | 1.72 ± 0.07 | 1.56 ± 0.10 | 5.06 ± 2.19 | 99.70 ± 0.16 |
| Vehicle + MK-801 | 0.68 ± 0.03 | 1.56 ± 0.16 | 1.10 ± 0.16* | 7.90 ± 0.86 | 71.50 ± 6.64* |
| JWS-USC-75-IX (0.1 mg/kg) + MK-801 | 0.66 ± 0.02 | 1.84 ± 0.16 | 1.07 ± 0.05* | 6.70 ± 1.63 | 87.08 ± 4.55† |
| JWS-USC-75-IX (0.3 mg/kg) + MK-801 | 0.70 ± 0.04 | 1.72 ± 0.21 | 1.22 ± 0.13* | 6.21 ± 1.44 | 92.83 ± 5.09† |
| JWS-USC-75-IX (1.0 mg/kg) + MK-801 | 0.69 ± 0.03 | 1.48 ± 0.17 | 1.05 ± 0.05* | 6.04 ± 1.41 | 85.88 ± 4.57† |
p < 0.05, statistically significant difference from vehicle-treated group.
p < 0.05, statistically significant difference from the vehicle-MK-801-treated group.
Nonhuman Primate Studies
Standard DMTS.
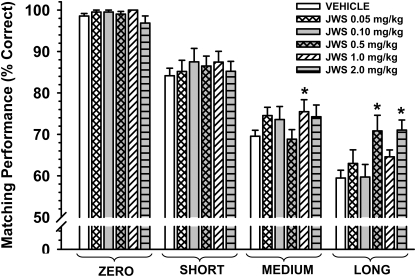
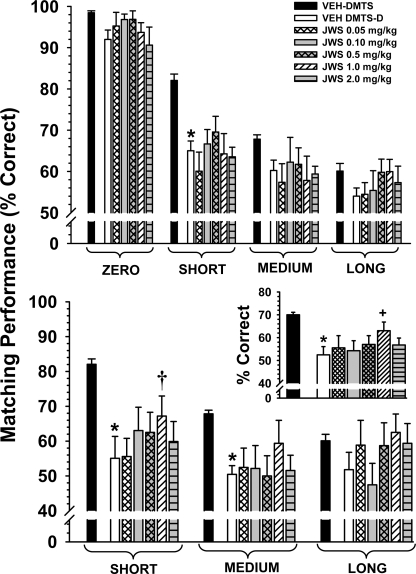
Figure 8 illustrates the dose-effect relationship for JWS in nine adult pigtail monkeys 30 min after drug administration. The data are presented for each drug dose associated with each of the four retention intervals. Accuracies in the standard DMTS task after vehicle administration approximated the criteria set forth in the Materials and Methods section: zero delay, 98.6%; short delay, 84.2%; medium delay, 69.6%; and long delay, 59.5% correct. There was not a significant main effect of dose [F(5,35) = 1.22, p = 0.32]; however, there was significant effect of delay [F(3,102) = 232.6, p < 0.001] and a significant dose × delay interaction [F(15,102) = 2.29, p = 0.008]. Post hoc analyses indicate that the 1.0 mg/kg dose significantly improved DMTS accuracy (p < 0.05) at the medium delay interval and that the 0.5 and 2.0 mg/kg doses improved accuracy at the long delays.
Fig. 8.
Effects of JWS on DMTS performance in monkeys. The dose-effect relationship for each delay in the DMTS task in nine adult pigtail macaques 30 min after the oral administration of JWS is shown. Each bar represents the mean (percentage correct) ± S.E.M. over 96 trials per session. The baseline (vehicle) was determined from the average of all vehicle sessions run throughout the study. *, p < 0.05 compared with vehicle baseline levels of DMTS accuracy.
DMTS-D.
The effects of JWS administration on the performance by nine adult monkeys in the distractor version of the DMTS task are presented in Fig. 9. Three data sets (designated as “treatments”) were compared in the analysis of the nondistractor trial component of the distractor-DMTS task. The first data set included baseline sessions of the standard DMTS task run both before and after distractor-DMTS sessions. The standard DMTS sessions were included to allow evaluation of any carryover effect of the distractor to the nondistractor trials in subsequent sessions. The second data set included sessions in which vehicle preceded nondistractor-DMTS sessions. The third data set included sessions in which JWS preceded nondistractor-DMTS sessions. In the nondistractor trials (Fig. 9, top) the main effect of treatment was statistically significant [F(6,42) = 5.85, p < 0.001] and there was significant effect of delay [F(3,126) = 83.42, p < 0.001]; however, the treatment × delay interaction was not significant [F(18,126) = 1.04, p = 0.42]. Post hoc analysis indicates that the distractor significantly (p < 0.05) impaired performance at the short delays (compared with standard DMTS-vehicle trials), but there were no significant effects of JWS.
Fig. 9.
The effect of JWS on the performance in the distractor version of the DMTS-D in monkeys. The dose-effect relationship for each delay in the DMTS-D task in nine adult pigtail macaques 30 min after the oral administration of JWS is illustrated. Top, nondistractor-related accuracies (72 trials) are plotted as a function of dose for each of four task delay intervals. Bottom, distractor-related accuracies (24 trials) are plotted as a function of dose for each of three task delay intervals. Mean accuracies associated with the standard DMTS task are included for comparison. Bottom inset, distractor-related accuracies averaged across delays. Each bar represents the mean ± S.E.M. VEH, vehicle. *, p < 0.05, significantly different compared with respective vehicle DMTS (nondistractor) mean; +, p < 0.05, significantly different compared with vehicle DMTS-D (distractor) mean. †, p < 0.09 compared with vehicle DMTS-D mean.
Three similar data sets to that described above were also compared with the distractor trial component of the distractor-DMTS task. In the distractor trials (Fig. 9, bottom) the main effect of treatment was also statistically significant [F(6,42) = 3.57, p < 0.01], and there was significant effect of delay [F(2,84) = 3.91, p < 0.05]; however, the treatment × delay interaction was not significant [F(12,84) = 1.15, p = 0.33]. Post hoc analysis indicates that the distractor significantly (p < 0.05) impaired performance at the short and medium delay intervals (compared with standard DMTS-vehicle trials) and that there was trend (p < 0.08) toward an attenuation of the distractor effect at short delays at the 1.0 mg/kg dose of JWS. A separate analysis was conducted in which the data were averaged across delays. In this analysis (one-way repeated measures ANOVA), the 1.0 mg/kg dose was associated with a significant (p < 0.05) attenuation of the effects of the distractor (see the inset at the bottom of Fig. 9).
Discussion
The results of this study can be summarized as follows. 1) The ranitidine analog JWS inhibits AChE and BChE at low (micromolar) concentrations and is a functional antagonist at M2 receptors. 2) JWS does not exhibit the classic features often observed in currently marketed antipsychotic drugs, such as the ability to attenuate amphetamine-enhanced locomotor activity, induce catalepsy, alter conditioned avoidance responding, or impair motor function. 3) JWS does seem to have the ability to improve information processing (specifically, sensorimotor gating) as indicated by its ability to improve PPI in nonimpaired adult rats and to attenuate deficits in three pharmacologic models of PPI impairment. 4) JWS also attenuated scopolamine-related impairments in an NOR task and improved 5C-SRTT performance in rats (in both a variable stimulus duration version of the task and an MK-801 impairment model), indicating its ability to improve recognition memory and sustained attention, respectively. 5) Likewise, in monkeys, JWS (depending on the dose) was associated with modest improvements in the performance of a DMTS (working/short-term memory) task and a distractor (attention-related) version of the DMTS task. Taken together, these data (combined with previously published results) indicate that JWS improves information processing and cognitive function in both rodent and nonhuman primate models.
The results of the pharmacological screen confirmed earlier work where the drug was found to have high affinity at M2 receptors and moderate affinity for AChE. It is noteworthy that we also observed some (albeit modest) activity at the σ1 receptor. This may be important because this receptor is also now considered a viable therapeutic target for both cognitive and psychiatric disorders (see Espallergues et al., 2007 for a review; Collier et al., 2007). In addition, there were no potent effects on any receptors that would normally be considered a major concern from an adverse side effect standpoint (α1, H1, prostaglandins, gut peptides, etc.). Therefore, it is also important to note that in the rodent studies conducted to date, we have observed no adverse effects with intraperitioneal doses up to 10 mg/kg in behavioral studies, and in earlier studies (Valli et al., 1992), the lethal dose in mice was found to be greater than 160 mg/kg i.p. (i.e., approximately 8-fold less toxic than tacrine and 200-fold less toxic than physostigmine).
In the initial set of behavioral experiments, JWS was evaluated for effects on locomotor activity and motor function, specifically in tasks that have commonly been used to screen compounds for potential antipsychotic activity. Amphetamine-induced hyperlocomotion is commonly used as a pharmacological model of psychosis in animals (Geyer and Ellenbroek, 2003). The model is thought to mimic the hyperdopaminergic tone thought to be present in many patients with schizophrenia (Kapur and Mamo, 2003). Although compounds with significant D2 receptor antagonist activity (i.e., such as both first- and second-generation antipsychotics) are active in this model (as might be expected), several agents from different drug classes have demonstrated activity in this model as well [e.g., H3 receptor antagonists (Akhtar et al., 2006), phosphodiesterase 10A inhibitors (Schmidt et al., 2008)]. As noted above, JWS was not active in this model. JWS was subsequently evaluated in a catalepsy procedure to assess its potential for inducing extrapyramidal symptoms (a major liability of most of the currently marketed antipsychotic drugs). Catalepsy refers to an inability of the test subject to modify a body posture imposed by the experimenter and is generally interpreted as similar to the extrapyramidal symptoms observed in humans (e.g., such as acute dystonia, akathisia, and parkinsonism; see Bürki, 1979). JWS did not induce catalepsy on its own or potentiate haloperidol-induced catalepsy (as phosphodiesterase 10A inhibitors can), and it did not attenuate catalepsy induced by haloperidol. JWS also did not affect the ability of rats to maintain balance on a rotarod. Finally, JWS was also not active in the conditioned avoidance response task, another method commonly used as a preclinical screen for potential antipsychotic (see Wadenberg and Hicks, 1999 for a review). Effective (currently marketed) antipsychotics seem to have the unique ability to selectively suppress CAR behavior—specifically, in a CAR task where animals are trained to respond to a stimulus within a certain time by moving from one place to another (avoidance), an antipsychotic agent produces a selective suppression of the avoidance response.
The observation that JWS was effective in three pharmacological impairment models of PPI and even improved PPI in nonimpaired rats was particularly interesting. Tests of PPI (defined as the reduction in startle response produced by a low-intensity stimulus presented before a high-intensity, startle-producing stimulus; see Graham et al., 1975) are useful for identifying novel therapeutic agents with the ability to improve sensory information-processing deficits, a common feature in several neuropsychiatric conditions. Auditory (sensory)-gating deficits in neuropsychiatric conditions are in fact thought to contribute to the deficits in attention, cognitive impairment, and even hallucinations in conditions such as schizophrenia (Adler et al., 1998). The pharmacological impairment models used in this study (apomorphine, MK-801, and scopolamine) have been previously used to provide evidence that the neurotransmitters dopamine (Mansbach et al., 1988), glutamate (Mansbach and Geyer, 1989), and acetylcholine (Jones and Shannon, 2000) are each likely to play an important role in normal sensory gating and PPI as well as disorders of these processes. The positive effects of JWS in all three pharmacologic impairment models suggest that JWS (via its multitarget effects) may be able to influence the activity of all three neurotransmitters.
The observation that JWS attenuated scopolamine-related decreases in PPI and OR performance was not altogether surprising because it is an AChEI, and its effect would presumably result in elevated synaptic acetylcholine levels that could overcome scopolamine antagonism at muscarinic receptors. However, the data do support the premise that cholinergic function is important for optimal OR performance and PPI (and thus a viable target in conditions where it is impaired). We recently reported that the AChEIs galantamine and donepezil can also attenuate scopolamine-related impairments in PPI (Hohnadel et al., 2007). In addition, PPI deficits induced by immunolesions of cholinergic neurons of the nucleus basalis were reversed by the AChEI rivastigmine (Ballmaier et al., 2002). Additional data to support the role of muscarinic receptors in PPI (and that these receptors could serve as targets for drug development in schizophrenia) have been reported in which xanomeline, an M1/M4 acetylcholine receptor agonist (Stanhope et al., 2001; Jones et al., 2005), normalized apomorphine-related impairments in PPI.
The positive effects of JWS observed in the spontaneous novel OR test complement the results of previous studies in which JWS improved water-maze learning and probe trial performance, passive avoidance, and delayed stimulus discrimination (Terry et al., 1999). OR (Ennaceur and Delacour 1988) is a rodent model of recognition memory, which by definition consists of two components, a recollective (episodic) component and a familiarity component (Squire et al., 2004). Recognition memory is demonstrated in the OR task when subjects explore a novel object more than a familiar one.
The JWS-related improvements in performance of the 5C-SRTT and the ability to attenuate impairments in the distractor version of the DMTS indicate the potential of JWS to improve attention and decrease distractibility, attractive features that could be useful for the treatment of multiple neurologic and psychiatric disorders. Performance of the rodent 5C-SRTT is considered to be analogous to the various versions of the human continuous performance test of sustained attention (Robbins 2002). Attention, as indexed by the accuracy measurement in addition to inhibitory response control (thought to be a form of executive functioning), is assessed. In the current study, JWS was able to improve accuracy (a variable stimulus duration version of the task) as well as to attenuate both the accuracy deficits and the elevated premature responses induced by MK-801. Premature responses, which occur during the intertrial interval before the target stimulus has been presented, are generally interpreted as a form of impulsive behavior. Thus, improvements in accuracy and a decrease in impulsive-like behaviors in a NMDA antagonist model could have special relevance for neuropsychiatric conditions such as schizophrenia. Finally, the positive effects of JWS in the DMTS task were encouraging because it can be used to assess behaviors that are relevant to human cognition such as attention, strategy formation, and working memory (Paule et al., 1998).
There are some limitations to this study that should be discussed. Although the salient pharmacological properties of JWS (M2 and AChE antagonist activity) would suggest efficacy in conditions such as AD, we did not evaluate JWS in AD animal models specifically. Although the scopolamine impairment procedure (used in this study) is a commonly used pharmacological model with relevance to AD (especially the cholinergic impairments), it would be interesting to evaluate JWS in transgenic mouse models of AD as well as aged animals. In addition, given that only specific doses of JWS were associated with statistically significant effects on DMTS performance in monkeys, it is unclear whether the positive effects would be potent enough to be observed clinically.
In conclusion, JWS (potentially via effects at several drug targets) improves information processing, attention, and memory-related task function in animal models. The data support the role of JWS as a prototypical representative of a novel class of therapeutic agents for disorders of cognition such as AD. The positive effects observed in the PPI and 5C-SRTT studies in rats, and the DMTS-D studies in monkeys, suggest that JWS might be useful in neuropsychiatric conditions not necessarily associated with advanced aged such as schizophrenia. The results of this study also support the potential value of developing single molecular entities with multiple therapeutic targets for neuropsychiatric disorders. Additional research is currently underway to explore further the multireceptor targeting properties and therapeutic potential of JWS analogs.
Acknowledgments
We thank Ashley Davis for administrative assistance in preparing this article. This article is dedicated to our close personal friend, mentor, and colleague, the late Dr. Jerry J. Buccafusco, whose contributions to the science of drug discovery and development for disorders of cognition spanned more than 25 years.
This work was supported by the National Institutes of Health National Institute on Aging [Grant AG032140] and the Institute for the Study of Aging [Grant 271225]. Salary support was also provided through a Merit Review Award from the Veterans Administration (to J.J.B.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.175422.
Abbreviations:
- AChEI
- acetylcholinesterase inhibitors
- NMDA
- N-methyl-d-aspartate
- AD
- Alzheimer's disease
- AChE
- acetylcholinersterase
- JWS
- JWS-USC-75-IX, 3-[[[2-[[(5-dimethylaminomethyl)-2-furanyl]methyl]thio]ethyl]amino]-4-nitropyridazine
- MK-801
- (−)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate
- DMSO
- dimethylsulfoxide
- CAR
- conditioned avoidance responding
- PPI
- prepulse inhibition
- NOR
- novel object recognition
- OR
- object recognition
- d2
- discrimination index
- 5C-SRTT
- five-choice serial reaction time task
- DMTS
- delayed match-to-sample
- DMTS-D
- delayed match-to-sample with distractor
- ANOVA
- analysis of variance
- BChE
- butyrylcholinesterase.
Authorship Contributions
Participated in research design: Terry, Buccafusco, and Callahan.
Conducted experiments: Herman, Callahan, Beck, Warner, Vandenhuerk, Bouchard, and Schwarz.
Contributed new reagents or analytic tools: Gao and Chapman.
Performed data analysis: Terry, Herman, and Callahan.
Wrote or contributed to the writing of the manuscript: Terry and Chapman.
References
- Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, Flach K, Nagamoto H, Bickford P, Leonard S, et al. (1998) Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Bull 24:189–202 [DOI] [PubMed] [Google Scholar]
- Akhtar M, Uma Devi P, Ali A, Pillai KK, Vohora D. (2006) Antipsychotic-like profile of thioperamide, a selective H3-receptor antagonist in mice. Fundam Clin Pharmacol 20:373–378 [DOI] [PubMed] [Google Scholar]
- Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, Gill R, Juszczak E, Yu LM, Jacoby R, et al. (2009) The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol 8:151–157 [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Casamenti F, Scali C, Mazzoncini R, Zoli M, Pepeu G, Spano PF. (2002) Rivastigmine antagonizes deficits in prepulse inhibition induced by selective immunolesioning of cholinergic neurons in nucleus basalis magnocellularis. Neuroscience 114:91–98 [DOI] [PubMed] [Google Scholar]
- Bürki HR. (1979) Extrapyramidal side-effects. Pharmacol Ther B 5:525–534 [DOI] [PubMed] [Google Scholar]
- Collier TL, Waterhouse RN, Kassiou M. (2007) Imaging sigma receptors: applications in drug development. Current Pharmaceutical Design 13:51–72 [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. (1961) A new and rapid colorimetric determination of acetylcholinesterase activity.Biochem Pharmacol 7:88–95 [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. (1988) A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31:47–59 [DOI] [PubMed] [Google Scholar]
- Espallergues J, Lapalud P, Christopoulos A, Avlani VA, Sexton PM, Vamvakides A, Maurice T. (2007) Involvement of the sigma1 (sigma1) receptor in the anti-amnesic, but not antidepressant-like, effects of the aminotetrahydrofuran derivative ANAVEX1–41. Br J Pharmacol 152:267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustace A, Coen R, Walsh C, Cunningham CJ, Walsh JB, Coakley D, Lawlor BA. (2002) A longitudinal evaluation of behavioural and psychological symptoms of probable Alzheimer's disease. Int J Geriatr Psychiatry 17:968–973 [DOI] [PubMed] [Google Scholar]
- Geyer MA, Ellenbroek B. (2003) Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry 27:1071–1079 [DOI] [PubMed] [Google Scholar]
- Graham FK, Putnam LE, Leavitt LA. (1975) Lead-stimulation effects of human cardiac orienting and blink reflexes. J Exp Psychol Hum Percept Perform 104:175–182 [PubMed] [Google Scholar]
- Gwee MC, Cheah LS. (1986) Actions of cimetidine and ranitidine at some cholinergic sites: implications in toxicology and anesthesia. Life Sci 39:383–388 [DOI] [PubMed] [Google Scholar]
- Hohnadel E, Bouchard K, Terry AV., Jr (2007) Galantamine and donepezil attenuate pharmacologically induced deficits in prepulse inhibition in rats. Neuropharmacology 52:542–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Jones CK, Shannon HE. (2000) Muscarinic cholinergic modulation of prepulse inhibition of the acoustic startle reflex. J Pharmacol Exp Ther 294:1017–1023 [PubMed] [Google Scholar]
- Jones CK, Eberle EL, Shaw DB, McKinzie DL, Shannon HE. (2005) Pharmacologic interactions between the muscarinic cholinergic and dopaminergic systems in the modulation of prepulse inhibition in rats. J Pharmacol Exp Ther 312:1055–1063 [DOI] [PubMed] [Google Scholar]
- Kapur S, Mamo D. (2003) Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 27:1081–1090 [DOI] [PubMed] [Google Scholar]
- Lai MK, Lai OF, Keene J, Esiri MM, Francis PT, Hope T, Chen CP. (2001) Psychosis of Alzheimer's disease is associated with elevated muscarinic M2 binding in the cortex. Neurology 57:805–811 [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. (1988) Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology 94:507–514 [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA. (1989) Effects of phencyclidine and phencyclidine biologs on sensorimotor gating in the rat. Neuropsychopharmacology 2:299–308 [DOI] [PubMed] [Google Scholar]
- Mash DC, Flynn DD, Potter LT. (1985) Loss of M2 muscarine receptors in the cerebral cortex in Alzheimer's disease and experimental cholinergic denervation. Science 228:1115–1117 [DOI] [PubMed] [Google Scholar]
- Michal P, Lysíková M, Tucek S. (2001) Dual effects of muscarinic M(2) acetylcholine receptors on the synthesis of cyclic AMP in CHO cells: dependence on time, receptor density and receptor agonists. Br J Pharmacol 132:1217–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemore-Risher ML, Buccafusco JJ, Terry AV., Jr (2010) Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicol Teratol 32:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minger SL, Esiri MM, McDonald B, Keene J, Carter J, Hope T, Francis PT. (2000) Cholinergic deficits contribute to behavioral disturbance in patients with dementia. Neurology 55:1460–1467 [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. (2005) Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10:79–104 [DOI] [PubMed] [Google Scholar]
- Paule MG, Bushnell PJ, Maurissen JP, Wenger GR, Buccafusco JJ, Chelonis JJ, Elliott R. (1998) Symposium overview: the use of delayed matching-to-sample procedures in studies of short-term memory in animals and humans. Neurotoxicol Teratol 20:493–502 [DOI] [PubMed] [Google Scholar]
- Quirion R. (1993) Cholinergic markers in Alzheimer disease and the autoregulation of acetylcholine release. J Psychiatry Neurosci 18:226–234 [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology 163:362–380 [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Bunsey MD, Giordano M, Norman AB. (1988) The catalepsy test: its ups and downs. Behav Neurosci 102:748–759 [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Chapin DS, Cianfrogna J, Corman ML, Hajos M, Harms JF, Hoffman WE, Lebel LA, McCarthy SA, Nelson FR, et al. (2008) Preclinical characterization of selective phosphodiesterase 10A inhibitors: a new therapeutic approach to the treatment of schizophrenia. J Pharmacol Exp Ther 325:681–690 [DOI] [PubMed] [Google Scholar]
- Schott Y, Decker M, Rommelspacher H, Lehmann J. (2006) 6-Hydroxy- and 6-methoxy-beta-carbolines as acetyl- and butyrylcholinesterase inhibitors. Bioorg Med Chem Lett 16:5840–5843 [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. (2004) The medial temporal lobe. Annu Rev Neurosci 27:279–306 [DOI] [PubMed] [Google Scholar]
- Stanhope KJ, Mirza NR, Bickerdike MJ, Bright JL, Harrington NR, Hesselink MB, Kennett GA, Lightowler S, Sheardown MJ, Syed R, et al. (2001) The muscarinic receptor agonist xanomeline has an antipsychotic-like profile in the rat. J Pharmacol Exp Ther 299:782–792 [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Bartoszyk GD. (2005) Selective serotonin 5-HT2A receptor antagonist EMD 281014 improves delayed matching performance in young and aged rhesus monkeys. Psychopharmacology 179:725–732 [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Gattu M, Buccafusco JJ, Sowell JW, Kosh JW. (1999) Ranitidine analog, JWS USC 75IX, enhances memory-related task performance in rats. Drug Dev Res 47:97–106 [Google Scholar]
- Terry AV, Jr, Gearhart DA, Warner SE, Zhang G, Bartlett MG, Middlemore ML, Beck WD, Jr, Mahadik SP, Waller JL. (2007) Oral haloperidol or risperidone treatment in rats: temporal effects on nerve growth factor receptors, cholinergic neurons, and memory performance. Neuroscience 146:1316–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations (2007) World Population Ageing, United Nations, New York [Google Scholar]
- U.S. CDC (2003) From the Centers for Disease Control and Prevention. Public health and aging: trends in aging–United States and worldwide. JAMA 289:1371–1373 [PubMed] [Google Scholar]
- Valli MJ, Tang Y, Kosh JW, Chapman JM, Jr, Sowell JW., Sr (1992) Synthesis and cholinergic properties of N-aryl-2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethylamino analogs of ranitidine. J Med Chem 35:3141–3147 [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Hicks PB. (1999) The conditioned avoidance response test re-evaluated: is it a sensitive test for the detection of potentially atypical antipsychotics? Neurosci Biobehav Rev 23:851–862 [DOI] [PubMed] [Google Scholar]
- Youdim MB, Buccafusco JJ. (2005) Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol Sci 26:27–35 [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhu D, Sheng R, Wu H, Hu Y, Wang F, Cai T, Yang B, He Q. (2009) BZYX, a novel acetylcholinesterase inhibitor, significantly improved chemicals-induced learning and memory impairments on rodents and protected PC12 cells from apoptosis induced by hydrogen peroxide. Eur J Pharmacol 613:1–9 [DOI] [PubMed] [Google Scholar]