Abstract
Surfactant protein-A (SP-A) and Toll-like receptor-4 (TLR4) proteins are recognized as pathogen-recognition receptors. An exaggerated activation of TLR4 induces inflammatory response, whereas SP-A protein down-regulates inflammation. We hypothesized that SP-A–TLR4 interaction may lead to inhibition of inflammation. In this study, we investigated interaction between native baboon lung SP-A and baboon and human TLR4-MD2 proteins by coimmunoprecipitation/immunoblotting and microwell-based methods. The interaction between SP-A and TLR4-MD2 proteins was then analyzed using a bioinformatics approach. In the in silico model of SP-A–TLR4–MD2 complex, we identified potential binding regions and amino acids at the interface of SP-A-TLR4. Using this information, we synthesized a library of human SP-A-derived peptides that contained interacting amino acids. Next, we tested whether the TLR4-interacting SP-A peptides would suppress inflammatory cytokines. The peptides were screened for any changes in the tumor necrosis factor-α (TNF-α) response against lipopolysaccharide (LPS) stimuli in the mouse JAWS II dendritic cell line. Different approaches used in this study suggested binding between SP-A and TLR4-MD2 proteins. In cells pretreated with peptides, three of seven peptides increased TNF-α production against LPS. However, two of these peptides (SPA4: GDFRYSDGTPVNYTNWYRGE and SPA5: YVGLTEGPSPGDFRYSDFTP) decreased the TNF-α production in LPS-challenged JAWS II dendritic cells; SPA4 peptide showed more pronounced inhibitory effect than SPA5 peptide. In conclusion, we identify a human SP-A-derived peptide (SPA4 peptide) that interacts with TLR4-MD2 protein and inhibits the LPS-stimulated release of TNF-α in JAWS II dendritic cells.
Introduction
The pathogen-pattern recognition receptors (PPRRs) are important components of innate immunity that sense pathogenic stimuli and regulate host immune responses. The surfactant protein-A (SP-A) and Toll-like receptor-4 (TLR4) have been identified as important PPRRs (Hoebe et al., 2006; Kawai and Akira, 2007; Kuroki et al., 2007; Pastva et al., 2007). The TLR4 is expressed as transmembrane receptor and is known as a “signaling PPRR” (Kawai and Akira, 2007). On the other hand, SP-A is synthesized by type II lung epithelial cells and secreted in the alveoli as a component of surfactant. SP-A is known as a “secretory PPRR” (Pastva et al., 2007). SP-A constitutes the majority of SPs and plays a critical role in the clearance of pathogens and down-regulation of the inflammatory response. On the other hand, TLR4 recognizes pathogen or pathogen-derived ligands and endogenous stress proteins and induces the inflammatory and adaptive immune responses. In a number of diseases including lung inflammatory conditions, an exaggerated activation of TLR4 has been found associated with nuclear factor κB and proinflammatory cytokine response (Guillot et al., 2004; He et al., 2009; Lv et al., 2009; Villar et al., 2010).
Published reports suggest that the bronchoalveolar lavage pools (extracellular pools) of SP-A are significantly reduced in lungs of infected patients and animal models (Alcorn et al., 2005; Kajikawa et al., 2005). In contrast, TLR4 expression is increased (Gagro et al., 2004; Kajikawa et al., 2005; Chang et al., 2006; Awasthi et al., 2008). The reduction in the amounts of SP-A and simultaneous increase in TLR4 expression corroborates well with the clinical condition of patients having fulminant infection and inflammation, respectively. In these clinical scenarios, the introduction of SP-A should facilitate clearance of pathogens and attenuate inflammation. However, currently available clinical surfactants do not contain SP-A or SP-D. Thus, a great need has been felt for designing a shorter fragment of SP-A and reformulating the surfactant.
It is noteworthy that published reports suggested that SP-A directly binds to TLR4 (Guillot et al., 2002; Yamada et al., 2006). The in vivo evidence of such an interaction has been lacking, and its functional relevance has not been fully elucidated. In this study, we determined the binding of SP-A to TLR4-MD2 in noninfected, normal baboon lung tissues by coimmunoprecipitation/immunoblotting and in vitro by a microwell-based method. Next, we used a bioinformatics approach to examine the interaction between SP-A and TLR4-MD2 proteins. In conjunction, potential binding regions were identified in an in silico model of the SP-A–TLR4–MD2 complex. Based on the information obtained from bioinformatics analysis, an SP-A-derived peptide library was synthesized. Studies were further extended to investigate the functional relevance of SP-A–TLR4 interaction in a dendritic cell system. Our aim was to investigate whether one property of SP-A, its interaction with TLR4, can be mimicked by an SP-A-derived peptide. We found that similar to native SP-A, an SP-A-derived peptide (SPA4) binds to TLR4-MD2 protein and inhibits the release of TNF-α in response to the most potent TLR4-ligand: Gram-negative bacteria-derived lipopolysaccharide (LPS).
Materials and Methods
Animals.
The animal studies were approved by the Institutional Animal Care and Use and Institutional Biosafety Committees at the University of Oklahoma Health Science Center, Oklahoma City, OK. Baboons (Papio anubis) were maintained at the Baboon Research Resource, University of Oklahoma Health Science Center. At the time of necropsy, lung tissue or bronchoalveolar lavage fluid specimens were obtained from normal healthy adult baboons. Gross and microscopic examinations of major viscera as well as the lung tissue specimens from these baboons showed no signs of inflammation or infection.
Preparation of Baboon Lung Tissue Homogenate.
The frozen lung tissue samples were homogenized in a buffer containing 1% Igepal CA630, 0.1% sodium dodecyl sulfate, and protease inhibitors (1 μM leupeptin, 1 mM ethylenediamine tetraacetic acid, 0.7 mg/liter pepstatin, and 0.2 mM phenylmethyl sulfonyl fluoride; Sigma-Aldrich, St. Louis, MO) at a concentration of 100 mg of tissue/ml buffer (Awasthi et al., 1999, 2001). The tissue homogenates were centrifuged to remove cell debris, and total protein concentration was measured in supernatants using the MicroBCA protein estimation kit (Pierce Biotechnology, Rockford, IL).
Western Blotting.
In our earlier studies, we recognized the cross-reactivity of anti-human SP-A- and anti-human TLR4-antibodies with their respective antigens in baboons and studied the expression of SP-A and TLR4 in lung tissue homogenates of fetal and adult baboons and neonate baboons having bronchopulmonary dysplasia (Awasthi et al., 1999, 2008). Here, using Western blotting, we confirmed the immunoreactivity of these antibodies with their respective antigens in baboon lung tissue homogenates to ensure the integrity of the antigens. Lysates of HEK293 cells stably transfected with human TLR4-cDNA (provided by Invitrogen, Carlsbad, CA), and purified human and baboon lung SP-A proteins served as positive controls.
The protein samples were prepared in SDS-PAGE sample buffer without dithiothreitol (DTT) + no heating (nonreducing), without DTT + heating at 100°C for 5 min (partially reducing) or with DTT + heating at 100°C for 5 min (reducing). The samples were loaded and separated on a SDS-PAGE gel (8% running and 5% stacking gel or Novex 4–20% Tris-glycine gel; Invitrogen). Separated proteins were then electro-transferred overnight onto a nitrocellulose membrane. The nonspecific sites were blocked by incubating the membrane in 7% skim milk diluted in Tris-buffered saline containing 0.4% Tween 20 (TBST). The membranes were then incubated with anti-human SP-A polyclonal antibody (Awasthi et al., 1999, 2001) or TLR4 antibody (eBioscience, San Diego, CA) (Awasthi et al., 2008), diluted 1:1000 in TBST, for 1 h at room temperature. The membrane was washed and then incubated with horseradish peroxidase-conjugated-anti-mouse or anti-rabbit IgG antibody (1:1000 diluted in TBST; Sigma-Aldrich). The immunoreactive bands were detected by Supersignal West Pico or Femto chemiluminescent substrate (Pierce Biotechnology).
Immunoprecipitation of Lung SP-A or TLR4 and Cross-Immunoblotting.
After confirming the reactivity of the antibodies and the integrity of TLR4 and SP-A proteins in baboon lung tissue homogenates, the physical binding between the two proteins was examined by immunoprecipitation/cross-immunoblotting. The SP-A and TLR4 proteins were immunoprecipitated from baboon lung tissue homogenates and cross-immunoblotted with anti-human TLR4 and SP-A antibodies, respectively. The SP-A (IP-SP-A) and TLR4 (IP-TLR4) were immunoprecipitated using the Primary Seize Immunoprecipitation kit (Pierce Biotechnology) according to the manufacturer's instructions. Approximately 200 μg of anti-human TLR4 or SP-A antibody (Awasthi et al., 2001, 2008) was conjugated to the AminoLink plus coupling gel column (Pierce Biotechnology) at 4°C overnight. Five hundred micrograms to 1 mg of total lung tissue homogenate protein was loaded into the columns, and the immunoprecipitation reaction was performed overnight at 4°C. IP-SP-A and IP-TLR4 were eluted from the antibody-bound column using ImmunoPure elution buffer. No calcium was added to the immunoprecipitation reaction at any step. In addition, none of the buffers in the kit contained calcium.
Various amounts of IP-TLR4 and IP-SP-A were run on SDS-PAGE gels. The separated proteins were then transferred on nitrocellulose membrane using the i-Blot system (Invitrogen). For cross-immunoblotting, IP-SP-A and IP-TLR4 were immunoblotted with anti-TLR4 and anti-SP-A antibodies, respectively, as described above. Positive controls included lung tissue homogenate protein, purified human SP-A, and lysate protein of HEK293 cells transfected with full-length human TLR4-cDNA. Negative controls included IP-SP-A and IP-TLR4 immunoblotted with a nonspecific antibody, and immunoprecipitates from columns where the lung tissue homogenate or the primary antibody had been omitted.
Purification and Characterization of Native Baboon SP-A.
We purified SP-A from bronchoalveolar lavage fluid of an adult baboon by a modification of the procedure described previously (Yang et al., 2005). The bronchoalveolar lavage fluid was collected from an adult baboon lung by instilling endotoxin-free, sterile normal saline (endotoxin-free 0.9% NaCl, 1.9–2 liters with approximately 90% recovery). The lavage fluid was centrifuged, and the supernatant was concentrated using a tangential flow filtration technique (10-kDa hollow fiber filter; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). The surfactant lipids were removed using isobutyl alcohol (1:5 ratio, lavage/isobutyl alcohol). The delipidated protein was centrifuged at 5000g for 15 min at room temperature, dried under nitrogen gas, and subsequently completely dried in a lyophilizer (Labconco, Kansas City, MO). The dried lavage residue was rehydrated in extraction buffer (25 mM Tris, pH 7.5, 0.15 M NaCl, and 20 mM octyl-β-d-glucoside) overnight at 4°C. Rehydrated surfactant was extracted six times with extraction buffer by vortex mixing and centrifugation at 20,000g for 30 min at 4°C. Insoluble SP-A was then suspended in solubilization buffer (5 mM HEPES, pH 7.5, 0.02% sodium azide) and dialyzed for 72 h against four changes of the solubilization buffer. Insoluble protein was removed by centrifugation at 50,000g for 30 min at 4°C, and supernatant was adjusted to 20 mM CaCl2 and 1 M NaCl to reprecipitate SP-A. Precipitated SP-A was pelleted by centrifugation at 50,000g for 30 min at 4°C and washed two times in 5 mM HEPES, pH 7.5, 20 mM CaCl2, and 1 M NaCl. The SP-A was suspended in 5 mM HEPES and 5 mM EDTA, pH 7.5 and dialyzed for 72 h against four changes of the solubilization buffer to remove EDTA. The purified SP-A was dialyzed against four changes of the endotoxin-free, highly purified water (Invitrogen) for 72 h to remove any remaining EDTA or salts (CaCl2 and NaCl). Finally, purified SP-A was lyophilized completely and resuspended in endotoxin-free Dulbecco's phosphate-buffered saline. The purified protein was filter-sterilized using a 0.2-μm low-protein binding, HT Tuffryn membrane filter (Pall Life Sciences, East Hills, NY) and stored frozen at −80°C. The protein concentration of purified SP-A was measured by the microBCA method (Pierce Biotechnology).
All the purification steps were performed under aseptic conditions using endotoxin-free solutions and reagents. The endotoxin concentration was measured using the Endpoint chromogenic limulus amebocyte lysate (LAL) assay (Charles River Laboratories, Inc., Wilmington, MA). The purity of the SP-A protein was confirmed by SDS-PAGE and Western blotting using the procedures described above.
Interaction between Purified Baboon Lung SP-A, SP-A-Peptides, and TLR4-MD2 Proteins Using a Microwell-Based Method.
The binding between the purified baboon lung SP-A, SP-A-peptides, and recombinant TLR4-MD2 and MD2 proteins was studied in vitro using a microwell-based method (Awasthi et al., 2004). The soluble recombinant TLR4-MD2 protein (R&D Systems, Minneapolis, MN) consisted of a mixture of recombinant human TLR4 and MD2 proteins. The recombinant extracellular domain of human TLR4 protein (Glu24-Lys631 amino acids) was joined with a DNA sequence encoding the signal peptide from human CD33 and a 10× histidine tag at the C terminus (GenBank accession no. O00206). For MD2 protein, a DNA sequence encoding the signal peptide from human CD33 was joined with the mature region of human MD-2 (mature region, Glu17-Asn160 amino acids) and a 10× histidine tag at the C terminus (GenBank accession no. Q9Y6Y9). The chimeric proteins were expressed in a mouse myeloma cell line, NS0 (R&D Systems). The proteins were obtained from the manufacturer in carrier-free condition and reconstituted in phosphate-buffered saline containing 0.1% low-endotoxin bovine serum albumin (BSA) (MP Biomedicals, Solon, OH). The MD2, an adaptor molecule for TLR4, is expressed by immune cells and is known to bind TLR4 in a noncovalent manner (Jain et al., 2008). Thus, the binding of SP-A to the recombinant MD2 protein (R&D Systems) was also studied.
For the binding assay, microwell ultra-high-protein binding Immunolon 4HBX strips (Thermo Fisher Scientific, Waltham, MA) were coated with soluble recombinant TLR4-MD2 protein or MD2 protein (R&D Systems; 250 ng per well, diluted in 0.1 M NaHCO3, pH 9.6) overnight at room temperature. The plates were washed three times, and nonspecific sites were blocked for 2 h at room temperature using phosphate-buffered saline containing 0.1% Triton X-100 and 3% nonfat milk (buffer A). The wells were washed and incubated for 3 h at 37°C with purified baboon lung SP-A (0.125–10 μg), SPA4 peptide (2–20 μg), or adult baboon lung tissue homogenate protein (10–100 μg) diluted in 20 mM Tris pH 7.4 buffer containing 0.15 M NaCl and 5 mM CaCl2 or an equal amount of BSA protein. The wells were washed with buffer A and incubated with anti-human SP-A antibody (1:1000 diluted in buffer A) for 2 h at room temperature followed by horseradish peroxidase-conjugated secondary antibody. The immune-complex was detected using 3,3′,5,5′-tetramethylbenzidine substrate system (Sigma-Aldrich). The reaction was stopped with 2 N-H2SO4 and read at 405 nm and/or 450 nm spectrophotometrically (Molecular Devices, Sunnyvale, CA).
JAWS II Dendritic Cell Culture.
The JAWS II dendritic cell line is an immortalized cell line derived from the bone marrow of C57BL/6 mice (American Type Culture Collection, Manassas, VA). The cells were maintained in α-modified minimum essential medium (Sigma-Aldrich) supplemented with 20% fetal bovine serum, 4 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin (Invitrogen), and 5 ng/ml of recombinant murine granulocyte macrophage-colony stimulating factor (Peprotech, Rocky Hill, NJ) (Awasthi and Cox, 2003). The culture medium was replaced with fresh medium every 48 h.
LPS Treatment of JAWS II Dendritic Cells with and without SP-A Peptides.
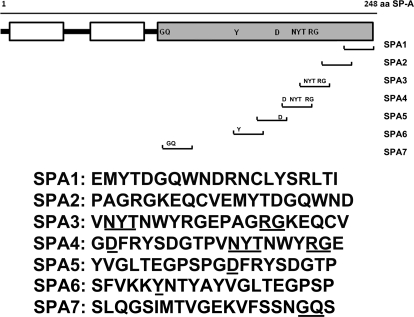
Based on the results of bioinformatics analysis (described under Results), we synthesized SP-A peptides (SPA1 to SPA7). The amino acid sequences were derived from the C-terminal carbohydrate recognition domain (CRD) of human SP-A corresponding to the TLR4-interacting sites identified in the in silico model of SP-A–TLR4–MD2 complex (Fig. 1). The 20-mer peptides were synthesized by Genscript Corp. (Piscataway, NJ), and mass spectroscopy and high-performance liquid chromatography analyses confirmed the characteristics and purity of synthesized peptides, respectively (data not shown). LAL test (Charles River Laboratories, Inc.) confirmed the absence of endotoxin in the peptide samples.
Fig. 1.
Synthetic peptides derived from C-terminal CRD region of human SP-A. The peptide sequences and their location in SP-A are shown. Underlined amino acids were recognized at the interface of the SP-A–TLR4 complex in the in silico analysis (Figs. 4 and 6).
In a separate investigation, we found that short-pulsing of baboon lung dendritic cells with purified lung SP-A and recombinant TLR4-MD2 protein leads to inhibition of TLR4-induced cytokine release against Escherichia coli (S. Awasthi, R. Madhusoodhanan, and R. Wolf, unpublished data). Thus, we questioned whether the SP-A-derived peptides from the TLR4-interacting regions would demonstrate similar effect. The JAWS II dendritic cells (1 million) were treated with SP-A peptides (1 or 10 μM) with or without E. coli-derived LPS (75 ng/ml, highly purified, low protein; Calbiochem, San Diego, CA). To observe the anti-inflammatory properties of the SP-A peptides, we treated the cells with peptides for 1 h before the addition of LPS or after 4-h incubation with LPS. The incubation was continued for a total of 5 h. Control cells were treated with vehicle, LPS (75 ng/ml), and SP-A peptides (1 and 10 μM) for 5 h. The cell-free supernatants were collected and stored at −80°C for further analysis.
Cytokine (TNF-α) Measurement.
The TNF-α levels were measured in cell-free supernatants of JAWS II dendritic cells treated with LPS with or without SP-A peptides by enzyme-linked immunosorbent assay as described previously (Awasthi and Cox, 2003).
Statistical Analysis.
The results were analyzed by Student's t test or analysis of variance for statistical significance using Prism software (GraphPad Software, Inc., San Diego, CA). At p < 0.05, the null hypothesis was rejected.
Results
The TLR4 and SP-A Are Coimmunoprecipitated from Baboon Lung Tissue Homogenates.
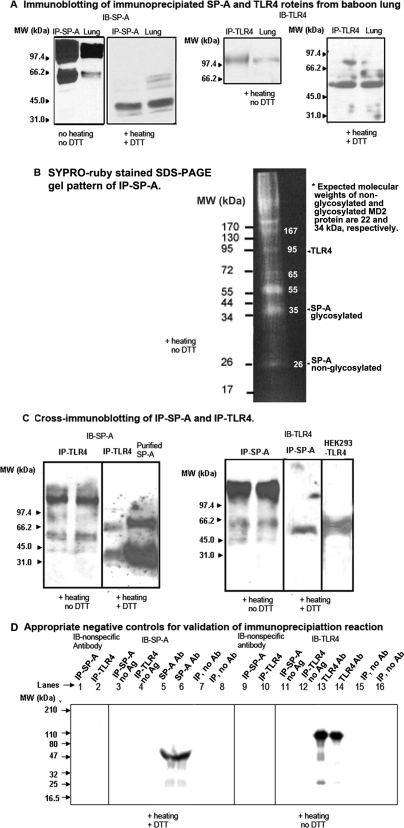
We have shown earlier that human SP-A and TLR4-specific antibodies react with baboon SP-A and TLR4 proteins, respectively (Awasthi et al., 1999, 2001, 2008). Using the same antibody clones, we confirmed the integrity of SP-A and TLR4 in baboon lung tissue homogenates. We identified the immunoprecipitation of specific proteins by immunoblotting the IP-SP-A and IP-TLR4 eluates from adult baboon lung tissue homogenates using SP-A- and TLR4-specific antibodies, respectively (Fig. 2A). The SDS-PAGE gel run of concentrated IP-SP-A shows additional protein bands besides SP-A, suggesting a number of SP-A-binding proteins (Fig. 2B). The lung tissue homogenate protein, lysate protein of HEK293 cells stably transfected with full-length TLR4, and purified SP-A protein were run simultaneously as positive controls to confirm the identity of the IP-SP-A and IP-TLR4. The sizes of the TLR4 and SP-A protein bands corresponded to the respective proteins in baboon lung tissue homogenates as described previously (Awasthi et al., 1999, 2001, 2008). Neither SP-A nor TLR4 were immunoprecipitated when a nonspecific antibody was used in the column (data not shown).
Fig. 2.
A, immunoblotting of immunoprecipitates (IP-SP-A and IP-TLR4) with anti-human SP-A (IB-SP-A) and TLR4 (IB-TLR4) antibodies, respectively, to confirm the immunoprecipitation of specific proteins from baboon lung. IP-SP-A, IP-TLR4, and adult baboon lung homogenate protein (40 μg) were run on 8% SDS-PAGE gel under nonreducing (no heating, no DTT) or partially reducing (+ heating, no DTT) or reducing (+ heating, + DTT) conditions. B, SYPRO-ruby-stained SDS-PAGE gel of IP-SP-A run under partially reducing (+ heating, no DTT) condition. Estimated molecular masses of major protein bands are shown within the gel image. Expected locations of SP-A, TLR4, and MD2 proteins are also marked. C, cross-immunoblotting of IP-SP-A and IP-TLR4 with anti-human-TLR4 (IB-TLR4) and SP-A (IB-SP-A) antibodies, respectively. Purified SP-A protein and lysate protein of HEK293 cells stably transfected with TLR4 (HEK293-TLR4) served as positive control. D, negative controls for immunoprecipitation reaction. Lanes 1, 2, 9, and 10: IP-SP-A and IP-TLR4 immunoblotted with nonspecific primary antibody. Lanes 3, 4, 11, and 12: IP-SP-A and IP-TLR4 without any antigen or lung tissue homogenate. Lanes 5 and 6: 1.5 and 1 μl of SP-A antibody, respectively. Lanes 13 and 14: 1.5 and 1 μl of TLR4 antibody, respectively. Lanes 7, 8, 15, and 16: IP reactions in the absence of immunoprecipitating antibodies in the columns. The numbers indicate molecular mass (kDa) of standard marker proteins.
Next, we hypothesized that if the SP-A and TLR4 proteins interact with each other, the two proteins may exist together in the lung and may be coimmunoprecipitated from lung tissue homogenates. The cross-immunoblotting results indicate that SP-A and TLR4 are coimmunoprecipitated from baboon lung specimens (Fig. 2C). A major protein band of >100 kDa was identified in both IP-TLR4 and IP-SP-A when the IP-eluates were separated on a partially reducing SDS-PAGE gel and cross-immunoblotted. Protein bands of 34 kDa (similar to SP-A monomer) and 66 kDa (SP-A dimer) were identified when IP-TLR4 was separated on reducing SDS-PAGE gel and immunoblotted with anti-SP-A antibody (Fig. 2C). A protein band of 55 kDa (TLR4) was recognized when IP-SP-A was separated on reducing SDS-PAGE gel and immunoblotted with anti-TLR4 antibody (Fig. 2C). The specificity of the immunoprecipitation reaction was validated using appropriate negative controls (Fig. 2D). These results suggested that the IP-TLR4 and IP-SP-A eluates did not contain any nonspecific protein or antibody fractions.
Characterization of Purified Native Baboon Lung SP-A.
To further elucidate the interaction between SP-A and TLR4, first we purified the native SP-A protein from bronchoalveolar lavage fluid specimens of a normal, healthy adult baboon (Awasthi et al., 1999, 2001). The solubility of purified baboon lung SP-A was 51% (Stenvall et al., 2005). The purity and identity of the native baboon lung SP-A was confirmed by SDS-PAGE and Western blotting (Supplemental Data 1). Under partially reducing conditions (heating and no DTT), SP-A separated as an oligomer, 90- to 100-kDa trimer, and a 66-kDa dimer on the SDS-PAGE gel. Under reducing condition (heating + DTT), purified SP-A ran mainly as 26-kDa monomer and 66-kDa partially reduced dimer. The purified baboon lung SP-A protein was also immunoblotted with SP-A antibody that identified the SP-A-specific protein bands (34 and 66 kDa). The SP-A exists in nonglycosylated (26 kDa) and glycosylated (31–36 kDa) forms. Earlier, we had shown that the antibody recognizes the 34-kDa protein band (Awasthi et al., 1999). Overall results indicate that only a minor population of the glycosylated form of SP-A (34 kDa) interacts with TLR4, and the majority of the SP-A (26 kDa) is either not involved in SP-A-TLR4 interaction or not detectable by this antibody (Supplemental Data 1). Because the TLR4-MD2 proteins are less abundant in the biological system and distributed throughout, it is difficult to obtain native TLR4-MD2 protein in sufficient quantity. Thus, we included recombinant human-TLR4-MD2 protein (R&D Systems).
Because TLR4 is a potent receptor for endotoxin, the presence of endotoxin can significantly influence the results. We prepared all the solutions and reagents in endotoxin-free water and performed all the assays in an aseptic environment. We measured the endotoxin concentration in the purified baboon SP-A preparation and the reconstituted TLR4-MD2 and MD2 proteins by the chromogenic LAL method. The endotoxin concentration was negligible in purified baboon SP-A (0.0003 ng/μg protein) and recombinant TLR4-MD2 and MD2 protein suspensions (≤0.006 ng/μg protein).
Lung SP-A and Recombinant TLR4-MD2 Proteins Interact In Vitro.
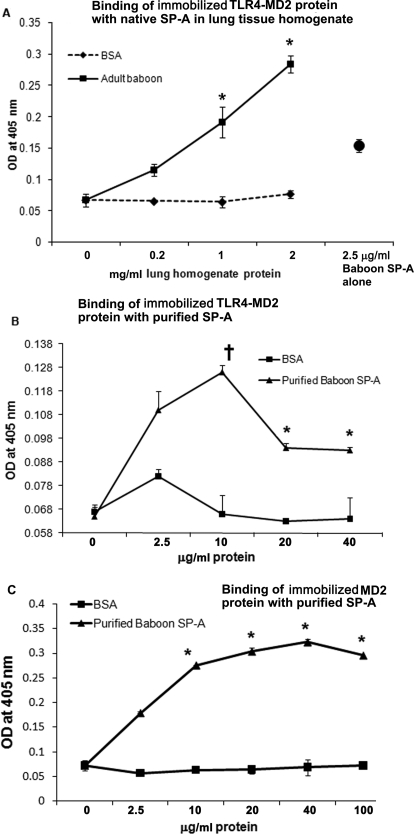
The immobilized TLR4-MD2 protein showed binding with purified baboon lung SP-A and SP-A protein present in native form in lung tissue homogenate (Fig. 3, A and B). Purified baboon lung SP-A was also found to bind to immobilized MD2 protein (Fig. 3C). In comparison, BSA (negative control) showed negligible binding to the TLR4-MD or MD2 protein.
Fig. 3.
A to C, binding between SP-A and recombinant TLR4-MD2 or MD2 protein by the microwell based-method. Various concentrations of lung tissue homogenate protein (0.2–2 mg/ml) (A) or purified SP-A protein (2.5–40 μg/ml) (B) were incubated with immobilized recombinant TLR4-MD2 protein (0.25 μg per well), and the complex was detected using SP-A-specific antibody. C, binding between purified baboon lung SP-A and immobilized recombinant MD2 protein (0.25 μg per well). Various concentrations of purified SP-A protein (2.5–100 μg/ml) were added. The wells were washed, and the complex was detected using SP-A-specific antibody. The binding of SP-A to BSA protein shows nonspecific binding. The results are representative of two experiments performed in triplicate. The error bars represent S.E.M. *, p < 0.05; †, p < 0.1 versus BSA control (t test).
Protein-Protein Docking and Prediction of Interacting Amino Acids at the Interface of the SP-A–TLR4–MD2 Protein Complex.
In previous sections, we experimentally characterized the interaction between SP-A and TLR4-MD2 proteins. In this section, we describe the bioinformatics approaches used to examine the interaction. We first describe how we obtained our data for bioinformatic analyses, then we describe the in silico docking of SP-A with the TLR4-MD2 and finally, the rendering of the docking interface.
SP-A Structure.
Under physiological conditions, SP-A exists as an octadecamer comprising of 6× trimer units (Palaniyar et al., 2000), and TLR4-MD2 as a dimer (Park et al., 2009). The trimeric crystal structure of neither the human SP-A nor the baboon SP-A is available in the Protein Data Bank (PDB; http://www.rcsb.org/pdb) (Berman et al., 2000). Head et al. (2003) solved the crystal structure of the trimeric carbohydrate recognition domain/neck domain of SP-A. However, the PDB file and X-ray structure in the PDB were available for the monomeric subunit of rat SP-A (PDB ID 1R13) (Head et al., 2003). Using bioinformatics approaches, it is possible to obtain the structure of trimer by docking three monomers to form a single complex. We used SymmDock (Schneidman-Duhovny et al., 2005a,b), an automated server that deduces the structure of homomultimer with cyclic symmetry when the structure of a monomeric subunit is available, for the above task. The SymmDock server returned 100 possible trimer complexes that differed in the arrangement of monomers, accompanied by a priority score. Of all the configurations returned by the server, only the top scoring complex was identical to structure of the trimer shown (Palaniyar et al., 2000; Head et al., 2003), and the rest had different arrangements.
TLR4-MD2 Structure.
For TLR4-MD2 proteins, the amino acid sequences and dimer crystal structure of human TLR4-MD2 complex are available in the PDB (PDB ID 3FXI). Although, the TLR4 and SP-A proteins are considered highly conserved proteins, we checked the SP-A, TLR4, and MD2 sequence homology between the respective animal species using CLUSTALW multiple alignment program (Protein Information Resource, Georgetown University Medical Center, Washington, DC). Only partial sequences were available for baboon SP-A and TLR4, and there was no information available on baboon MD2. The alignment results suggest that the SP-A, TLR4, and MD2 proteins are highly conserved among different species (including mouse, rat, macaca, baboon, and human) (Supplemental Data 2).
Protein-Protein Docking.
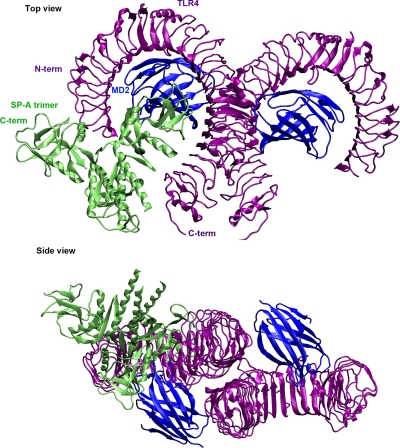
Next, the protein-protein docking was carried out using Global Range Molecular Matching (GRAMM-X) methodology (Tovchigrechko and Vakser, 2006) on a public web server by submitting the PDB files (trimer assembly of SP-A and dimer receptor-adaptor molecule complex of TLR4 and MD2). GRAMM-X represents a new implementation of original GRAMM methodology that uses a smoothed Lennard-Jones potential on a fine grid during the global search fast Fourier transformation stage, followed by refinement optimization in continuous coordinates and rescoring with several knowledge-based potential terms (Tovchigrechko and Vakser, 2006). We examined the top 100 docked configurations to select most plausible configurations. Results from published studies (Yamada et al., 2006) and our laboratory data were considered to set the inclusion and exclusion criteria for the selection of the most plausible model of the SP-A–TLR4–MD2 complex. First, we discarded 90 configurations that did not show the MD2 adaptor molecule interacting with SPA in the SP-A–TLR4–MD2 complex, because the microwell-based assay results indicated binding between the SP-A and MD2 adaptor molecule (Fig. 3). However, identification of in vitro interactions between purified SP-A and recombinant MD2 protein does not ensure that the SP-A binds to the MD2 present as a TLR4-MD2 complex in lung. In the remaining 10 configurations, some were the same configurations with the SP-A docked to a different monomer of the TLR4-MD2 dimer. Finally, only three distinct configurations remained. Of these three configurations, we chose the configuration that had the highest area of contact between the molecules, which also happened to be the configuration ranked one. It is a model in which the C-terminal portion of SP-A binds to the extracellular domain of TLR4 and MD2 (Fig. 4).
Fig. 4.
The C-terminal portion of SP-A binds to the extracellular domain of the TLR4-MD2 complex. First, the structure of SP-A trimer was predicted by the SymmDock program from the monomeric crystal structure (PDB ID 1R13). Next, the predicted trimer was used to dock with the TLR4-MD2 complex (PDB ID 3FXI) using the GRAMM-X web server. The above configuration is the most likely interaction model based on the GRAMM-X server ranking and detailed analysis.
Identification of Amino Acids at the Interface of In Silico Model of the SP-A–TLR4–MD2 Protein Complex.
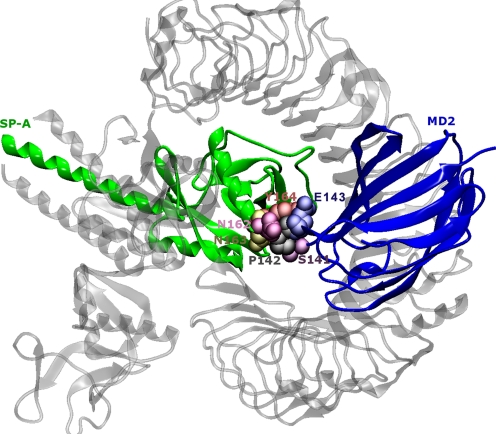
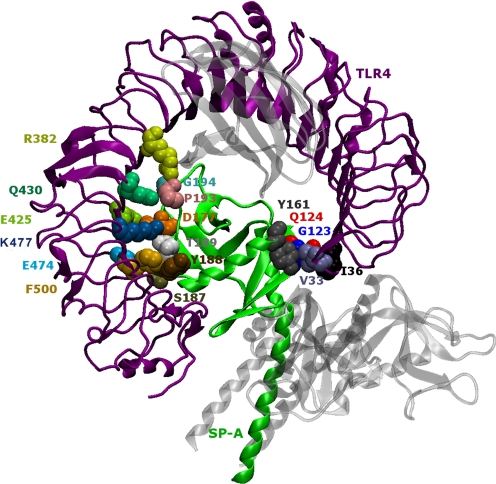
To examine the binding interface of the complex and identify the amino acids at the SP-A–TLR4 and SP-A–MD2 interfaces, we input the structures into another server called Knowledge-Based Fade and Contacts [comprised of Fast Atomic Density Evaluator (K-Fade): shape specificity features and K-Con: biochemical contacts such as intermolecular hydrogen bonds and atomic contacts] (Darnell et al., 2007). The server predicts the binding hotspots and the associated prediction confidence based on the shape specificity features and biochemical contact features of the residues at the interface. The predicted docking configuration of the SP-A–TLR4–MD2 complex with high confidence (K-Fade >0.9 or K-Con >0.9) have been highlighted in Figs. 5 and 6 using Van der Waals representation. The rendering was carried out using the Visual Molecular Dynamics program (Humphrey et al., 1996). The amino acids (SP-A: Asn162-Asn163-Tyr164; MD2: Ser141-Pro142-Glu143) in the selected docked configuration were highlighted using a Van der Waals representation (Fig. 5). In the illustration (Fig. 5), the other parts of the complex (two chains of SP-A and TLR4) are rendered transparent to focus on the SP-A–MD2 interaction site. According to the prediction from the Knowledge-Based Fade and Contacts server, the SP-A and TLR4 proteins interact at four different places (Fig. 6). The amino acids involved at the interface of TLR4 and SP-A (K-Fade >0.9 or K-Con >0.9) are listed in the Table 1.
Fig. 5.
The amino acids that are likely to interact in the docked model of the SP-A–TLR4–MD2 complex as shown in Fig. 4. In the illustration here, the other parts of the complex (two chains of SP-A and TLR4) are rendered transparent to focus on the SP-A–MD2 interaction site.
Fig. 6.
The docked model of the SP-A–TLR4–MD2 complex, as shown in Fig. 4, shows that SP-A interacts with TLR4 in the SP-A–TLR4–MD2 complex in at least four different places. The second monomer of the TLR4-MD2 dimer has been removed from the original model here for clarity. In addition, the noninteracting chains of SP-A and MD2 molecule have been rendered transparent.
TABLE 1.
Amino acids identified at the SP-A-TLR4 interface
| Molecule | Amino Acid | Residue No. | K-Fade Confidence | K-Con Confidence |
|---|---|---|---|---|
| TLR4 (human TLR4) | Val (V) | 33 | 1 | 1 |
| Ile (I) | 36 | 0.6 | 0.92 | |
| Arg (R) | 382 | 1 | 1 | |
| Glu (E) | 425 | 1 | 1 | |
| Gln (Q) | 430 | 0.93 | 0.92 | |
| Glu (E) | 474 | 0.9 | 1 | |
| Lys (K) | 477 | 0.94 | 0.83 | |
| Phe (F) | 500 | 1 | 0.91 | |
| SP-A (rat-SP-A) | Gly (G) | 123 | 1 | 1 |
| Gln (Q) | 124 | 1 | 1 | |
| Tyr (Y) | 161 | 1 | 1 | |
| Asp (N) | 177 | 0.92 | 1 | |
| Ser (S) | 187 | 1 | 0.85 | |
| Tyr (Y) | 188 | 1 | 0.81 | |
| Thr (T) | 189 | 0.91 | 0.82 | |
| Pro (P) | 193 | 1 | 1 | |
| Gly (G) | 194 | 1 | 1 |
Functional Screening of SP-A Library Reveals a Peptide (SPA4) that Reduces LPS-Induced TNF-α Secretion.
Based on the in silico observations and homology to respective SP-A regions between rat and humans, the SP-A peptides derived from the C-terminal CRD of human SP-A were synthesized. SP-A peptides were tested for purity by mass spectrometry (Genscript) and for endotoxin contamination by LAL test.
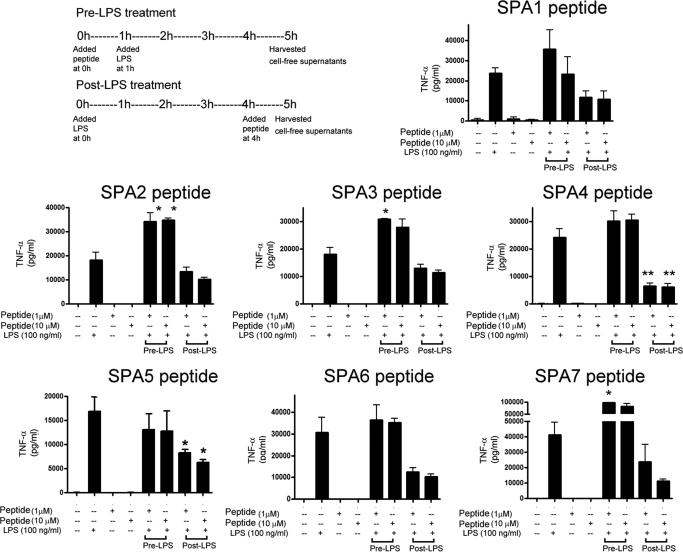
Because an exaggerated activation of TLR4 is directly linked to secretion of proinflammatory cytokine (TNF-α) and SP-A in down-regulating TLR4-induced TNF-α in lung dendritic cells (S. Awasthi, R. Madhusoodhanan, and R. Wolf, unpublished data), SP-A peptides were screened for any changes in LPS-induced TNF-α cytokine secretion in JAWS II dendritic cells. We included pre-LPS and post-LPS inflammation models in this study to investigate whether the peptides affect the LPS-mediated responses, prophylactically or therapeutically. We found that in the pre-LPS model, the SPA2, SPA3, and SPA7 peptides increased the TNF-α secretion against LPS challenge, and the rest of the peptides did not show any effect. On the other hand, in the post-LPS model, two peptides (SPA4 and SPA5) significantly inhibited the secretion of TNF-α at both 1 and 10 μM concentrations (Fig. 7). However, the SPA4 peptide had a more distinct effect on LPS-induced TNF-α than the SPA5 peptide (mean values 6448 versus 8284 pg/ml at 1 μM concentration, and 6101 versus 6319 pg/ml at 10 μM concentration). Coincidentally, the SPA4 peptide contains most of the amino acids recognized at the interface of SP-A and TLR4 in the in silico model of the SP-A–TLR4–MD2 complex, and the SPA5 peptide contains the first 10 amino acids of the SPA4 peptide.
Fig. 7.
Effect of synthetic SP-A peptides on LPS stimulated-TNF-α release by JAWS II dendritic cells. The experimental schematic is shown for pre-LPS and post-LPS treatment of cells with SP-A-peptides. The control cells were treated with vehicle control, SP-A-peptides (1 and 10 μM), or LPS (75 ng/ml) alone for 5 h. The cell-free supernatants were collected after 5 h of stimulation. The results are from three experiments performed in triplicate. The error bars represent S.E.M. *, p < 0.05 and **, p < 0.001 compared with TNF-α levels in cell-free supernatants of LPS-treated cells (analysis of variance).
SPA4 Peptide Binds to TLR4 and Blocks the LPS-Induced TLR4 Expression.
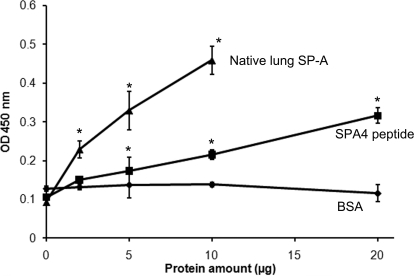
Next, we confirmed the binding of the SPA4 peptide with recombinant TLR4-MD2 protein by in vitro microwell-based binding assay. The binding results show that similar to purified native SP-A, the SPA4 peptide binds to TLR4-MD2 protein (Fig. 8). Binding of the SPA4 peptide to the TLR4-MD2 protein was observed as less efficient than the whole native SP-A protein, which exists as octadecamer (composed of six trimers). The SPA4 peptide, however, represents a small portion of the TLR4-interacting region of SP-A derived from a monomer. Because a polyclonal antibody was used to detect the binding of SP-A and SPA4 peptide with TLR4-MD2 proteins, the epitope detection may differ depending on whether it is a fragment (SPA4 peptide) or a full-length protein (purified baboon lung SP-A).
Fig. 8.
Binding between SPA4 peptide and recombinant-TLR4-MD2 protein by the microwell based-method. Native SP-A purified from baboon lung was included as control. Various amounts of purified native SP-A protein (2–10 μg) or SPA4 peptide (2–20 μg) were incubated with immobilized recombinant TLR4-MD2 protein (0.25 μg per well), and the complex was detected using SP-A-specific antibody. The results are from one representative experiment of three experiments performed in triplicate. The error bars represent S.E.M. The binding of SP-A or SPA4 peptide to BSA protein shows nonspecific binding. *, p < 0.05 compared with 0 μg of protein (t test).
Discussion
In lung, SP-A is synthesized by type II lung epithelial cells and is secreted in alveoli as a component of surfactant (King and Clements, 1972). SP-A plays a critical role in pathogen opsonization, clearance, down-regulation of inflammation, and maintenance of lung function. Thus, it is reasonable to imagine that administration of SP-A should enhance clearance of pathogens and inhibit inflammation. Unfortunately, currently available surfactants do not contain SP-A, because it is a large and hydrophilic protein and cannot be mixed efficiently with surfactant lipids. It is important to search for smaller fragments of SP-A. Unavailability of such an SP-A-derived fragment has been associated with the lack of an appropriate model to mimic such a complex scenario.
Since the discovery of TLR4 as a pathogen-recognition receptor that is expressed mainly by the antigen-presenting cells (Basu and Fenton, 2004), it is now established that an exaggerated expression and activity of TLR4 leads to deleterious inflammatory response. A basal activity is, however, important for antigen presentation and adaptive immunity. Reduction in SP-A and simultaneous increase in TLR4 expression have been reported in different models of lung infection and inflammation (Gagro et al., 2004; Alcorn et al., 2005; Kajikawa et al., 2005; Chang et al., 2006). The reduction in SP-A amounts and concomitant increase in TLR4 expression corroborates with the clinical condition of patients with lung infection where reduced pathogen clearance is observed with robust inflammation.
A number of SP-A-binding proteins and receptors have been recognized (reviewed in Chroneos et al., 2010); however, their functions and expression by cell type remain unexplored (Strayer et al., 1993; Stevens et al., 1995; Wissel et al., 1996; Gil et al., 2009). The binding of SP-A to the TLR4 protein has also been shown to occur under in vitro condition (Yamada et al., 2006); however, the in vivo evidence had been lacking and functional relevance remained largely unexplored. The anti-inflammatory effects of SP-A have been established; however, the mechanism is not known. We believe that down-regulation of the inflammatory response may be via interaction between SP-A and TLR4. Thus a smaller fragment of SP-A belonging to the TLR4-interacting region should inhibit the TLR4-mediated inflammatory response while maintaining the basic functions of antigen-presenting cells.
In this study, we found that SP-A and TLR4 proteins are coimmunoprecipitated from baboon lung tissue homogenates. This is the first report where such an interaction between SP-A and TLR4 has been shown to exist in the lung by immunoprecipitation/immunoblotting and microwell-based methods using lung tissue homogenates and purified lung SP-A. Earlier, interaction between SP-A and TLR4 was studied with purified or recombinant forms of proteins by ligand-blot, microwell-based binding assay, and BIAcore methods (Yamada et al., 2006; Chroneos et al., 2010). Bioinformatics simulation studies further support the interaction between SP-A and TLR4-MD2 protein. Although several aspects of TLR4 and SP-A binding are not clearly understood, it is clear that the lung microenvironment may significantly influence their interaction. It should be noted that in the antibody-based methods used here, the kinetics and characteristics of binding between the two proteins depend on the antigen-antibody affinity. The specific binding sites of both the SP-A and TLR4 proteins and the kinetic parameters of the native-SP-A-TLR4 interaction needed further investigation. It is important to note that the native SP-A molecule (ligand) is quite large (octadecamer) because of the oligomerization of trimers (Pastva et al., 2007), and TLR4 protein is a homomer and associates with other adaptor (MD2) and signaling receptors for its activity (Re and Strominger, 2002). Moreover, we also found that SP-A can bind to the TLR4 adaptor molecule MD2 as well. Thus, we considered computer modeling of the SP-A–TLR4–MD2 complex; an in silico model of the SP-A–TLR4–MD2 complex was obtained where the binding features fitted best with the results from immunobiochemical assays (Figs. 2 and 3). The selected in silico model was analyzed further to identify potential binding sites and amino acids.
As identified earlier, the functional significance of such an interaction is very difficult to assess in vivo. The functional relevance of such an interaction can be better examined under in vitro conditions in a controlled environment using a ell culture system and the appropriate dosage of effector molecules. Thus, we used the JAWS II dendritic cell system that we established to investigate the effects of SP-A peptides derived from TLR4-interacting region on cytokine response against a well known inflammatory stimuli: LPS. We found that the SPA4 peptide encodes most of the amino acids belonging to the TLR4-interacting region in in silico model, binds to TLR4-MD2 protein, and reduces LPS-induced TLR4 expression and cytokine response.
These results suggest that SP-A blocks the TLR4-MD2-mediated intracellular signaling and cytokine release against infectious stimuli. In a human monocytes culture system, Henning et al. (2008) found that SP-A did not affect TLR4 expression, but it down-regulated the TLR4-mediated signaling against LPS. However, based on the information on the interacting amino acids at the SP-A-TLR4 interface in the computer-simulated SP-A–TLR4–MD2 complex model, and screening of the peptides, we identify one peptide (SPA4) that suppresses LPS-induced TNF-α release. More detailed investigations will be the focus of future studies, including studies to elucidate the mechanisms of SPA4 peptide-mediated effects on host-pathogen interaction and inflammation in different in vitro and in vivo models.
We are continuing to investigate the possibility that the instillation of exogenous SP-A (or SPA4 peptide) will control TLR4-mediated inflammation. Further understanding of the SP-A–TLR4–MD2 interaction and signaling mechanisms may help in designing improved immunotherapeutic interventions against fatal respiratory conditions.
Supplementary Material
Acknowledgments
We thank Dr. Roman Wolf (Baboon Resources, University of Oklahoma Health Science Center) for providing intact lung from routinely culled animal and Dr. Shrinkant Anant (Department of Cell Biology, University of Oklahoma Health Science Center) for helpful discussions.
This work was supported by the National Institutes of Health National Center for Research Resources [Grant P40-RR012317]; the Presbyterian Health Foundation [Grant PHF1539]; and the College of Pharmacy Seed Grant Program, University of Oklahoma Health Science Center, Oklahoma City [Grant PSXA1].
Part of this work was presented previously: Awasthi S, Brown K, Wolf R, and White G (2008) Interaction between surfactant protein-A and toll-like receptor-4 in lung, at the American Association of Immunologists Meeting; 2008 April 5–9; San Diego, CA. American Association of Immunologists, Bethesda, MD. Awasthi S, Wolf R, White G, and Awasthi V (2008) Surfactant protein-A interacts with TLR4 and affects the phagocytic function of dendritic cells, at the American Association of Immunologists Meeting; 2009 May 8–12; Seattle, WA. American Association of Immunologists, Bethesda, MD.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army or of the U.S. Department of Defense.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.173765.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
Abbreviations:
- PPRR
- pathogen-pattern recognition receptor
- SP
- surfactant protein
- TLR4
- Toll-like receptor-4
- IP-SP-A
- immunoprecipitated SP-A
- IP-TLR4
- immunoprecipitated TLR4
- IB-SP-A
- immunoblotted SP-A
- IB-TLR4
- immunoblotted TLR4
- LPS
- lipopolysaccharide
- DTT
- dithiothreitol
- PAGE
- polyacrylamide gel electrophoresis
- CRD
- carbohydrate recognition domain
- TNF-α
- tumor necrosis factor-α
- HEK
- human embryonic kidney
- TBST
- Tris-buffered saline containing 0.4% Tween 20
- LAL
- limulus amebocyte lysate
- BSA
- bovine serum albumin
- PDB
- Protein Data Bank
- GRAMM
- Global Range Molecular Matching.
Authorship Contributions
Participated in research design: S. Awasthi, King, and V. Awasthi.
Conducted experiments: S. Awasthi, Brown, King, and V. Awasthi.
Contributed new reagents or analytic tools: S. Awasthi and V. Awasthi.
Performed data analysis: S. Awasthi, Brown, and Bondugula.
Wrote or contributed to the writing of the manuscript: S. Awasthi, King, V. Awasthi, and Bondugula.
Other: S. Awasthi acquired funding for the research.
References
- Alcorn JL, Stark JM, Chiappetta CL, Jenkins G, Colasurdo GN. (2005) Effects of RSV infection on pulmonary surfactant protein SP-A in cultured human type II cells: contrasting consequences on SP-A mRNA and protein. Am J Physiol Lung Cell Mol Physiol 289:L1113–L1122 [DOI] [PubMed] [Google Scholar]
- Awasthi S, Coalson JJ, Crouch E, Yang F, King RJ. (1999) Surfactant proteins A and D in premature baboons with chronic lung injury (Bronchopulmonary dysplasia). Evidence for an inhibition of secretion. Am J Respir Crit Care Med 160:942–949 [DOI] [PubMed] [Google Scholar]
- Awasthi S, Coalson JJ, Yoder BA, Crouch E, King RJ. (2001) Deficiencies in lung surfactant proteins A and D are associated with lung infection in very premature neonatal baboons. Am J Respir Crit Care Med 163:389–397 [DOI] [PubMed] [Google Scholar]
- Awasthi S, Cox RA. (2003) Transfection of murine dendritic cell line (JAWS II) by a nonviral transfection reagent. Biotechniques 35:600– 602,, 604 [DOI] [PubMed] [Google Scholar]
- Awasthi S, Cropper J, Brown KM. (2008) Developmental expression of Toll-like receptors-2 and -4 in preterm baboon lung. Dev Comp Immunol 32:1088–1098 [DOI] [PubMed] [Google Scholar]
- Awasthi S, Magee DM, Coalson JJ. (2004) Coccidioides posadasii infection alters the expression of pulmonary surfactant proteins (SP)-A and SP-D. Respir Res 5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Fenton MJ. (2004) Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol 286:L887–L892 [DOI] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. (2000) The Protein Data Bank. Nucleic Acids Res 28:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JS, Huggett JF, Dheda K, Kim LU, Zumla A, Rook GA. (2006) Myobacterium tuberculosis induces selective up-regulation of TLRs in the mononuclear leukocytes of patients with active pulmonary tuberculosis. J Immunol 176:3010–3018 [DOI] [PubMed] [Google Scholar]
- Chroneos ZC, Sever-Chroneos Z, Shepherd VL. (2010) Pulmonary surfactant: an immunological perspective. Cell Physiol Biochem 25:13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell SJ, Page D, Mitchell JC. (2007) An automated decision-tree approach to predicting protein interaction hot spots. Proteins 68:813–823 [DOI] [PubMed] [Google Scholar]
- Gagro A, Tominac M, Krsulović-Hresić V, Baće A, Matić M, Drazenović V, Mlinarić-Galinović G, Kosor E, Gotovac K, Bolanca I, et al. (2004) Increased Toll-like receptor 4 expression in infants with respiratory syncytial virus bronchiolitis. Clin Exp Immunol 135:267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M, McCormack FX, Levine AM. (2009) Surfactant protein A modulates cell surface expression of CR3 on alveolar macrophages and enhances CR3-mediated phagocytosis. J Biol Chem 284:7495–7504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M. (2002) Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol 168:5989–5992 [DOI] [PubMed] [Google Scholar]
- Guillot L, Medjane S, Le-Barillec K, Balloy V, Danel C, Chignard M, Si-Tahar M. (2004) Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J Biol Chem 279:2712–2718 [DOI] [PubMed] [Google Scholar]
- He Z, Zhu Y, Jiang H. (2009) Inhibiting toll-like receptor 4 signaling ameliorates pulmonary fibrosis during acute lung injury induced by lipopolysaccharide: an experimental study. Respir Res 10:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head JF, Mealy TR, McCormack FX, Seaton BA. (2003) Crystal structure of trimeric carbohydrate recognition and neck domains of surfactant protein A. J Biol Chem 278:43254–43260 [DOI] [PubMed] [Google Scholar]
- Henning LN, Azad AK, Parsa KV, Crowther JE, Tridandapani S, Schlesinger LS. (2008) Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol 180:7847–7858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebe K, Jiang Z, Georgel P, Tabeta K, Janssen E, Du X, Beutler B. (2006) TLR signaling pathways: opportunities for activation and blockade in pursuit of therapy. Curr Pharm Des 12:4123–4134 [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38, 27–28 [DOI] [PubMed] [Google Scholar]
- Jain V, Halle A, Halmen KA, Lien E, Charrel-Dennis M, Ram S, Golenbock DT, Visintin A. (2008) Phagocytosis and intracellular killing of MD-2 opsonized gram-negative bacteria depend on TLR4 signaling. Blood 111:4637–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa O, Frevert CW, Lin SM, Goodman RB, Mongovin SM, Wong V, Ballman K, Daubeuf B, Elson G, Martin TR. (2005) Gene expression of Toll-like receptor-2, Toll-like receptor-4, and MD2 is differentially regulated in rabbits with Escherichia coli pneumonia. Gene 344:193–202 [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. (2007) TLR signaling. Semin Immunol 19:24–32 [DOI] [PubMed] [Google Scholar]
- King RJ, Clements JA. (1972) Surface active materials from dog lung. II. Composition and physiological correlations. Am J Physiol 223:715–726 [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Takahashi M, Nishitani C. (2007) Pulmonary collectins in innate immunity of the lung. Cell Microbiol 9:1871–1879 [DOI] [PubMed] [Google Scholar]
- Lv T, Shen X, Shi Y, Song Y. (2009) TLR4 is essential in acute lung injury induced by unresuscitated hemorrhagic shock. J Trauma 66:124–131 [DOI] [PubMed] [Google Scholar]
- Palaniyar N, McCormack FX, Possmayer F, Harauz G. (2000) Three-dimensional structure of rat surfactant protein A trimers in association with phospholipid monolayers. Biochemistry 39:6310–6316 [DOI] [PubMed] [Google Scholar]
- Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195 [DOI] [PubMed] [Google Scholar]
- Pastva AM, Wright JR, Williams KL. (2007) Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc 4:252–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F, Strominger JL. (2002) Monomeric recombinant MD-2 binds toll-like receptor 4 tightly and confers lipopolysaccharide responsiveness. J Biol Chem 277:23427–23432 [DOI] [PubMed] [Google Scholar]
- Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. (2005a) Geometry-based flexible and symmetric protein docking. Proteins 60:224–231 [DOI] [PubMed] [Google Scholar]
- Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ. (2005b) PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res 33:W363–W367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvall M, Steen J, Uhlén M, Hober S, Ottosson J. (2005) High-throughput solubility assay for purified recombinant protein immunogens. Biochim Biophys Acta 1752:6–10 [DOI] [PubMed] [Google Scholar]
- Stevens PA, Wissel H, Sieger D, Meienreis-Sudau V, Rüstow B. (1995) Identification of a new surfactant protein A binding protein at the cell membrane of rat type II pneumocytes. Biochem J 308:77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer DS, Yang S, Jerng HH. (1993) Surfactant protein A-binding proteins. Characterization and structures. J Biol Chem 268:18679–18684 [PubMed] [Google Scholar]
- Tovchigrechko A, Vakser IA. (2006) GRAMM-X public web server for protein-protein docking. Nucleic Acids Res 34:W310–W314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J, Cabrera NE, Casula M, Flores C, Valladares F, Díaz-Flores L, Muros M, Slutsky AS, Kacmarek RM. (2010) Mechanical ventilation modulates TLR4 and IRAK-3 in a non-infectious, ventilator-induced lung injury model. Respir Res 11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissel H, Looman AC, Fritzsche I, Rüstow B, Stevens PA. (1996) SP-A-binding protein BP55 is involved in surfactant endocytosis by type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 271:L432–L440 [DOI] [PubMed] [Google Scholar]
- Yamada C, Sano H, Shimizu T, Mitsuzawa H, Nishitani C, Himi T, Kuroki Y. (2006) Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J Biol Chem 281:21771–21780 [DOI] [PubMed] [Google Scholar]
- Yang CH, Szeliga J, Jordan J, Faske S, Sever-Chroneos Z, Dorsett B, Christian RE, Settlage RE, Shabanowitz J, Hunt DF, et al. (2005) Identification of the surfactant protein A receptor 210 as the unconventional myosin 18A. J Biol Chem 280:34447–34457 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.